Abstract
Background
The effect of bone marrow-derived mononuclear cells (BM-MNCs) after acute myocardial infarction (AMI) on myocardial function indices such as left ventricular ejection fraction has been widely studied. However, the effect of this intervention on major adverse cardiovascular events (MACE) was not the principal purpose of most investigations and its role is unclear. The aim of this study was to investigate the possible long-term clinical efficacy of BM-MNCs on MACE after AMI.
Methods
A comprehensive search was conducted through electronic databases for potentially eligible randomized trials investigating the impact of BM-MNC therapy following acute MI on clinical outcomes. Risk of bias of the eligible studies was assessed using the Cochrane Collaboration’s tool. The effect of treatment was displayed by risk ratio (RR) and its 95% confidence interval (CI) using random-effects model.
Results
Initial database searching found 1540 records and 23 clinical trials with a total of 2286 participants eligible for meta-analysis. Injection of BM-MNCs was associated with lower risk of composite end points of hospitalization for congestive heart failure (CHF), re-infarction, and cardiac-related mortality (91/1191 vs. 111/812, RR = 0.643, 95% CI = 0.489 to 0.845, p = 0.002). This effect was derived from both reduction of CHF (47/1220 vs. 62/841, RR = 0.568, 95% CI = 0.382 to 0.844, p = 0.005) and re-infarction rate (23/1159 vs. 30/775, RR = 0.583, 95% CI = 0.343 to 0.991, p = 0.046), but not cardiac-related mortality (28/1290 vs. 31/871, RR = 0.722, 95% CI = 0.436 to 1.197, p = 0.207).
Conclusion
This is the first meta-analysis focused on the cardiovascular outcomes of stem cell therapy after AMI and it revealed that transplantation of BM-MNCs may reduce composite endpoint of hospitalization for CHF, re-infarction, and cardiac related mortality driven mainly by reducing reinfarction and hospitalization for heart failure rates but not cardiovascular mortality.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-022-02701-x.
Keywords: Stem cell, Myocardial infarction, Heart failure, Bone marrow mononuclear cell
Background
Myocardial infarction (MI) represents the leading cause of mortality worldwide [1]. With a reduction in the rate of mortality due to MIs in recent decades, the incidence of heart failure (HF) has been on the rise [2]. This incidence ranges between 14 and 36% among those hospitalized due to an acute MI (AMI) [3]. HF exerts a considerable effect on healthcare systems in America, accounting for 6 million cases, 300,000 deaths, and roughly 40 billion USD worth of costs every year [4]. Despite the therapeutic efforts [5], post-MI HF still leads to a high rate of morbidity and mortality [6, 7]. Although we have been successful in prolonging the life of HF patients and relieving symptoms, we are yet to regenerate the infarcted cardiac tissues. Hence, a gap exists in the literature as restoring the standard histological architecture of the heart should theoretically lead to improved outcomes for patients with MI-induced HF [6]. This may be possible using stem cell-based therapies [8].
For over two decades, autologous cell-based treatments using bone marrow mononuclear cells (BM-MNC) have been assessed in managing cardiovascular diseases through preclinical and clinical studies. However, phase III trials have been infrequent and most of them have only assessed paraclinical outcomes such as left ventricular ejection fraction (LVEF); also, the infarct size and trials with clinical endpoints are rare.
The BAMI trial was the first phase III trial conducted to clarify whether or not post-MI intracoronary transplantation of BM-MNCs would reduce all-cause mortality [9]. All-cause mortality after two years was 3.26% [n = 6; 95% confidence interval (CI) 1.48–7.12%] with BM-MNCs compared to 3.82% (n = 7; 95% CI 1.84–7.84%) with optimal medical therapy. Importantly, the investigators noticed that only five patients (2.7%, 95% CI 1.0–5.9%) who received BM-MNCs were hospitalized due to HF during the two years of follow-up compared with 15 patients (8.1%, CI 4.7–12.5%) who received optimal medical therapy (HR: 0.33, 95% CI: 0.12–0.88), representing the sole clinical benefit observed. Since the effect of BM-MNC transplantation after AMI on major cardiovascular outcomes is poorly studied and to the best of our knowledge no meta-analysis has focused specifically on this issue, in this meta-analysis, we have investigated the clinical outcomes of patients who had undergone autologous BM-MNC transplantation after AMI.
Methods
This meta-analysis was registered in PROSPERO (CRD42022295741) and it was prepared and reported using the recommendations made by the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [10].
Eligibility criteria
Potential eligible studies were all the randomized controlled trials which performed autologous transplantation of BM-MNCs following a successful coronary angioplasty using stent implantation in patients diagnosed with acute ST-segment elevation MI. Patients undergoing stem cell therapy who were compared with a control group of acute MI patients receiving standard therapy with or without intracoronary injection of placebo were considered for inclusion. Studies were excluded if they did not include a control arm, were not randomized, had less than 6 months of follow-up, used any other stem cells than bone-marrow mononuclear cells as the stem cell therapy, and did not compare long term adverse clinical events including hospitalization due to CHF, recurrent MI, and composite endpoints (cardiac death, CHF, and MI) between the intervention and control groups. The primary outcomes were major adverse cardiovascular events (MACE) including rehospitalization for CHF, recurrence of MI, cardiac-related death, and the composite endpoints separately. The clinical outcomes were assessed at the longest available follow up (at least 6 months). Comparisons of the left ventricular function indices including LVEF, left ventricular end-diastolic volume (LVEDV), and left ventricular end-systolic volume (LVESV) between 3 and 12 months after stem cell therapy in the intervention group and the control arm were listed as secondary outcomes of interest.
Search strategy for identification of eligible studies
We conducted a comprehensive search through PubMed, Embase, and Cochrane Central Register of Controlled Trials which were last performed on 5 April 2021 by using one or a combination of keywords including “myocardial infarction”, “coronary artery disease”, “stem cell”, “mononuclear cell”, “bone marrow”, and “heart failure”. Articles in English with no further restriction in sample size or time frame were screened for eligibility. Bibliographies were screened to find any other relevant studies. All the abstracts and titles of the identified studies were screened by two independent reviewers (AA and AH), and the full texts of the possible eligible ones were considered suitable for meta-analysis if they met the inclusion criteria. In any case of discrepancy, disagreements were resolved by discussion with a third investigator (HH).
Data extraction
A reviewer (AH) independently collected the study information including trial characteristics (authors, trial name, and year of publication), sample size of the intervention and control groups, information regarding the features of intervention (stem cell injection time, dose of injection, route of injection), primary outcomes of both control and intervention arms (rehospitalization due to CHF, reinfarction, cardiac-related mortality, and composite of hospitalization, MI and cardiac death either stated in the study or calculated by the reviewer), and characteristics of secondary outcomes (LVEF, LVEDV, LVESV, change in the mentioned markers over the follow up period, and the modality used for measurement of left ventricular (LV) indices. If the change in the mentioned markers was measured for multiple times over the follow up period, values at 6 months of follow up were extracted for analyses. Then, a second investigator (AA) evaluated the accuracy and consistency of the extracted data. Disagreements were solved by discussion between the authors.
Risk of bias and quality appraisal
The quality of the selected studies was assessed by two authors (AA and AH) independently, using the Cochrane Collaboration’s tool for assessing risk of bias in randomized trials [11]. We evaluated the studies for selection, performance, detection, attrition, and reporting bias with Review Manager (RevMan 5.1.7) Software and rated the status of bias as low, unclear, or high risk. If there were any disagreements, the authors resolved them by discussion.
Statistical analysis
The extracted data from the enrolled eligible studies were entered to a pre-designed Microsoft Excel spreadsheet and all the analyses were conducted using Stata software version 13 (StataCorp LP, College Station, TX, USA). For the primary endpoints (hospitalization for CHF, myocardial reinfarction, cardiac-related mortality, and composite endpoints), we reported the risk ratio (RR) and its 95% confidence interval (CI) as the treatment effect. Also, subgroup analyses for the primary endpoints were made according to the time of stem cell injection (early group defined as patients receiving stem cell < 11 days and late group ≥ 11 days) and dosage of therapy (≥ 108: high dose and < 108: low dose) to study the effect of time and dose. Moreover, we expressed continuous data for secondary endpoints as weighted mean difference (WMD) and 95% CI. The publication bias was assessed using funnel plots and Egger’s test. The I2 values were calculated for measuring the amount of heterogeneity. Random-effects model was used for all the analyses. The studies were different regarding the time and dosing of BM-MNCs transplantation. Also, some studies divided the intervention group into subgroups with different times of injection (early or late) and injection dose (high dose and low dose). Since timing and dosage of stem cell therapy could impact the clinical outcomes of the participants, we grouped the studies according to the time of injection (≥ 11 days after revascularization as the late group or < 11 days defined as the early group) and dosage of stem cell therapy (high dose group was defined as a median or mean of ≥ 108 BM-MNC injected and lower number of cells was considered as low dose group) and analyzed the primary outcomes of interest between the subgroups. Furthermore, different modalities including echocardiography, LV angiography, cardiac magnetic resonance (CMR) imaging, and Single-photon emission computed tomography (SPECT) were used for measurement of LV indices among the studies. Thus, in addition to the main analysis, we compared the LV indices in subgroups for each modality. Random-effects meta-regression was performed to explore the potential linear associations between baseline characteristics (age and gender) and primary outcomes of interest.
Results
Literature search and study characteristics
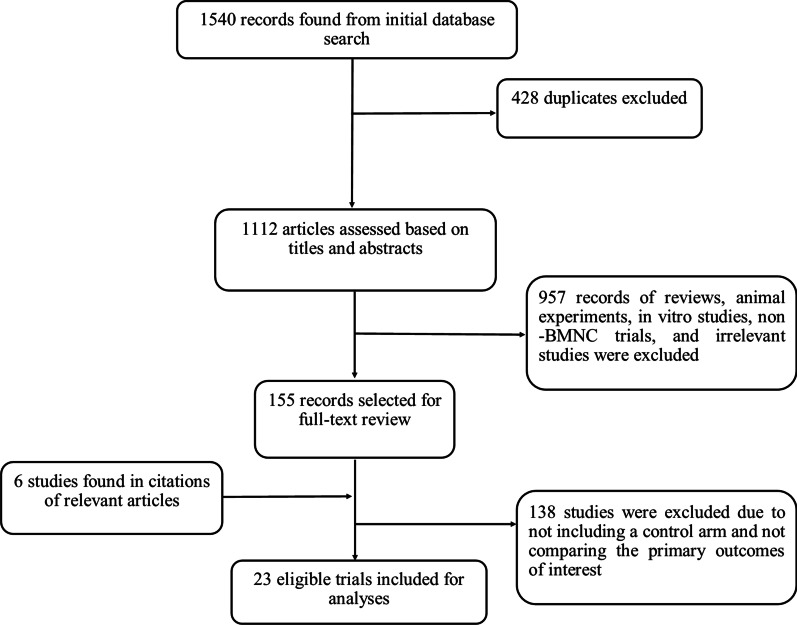
We identified a total of 1540 records through electronic searches of PubMed, Embase, and Cochrane database. After removal of the duplicates, titles and abstracts of 1112 records were screened for potential eligibility. At this stage, 957 records were excluded (letters, reviews, in vitro and animal studies, and irrelevant topics), and full texts of 155 articles were selected for screening. After detailed assessment of the potentially eligible studies, 23 trials met all the inclusion criteria and were considered eligible for the meta-analyses (Fig. 1) [9, 12–33]. The eligible studies included a total of 2286 participants (1402 receiving BM-MNC therapy and 884 in the placebo group). The most frequent comorbidities of the participants included hypertension, hyperlipidemia, and diabetes. No inclusion or exclusion criteria was set for pre-existing conditions of the patients. The injection time of BM-MNCs ranged from 24 h to 3 months after AMI. All the trials measured the primary outcomes of interest of this review (CHF needing hospitalization, reinfarction, and mortality), and their follow up period ranged from 6 to 60 months. For the secondary outcomes (LV function indices), the follow ups ranged from 3 to 12 months. Six studies divided the patients receiving BM-MNCs into different subgroups: one trial giving BM-MNCs based on normoxia or hypoxia-preconditioning of the stem cells [14], two studies based on the time of injection [15, 22], one based on the dosage (low dose and high dose group) [18], one according to the type of mononuclear cell [29], and one based on the dosage and the status of radiation given to the cells [30]. In all the included trials, the route of injection was intracoronary. Characteristics of the trials are summarized in Table 1.
Fig. 1.
Flow diagram of the eligible studies included in the meta-analysis
Table 1.
Characteristics of the included studies
| Study | Trial Name | Country | Sample size | Mean age | Male (%) | Baseline LVEF | Injection time interval(d) | Modality | F/U for clinical events(m) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BM-MNC | Control | BM-MNC | Control | BM-MNC | Control | BM-MNC | Control | ||||||
| Meyer et al. [27] | BOOST | Germany | 30 | 30 | 53.4 ± 14.8 | 59.2 ± 13.5 | 67 | 73 | 50 ± 10 | 51.3 ± 9.3 | 4.8 ± 1.3 | CMR | 61 |
| Assmus et al. [12] | REAPIR-AMI | Germany | 101 | 103 | 55 ± 11 | 57 ± 11 | 82 | 82 | 47.5 ± 10 | 46.7 ± 10.3 | 4.4 ± 1.3 | LV angiography | 60 |
| Beitnes et al. [32] | ASTAMI | Norway | 50 | 50 | 58.1 ± 8.5 | 56.7 ± 9.6 | 84 | 84 | 45.7 ± 9.4 | 46.9 ± 9.6 | 4–8 | Echo/CMR | 36 |
| Benedek et al. [33] | - | Romania | 9 | 9 | 53.55 ± 15.08 | 61 ± 10.06 | 77.77 | 55.55 | 41.66 ± 3.5 | 39.7 ± 3 | 21–90 | Echo | 48 |
| Delewi et al. [13] | HEBE | Netherlands | 69 | 65 | 56 ± 9 | 55 ± 10 | 84 | 86 | 43.7 ± 9 | 42.4 ± 8.3 | 8 | CMR | 60 |
| Hu et al. [14] | CHINA-AMI | China | 22 | 14 | 60.45 ± 11.4 | 60.62 ± 10.85 | 86.5 | 64 | 53.8 ± 11.5 | 57.1 ± 11.6 | 5 | Echo/SPECT | 12 |
| Huang et al. [15] | - | China | 79 | 25 | 58.55 ± 8.72 | 58.8 ± 8.4 | 91 | 88 | 43.65 ± 5.21 | 43.5 ± 3.5 | 1–30 | Echo/SPECT | 12 |
| Huikuri et al. [16] | FINCELL | Finland | 40 | 40 | 60 ± 10 | 59 ± 10 | 90 | 85 | 59 ± 11 | 62 ± 12 | 2–6 | LV angiography/echo | 6 |
| Lamriault et al. [17] | BONAMI | France | 59 | 42 | 56 ± 12 | 55 ± 11 | 80.8 | 89.8 | 38.1 ± 7.9 | 39.8 ± 7 | 9.3 ± 1.7 | Echo | 12 |
| Mathur et al. [9] | BAMI | UK | 185 | 190 | 59 ± 11 | 60 ± 11 | 83.78 | 77.37 | 39 ± 5 | 39 ± 5 | 2–8 | Echo | 24 |
| Meluzin et al. [18] | - | Czech | 40 | 20 | 54 ± 2 | 55 ± 2 | 92.5 | 90 | 40.5 ± 8.94 | 40 ± 8.94 | 3–8 | Echo/SPECT | 12 |
| Plewka et al. [19] | - | Poland | 40 | 20 | 56 ± 9 | 56 ± 9 | 67 | 75 | 35 ± 6 | 33 ± 7 | 7 | Echo | 24 |
| San Roman et al. [20] | TECAM | Spain | 30 | 31 | 54 ± 11 | 57 ± 11 | 97 | 90 | 49 ± 8 | 47 ± 8 | 3–5 | CMR/LV angiography | 12 |
| Skalicka et al. [21] | - | Czech | 17 | 10 | 61 ± 14 | 54 ± 10 | 71 | 100 | 39.2 ± 9.2 | 39.4 ± 5.6 | 4–11 | Echo | 24 |
| Sürder et al. [22] | - | Switzerland | 133 | 67 | 58.53 ± 14.77 | 56 ± 14.5 | 84 | 83.6 | 36.4 ± 8.9 | 40 ± 9.9 | 5–28 | CMR | 12 |
| Traverse et al. [23] | TIME (phase I) | USA | 30 | 10 | 52.5 ± 15.56 | 57.5 ± 3.7 | 83 | 60 | 49 ± 9.5 | 48.6 ± 8.5 | 3–10 | CMR/Echo | 6 |
| Traverse et al. [24] | LateTIME | USA | 58 | 29 | 57.6 ± 11 | 54.6 ± 11 | 79 | 90 | 48.7 ± 12 | 45.3 ± 9.9 | 14–21 | CMR | 6 |
| Traverse et al. [25] | TIME | USA | 58 | 27 | 55.9 ± 11 | 56.4 ± 10.4 | 88 | 86 | 45.9 ± 9.4 | 46.9 ± 8.7 | 3–7 | CMR | 24 |
| Wöhrle et al. [26] | - | Germany | 29 | 13 | 61 ± 8.1 | 61.1 ± 9.3 | 90 | 62 | 53.5 ± 9.3 | 55.7 ± 9.4 | 5–7 | CMR | 6 |
| Piepoli et al. [28] | Cardiac study | Italy | 19 | 19 | 63.1 ± 2.4 | 67 ± 2.7 | 68.4 | 68.4 | 38.9 ± 1.3 | 38.4 ± 1.5 | 4–7 | Echo/SPECT | 12 |
| Tendera et al. [29] | REGENT | Poland | 160 | 40 | 56.5 ± 29.98 | 59 ± 26.67 | 67 | 75 | 36 ± 21.2 | 39 ± 15.56 | 3–12 | CMR | 6 |
| Wollert et al. [30] | BOOST-2 | Germany | 127 | 26 | 55.46 ± 9.83 | 55 ± 9 | 85 | 92 | 44.3 ± 8.48 | 47.8 ± 6.7 | 7.1 ± 2.6 | CMR | 6 |
| Yao et al. [31] | - | China | 27 | 12 | 51.7 ± 6.4 | 52.7 ± 7.8 | 81 | 92 | 33.2 ± 3.9 | 32.3 ± 2 | 3d-3 m | CMR | 12 |
Risk of bias in individual studies
The quality assessment of the enrolled studies was performed, as illustrated in Additional file 1: Fig. S1. Sixteen trials [9, 12–14, 16, 17, 20, 22–25, 27, 28, 30–32] reported their method for random sequence generation and seven trials [15, 18, 19, 21, 26, 29, 33] did not mention a clear method of randomization. Also, eleven trials were at low risk for proper statement of allocation concealment [9, 12, 14–16, 18, 23–25, 31, 32]. Eight trials did not perform blinding of either participants or personnel [9, 13, 15, 17, 20–22, 29] and seven were unknown regarding the blinding process [18, 19, 27, 28, 31–33]. Masking was not done or was unclear for outcome assessors of four trials [15, 19, 29, 33]. Six studies [15, 19–22, 30] were high risk for attrition bias and two trials [18, 28] were at unclear risk. Out of all the included trials, only three [18, 26, 31] were unclear regarding selective reporting of outcomes. Also, we evaluated the possibility of bias by assessing funnel plots and Egger’s test. For the primary outcomes of interest (rehospitalization for CHF and composite endpoints), p-value did not reach a significant level (p = 0.082 and p = 0.120, respectively), and funnel plots showed symmetrical distribution (Additional file 1: Figs. S2, S3).
Hospitalization for heart failure
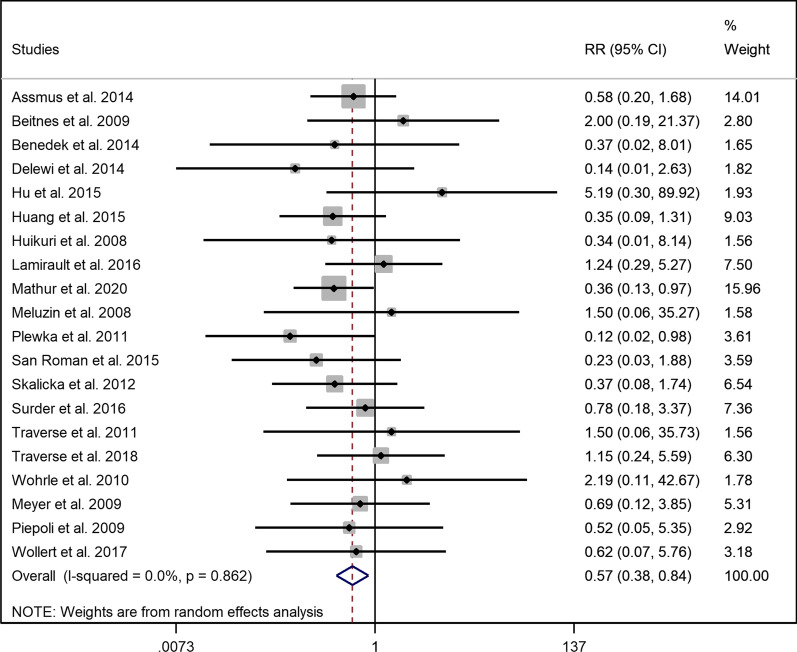
Twenty trials reported the number of cases needing rehospitalization due to CHF in both intervention and placebo groups during their follow-up (Duration of follow-up period ranged from 6 to 61 months). Overall, there was a significantly lower risk of hospitalization for CHF in the intervention group compared to the control group which received placebo (RR = 0.568, 95% CI = 0.382 to 0.844, p = 0.005, I2 = 0.00%) (Fig. 2). Subgroup analysis showed that early injection of BM-MNCs could lower the risk of hospitalization (RR 0.539, 95% CI = 0.354 to 0.819, p = 0.004, I2 = 0.00%), whereas there was no significant difference of hospitalization compared to the control group in the intervention group with late injection of BM-MNCs (RR = 0.810, 95% CI = 0.298 to 2.198, p = 0.678, I2 = 0.00%) (Additional file 1: Fig. S4). Also, there was no evidence for a difference in hospitalization in the low dose group (RR = 0.998, 95% CI = 0.364 to 2.735, p = 0.997, I2 = 0.00%) contrary to high dose group; the risk of hospitalization was significantly lower (RR = 0.518, 95% CI = 0.337 to 0.798, p = 0.003, I2 = 0.00%) (Additional file 1: Fig. S5).
Fig. 2.
Forest plot demonstrating relative risk of hospitalization for CHF compared between the intervention and control groups (RR: Risk ratio)
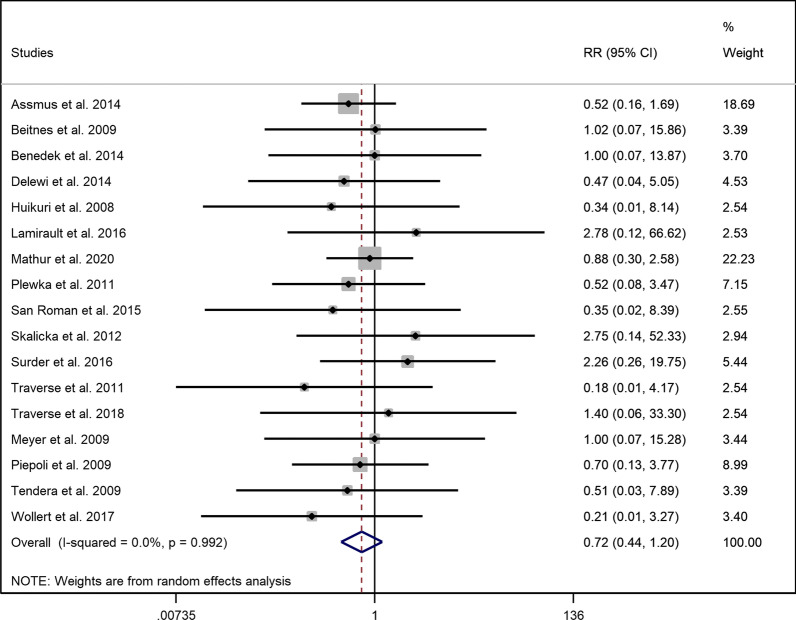
Myocardial reinfarction
Incidence of myocardial reinfarction was reported in eighteen enrolled studies. Two trials stated no recurrence of MI in their study [14, 33]. Similar to hospitalization for CHF, there was a significant difference regarding the occurrence of reinfarction between the intervention and placebo group (RR = 0.583, 95% CI = 0.343 to 0.991 p = 0.046) with no evidence of heterogeneity (I2 = 0.00%) (Fig. 3). Subgroup analysis of early and late injection of the stem cells revealed that both results of early and late injection were not different compared to the control group (early: RR = 0.585, 95% CI = 0.339 to 1.008, p = 0.054, I2 = 0.00% and late: RR = 0.555, 95% CI = 0.113 to 2.741, p = 0.470, I2 = 0.00% (Additional file 1: Fig. S6)). Moreover, there was evidence for a difference in the risk of MI in the group with high dose of injection in contrast to the group with low dose of injection (High dose: RR = 0.566, 95% CI = 0.326 to 0.984, p = 0.044, I2 = 0.00% and low dose: RR = 1.309, 95% CI = 0.149 to 11.490, p = 0.808, I2 = 25.00% (Additional file 1: Fig. S7)).
Fig. 3.
Forest plot demonstrating relative risk of myocardial reinfarction between the intervention and control group (RR: Risk ratio)
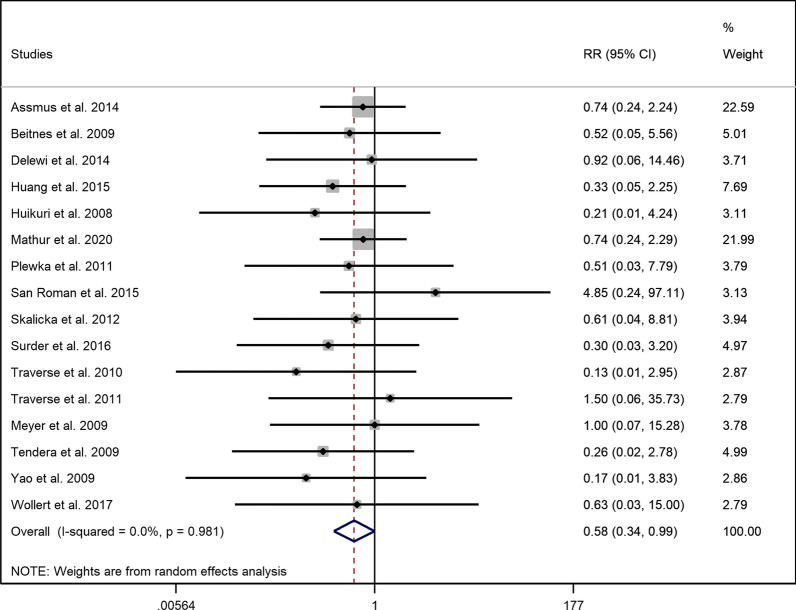
Cardiac-related mortality
Twenty-one studies stated the number of cardiac-related mortality in their trials although in some studies the incidence of cardiac-related and all-cause mortality was not differentiated. The incidence of cardiac death appeared to be not significantly different between the two study groups (RR = 0.722, 95% CI = 0.436 to 1.197, p = 0.207, I2 = 0.00%) (Fig. 4). Subgroup analysis of cardiac death in both early and late injection also remained insignificant with no evidence of heterogeneity (I2 = 0.00%) (Early: RR = 0.750, 95% CI = 0.444 to 1.265, p = 0.280 and late: RR = 0.693, 95% CI = 0.136 to 3.533, p = 0.659 (Additional file 1: Fig. S8)). Similarly, no difference was found regarding the risk of cardiac death in the high and low dose group compared to controls (High dose: RR = 0.701, 95% CI = 0.413 to 1.189, p = 0.187 and low dose: RR = 1.001, 95% CI = 0.176 to 5.679, p = 0.999 (Additional file 1: Fig. S9) (I2 = 0.00% in both analyses)).
Fig. 4.
Forest plot demonstrating relative risk of cardiac-related mortality between the intervention and control group (RR: Risk ratio)
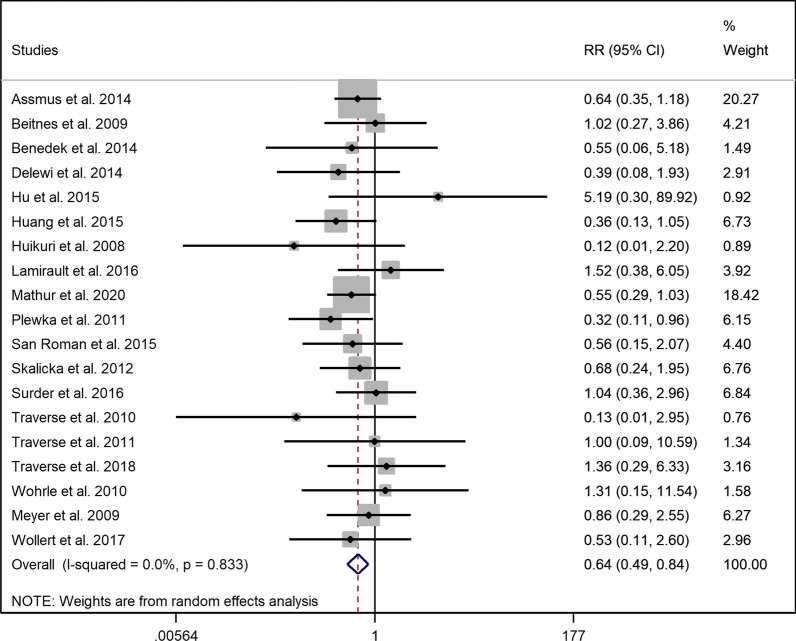
Composite endpoints (hospitalization for heart failure, myocardial reinfarction, and cardiac-related mortality)
As defined before, the composite endpoints could be calculated in nineteen trials. There was evidence that autologous injection of BM-MNCs could lower the risk of composite endpoints in the intervention group when compared to the control arm (RR = 0.643, 95% CI = 0.489 to 0.845, p = 0.002), and there was no evidence of heterogeneity (I2 = 0.00%) (Fig. 5). Patients who had received BM-MNCs earlier than 11 days (early group) had a lower risk of composite endpoint (RR = 0.636, 95% CI = 0.479 to 0.845, p = 0.002, I2 = 0.00%), whereas there was no evidence of lower risk of composite endpoints in the late group who received stem cell therapy compared to standard therapy (RR = 0.748, 95% CI = 0.340 to 1.644, p = 0.470, I2 = 0.00%) (Additional file 1: Fig. S10). Furthermore, patients who received high doses of mononuclear cells were at lower risks of composite end points (RR = 0.609, 95% CI = 0.455 to 0.816, p = 0.001) although those in the low dose group were not significantly different compared to the controls (RR = 0.936, 95% CI = 0.432 to 2.028, p = 0.868) (Additional file 1: Fig. S11), both with no evidence of heterogeneity (I2 = 0.00%).
Fig. 5.
Forest plot demonstrating relative risk of composite endpoints between intervention and control group (RR: Risk ratio)
Left ventricular ejection fraction
Twenty-one studies measured the change in LVEF in the follow-up period (ranging from 3 to 12 months). LVEF improved significantly in patients in the intervention group compared to the control arm (WMD = 1.695%, 95% CI = 0.681 to 2.710, p = 0.001) and high level of heterogeneity (I2 = 65.1%) (Additional file 1: Fig. S12). Publication bias was not significant according to both Begg’s test (p = 0.880) and Egger’s test (p = 0.208).
Echocardiography
Ten trials used echocardiography for measuring LVEF [14–17, 19, 21, 23, 28, 32, 33] and three of them measured the change of LVEF from baseline in the follow up [14, 15, 34] (We used the data from a serial publication for one of the trials (Beitnes et al. [32]) for change of LVEF). An improvement in LVEF associated with cell therapy was found (WMD = 1.550%, 95% CI = 0.408 to 2.692, p = 0.008, I2 = 37.6%).
CMR
CMR imaging was used for measurement of LV markers in 13 studies [13, 20, 22–26, 29–32]. After exclusion of the studies with low correlation with others, pooled analysis showed no significant difference in LVEF associated with stem cell therapy (WMD = 0.981%, 95% CI = -0.966 to 2.929, p = 0.323, I2 = 76.3%).
LV angiography
Analysis of change in the LVEF of the studies using LV angiography [12, 16, 20] showed evidence of improvement in LVEF linked with BM-MNCs injection (WMD = 3.192%, 95% CI = 0.509 to 5.874, p = 0.020, I2 = 23.0%).
SPECT
Two studies used SPECT for measuring LVEF values in baseline and follow-ups [18, 28], and the observed change was found to be not significant (WMD = 3.036%, 95% CI = − 1.285 to 7.357, p = 0.168, I2 = 18.6%) (Additional file 1: Fig. S13).
Other echocardiographic parameters
There was not a significant correlation between stem cell therapy and improvement in LVEDV (WMD = -2.940, 95% CI = − 6.505 to 0.625, p = 0.106, I2 = 54.1%) (Additional file 1: Figs. S14, S15) and low possibility of publication bias according to Egger’s test (p = 0.211). Moreover, there was an association between stem cell therapy and changes in LVESV (WMD = − 2.376, 95% CI = − 3.534 to − 1.218, p < 0.001, I2 = 0.00%) (Additional file 1: Fig. S16). When we sequentially removed each study from the main analysis, we observed that summary WMD changed after excluding the Yao et al. study [31] (WMD = − 4.146, 95% CI = − 6.348 to − 1.944, p < 0.001, I2 = 0.00%) (Additional file 1: Fig. S17). The subgroup analysis of LVESV for each imaging modality is also presented in Additional file 1: Fig. S18.
Meta-regression analysis
Meta-regression analyses were performed between primary outcomes (hospitalization for HF, MI recurrence, mortality, and composite end-points) and age and gender. No association was found between age and long-term clinical efficacy of BM-MNC therapy (Hospitalization: p = 0.83, Recurrence of MI: p = 0.91, Mortality: p = 0.69, Composite end-points: p = 0.97) (Additional file 1: Fig. S19). Also, there was no statistically significant trend for primary end-points and gender (Hospitalization: p = 0.71, Recurrence of MI: p = 0.70, Mortality: p = 0.93, Composite end-points: p = 0.85) (Supplementary Fig. 20).
Discussion
In this study, we have shown that transplantation of BM-MNCs after AMI improves both myocardial performance indices, such as LVEF and cardiovascular outcomes, mainly by reducing the rehospitalization rate for CHF and reinfarction rates. This treatment has not been shown to have an effect on reduction of cardiovascular death. To the best of our knowledge, this is the only meta-analysis in the field which has focused on the effect of cell therapy on MACE.
In the last two decades, many trials have been conducted to acquire a better understanding about the possible effects of stem cells transplantation on myocardial performance indices such as LVEF and scar size. However, studies focusing on clinical outcomes are rare. The BAMI trial was the first phase III trial conducted to clarify whether post-MI intracoronary transplantation of BM-MNCs would reduce all-cause mortality or not. Although the trial was designed to involve 3000 patients, it was stopped prematurely due to futility after the enrollment of 375 patients. Among them, 185 received BM-MNCs (intracoronary infusion) 2–8 days following primary percutaneous coronary intervention (PPCI), and the remaining 190 patients received optimal medical therapy as the control group. All-cause mortality after two years was 3.26% [n = 6; 95% confidence interval (CI): 1.48–7.12%] with BM-MNCs compared to 3.82% (n = 7; 95% CI: 1.84–7.84%) with optimal medical therapy [9]. The main reason behind such results was a significant reduction in post-AMI mortality. At the start of the project in 2011, the literature held that following an AMI, the mortality rate from all causes after two years would be approximately 12% among those with an LVEF ≤ 45% post-reperfusion therapy [3]. However, the researchers noticed a 3.85% mortality rate while conducting the study, reflecting the evolution of primary angioplasty procedures in those years. Our findings in this meta-analysis confirm the BAMI findings.
Post-MI heart failure appears to be a strong predictor of mortality [35]. Thus, this meta-analysis aimed to explore the potential impact of BM-MNC therapy on clinical outcomes including hospitalization for CHF. We found that transplantation of the mononuclear cells following reperfusion therapy in the setting of acute MI could significantly decrease the risk of rehospitalization due to decompensated heart failure. It should be noted that the subgroup analyses revealed that high dose (≥ 108 cells) and early injection (< 11 days) of the stem cells lowered the risk of hospitalization, whereas there was no evidence of association between low dose and late injection of BM-MNCs and lower risk of hospitalization. In to the same line with our findings, BAMI investigators noticed that only five patients (2.7%, 95% CI 1.0–5.9%) who received BM-MNCs were hospitalized due to HF during the two years of follow-up compared with 15 patients (8.1%, CI 4.7–12.5%) who received optimal medical therapy (HR: 0.33, 95% CI: 0.12–0.88), representing the sole clinical benefit observed. Results from our meta-analysis and BAMI showed that taking mortality as an endpoint for stem cell therapy trials was futile, and the best clinical endpoint to assess was HF incidence.
Our results also demonstrated that injection of the mononuclear cells could cause favorable effects on LV function indices including LVEF and LVESV although there was no statistical improvement in LVEDV in the BM-MNC group when compared to the placebo group. Results regarding the effect of BM-MNC transplantation on LVEF are controversial. These controversies are mainly derived from the different protocols used in these studies. The number of the cells transplanted, route of delivery, transplantation time from AMI, age, baseline LVEF, and the method used for measuring LVEF are all affecting these outcomes. In general, most meta-analyses have shown at least a modest effect on LVEF. In a Cochrane meta-analysis, it was shown that BM-MNCs could achieve a 2.72% improvement in LVEF [36]. In a patient level data meta-analysis, it was shown that this effect might be improved in younger patients (< 55 years) and those with lower values of LVEF in the time of admission (LVEF < 40) [37]. Meanwhile, in some trials, BM-MNC therapy failed to improve the LV function including LVEF, regional LV function, and wall motion in the infarct zone [24, 25]. Although the effect of BM-MNC infusion on LVEF seems to be small, it should be noted that other treatment modalities such as beta blocker therapy or direct revascularization also have a relatively small influence on LVEF improvement [37]. Thus, a more important question would be the long-term effects of this treatment on clinical outcomes and that is where our study has focused on.
Limitations
There were some limitations to our analysis that should be taken into account. As with any meta-analysis, limitations to the method include heterogeneity across trials. In particular, there are differences in terms of treatment characteristics including the cell dosage used, cell isolation protocols, storage methods, and image modalities. Furthermore, the primary outcome of many studies was LVEF, and these studies were not designed specifically to monitor major cardiovascular events.
Conclusion
In conclusion, injection of BM-MNC in patients with acute MI may contribute to lower risk of long-term hospitalization for CHF and recurrence of MI, especially when administered in high doses and shortly after the reperfusion therapy. However, despite a lower numerical rate of cardiovascular mortality this treatment does not reach statistical significance. BM-MNC therapy could also result in significant improvements in LV function indices including LVEF and LVESV in the follow up period compared to the patients receiving standard therapy. The results of this meta-analysis showed that transplantation of BM-MNCs can have a substantial effect on clinical and paraclinical outcomes.
Supplementary Information
Additional file 1: Supplementary material.
Acknowledgements
This article is extracted from Alireza Hosseinpour’s thesis for his medical doctorate diploma and has been registered with No. 23505 in the records of the research deputy of Shiraz University of Medical Sciences. The authors would like to thank Center for Development of Clinical Research of Nemazee Hospital and Dr. Nasrin Shokrpour for editorial assistance.
Abbreviations
- MI
Myocardial infarction
- AMI
Acute myocardial infarction
- HF
Heart failure
- CHF
Congestive heart failure
- BM-MNC
Bone marrow mononuclear cell
- LVEF
Left ventricular ejection fraction
- LVEDV
Left ventricular end-diastolic volume
- LVESV
Left ventricular end-systolic volume
- RR
Risk ratio
- CI
Confidence interval
- WMD
Weighted mean difference
- MACE
Major adverse cardiovascular events
- CMR
Cardiac magnetic resonance
- SPECT
Single-photon emission computed tomography
- PPCI
Primary percutaneous coronary intervention
Author contributions
AA contributed to concept and design of the study. AK assisted in statistical analysis and administrative support. The first draft of the manuscript was written by AH. AA, AH, and HH contributed to manuscript writing and preparing the final version of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by a grant number 23505 from vice chancellor of research from Shiraz University of Medical Sciences.
Availability of data and materials
The data underlying this article will be shared on reasonable request to the corresponding author.
Declarations
Ethics approval and consent to participate
The study protocol has been approved by local ethical committee with an ID of IR.SUMS.MED.REC.1400.225.
Consent for publication
Not applicable.
Competing interests
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Armin Attar, Email: attarar@sums.ac.ir, Email: attar_armin@yahoo.com.
Alireza Hosseinpour, Email: alireza.hosseinpour1997@gmail.com.
References
- 1.Organization WH. The global burden of disease: 2004 update. Geneva: World Health Organization; 2008. [Google Scholar]
- 2.Velagaleti RS, Pencina MJ, Murabito JM, Wang TJ, Parikh NI, D'Agostino RB, et al. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118(20):2057–2062. doi: 10.1161/CIRCULATIONAHA.108.784215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hellermann JP, Jacobsen SJ, Gersh BJ, Rodeheffer RJ, Reeder GS. Heart failure after myocardial infarction: a review. Am J Med. 2002;113(4):324–330. doi: 10.1016/S0002-9343(02)01185-3. [DOI] [PubMed] [Google Scholar]
- 4.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics–2012 update: a report from the American heart association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e318245fac5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Colvin MM, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 accf/aha guideline for the management of heart failure: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. Circulation. 2016;134(13):e282–e293. doi: 10.1161/CIR.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 6.Juillière Y, Cambou JP, Bataille V, Mulak G, Galinier M, Gibelin P, et al. Heart failure in acute myocardial infarction: a comparison between patients with or without heart failure criteria from the FAST-MI registry. Revista espanola de cardiologia. 2012;65(4):326–333. doi: 10.1016/j.recesp.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 7.Lewis EF, Moye LA, Rouleau JL, Sacks FM, Arnold JM, Warnica JW, et al. Predictors of late development of heart failure in stable survivors of myocardial infarction: the CARE study. J Am Coll Cardiol. 2003;42(8):1446–1453. doi: 10.1016/S0735-1097(03)01057-X. [DOI] [PubMed] [Google Scholar]
- 8.Braunwald E. Cell-based therapy in cardiac regeneration: an overview. Circ Res. 2018;123(2):132–137. doi: 10.1161/CIRCRESAHA.118.313484. [DOI] [PubMed] [Google Scholar]
- 9.Mathur A, Fernández-Avilés F, Bartunek J, Belmans A, Crea F, Dowlut S, et al. The effect of intracoronary infusion of bone marrow-derived mononuclear cells on all-cause mortality in acute myocardial infarction: the BAMI trial. Eur Heart J. 2020;41(38):3702–3710. doi: 10.1093/eurheartj/ehaa651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assmus B, Leistner DM, Schächinger V, Erbs S, Elsässer A, Haberbosch W, et al. Long-term clinical outcome after intracoronary application of bone marrow-derived mononuclear cells for acute myocardial infarction: migratory capacity of administered cells determines event-free survival. Eur Heart J. 2014;35(19):1275–1283. doi: 10.1093/eurheartj/ehu062. [DOI] [PubMed] [Google Scholar]
- 13.Delewi R, van der Laan AM, Robbers LF, Hirsch A, Nijveldt R, van der Vleuten PA, et al. Long term outcome after mononuclear bone marrow or peripheral blood cells infusion after myocardial infarction. Heart. 2015;101(5):363–368. doi: 10.1136/heartjnl-2014-305892. [DOI] [PubMed] [Google Scholar]
- 14.Hu X, Huang X, Yang Q, Wang L, Sun J, Zhan H, et al. Safety and efficacy of intracoronary hypoxia-preconditioned bone marrow mononuclear cell administration for acute myocardial infarction patients: the CHINA-AMI randomized controlled trial. Int J Cardiol. 2015;184:446–451. doi: 10.1016/j.ijcard.2015.02.084. [DOI] [PubMed] [Google Scholar]
- 15.Huang R, Yao K, Sun A, Qian J, Ge L, Zhang Y, et al. Timing for intracoronary administration of bone marrow mononuclear cells after acute ST-elevation myocardial infarction: a pilot study. Stem Cell Res Ther. 2015;6(1):112. doi: 10.1186/s13287-015-0102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huikuri HV, Kervinen K, Niemelä M, Ylitalo K, Säily M, Koistinen P, et al. Effects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarction. Eur Heart J. 2008;29(22):2723–2732. doi: 10.1093/eurheartj/ehn436. [DOI] [PubMed] [Google Scholar]
- 17.Lamirault G, de Bock E, Sébille V, Delasalle B, Roncalli J, Susen S, et al. Sustained quality of life improvement after intracoronary injection of autologous bone marrow cells in the setting of acute myocardial infarction: results from the BONAMI trial. Qual Life Res Int J Qual Life Asp Treat Care Rehabilit. 2017;26(1):121–125. doi: 10.1007/s11136-016-1366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meluzín J, Janousek S, Mayer J, Groch L, Hornácek I, Hlinomaz O, et al. Three-, 6-, and 12-month results of autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction. Int J Cardiol. 2008;128(2):185–192. doi: 10.1016/j.ijcard.2007.04.098. [DOI] [PubMed] [Google Scholar]
- 19.Plewka M, Krzemińska-Pakuła M, Peruga JZ, Lipiec P, Kurpesa M, Wierzbowska-Drabik K, et al. The effects of intracoronary delivery of mononuclear bone marrow cells in patients with myocardial infarction: a two year follow-up results. Kardiol Pol. 2011;69(12):1234–1240. [PubMed] [Google Scholar]
- 20.San Roman JA, Sánchez PL, Villa A, Sanz-Ruiz R, Fernandez-Santos ME, Gimeno F, et al. Comparison of different bone marrow-derived stem cell approaches in Reperfused STEMI. A multicenter, prospective, randomized, open-labeled TECAM Trial. J Am Coll Cardiol. 2015;65(22):2372–82. doi: 10.1016/j.jacc.2015.03.563. [DOI] [PubMed] [Google Scholar]
- 21.Skalicka H, Horak J, Kobylka P, Palecek T, Linhart A, Aschermann M. Intracoronary injection of autologous bone marrow-derived mononuclear cells in patients with large anterior acute myocardial infarction and left ventricular dysfunction: a 24- month follow up study. Bratisl Lek Listy. 2012;113(4):220–227. doi: 10.4149/bll_2012_051. [DOI] [PubMed] [Google Scholar]
- 22.Sürder D, Manka R, Moccetti T, Lo Cicero V, Emmert MY, Klersy C, et al. Effect of bone marrow-derived mononuclear cell treatment, early or late after acute myocardial infarction: twelve months CMR and long-term clinical results. Circ Res. 2016;119(3):481–490. doi: 10.1161/CIRCRESAHA.116.308639. [DOI] [PubMed] [Google Scholar]
- 23.Traverse JH, McKenna DH, Harvey K, Jorgenso BC, Olson RE, Bostrom N, et al. Results of a phase 1, randomized, double-blind, placebo-controlled trial of bone marrow mononuclear stem cell administration in patients following ST-elevation myocardial infarction. Am Heart J. 2010;160(3):428–434. doi: 10.1016/j.ahj.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA. 2011;306(19):2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Traverse JH, Henry TD, Pepine CJ, Willerson JT, Chugh A, Yang PC, et al. TIME trial: effect of timing of stem cell delivery following ST-elevation myocardial infarction on the recovery of global and regional left ventricular function: final 2-year analysis. Circ Res. 2018;122(3):479–488. doi: 10.1161/CIRCRESAHA.117.311466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wöhrle J, Merkle N, Mailänder V, Nusser T, Schauwecker P, von Scheidt F, et al. Results of intracoronary stem cell therapy after acute myocardial infarction. Am J Cardiol. 2010;105(6):804–812. doi: 10.1016/j.amjcard.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 27.Meyer GP, Wollert KC, Lotz J, Pirr J, Rager U, Lippolt P, et al. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled BOOST trial. Eur Heart J. 2009;30(24):2978–2984. doi: 10.1093/eurheartj/ehp374. [DOI] [PubMed] [Google Scholar]
- 28.Piepoli MF, Vallisa D, Arbasi M, Cavanna L, Cerri L, Mori M, et al. Bone marrow cell transplantation improves cardiac, autonomic, and functional indexes in acute anterior myocardial infarction patients (Cardiac Study) Eur J Heart Fail. 2010;12(2):172–180. doi: 10.1093/eurjhf/hfp183. [DOI] [PubMed] [Google Scholar]
- 29.Tendera M, Wojakowski W, Ruzyłło W, Chojnowska L, Kepka C, Tracz W, et al. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre myocardial regeneration by intracoronary infusion of selected population of stem cells in acute myocardial infarction (REGENT) trial. Eur Heart J. 2009;30(11):1313–1321. doi: 10.1093/eurheartj/ehp073. [DOI] [PubMed] [Google Scholar]
- 30.Wollert KC, Meyer GP, Müller-Ehmsen J, Tschöpe C, Bonarjee V, Larsen AI, et al. Intracoronary autologous bone marrow cell transfer after myocardial infarction: the BOOST-2 randomised placebo-controlled clinical trial. Eur Heart J. 2017;38(39):2936–2943. doi: 10.1093/eurheartj/ehx188. [DOI] [PubMed] [Google Scholar]
- 31.Yao K, Huang R, Sun A, Qian J, Liu X, Ge L, et al. Repeated autologous bone marrow mononuclear cell therapy in patients with large myocardial infarction. Eur J Heart Fail. 2009;11(7):691–698. doi: 10.1093/eurjhf/hfp062. [DOI] [PubMed] [Google Scholar]
- 32.Beitnes JO, Hopp E, Lunde K, Solheim S, Arnesen H, Brinchmann JE, et al. Long-term results after intracoronary injection of autologous mononuclear bone marrow cells in acute myocardial infarction: the ASTAMI randomised, controlled study. Heart. 2009;95(24):1983–1989. doi: 10.1136/hrt.2009.178913. [DOI] [PubMed] [Google Scholar]
- 33.Benedek I, Bucur O, Benedek T. Intracoronary infusion of mononuclear bone marrow-derived stem cells is associated with a lower plaque burden after four years. J Atheroscler Thromb. 2014;21(3):217–229. doi: 10.5551/jat.19745. [DOI] [PubMed] [Google Scholar]
- 34.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 35.Bahit MC, Kochar A, Granger CB. Post-myocardial infarction heart failure. JACC Heart Fail. 2018;6(3):179–186. doi: 10.1016/j.jchf.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Fisher SA, Zhang H, Doree C, Mathur A, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD006536.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delewi R, Hirsch A, Tijssen JG, Schächinger V, Wojakowski W, Roncalli J, et al. Impact of intracoronary bone marrow cell therapy on left ventricular function in the setting of ST-segment elevation myocardial infarction: a collaborative meta-analysis. Eur Heart J. 2014;35(15):989–998. doi: 10.1093/eurheartj/eht372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary material.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.