Abstract
Opioid use disorders (OUD) and overdose represent a public health threat, resulting in thousands of deaths annually worldwide. Vaccines offer a promising treatment for OUD and potentially the prevention of fatal overdoses. The Oxy(Gly)4-sKLH Conjugate Vaccine, Adsorbed (Oxy(Gly)4-sKLH) has shown promising pre-clinical efficacy at reducing the behavioral and pharmacological effects of oxycodone. To support its clinical evaluation, a GLP toxicology study was performed to address the safety of Oxy(Gly)4-sKLH. Sprague Dawley rats were vaccinated with either aluminum adjuvant (alum) or vaccine adsorbed on alum. Low and high doses of Oxy(Gly)4-sKLH, equivalent to a 1X or 47X human dose, respectively, were administered every two weeks for a total of four vaccinations. Both vaccine doses induced high antibody titers. Vaccine-related toxicity was assessed postmortem in one experimental group after receiving the fourth immunization of the vaccine’s high dose. For the remaining experimental groups, rats were challenged with 1.5 mg/kg/day s.c. oxycodone for 7 days after the fourth vaccination to assess whether concurrent exposure to oxycodone in vaccinated animals resulted in toxicity. All rats, except a subset of the aluminum control and the high dose vaccine groups, were sacrificed following oxycodone exposure. These subsets were allowed a four weeks recovery period prior to euthanasia. In this study, no Oxy(Gly)4-sKLH-related hematology, clinical chemistry, urinalysis, body weight, organ weight, or anatomic pathology toxicological findings were observed. These results demonstrate that the Oxy(Gly)4-sKLH vaccine is well tolerated, is immunogenic even at low doses, and does not produce undesired side effects in rats.
Keywords: opioid, oxycodone, safety, toxicity, opioid use disorder, vaccine, FDA, IND, GLP
Graphical Abstract
INTRODUCTION
Globally, 27 million people have been diagnosed with an opioid use disorder (OUD) and over 92,000 deaths are attributed to opioid overdose annually (1). In the U.S., the prevalence of OUD and the incidence of fatal overdoses associated to the illicit use of opioids have now reached alarming proportions, which led the U.S. Department of Health and Human Services to declare an opioid epidemic in 2017. In the last decade, the recent decline in the U.S. life expectancy has been partially attributed to the opioid epidemic (2). Overuse and misuse of opioids in addition to large numbers of opioid-related overdose deaths were initially caused largely by excessive prescribing of opioid analgesics such as oxycodone and hydrocodone in the 1990s (3). The cause of opioid overdose deaths then evolved due to the widespread availability of illicit opioids including heroin, synthetic opioids such as fentanyl, and street mixtures containing heroin or psychostimulants laced with fentanyl or its potent analogs (4). Currently, the U.S. has the highest number of opioid overdose-related deaths in the world (5). In addition, OUD can contribute to mortality, poor quality of life, generally exacerbate other underlying conditions, and increases the risk of transmitting blood borne pathogens (e.g., HIV or hepatitis) and other infectious diseases (6, 7). During the COVID-19 pandemic, isolation resulting from quarantine or shelter-in-place orders as well as limited access to medication or counseling has increased the overall overdose incidence in the United States (8).
Current FDA approved treatments of OUD include opioid receptor replacement pharmacotherapies such as the agonist methadone, the partial agonist buprenorphine, and the antagonist naltrexone (10,11). While there is a large amount of evidence supporting their effectiveness in managing opioid addiction, there are also many limitations associated with long-term maintenance therapy with these treatments such as adverse side effects and abuse potential, leading to low therapy retention rates. For instance, naltrexone requires detoxification prior to use, while agonists (i.e., methadone and buprenorphine) are associated with physical dependence, possible diversion for illicit use, and withdrawal symptoms if discontinued. Furthermore, treatment with opioid receptor antagonists complicate pain management with other opioids. Considering the burden of OUD on the public health and social system, this advocates for an urgent need to develop new approaches for treatment of OUD and prevention of opioid overdose.
In the pre-clinical literature, vaccines have emerged as a promising treatment strategy for OUD (9, 10). Vaccines elicit high affinity opioid-specific antibodies (11, 12), which bind to their targeted opioid in blood, reduce their distribution to brain, and subsequently reduce opioid-induced behavioral and pharmacological effects. An anti-oxycodone vaccine consisting of an oxycodone-based hapten containing a tetraglycine linker conjugated to the highly immunogenic keyhole limpet hemocyanin protein carrier subunit (sKLH) and adsorbed to aluminum adjuvant (Oxy(Gly)4-sKLH Conjugate Vaccine, Adsorbed; Oxy(Gly)4-sKLH) has been shown to significantly reduce oxycodone-induced rewarding and antinociceptive effects as well as opioid-induced respiratory depression and bradycardia (13, 14).
The overall toxicity profile of vaccines against OUD has not yet been fully explored, and reports have focused on specific formulations. Preclinical testing of a vaccine formulation containing the Oxy(Gly)4-sKLH has demonstrated its safety and efficacy (15-18). In a previous toxicology study, mice were injected subcutaneously with either sKLH, Oxy(Gly)4-sKLH, or the vaccine along with an anti-IL-4 mAb (18). No significant differences were reported in the Oxy(Gly)4-sKLH vaccine group compared to other treatment groups in terms of overall toxicology tests and organ histopathology performed (14). KLH has an acceptable clinical safety profile (19, 20). It has been approved in the European Union as an immunotherapy for bladder cancer and has been used as a carrier protein for vaccines in several clinical trials (21, 22).
Prior to the use of Oxy(Gly)4-sKLH in a clinical setting, a complete toxicological evaluation is required to support the IND application. To this end, we conducted an 8-week toxicology study in rats following Good Laboratory Practices (GLP) complying with the regulatory guidance of the Center for Biologics Evaluation and Research (CBER). This study evaluated two doses of Oxy(Gly)4-sKLH, a low dose (1.43 μg/animal; equivalent to the highest intended human dose in the Phase 1 clinical trial on a body weight basis) and a high dose (68 μg/animal; equivalent to approximately a 40X human dose, which has routinely been used in previous rodent pre-clinical studies from our group (13, 14)). During the toxicology study, animals were challenged with an oxycodone dose that was larger than the most commonly abused dose in humans (mg/kg equivalent) to evaluate the potential for toxicity of drug:antibody complexes in presence of the target drug. Additional groups of rats (high dose and alum control groups) were included as recovery groups to assess the severity and the reversibility of any possible side effects and kept under observation for four additional weeks after completing the oxycodone challenge. This study included the assessment of clinical pathology (serum chemistry, hematology, coagulation, urinalysis), postmortem analysis (gross necropsy, bone marrow, organ weights, histopathology, and serum drug concentrations), various clinical observations (cage-side, injection site, clinical, body weight, food consumption, functional tests, and ophthalmology) and vaccine-induced antibody response. Results showed that the intramuscular administration of the Oxy(Gly)4-sKLH vaccine was tolerable even at high doses with no evidence of adverse effects in rats. Moreover, the administration of oxycodone in vaccinated rats was not associated with any major adverse effects. These findings enabled initiation of clinical trials to evaluate the Oxy(Gly)4-sKLH in human subjects with an OUD (NCT04458545).
MATERIALS AND METHODS:
Ethics.
All protocols were designed based on the principles of the Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER)/International Conference on Harmonization (ICH) Harmonized Tripartite Guidelines ICH-M3(R2), Nonclinical Safety Studies for the conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals (CDER, January 2010). This GLP toxicology study was performed by Covance Inc. which is certified by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). All studies were compliant with applicable animal welfare acts and approved by the Covance Institutional Animal Care and Use Committee (IACUC).
Animals.
Sprague Dawley rats were purchased from Charles River Laboratories (Raleigh, North Carolina) and studied under GLP-compliant protocols. Animals were group-housed (up to three animals of the same sex/cage) in polycarbonate cages on hardwood chip bedding and provided with water at ad libitum and certified rodent diet #2014C (Envigo RMS, Inc) ad libitum unless fasted for study procedures. All animals within a cage were in the same vaccine treatment group. Animals were individually housed, as needed, in stainless steel or polycarbonate cages as needed for study-related purposes (e.g., urinalysis sample collection). Animals were acclimated to the testing facility for at least 12 days prior to initiation of dosing. At the initiation of dosing, rats were 7 weeks old with body weights ranging from 245 to 307 g for males and 182 to 231 g for females. A standard 12-hour light/12-hour dark cycle was used.
Drugs.
Oxycodone (Sigma-Aldrich, Saint Louis, MO, lot CLBX4974) was prepared as a 1.47 mg/ml solution in phosphate buffered saline (PBS; Gibco, Grand Island, NY, lot 1967654). Aluminum hydroxide (alum; Alhydrogel, Brenntag Biosector, Essen, Germany, lot 85644), the adjuvant control, was dissolved in PBS with 0.1 mg/mL polysorbate 80 (Avantor, Radnor, PA, batch 0000229418).
Vaccine formulations.
This study tested a vaccine formulation (Oxy(Gly)4-sKLH, adsorbed) consisting of an oxycodone-based hapten (Oxy(Gly)4-OH) conjugated to the sKLH via carbodiimide coupling chemistry (EDAC, N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride, Sigma-Aldrich), and adsorbed to alum (Alhydrogel, Brenntag Biosector, Essen, Germany, lot 85644) as previously described (23). The hapten was synthesized by Cambrex (Durham, NC), while the drug product (hapten-protein conjugate adsorbed to aluminum adjuvant) was generated by Goodwin Biotechnology, Inc. (GBI; Plantation, FL). In this study, the drug product was used as provided by GBI for groups receiving the high dose formulation, while the lower dose was obtained by a 10X dilution in PBS at Covance. Quality control testing included protein concentration, aluminum content, adsorption, sterility, endotoxin, conductivity, and in vivo potency. The drug product lot used in this study consisted of the engineering batch, which was made at GBI using the same manufacturing process and release specifications used for the GMP-grade batch used in the clinical trial.
Study design.
Female and male Sprague Dawley rats were first identified with a microchip and then randomly assigned to the treatment groups such that body weight was balanced across groups as summarized in Figure 1. The vaccine doses were selected based on the proposed human doses for the phase 1 clinical trial. The doses were 1.43 μg/animal, which is equivalent to the proposed human dose, and 68 μg/animal, which is approximately 40X higher than the proposed human dose on a dose per body weight basis. Assuming a 300 g rat body weight, these vaccine doses translate to 4.8 μg/kg and 227 μg/kg for the low and high doses, respectively. In a human weighing 70 kg, the highest proposed human dose (400 μg) would be 5.7 μg/kg. The vaccine doses chosen satisfy the WHO recommendation of testing doses that are equivalent or higher than the proposed highest human doses on a mg/kg basis (24). These vaccine doses elicited moderate to very high levels of oxycodone specific antibodies in rats. Furthermore, the vaccination regimen used in this toxicology study consisted of the same number of doses and dosing interval as those proposed for the clinical study. A volume of 0.15 mL/animal of alum control (placebo) or vaccine was delivered intramuscularly (IM) on Days 1, 15, 29, and 43 (immunization phase). One week after the last vaccination (Day 49), all rats in Group 4 were euthanized. Rats in Groups 1-3 received once-daily subcutaneous (s.c) injections of oxycodone (1.47 mg/kg) for a week (oxycodone challenge phase). At the end of the oxycodone challenge phase (Day 58), all rats in Group 2 and 10 rats/sex in Groups 1 and 3 were anesthetized with isoflurane, exsanguinated, and necropsied. The remaining rats (5 rats/sex) in Groups 1 and 3 underwent a four-week recovery phase after the end of the oxycodone challenge phase and were then euthanized on Day 87. The recovery groups were meant to assess the reversibility, persistence, or delayed occurrence of any adverse effects.
Figure 1. Experimental design of the GLP toxicology study for OXY-sKLH.
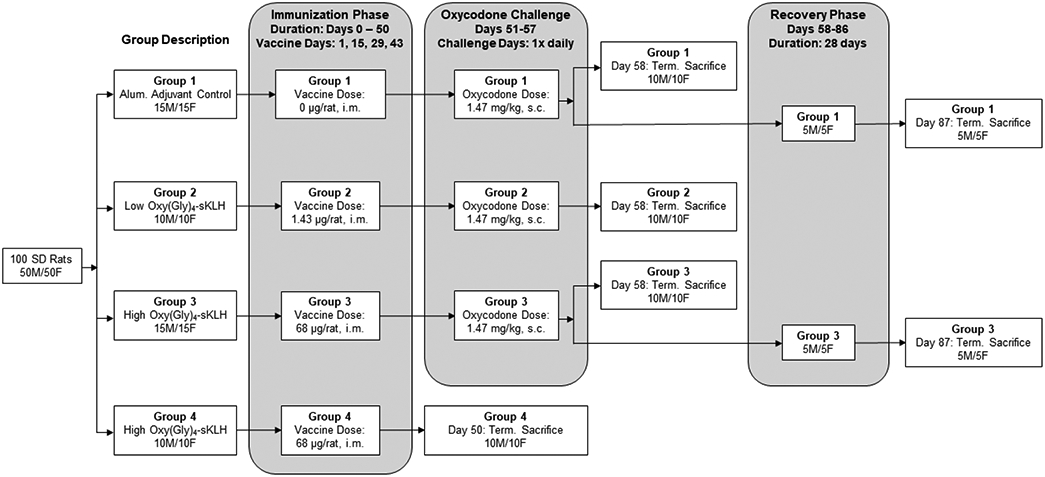
Groups were administered vaccine or alum adjuvant control via IM injection as designated. Group 1 received alum, Group 2 received 1.43 μg/animal/vaccination, and Groups 3 and 4 received 68 μg/animal/vaccination. Animals were vaccinated every 2 weeks for a total of 4 vaccinations. Animals in Group 4 were sacrificed one week after last immunization (Day 50) while all other groups were then challenged with oxycodone (1.47 mg/kg, s.c.) daily for 7 days. Ten male and female animals from each of the remaining groups (Groups 1 – 3) were sacrificed after the oxycodone challenge (Day 58). A subset (5 male and 5 female) of Groups 1 and 3 were kept under observation for an additional 4 week recovery period and sacrificed at the end of the recovery phase (Day 87).
Clinical and dermal/dose site observation.
Animals were observed twice daily (AM and PM) throughout the study for mortality and abnormalities. Clinical observations for general welfare and other clinical signs were performed at least once daily throughout the duration of study. Detailed observations for each animal were conducted once prior to vaccination, and on a weekly basis until the end of the study (before and 30 minutes post-dose). Abnormal findings were recorded. Dermal irritation was evaluated at the sites of injection on Day 1 (pre-dosing) and on the day of the scheduled euthanasia. During the immunization phase, IM dose sites were observed prior to control or vaccine dosing and at approximately 1, 6-, and 24-hours post-dose on Days 1, 15, 29, and 43. Subcutaneous dose sites were observed prior to the first oxycodone dose and approximately 45 minutes after each dose on Days 51 through 57 during the oxycodone challenge phase. Dose site and dermal observations were scored using a modified Draize method. Erythema and edema were scored on a 5-point scale (none to severe) while edema, atonia, desquamation, fissuring, and eschar were assessed as present or absent. All scores were assigned based in the most severely affected area using a modified Draize technique (26).
Body weights and food consumption.
All animals were weighed on Day 1 then weekly for the remainder of the study. Body weights were reported as grams. Body weight change is the difference between weekly body weight measurements. Food consumption was measured per cage (up to 3 rats/cage) on a weekly basis throughout the study. Food consumption data were reported as grams/animal/day.
Ophthalmic examination.
All animals were examined by a veterinarian prior to the immunization phase and once during week 7 of the immunization phase. Prior to examination, pupils were dilated with a mydriatic agent and observations were made using an indirect ophthalmoscope.
Blood and urine collection.
Urine samples and blood for clinical chemistry, hematology and blood coagulation and were collected from fasted animals on Day 50 (Group 4), Day 58 (Groups 1 through 3), and Day 87 (recovery Groups 1 and 3). Note that urine was collected chilled during the overnight period prior to the day of euthanasia. Blood was collected via a jugular vein. Sodium citrate was used as the anticoagulant for coagulation tests, potassium EDTA was used for hematology tests, and samples for clinical chemistry were collected without anticoagulant. All methods were per Covance standard protocols. Blood samples for immunogenicity testing were collected in serum separator tubes before vaccination, one week after the last vaccine dose, and on the day of sacrifice at the end of the recovery phase. The serum was harvested and stored at approximately −20C until the serum was analyzed for oxycodone-specific titers.
Terminal necropsy and tissue histopathology.
Rats that had been fasted overnight were anesthetized with isoflurane, exsanguinated, and necropsied. Macroscopic examination of the external features of the carcass were performed. Bone marrow smears were prepared from a femur of each animal at scheduled sacrifice. Tissues collected during necropsy were placed in 10% neutral-buffered formalin and shipped to Histo-Scientific Research Laboratories (Mount Jackson, VI) for paraffin embedding and hematoxylin and eosin (H&E) staining. All analyses were conducted by a certified pathologist blinded to the group treatment.
Evaluation of oxycodone specific antibody titer.
Serum antibody analysis was performed via indirect enzyme-linked immunosorbent assay (ELISA) under non-GLP conditions as described previously (17). Briefly, 96-well plates were coated with 5 ng/well of oxycodone-BSA (OXY-BSA) conjugate or unconjugated BSA as a negative control. Conjugates were made in 0.5 M carbonate coating buffer pH 9.6 (Sigma, C3041-100CAP). Plates were blocked with 1% gelatin. Various sera dilutions in 0.05 M PBST were added to the wells, washed, and HRP-conjugated goat anti-rat IgG (Jackson ImmunoResearch Laboratories) was added to the wells to assess oxycodone-specific serum IgG antibody. The following day, ophenylenediamine (OPD, SIGMAFAST™ tablet set, Sigma Life Sciences, St Louis, MO) was prepared, added to the wells, and the plates were incubated in the dark at room temperature. Thirty minutes later, stop solution of 2% oxalic acid was added to the wells and plates were read at 492 nm wavelength on a Tecan Infinite M1000 PRO Microplate Reader. Titers were calculated as the dilution producing 50% maximal binding. Ranges were reported as the geometric mean divided by the standard deviation factor (SD factor) to the geometric mean multiplied by the SD
Quality Assurance.
Quality assurance was conducted by the Covance GLP Quality Assurance Unit (QAU). It was performed in accordance with the United States Food and Drug Administration: Good Laboratory Practice (GLP) for Nonclinical Laboratory Studies, Code of Federal Regulations, Title 21 Part 58.
Data Analysis.
Data analysis, unless otherwise specified, was performed by Covance using Statistical Analysis Software (SAS; Cary, NC) and independently verified by the Pravetoni lab using Prism (v. 9.02; Graphpad, La Jolla, CA). Statistical information presented here are as reported by Covance. Data for each sex were analyzed separately. Levene’s test was performed first to test for equality of variances between groups. The analysis of variance (ANOVA) and post hoc pairwise comparisons were used to analyze group differences for absolute body weight, body weight change (the difference between weekly body weights), quantitative food consumption, continuous clinical pathology values, terminal body weight, absolute organ weight, organ:body weight percentage, and organ:brain weight percentage. Dunnett’s test was used for pairwise comparisons to a single control or combined identical controls, for all other comparisons a t-test was used. Oxycodone-specific serum antibody titers were analyzed using descriptive statistics to determine geometric mean for each group separately (Prism, v. 9.02; Graphpad; La Jolla, CA). Oxycodone-specific antibody titers were analyzed using a one-way ANOVA paired with Tukey’s multiple comparison test for all groups compared to the appropriate control except for recovery groups where a paired-t test was used. Level of significance was set at p ≤ 0.05 for all analyses.
RESULTS
Systemic administration of Oxy(Gly)4-sKLH vaccine did not result in significant clinical signs or mortality.
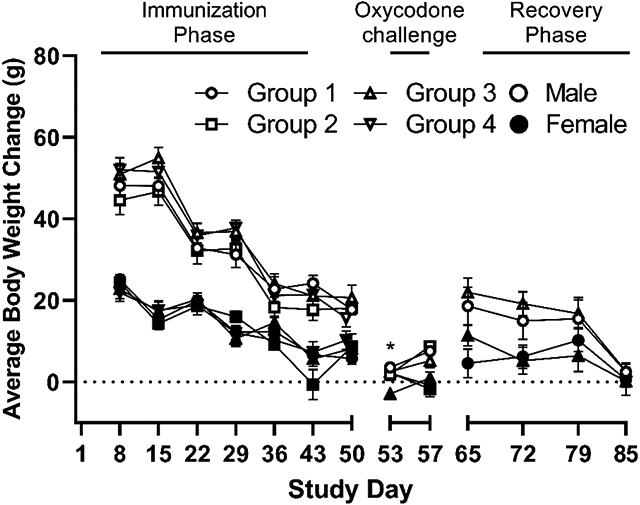
All animals remained healthy for the duration of the study and survived until their scheduled sacrifice day without need for veterinary treatment. A decrease in body weight change (Supplemental Figure S1) for males and females during the oxycodone challenge week was observed in all groups including the adjuvant controls. A statistically significant decrease in body weight change (Supplemental Figure S1) was detected for Group 3 females (68 μg/animal; p ≤ 0.05) between Day 50 and 53. No concomitant significant difference was observed for body weight (Figure 2, Panel A) or body weight change at any other time during the study. For these reasons, these differences were considered incidental and non-vaccine related. Overall, all body weight and body weight change observations were deemed to be consistent with the alum control group, incidental, and not considered vaccine related. Similarly, no vaccine-related changes in food consumption were observed in male or female rats treated with either dose of Oxy(Gly)4-sKLH vaccine compared to alum adjuvant controls either prior to or post oxycodone treatment (Figure 2, panel B).
Figure 2. Effect of vaccine on body weight and food consumption.
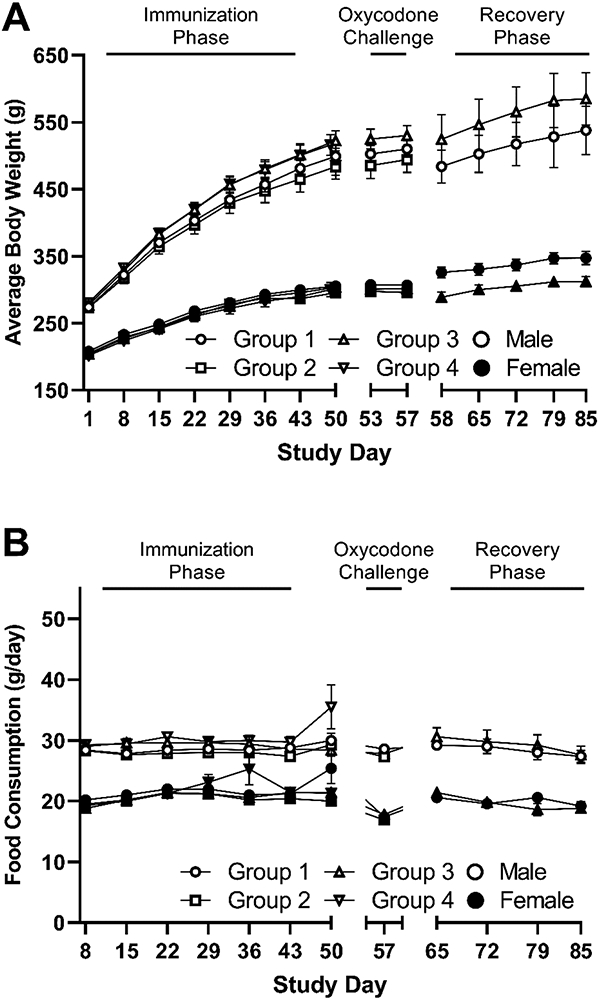
A) Body weights and B) food consumption were measured weekly from Day 1 throughout the study. For Group 4, N=10/sex through Day 49, Groups 1 and 3, N=15/sex through Day 58, and for Group 2, N=10/sex through Day 57. The recovery phase for Groups 1 and 3, N=5/sex through Day 85. Increases in body weight observed in all groups are consistent with the typical growth pattern of Sprague Dawley rats. Data are presented as mean ± SEM.
Detailed observations of the animals’ eyes, skin, and hair were also performed at various time points throughout the study. No abnormal ophthalmic observations were recorded for any animals in the study. Due to the lack of remarkable ophthalmic observations at Week 7, an examination during the recovery phase was not warranted. Thinning of the hair coat and the presence of skin scabs observed in a few of the rats were not considered Oxy(Gly)4-sKLH vaccine-related (Table 1). IM administration of Oxy(Gly)4-sKLH vaccine did not result in any abnormal dermal signs related to the vaccine.
Table 1. Summary of clinical and ophthalmic findings.
Summary of all cage-side clinical observations over the in-life portion of the study and the ophthalmic examination performed during Week 7. Cage-side observations were performed once daily during the in-life portion of the study and detailed observations were conducted weekly, as applicable, throughout the study. An ophthalmic examination was performed prior to Day 0 and during Week 7 (prior to the Oxycodone Challenge Phase). Data in the Normal column are the number of animals considered to have no remarkable non-normal observations over the course of the study. Data for the pelage, skin, and ophthalmic lesions column are presented as the total number of non-normal observations for all animals in the treatment group at any time throughout the study.
| Group Details | Clinical | Ophthalmic | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Group # |
Sex | Phase | N | Normal1 | Pelage (Thinning2) |
Skin (Scab3) |
Lesions (Week 7) |
| 1 | M | Dosing | 15 | 15 | 0 | 0 | 0 |
| 2 | M | Dosing | 10 | 10 | 0 | 1 | 0 |
| 3 | M | Dosing | 15 | 15 | 0 | 0 | 0 |
| 4 | M | Dosing | 10 | 10 | 1 | 0 | 0 |
| 1 | F | Dosing | 15 | 15 | 3 | 1 | 0 |
| 2 | F | Dosing | 10 | 10 | 0 | 0 | 0 |
| 3 | F | Dosing | 15 | 15 | 0 | 0 | 0 |
| 4 | F | Dosing | 10 | 10 | 0 | 0 | 0 |
| 1 | M | Recovery | 5 | 5 | 0 | 1 | - |
| 3 | M | Recovery | 5 | 5 | 0 | 0 | - |
| 1 | F | Recovery | 5 | 5 | 0 | 0 | - |
| 3 | F | Recovery | 5 | 5 | 0 | 0 | - |
No remarkable observations
Thinning was observed on head or shoulder areas
Scabs observed on the dorsal thoracic or thigh areas
No biologically relevant effects of vaccine treatment were observed for any clinical chemistry, hematological, or coagulation parameters.
Among the clinical chemistry parameters tested only one statistically significant difference between vaccinated rats and control animals was observed for all parameters evaluated. Alanine aminotransferase (ALT) was significantly decreased in male low (Group 2) and high dose (Group 3) animals compared to male control animals (Supplemental Tables 1A and 1B for male and female, respectively). However, all individual animal measurements were within the normal reference range. Some minor differences were observed for female Group 4 rats (high dose Oxy(Gly)4-sKLH euthanized on Day 50) compared to control female rats (alum adjuvant euthanized on Day 58) including minimally higher mean urea nitrogen, total protein, and globulin concentrations and slightly lower mean albumin:globulin ratios. Of note is that all individual Group 4 animal measurements, except for a single urea nitrogen measurement, were within the normal range. No significant change in clinical chemistry was considered related to Oxy(Gly)4-sKLH vaccination in treated animals compared to control animals during the course of the study.
Statistically significant differences between vaccinated rats and control animals were found for only two hematology parameters for all parameters evaluated (Supplemental Tables 1C and 1D for male and female, respectively). The mean eosinophil concentration (EOS) in females administered the low dose of Oxy(Gly)4-sKLH followed by oxycodone challenge (Group 2) was higher compared to the concurrent alum adjuvant control females. Additionally, the mean cell hemoglobin (MCHC) in Group 3 males (high dose of Oxy(Gly)4-sKLH followed by oxycodone challenge) was higher compared to the concurrent alum adjuvant control males. However, the mean values for both parameters and almost all individual animal values were within the normal range of the species.
No significant differences between vaccinated and control animals were observed in any of the coagulation parameters (Supplemental Tables 1D and 1E for male and female, respectively). Overall, no biologically relevant effects of vaccine treatment were observed for any clinical chemistry, hematological, or coagulation parameters.
The effect of Oxy(Gly)4-sKLH vaccine of the urinalysis profile of Sprague Dawley rats.
Higher urine volumes were observed in both male and female Group 4 animals (68 μg/animal Oxy(Gly)4-sKLH) euthanized on Day 50 compared to Group 3 animals (68 μg/animal Oxy(Gly)4-sKLH + 1.47mg/kg oxycodone) euthanized on Day 58 (Supplemental Tables 2A and 2B for male and female, respectively). Animals treated with oxycodone are typically lethargic and may not be actively drinking. Similar urine volumes were observed for all groups subjected to the oxycodone challenge. Other minor differences in vaccine-treated animals compared to the control (Supplemental Tables 2C and 2D for male and female, respectively) were consistent with normal variations in this animal species.
The administration of Oxy(Gly)4-sKLH vaccine in Sprague Dawley rats revealed no organ specific toxicity.
In general, organ weight (organ weight corrected by body weight presented; Supplemental Tables 3A and 3B for male and female, respectively) was comparable between the control rats and those administered Oxy(Gly)4-sKLH vaccine. While some statistically significant differences were observed in vaccine-treated groups compared to control, they were consistent with normal variations and were not considered vaccine related. The observed differences between vaccine- and control-treated animals were characterized by various inconsistencies such as lack of dose-relationship and lack or correlative findings in the clinical pathology results or upon microscopic examination of the organs.
Immunogenicity and persistence of oxycodone-specific antibodies titer.
Significantly higher anti-oxycodone IgG antibodies titers were observed in both male and female animals that received either the low (1.43 μg; Group 2) or high (68 μg; Groups 3 & 4) dose of adjuvanted Oxy(Gly)4-sKLH compared to those that received alum adjuvant (Figure 3A & Table 3) when assessed after the immunization phase (day 50). The titers in the high dose groups (Groups 3 & 4) were 7 to 12 times greater compared to the low dose group (Group 2) demonstrating a dose-response relationship, For male and female recovery animals (Group 3), titers measured on Day 50 (post-immunization phase, pre-oxycodone challenge) were not significantly different compared to oxycodone specific IgG titers measured on study day 87 (post-recovery phase). These results indicate that 68 μg/animal dose of Oxy(Gly)4-sKLH in alum induce high IgG titers that persist up to 37 days after the last vaccination, suggesting long serum half-life of oxycodone-specific antibodies. Moreover, the administration of oxycodone does not boost pre-challenge levels of oxycodone-specific antibodies (Figure 3B).
Figure 3. Immune response and the persistence of oxycodone specific antibodies.
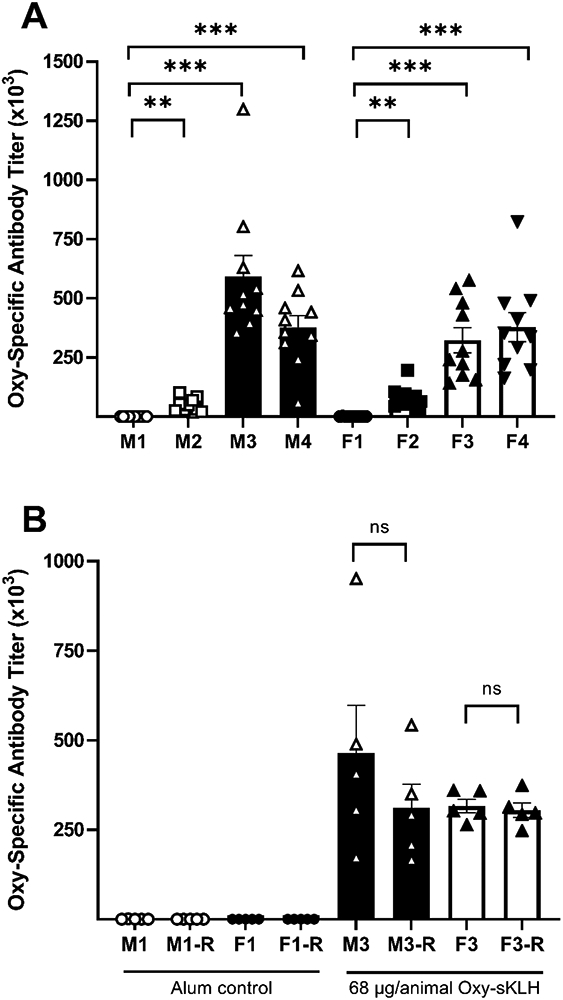
Male (M) and female (F) Sprague Dawley rats received four IM injections of either alum adjuvant (Group 1; M1 or F1), 1.43 (Group 2; M2 or F2), or 68 (Groups 3 and 4; M3 or F3 and M4 or F4, respectively) μg/animal Oxy(Gly)4-sKLH vaccine on Days 1, 15, 29, and 43 of the study. A) Oxycodone-specific serum IgG antibody titers were evaluated by ELISA on Day 50 for 10 animals per sex per group. B) Following oxycodone challenge 5 animals per sex from Groups 1 (M1-R or F1-R) and 3 (M3-R or F3-R) were allowed 4 weeks recovery and their oxycodone-specific serum IgG antibody titers were evaluated on Day 87 of the study. Difference in antibodies titers compared to the control from Day 50 (panel A) were analyzed using a Brown-Forsythe and Welch ANOVA with Tukey’s multiple comparison post-hoc test. Recovery Group 3 titers from Day 87 (panel B) were compared to Group 1 titers using a Welch’s t-test. Comparisons between Day 50 and Day 87 titers for Group 3 were made using a paired t-test with the paired Day 50 and Day 87 data from the same animals. Data are presented as mean ± SEM with individual animal responses shown. Statistical symbols (brackets indicate significance between groups): **: p≤0.01, ***: p≤0.001 compared to the gender matched Group 1, ns=not significant compared to gender and group matched Day 50 and Day 87 time points.
Table 3. Mean IgG titer values for oxycodone specific antibodies.
Male (M) and female (F) rats received four I injections of either alum adjuvant (Group 1), 1.43 (Group 2), or 68 (Groups 3 & 4) μg/animal Oxy(Gly)4-sKLH vaccine on Days 1, 15, 29, and 43 of the study. Oxycodone-specific serum IgG antibody titers were evaluated by ELISA on Day 50 (Dosing Phase) for 10 animals per group. Following oxycodone challenge 5 animals per sex from Groups 1 and 3 were allowed 4 weeks recovery and their oxycodone-specific serum IgG antibody titers were evaluated on Day 87 (Recovery Phase) of the study.
| Group Details | Dosing Phase | Recovery Phase | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Group # |
Sex | Figure1 | N | Mean | SEM | Mean | SEM | ||
| 1 | M | 3A | 10 | 298 | ± | 70 | - | - | |
| 2 | M | 3A | 10 | 48150 | ± | 9108 | - | - | |
| 3 | M | 3A | 10 | 591559 | ± | 88242 | - | - | |
| 4 | M | 3A | 10 | 376758 | ± | 49284 | - | - | |
| 1 | F | 3A | 10 | 378 | ± | 175 | - | - | |
| 2 | F | 3A | 10 | 80376 | ± | 14893 | - | - | |
| 3 | F | 3A | 10 | 322839 | ± | 52660 | - | - | |
| 4 | F | 3A | 10 | 377542 | ± | 60966 | - | - | |
| 1 | M | 3B | 5 | 479 | ± | 259 | 224 | ± | 24 |
| 3 | M | 3B | 5 | 465075 | ± | 132544 | 311291 | ± | 65980 |
| 1 | F | 3B | 5 | 241 | ± | 40 | 200 | ± | 0 |
| 3 | F | 3B | 5 | 316018 | ± | 18841 | 304521 | ± | 20456 |
Figure with associated data
DISCUSSION
This eight-week GLP toxicology study in rats was conducted to enable a Phase I clinical trial of the Oxy(Gly)4-sKLH vaccine, and yielded three main findings. First, the alum adjuvanted Oxy(Gly)4-sKLH vaccine was well tolerated at both the low and high vaccine formulations representing the 1X or 40X human dose, respectively. Second, no adverse effects or toxicity related to Oxy(Gly)4-sKLH were evident in any of the animals before or after oxycodone administration or after the 4 week recovery period. Third, the immune response as measured with IgG antibody titers appeared to be dose-related, long-lasting, and unaffected by oxycodone administration. The findings demonstrated that Oxy(Gly)4-sKLH was safe and well-tolerated, subsequently leading to the FDA approving initiation of a Phase I clinical trial (NCT04458545).
Vaccines targeting nicotine and cocaine have been deemed safe enough to proceed to clinical trials and there have been several clinical trials assessing these vaccines in humans (27-29). However, unlike the anti-nicotine and anti-cocaine vaccines, published clinical data available with regards to opioid-specific vaccines is limited to one study conducted in Iran (31). Previous preclinical testing of the oxycodone vaccine has evaluated the selectivity of vaccine induced drug specific antibodies and possible cross reactivity with opioids other than the targeted drug as well as endogenous opioids. Measuring the polyclonal antibody affinity using competitive binding ELISA showed that oxycodone induced antibodies have negligible binding to all endogenous opioids tested (18). Pre-clinically, a morphine conjugate vaccine was evaluated for safety using Lohmann specific pathogen free eggs. The vaccine was found to cause no adverse effect on the growth or the evolution of those eggs (30). When that morphine vaccine was tested in humans, it was reported to be well-tolerated with no serious drug related side effects (31). The results of the current GLP toxicity study presented here agree with what has been observed with similar hapten:protein conjugate vaccines suggesting that these vaccines, which target small exogenous compounds, are safe and well tolerated.
An important aspect of this study design was to address the safety concerns related to the formation of oxycodone:antibody complexes upon the administration of oxycodone in vaccinated subjects displaying oxycodone specific polyclonal antibodies. In this context, it is foreseeable that antibodies bound to oxycodone could result in adverse effects, toxicity, or potential organ damage due to accumulation of oxycodone:antibody complexes. While drug:antibody complex formation is sometimes associated with immunotoxicity due to complement system activation, these concerns are mitigated by pre-clinical studies demonstrating the lack of involvement of Fc receptors in the efficacy of vaccines and monoclonal antibodies against OUD (32). However, there is still a potential risk of immune complex tissue disposition and subsequent tissue damage (33). Designing this assessment had to consider several factors. In people with OUD who use oxycodone, the most commonly used dose is 80 mg (1.1 mg/kg for a 70 kg human, oral; self-reported) (34-37). Because orally administered oxycodone has low bioavailability in the rat, the subcutaneous route was chosen as it has a similar bioavailability to human oral administration (38). A daily dose of 1.47 mg/kg via the subcutaneous route is approximately 50% greater than the typically abused equivalent human dose of 80 mg, oral. In this study, the lack of significant differences in hematology, clinical chemistry, urinalysis, organ weight, and anatomical pathology in rats administered either the low or high vaccine dose provides evidence that administration of oxycodone in vaccinated subjects will not give rise to immunotoxicity or other side effects.
Both doses of the Oxy(Gly)4-sKLH vaccine were determined to be well-tolerated with no vaccine-related adverse effects or toxicity. When certain parameters were found to be both outside the normal reference range and different from control, it was not considered to be biologically relevant. For example, the ALT values were found to be low. However, low levels of ALT, without concomitant changes in other liver parameters or abnormal histopathology, are not considered biologically relevant (39). Additionally, many differences were considered biologically insignificant and characterized as small in magnitude (less than 10% difference between control and test groups), having a lack of dose relationship, inconsistency between sexes, and the absence of correlative findings in the anatomic pathology results. The increased urine volumes observed in Group 4 compared to Group 3 were likely due to the animals being treated differently (e.g., no oxycodone treatment, euthanasia on a different day, etc.). Due to the lack of any correlative findings, these differences were considered unrelated to the vaccination with Oxy(Gly)4-sKLH. These data are in line with previous studies performed in our laboratory and by other teams to demonstrate the safety of immunotherapy and other biologics for the treatment of opioid or substance use disorders (10, 15, 30, 40). A previous report of a nanoparticle-based nicotine vaccine did not find any significant toxicity in murine heart, liver, and kidneys as assessed via microscopy (41).
In addition to assessing safety, another goal of this study was to assess immunogenicity. The titers were observed to be dose-dependent with the high dose of Oxy(Gly)4-sKLH vaccine producing 7 to 12 times higher oxycodone-specific titers than the low dose. Importantly, the low dose of the Oxy(Gly)4-sKLH vaccine, which is equivalent to the proposed human vaccine dose, produced significant levels of oxycodone-specific titers. Based upon these data, it is expected that Oxy(Gly)4-sKLH will produce measurable levels of oxycodone-specific titers in humans. Finally, administration of oxycodone had no apparent effect on the level of oxycodone-specific titers in rats immunized with Oxy(Gly)4-sKLH. This is consistent with what has been demonstrated previously (16). While this finding suggests that oxycodone administration does not boost antibody production, ultimately this observation will require further evaluation of the underlying adaptive immune response (e.g., memory B cell lymphocytes) as well as careful analysis of clinical data to determine potential long-term immune-related effects of vaccination against opioids.
In addition to the clinical trials for related vaccines, there is a relatively large amount of pre-clinical literature suggesting that vaccines targeting heroin or prescription opioids are safe (9, 40). Additionally, a cocaine vaccine in alum has been tested in rhesus monkeys and found to be safe, well tolerated with no significant side effects (42). These preclinical safety data in rodents and non-human primates were well translated into humans which was evident when the Nicotine–Qb VLP conjugate vaccine in alum was tested in phase I and II clinical trials (43, 44). Also, the cocaine vaccine with alum adjuvant resulted in no safety concerns during clinical testing (45). Finally, it was previously reported that a morphine-based conjugate vaccine was safe in a Phase I clinical study, but few details are available (31). Based on the similar vaccine component and lack of toxicity in early anti addiction vaccine clinical trials, this oxycodone vaccine is expected to have a comparable safety profile in humans.
In addition to other pre-clinical and clinical data, the components of the vaccine assessed in this GLP toxicology have been shown to be generally safe. Keyhole limpet hemocyanin (KLH) is the carrier protein the oxycodone hapten has been conjugated to. In previous studies, KLH has been shown to be safe (46). KLH is a common carrier protein for several vaccines in clinical trials, and it has been approved in Europe for treatment of bladder cancer. KLH has been safely administered to humans (10 μg to 5000 μg) and a tau-KLH conjugate vaccine adsorbed to alum has been extensively studied for immunotherapy of Alzheimer’s disease pre-clinically and in Phase 1 human clinical trials, with a good safety and toxicity profile (47-52).
Opioid use disorder is a chronic, complex, and complicated disorder which can be very difficult to treat. Given the alarming number of deaths associated with opioids during the opioid epidemic, more treatments are urgently needed. Oxy(Gly)4-sKLH has demonstrated high efficacy at selectively reducing effects of oxycodone, suggesting that it may be an effective therapy in humans. The findings from this study demonstrated that Oxy(Gly)4-sKLH is safe when administered and in the presence of chronic oxycodone doses, which has allowed for the initiation of the first in-human Phase I clinical trial to assess its safety and efficacy in humans.
Supplementary Material
Table 2. Summary of dermal dose site findings.
Summary of all dose site dermal observations over the in-life portion of the study. Injection sites A and B (left and right thigh muscle, respectively) comprised the IM vaccine and adjuvant control dosing locations. Injection sites C, D, E, and F (upper left, upper right, lower left, and lower right dorsal regions, respectively) comprised the subcutaneous (SC) Oxycodone challenge dosing sites. All dosing sites were scored using a modified Draize technique and the signs scored included atonia, desquamation, edema, erythema, eschar, and fissuring. Observations for sites A and B were performed prior to dosing and approximately 1, 6, and 24 hours post dosing on Days 1, 15, 29, and 43. Observations for dose sites C, D, E and F were performed prior to the first oxycodone dose and once after each dose at approximately 45 ± 15 minutes on Days 51 through 57 of the dosing phase. Data are presented as the total number of non-normal signs observed per the total number of observations for all animals in the treatment group at any time throughout the study.
| Group Details | Vaccination Sites1 |
Oxycodone Challenge Sites2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Group # |
Sex | Phase | N | A | B | C | D | E | F3 |
| 1 | M | Dosing | 15 | 0/105 | 0/60 | 0/30 | - | 0/68 | 0/2 |
| 2 | M | Dosing | 10 | 0/70 | 1/40 | 0/20 | - | 0/50 | - |
| 3 | M | Dosing | 15 | 0/105 | 0/60 | 0/30 | - | 0/69 | - |
| 4 | M | Dosing | 10 | 0/70 | 0/30 | - | - | - | - |
| 1 | F | Dosing | 15 | 0/105 | 0/60 | 0/30 | 0/60 | 0/15 | - |
| 2 | F | Dosing | 10 | 0/70 | 0/40 | 0/20 | 0/40 | 0/10 | - |
| 3 | F | Dosing | 15 | 0/105 | 0/60 | 0/30 | 0/60 | 0/15 | - |
| 4 | F | Dosing | 10 | 0/90 | 0/50 | - | - | - | - |
IM sites on the left (site A) or right (site B) thigh
SC sites in the upper left (site C), upper right (site D), lower left (site E), and lower right (site F) dorsal regions
Only 1 animal in Group 1 (male) was injected in site F
Highlights:
Opioid use disorder is a global problem and better treatments are needed.
This study assessed the safety and toxicity profile of an anti-oxycodone vaccine.
No vaccine-induced adverse effects or toxicity were observed.
Results from this study enabled initiation of a Phase I clinical trial (NCT04458545).
Acknowledgment and funding:
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants U01DA038876 (M.P. and P.R.P and UG3DA047711 (M.P.)]. S.W. received compensation as an independent consultant on this study and he is the sole proprietor of Winston Biopharmaceutical Consulting. The authors declare no conflicts of interest.
Abbreviations:
- GLP
Good Laboratory Practices
- GMP
Good Manufacturing Practices
- IM
intramuscular
- OUD
opioid use disorder
- sKLH
subunit keyhole limpet hemocyanin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- 1.Strang J, Volkow ND, Degenhardt L, Hickman M, Johnson K, Koob GF, et al. Opioid use disorder. Nat Rev Dis Primers. 2020;6(1):3. [DOI] [PubMed] [Google Scholar]
- 2.Volkow ND. Stigma and the Toll of Addiction. N Engl J Med. 2020;382(14):1289–90. [DOI] [PubMed] [Google Scholar]
- 3.Hall W, Degenhardt L, Hickman M. Generational trends in US opioid-overdose deaths. Nat Med. 2020;26(5):651–2. [DOI] [PubMed] [Google Scholar]
- 4.Armenian P, Vo KT, Barr-Walker J, Lynch KL. Fentanyl, fentanyl analogs and novel synthetic opioids: A comprehensive review. Neuropharmacology. 2018;134(Pt A):121–32. [DOI] [PubMed] [Google Scholar]
- 5.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and Opioid-Involved Overdose Deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagemeier NE. Introduction to the opioid epidemic: the economic burden on the healthcare system and impact on quality of life. Am J Manag Care. 2018;24(10 Suppl):S200–S6. [PubMed] [Google Scholar]
- 7.Zibbell JE, Asher AK, Patel RC, Kupronis B, Iqbal K, Ward JW, et al. Increases in Acute Hepatitis C Virus Infection Related to a Growing Opioid Epidemic and Associated Injection Drug Use, United States, 2004 to 2014. Am J Public Health. 2018;108(2):175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander GC, Stoller KB, Haffajee RL, Saloner B. An Epidemic in the Midst of a Pandemic: Opioid Use Disorder and COVID-19. Ann Intern Med. 2020;173(1):57–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pravetoni M Biologics to treat substance use disorders: Current status and new directions. Hum Vaccin Immunother. 2016;12(12):3005–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olson ME, Janda KD. Vaccines to combat the opioid crisis: Vaccines that prevent opioids and other substances of abuse from entering the brain could effectively treat addiction and abuse. EMBO Rep. 2018;19(1):5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bremer PT, Janda KD. Conjugate Vaccine Immunotherapy for Substance Use Disorder. Pharmacol Rev. 2017;69(3):298–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baruffaldi F, Raleigh MD, King SJ, Roslawski MJ, Birnbaum AK, Hassler C, et al. Formulation and Characterization of Conjugate Vaccines to Reduce Opioid Use Disorders Suitable for Pharmaceutical Manufacturing and Clinical Evaluation. Mol Pharm. 2019;16(6):2364–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pravetoni M, Vervacke JS, Distefano MD, Tucker AM, Laudenbach M, Pentel PR. Effect of currently approved carriers and adjuvants on the pre-clinical efficacy of a conjugate vaccine against oxycodone in mice and rats. PLoS One. 2014;9(5):e96547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pravetoni M, Raleigh MD, Le Naour M, Tucker AM, Harmon TM, Jones JM, et al. Co-administration of morphine and oxycodone vaccines reduces the distribution of 6-monoacetylmorphine and oxycodone to brain in rats. Vaccine. 2012;30(31):4617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raleigh MD, Peterson SJ, Laudenbach M, Baruffaldi F, Carroll FI, Comer SD, et al. Safety and efficacy of an oxycodone vaccine: Addressing some of the unique considerations posed by opioid abuse. PLoS One. 2017;12(12):e0184876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raleigh MD, King SJ, Baruffaldi F, Saykao A, Hamid FA, Winston S, et al. Pharmacological mechanisms underlying the efficacy of antibodies generated by a vaccine to treat oxycodone use disorder. Neuropharmacology. 2021;195:108653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pravetoni M, Pentel PR, Potter DN, Chartoff EH, Tally L, LeSage MG. Effects of an oxycodone conjugate vaccine on oxycodone self-administration and oxycodone-induced brain gene expression in rats. PLoS One. 2014;9(7):e101807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laudenbach M, Baruffaldi F, Robinson C, Carter P, Seelig D, Baehr C, et al. Blocking interleukin-4 enhances efficacy of vaccines for treatment of opioid abuse and prevention of opioid overdose. Scientific reports. 2018;8(1):5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lammers RJ, Witjes WP, Janzing-Pastors MH, Caris CT, Witjes JA. Intracutaneous and intravesical immunotherapy with keyhole limpet hemocyanin compared with intravesical mitomycin in patients with non-muscle-invasive bladder cancer: results from a prospective randomized phase III trial. J Clin Oncol. 2012;30(18):2273–9. [DOI] [PubMed] [Google Scholar]
- 20.Lamm DL, Dehaven JI, Riggs DR. Keyhole limpet hemocyanin immunotherapy of bladder cancer: laboratory and clinical studies. Eur Urol. 2000;37 Suppl 3:41–4. [DOI] [PubMed] [Google Scholar]
- 21.Hogenesch H Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunol. 2012;3:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol. 2004;82(5):497–505. [DOI] [PubMed] [Google Scholar]
- 23.Pravetoni M, Le Naour M, Harmon TM, Tucker AM, Portoghese PS, Pentel PR. An oxycodone conjugate vaccine elicits drug-specific antibodies that reduce oxycodone distribution to brain and hot-plate analgesia. J Pharmacol Exp Ther. 2012;341(1):225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Technical Report Series 927: 54th report; Annex 1: WHO Guidelines on nonclinical evaluation of vaccines. Typeset in Hong Kong Printed in Singapore: WHO; 2005. Report No.: 9241209275. [Google Scholar]
- 25.Raleigh MD, Pravetoni M, Harris AC, Birnbaum AK, Pentel PR. Selective effects of a morphine conjugate vaccine on heroin and metabolite distribution and heroin-induced behaviors in rats. J Pharmacol Exp Ther. 2013;344(2):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.United S, Association of Food and Drug Officials of the United S. Appraisal of the safety of chemicals in foods, drugs, and cosmetics. Austin, Tex.: Association of Food & Drug Officials of the United States; 1959. 107 p. p. [Google Scholar]
- 27.Haney M, Kosten TR. Therapeutic vaccines for substance dependence. Expert Rev Vaccines. 2004;3(1):11–8. [DOI] [PubMed] [Google Scholar]
- 28.Kinsey BM, Kosten TR, Orson FM. Anti-cocaine vaccine development. Expert Rev Vaccines. 2010;9(9):1109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henningfield JE, Fant RV, Buchhalter AR, Stitzer ML. Pharmacotherapy for nicotine dependence. CA Cancer J Clin. 2005;55(5):281–99; quiz 322-3, 5. [DOI] [PubMed] [Google Scholar]
- 30.Farhangi A, Akbarzadeh A, Mehrabi MR, Chiani M, Saffari Z, Ghassemi S, et al. Safety of human therapeutic morphine vaccine employing Lohmann specific pathogen free eggs. Pak J Biol Sci. 2010;13(21):1047–51. [DOI] [PubMed] [Google Scholar]
- 31.Akbarzadeh A, Mehraby M, Zarbakhsh M, Farzaneh H. Design and synthesis of a morphine-6-succinyl-bovine serum albumin hapten for vaccine development. Biotechnol Appl Biochem. 1999;30(2):139–46. [PubMed] [Google Scholar]
- 32.Huseby Kelcher AM, Baehr CA, Hamid FA, Hart GT, Pravetoni M. Contribution of Antibody-Mediated Effector Functions to the Mechanism of Efficacy of Vaccines for Opioid Use Disorders. J Immunol. 2021;207(3):860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vahle JL. Immunogenicity and Immune Complex Disease in Preclinical Safety Studies. Toxicol Pathol. 2018;46(8):1013–9. [DOI] [PubMed] [Google Scholar]
- 34.Jones JD, Vosburg SK, Manubay JM, Comer SD. Oxycodone abuse in New York City: characteristics of intravenous and intranasal users. Am J Addict. 2011;20(3):190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walsh SL, Nuzzo PA, Lofwall MR, Holtman JR. The relative abuse liability of oral oxycodone, hydrocodone and hydromorphone assessed in prescription opioid abusers. Drug Alcohol Depend. 2008;98(3):191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tompkins DA, Lanier RK, Harrison JA, Strain EC, Bigelow GE. Human abuse liability assessment of oxycodone combined with ultra-low-dose naltrexone. Psychopharmacology (Berl). 2010;210(4):471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butler SF, Black RA, Cassidy TA, Dailey TM, Budman SH. Abuse risks and routes of administration of different prescription opioid compounds and formulations. Harm Reduct J. 2011;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan S, Edwards SR, Wyse BD, Smith MT. Sex differences in the pharmacokinetics, oxidative metabolism and oral bioavailability of oxycodone in the Sprague-Dawley rat. Clin Exp Pharmacol Physiol. 2008;35(3):295–302. [DOI] [PubMed] [Google Scholar]
- 39.Cornell University College of Veterinary Medicine. ECLINPATH n.d. [Online veterinary clinical pathology textbook]. Available from: https://eclinpath.com/.
- 40.Pravetoni M, Comer SD. Development of vaccines to treat opioid use disorders and reduce incidence of overdose. Neuropharmacology. 2019;158:107662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu Y, Smith D, Zhao Z, Harmon T, Pentel PR, Ehrich M, et al. Alum as an adjuvant for nanoparticle based vaccines: A case study with a hybrid nanoparticle-based nicotine vaccine. Nanomedicine. 2019;20:102023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koetzner L, Deng S, Sumpter TL, Weisslitz M, Abner RT, Landry DW, et al. Titer-dependent antagonism of cocaine following active immunization in rhesus monkeys. J Pharmacol Exp Ther. 2001;296(3):789–96. [PubMed] [Google Scholar]
- 43.Maurer P J GT Willers J Rohner F Lindman Y Roubicek K Renner WA Muller P Bachmann MF A therapeutic vaccine for nicotine dependence: preclinical efficacy, and Phase I safety and immunogenicity. European journal of immunology. 2005;35(7):2031–40. [DOI] [PubMed] [Google Scholar]
- 44.Wagena EJ, de Vos A, Horwith G, van Schayck CP. The immunogenicity and safety of a nicotine vaccine in smokers and nonsmokers: results of a randomized, placebo-controlled phase 1/2 trial. Nicotine Tob Res. 2008;10(1):213–8. [DOI] [PubMed] [Google Scholar]
- 45.Haney M, Gunderson EW, Jiang H, Collins ED, Foltin RW. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biological psychiatry. 2010;67(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swaminathan A, Lucas RM, Dear K, McMichael AJ. Keyhole limpet haemocyanin - a model antigen for human immunotoxicological studies. British journal of clinical pharmacology. 2014;78(5):1135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kontsekova E, Zilka N, Kovacech B, Novak P, Novak M. First-in-man tau vaccine targeting structural determinants essential for pathological tau-tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer's disease model. Alzheimer's research & therapy. 2014;6(4):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kontsekova E, Zilka N, Kovacech B, Skrabana R, Novak M. Identification of structural determinants on tau protein essential for its pathological function: novel therapeutic target for tau immunotherapy in Alzheimer's disease. Alzheimer's research & therapy. 2014;6(4):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Novak P, Schmidt R, Kontsekova E, Kovacech B, Smolek T, Katina S, et al. FUNDAMANT: an interventional 72-week phase 1 follow-up study of AADvac1, an active immunotherapy against tau protein pathology in Alzheimer's disease. Alzheimer's research & therapy. 2018;10(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fenstermaker RA, Ciesielski MJ, Qiu J, Yang N, Frank CL, Lee KP, et al. Clinical study of a survivin long peptide vaccine (SurVaxM) in patients with recurrent malignant glioma. Cancer Immunol Immunother. 2016;65(11):1339–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milgrom H, Kesler K, Byron M, Harbeck R, Holliday R, Leung DY. Response to cutaneous immunization with low-molecular-weight subunit keyhole limpet hemocyanin. Int Arch Allergy Immunol. 2012;157(3):269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elsamadicy AA, Chongsathidkiet P, Desai R, Woroniecka K, Farber SH, Fecci PE, et al. Prospect of rindopepimut in the treatment of glioblastoma. Expert Opin Biol Ther. 2017;17(4):507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


