Abstract
Background
Ultrasound (US) is an alternative to magnetic resonance enterography, and has the potential to significantly reduce waiting times, expedite clinical decision-making and improve patient experience. Point of care US is an advantage of the US imaging modality, where same day scanning, interpretation and treatment decisions can be made.
Aim
To systematically scope the literature on point of care US use in small bowel Crohn’s disease, generating a comprehensive list of factors relating to the current understanding of clinical utility of this imaging modality.
Methods
Searches included MEDLINE, EMBASE, Cochrane Library, Cumulative Index to Nursing and Allied Health Literature, PsycINFO, clinicaltrial.gov,‘TRIP’ and Epistemonikos. Reference lists of included studies were hand searched. Search terms were searched for as both keywords and subject headings (MeSH) as appropriate. Searches were performed with the ‘suggested search terms’ and ‘explode’ selection, and restricted to ‘human’, ‘adult’ and ‘English language’ publications. No date limits were applied to be as inclusive as possible. Two investigators conducted abstract and full-text review. No formal quality appraisal process was undertaken; however, quality of sources was considered when reporting findings. A narrative synthesis was conducted.
Results
The review included 42 sources from the UK, Europe, Japan, Canada and the USA. Small bowel ultrasound (SBUS) has been shown to be as accurate in detecting the presence of small bowel Crohn’s disease, is quicker, safer and more acceptable to patients, compared with magnetic resonance enterography. SBUS is used widely in central Europe and Canada but has not been embraced in the UK. Further research considering economic evaluation, clinical decision-making and exploration of perceived barriers to future implementation of SBUSs is required.
Keywords: inflammatory bowel disease, Crohn's disease, ultrasonography, clinical decision making, IBD clinical
Significance of this study.
What is already known on this topic
Small bowel ultrasound (SBUS) has been shown to have a relatively comparable accuracy to magnetic resonance enterography in detecting the presence of small bowel Crohn’s disease. SBUS and point of care ultrasound (POCUS) are used widely in central Europe, Canada and some parts of the USA, but have not been embraced in the UK and other parts of the world.
What this study adds
This study consolidates and comprehensively presents what is known regarding the clinical utility of SBUSs and POCUS for use in Crohn’s disease. This study gives an insight into the future directions of research in this field.
How might it impact on clinical practice in the foreseeable future
This study is the first step in a programme of work to investigate barriers and enablers to implementation of a SBUS, point of care, service for Crohn’s disease in the National Health Service. Through this work, we have been able to better direct our research to investigate stakeholder perceptions of barriers to implementation, clinical decision-making behaviours and cost-effectiveness studies.
Introduction
The UK prevalence of Crohn’s disease (CD) is one of the highest world wide.1 The mean cost per patient-year during follow-up has been reported as €3542 (median €717 (214–3512)) for patients with CD, with an overall annual cost to the National Health Service (NHS) of up to £470 million.2
Assessing treatment response with more objective measures and a wider array of biological therapies has significantly increased the projected inflammatory bowel disease (IBD) healthcare burden for the next decade.3 4 To ensure cost-effective IBD practice, complex and expensive pharmacological interventions should be targeted at patients most likely to benefit.5
Cross-sectional imaging is used to diagnose and monitor disease activity in small bowel CD (SBCD).6 Magnetic resonance enterography (MRE), with oral preparation and intravenous contrast is a standard of care modality in the UK for assessment and monitoring of SBCD.6 However, waiting times for an NHS MRE may be up to 4 weeks or in some instances longer, with reporting is then undertaken at a later date. Additionally, the use of gadolinium as contrast agent has a risk of allergy, is expensive and has been implicated with long-term brain deposition in exposed patients.7 The European Crohn’s and Colitis Organisation (ECCO) and the European Society of Gastrointestinal and Abdominal Radiology (ECCO-ESGAR) guidelines have already negated some of the risks posed by the use of gadolinium, by stating that gadolinium should be used on a case-by-case basis.8 Some centres are moving away from its use and have shown no significant decrease in accuracy.9 However, there is still a clinical need to find quicker, more tolerable and cheaper alternatives for monitoring patients with IBD.
Abdominal ultrasound (US) is an alternative to MRE, with the potential to reduce waiting times, speed up clinical decision-making and improve patient experiences and outcomes.10 Point of care (abdominal) US (POCUS) is an advantage of the US imaging modality, where same day scanning and interpretation can be undertaken.
This review is undertaken as the first step in investigating the use of POCUS for assessment of disease activity in SBCD. Due to the vastness of the existing evidence and the objective of this review, it was decided that a scoping review, rather than a systematic literature review, was more appropriate.11 The objective was to systematically scope the literature on POCUS use in SBCD, identify specific characteristics and expand the current understanding of the clinical utility of POCUS for patients with SBCD.
Multidimensional model of clinical utility
Clinical utility can be described as a multidimensional judgement about the usefulness, benefits and drawbacks of an intervention. The model of dimensions of clinical utility presented by Smart12 (figure 1) provides a frame work for assessing the clinical utility of a new technology or technique, asking whether the innovation is appropriate, accessible, practicable and acceptable for the purposes of the task intended. In this scoping review, factors were identified and grouped into themes in relation to the factors of clinical utility.
Figure 1.
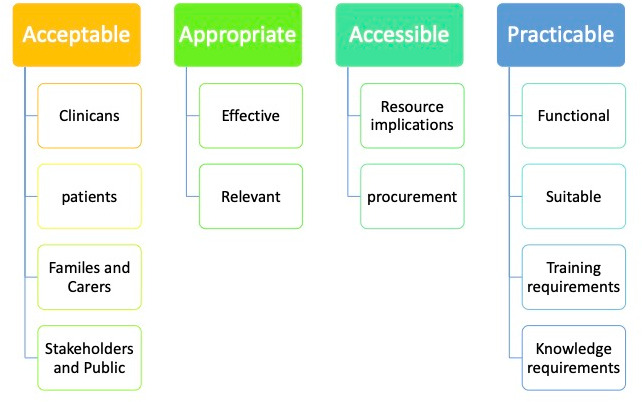
Factors of Clinical utility from Smart.27 The model of dimensions of clinical utility presented by Smart12 encompasses elements of work practice alongside other factors such as economic considerations, stakeholder acceptability and future planning for interventions and services. Assessing the clinical utility of a new technology or technique involves asking whether the innovation is appropriate, accessible, practicable and acceptable for the purposes of the task intended.12 58 59
Methods
Preliminary searches of MEDLINE, Cochrane Database of Systematic Reviews and JBI Evidence Synthesis were conducted, no current systematic reviews or scoping reviews on the same topic were identified. Methods for this study were developed based on established scoping review methodology.13 14 The research question was ‘What evidence is currently available on the clinical utility of POCUS for the diagnosis and management of SBCD?’.
Inclusion criteria
Searches of electronic databases of published literature included MEDLINE, EMBASE, the Cochrane Library, Cumulative Index to Nursing and Allied Health Literature and PsycINFO. Searches were also conducted of clinicaltrial.gov for current clinical trials, ‘TRIP’ and Epistemonikos. Reference lists of included studies, grey literature and non-indexed sources were hand searched to identify additional sources of relevance.
Search terms were searched as keywords in title and/or abstract and subject headings (MeSH) as appropriate. Search terms (table 1) were determined through consideration of previously reviewed literature and preliminary searches of Google Scholar. The Boolean operator ‘OR’ was used within each facet to maximise searches, with the operator ‘AND’ used between facets to combine terms, truncation of terms was used to be as inclusive as possible. Searches were performed with ‘suggested search terms’ and ‘explode’ selection, included any type of study design, and restricted to ‘human’, ‘adult’ and ‘English language’ publications. No date limits were applied to be as inclusive as possible.
Table 1.
Key search terms
| Crohn’s disease (MeSH) | Small bowel | Ultrasound (MeSH) |
| Crohn’s disease | Ileal | Ultrasound |
| Crohn’s | Ileum | US |
| CD | Ileitis | Sonography |
| Crohn* | Echography | |
| Inflammatory bowel disease | Point of care ultrasound | |
| IBD | POCUS | |
| Ultrasonography |
CD, Crohn’s disease; IBD, inflammatory bowel disease; POCUS, point of care ultrasound; US, ultrasound.
Two investigators (SJR and GM) independently screened the title and abstract of all retrieved citations for inclusion against inclusion criteria. Each author reviewed each title and abstract, if both agreed to include the full text for review it was included, if both chose to exclude it was excluded. There were no disagreements which led to the need for a third author deliberation. No formal quality appraisal process was undertaken; however, quality of sources was considered when reporting findings.
The two investigators (SJR and GM) then each independently assessed all full-text articles to determine if they met inclusion criteria. There were no disagreements about study eligibility at the full-text review stage that required discussion with a third investigator. Reasons for exclusion of full-text sources were recorded and reported in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses15 flow diagram (figure 2). A narrative synthesis was conducted to explore relationships within and across the included sources.
Figure 2.
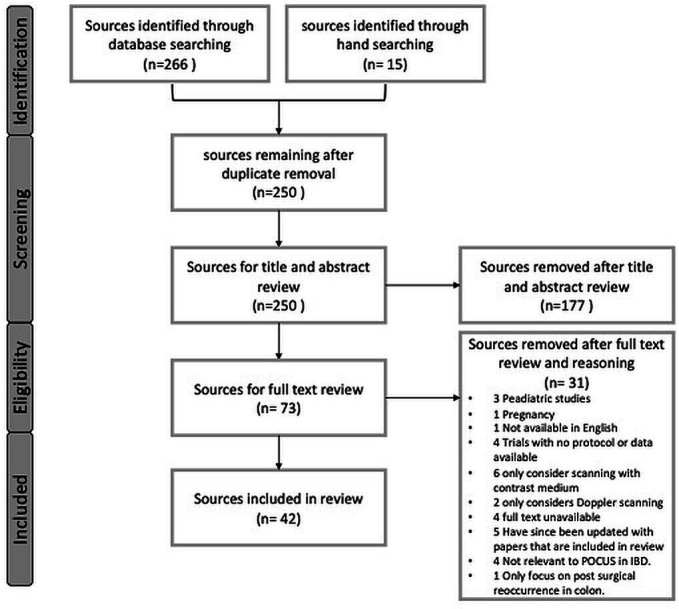
PRISMA flow diagram—supplemental material. The flow diagram depicts the flow of sources through the different phases of screening for inclusion and exclusion. We included 42 sources in our scoping review. Reasons for full-test exclusion are detailed in the PRISMA flow diagram. IBD, inflammatory bowel disease; POCUS, point of care ultrasound; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Results
The review included 42 sources (online supplemental table 1). A common view across 24 of the included sources was that US is non-invasive test that is acceptable to and well-tolerated by patients, is safe and is inexpensive.8 10 16–37
flgastro-2021-101897supp001.pdf (52.8KB, pdf)
Only four sources directly mention the use of POCUS,10 30 36 38 the remainder discuss the use of SBUS. For the purposes of this review, we consider the use of SBUS without contrast agents, minimal or no bowel preparation and not the use of specialised tests such as Doppler or elastography scanning.
In central Europe and Canada, SBUS is widely used, often performed by gastroenterologists. This allows gastroenterologists to have a whole view of patient management, reducing waiting times for clinical decision-making.34 36
The METRIC study showed that both SBUS and MRE had a diagnostic accuracy above 90% for detecting SBCD. Sensitivity of SBUS for small bowel disease presence and extent were 92% and 70%, respectively.39 Sensitivity and specificity were significantly greater for MRE, with a 10% and 14% difference for extent and a 5% and 12% difference for presence.39 It was also found that there was substantial sonographic agreement for the presence of SBCD, both in newly diagnosed and relapsed disease.40 Agreement for SBCD extent was inferior to that of presence alone; this is in contrast to previous work by Parente et al,41 who reported near perfect agreement for segmental localisation.
The most prominent parameter for detection of inflammation throughout the reviewed sources was bowel wall thickness (BWT), which correlates well with clinical disease activity markers.8 10 17–22 24 25 27–29 32 34 35 37 38 42–46 The most common cut-off value was BWT exceeding 3 mm being considered pathological and a BWT of 2 mm or less considered normal.31 32 42
A number of SBUS scores have been developed, most lack validation, were developed from small sample sizes or are limited to quantification of ‘damage’ or the risk of surgery.25 47 Novak et al 25 have developed a promising, simple US score for identifying CD activity comparing BWT to endoscopic activity, however the results reported have not yet been externally validated.25
Fraquelli et al 34 notes that the use of SBUS in different clinical settings may impact on the utility of SBUS. In specialist centres where the pretest probability of IBD is elevated, US would be used to ‘rule in’ the disease. Alternatively, in primary care SBUS would be a useful tool to ‘rule out’ the disease.48
Paredes et al 49 used SBUS for assessing changes induced with an antitumour necrosing factor (TNF) therapy in CD. The study reported a significant reduction in BWT in patients receiving anti-TNF therapy, however, ‘resolution’ of inflammation visible on SBUS was only achieved in 29% of subjects.34 Results from Ripolles et al 45 showed that SBUS may be able to predict the 1-year response to anti-TNF therapy after 12 weeks of treatment with 85% (22/26) of patients showing a sonographic response at 12 and 52 weeks. Moreover, in the majority of patients (96%), clinical and biological response corresponded to sonographic response. Multiple authors suggest that SBUS may have a role in supporting MRE as a useful examination for monitoring the response to treatment in CD patients.23 29 34 38 50
The METRIC39 study found no major difference between MRE and SBUS on therapeutic decision-making. Both tests agreed with a final therapeutic decision based on all tests in >75% of cases. Very little further investigation into the impact of the use of SBUS on the clinical decision-making behaviours of clinicians has been undertaken, nor exploration of the confidence of clinical decisions made using each imaging modality.
Multiple sources refer to SBUS being inexpensive, however there is little empirical evidence within the included sources to support this claim.20–23 26 39 51 The METRIC39 study presents data on a cost-utility analysis of MRE versus SBUS indicating a trend towards SBUS over MRE. However, given the small non-significant differences in costs and QALYs between the two options, it was not possible to endorse US or MRE on cost-effectiveness grounds.
The benefits of POCUS being performed by a member of the clinical IBD team include increased capacity for real-time interpretation of findings, expediting decisions concerning disease management and strengthening the rapport between healthcare professionals (HCPs) and patients.35 36 38 Many centres have standalone IBD US lists. These lists may be advantageous in expanding capacity to perform SBUS, particularly in centres where gastroenterologists are not trained in SBUS. This may also maximise healthcare resource allocation via predictable patient bookings.36
Over the last few years, outside of the UK, the widespread availability of US technology and the increasing expertise of practitioners has boosted the uptake and role of US in assessing patients with IBD.31 34 39 43 Throughout the included sources, results reported were from SBUS being performed by individuals with extensive experiences of SBUS.16 17 19–21 26 28–30 37 44 45 48 For example, Taylor et al 39 report that the team involved in the METRIC study had an average of 8 years (4–11) experience of interpreting US. Despite SBUS typically being performed using standard devices and techniques, the uptake is not widespread or universal. Multiple authors have speculated this is due to lack of training availability and the substantial training and experience requirements of those preforming the test.34 52 However, interobserver agreement between sonographers with variable experience in SBUS has been reported in preliminary studies showing satisfactory results.10 16 17 34 36 37 40 42 48 With appropriate training, transabdominal US can be performed by specialist gastroenterologists in clinic as part of routine care.30 Gastroenterologist-performed SBUS is yet to establish universal acceptance.53 The benefit of SBUS being performed within a radiology department by a dedicated sonographer or radiologist is the potential for increased diagnostic accuracy in detecting pathology.36
SBUS and MRE are the most preferred imaging modalities by patients with CD.39 SBUS is well tolerated by patients with IBD.8 26 MRE recovery time has been shown to be significantly longer than US, with 15 participants out of 149 (10%) reporting immediate recovery following MRE compared with 102/147 (69%) for US.54 The proportion of participants willing to repeat MRE was 127/147 (91%). This was lower than for US where 133/135 (99%) were happy to repeat the test.54 Overall 128/145 patients rated MRE as very or fairly acceptable, while 144/146 (99%) participants rated US as very or fairly acceptable. Issues reported by patients concerning MRE mainly reflected ingesting contrast, repeated breath holds and the after-effects of contrast such as diarrhoea and bloating. Perceived scan burden was significantly higher for MRE than SBUS. One important finding is that patients rated diagnostic accuracy as the most important attribute and more important than the challenges related to discomfort of undergoing scans.55 None of the included sources presented findings related to preferences of HCPs or patients as to where and when SBUS should be delivered.
Discussion
Mucosal healing, defined by the absence of ulcerations, is recommended as the therapeutic goal in clinical practice. MRE is the current standard for assessing SBCD, however. It is expensive, time consuming and poorly tolerated by patients.7 30
Meta-analyses suggest that MRE and SBUS have similar accuracy for diagnosing and staging SBCD.56 SBUS could be a good alternative to more invasive and expensive imaging techniques. Besides being quick, well-tolerated and readily available, SBUS is reported and interpreted at the time of scanning and allows for expedited clinical decision-making.10
POCUS is reported as having impact on clinical decision-making in routine IBD care by expediting clinical decision-making.10 30 36 However, there is no current evidence on the impact that SBUS has on the nature of clinical decision-making behaviours, or confidence of HCPs making those clinical decisions.
Multiple sources referred to SBUS as inexpensive. However, none of the included sources presented clear data relating to cost or cost effectiveness of SBUS or POCUS. More data on the cost effectiveness of SBUS are needed to encourage the implementation of SBUS in IBD services.10 SBUS involves the use of standard US equipment that is readily available in most hospitals, however increasing scanning capacity also involves increased resources such as staffing and training. SBUS is often seen as having limited clinical utility due to operator dependence.36 However, this criticism is perhaps more reflective of a previous lack of identifiable international performance and training standards.36 NHS radiology workforce is short staffed by 33%, and is already at a deficit before considering the backlog following COVID-19.57 ECCO-ESGAR guidelines describe the dedicated training in bowel US process, and that SBUS should be performed following training in general abdominal US.8
Although various SBUS activity scores are available, the methodology for development was insufficient in most studies. There are several scoring systems for disease activity assessment using SBUS in CD, however until recently none had been completely validated.
There is no current work to investigate patient or HCPs preferences or service delivery. There are also questions relating to HCP perceptions of acceptability related to the diagnostic accuracy and confidence in basing clinical decisions on SBUS. It would seem prudent to investigate broader stakeholder perceptions of the use of POCUS in order to better understand perceived barriers and enablers to POCUS implementation in world-wide healthcare systems and recognise and manage preferences for future service delivery.
Limitations
Scoping reviews do not formally evaluate the quality of evidence gathering information from a wide range of study designs and methods, providing a descriptive account of available information leading to broad overview of the available literature. The outcomes represent an accurate response to the research question. Continuous conversations between authors occurred throughout to ensure a unanimous decision regarding article searches, thus limiting any potential bias. The scope of background information collected, disease activity levels, depth of data relating to the use of SBUS/POCUS vary vastly between sources.
Conclusions
SBUS has been shown have a relatively comparable accuracy to MRE in detecting presence of SBCD. SBUS and POCUS are used widely in central Europe, Canada and some parts of the USA, but has not been embraced in the UK and other parts of the world. The resources required in terms of equipment are readily available in most hospitals. Resource implications for future implementation include training of gastroenterologists and staffing of supporting radiology departments
Multiple sources reported SBUS as an inexpensive test, however there is scant literature to support this. Further research in this area would better inform decision-makers regarding future intervention implementation.
SBUS is reported as being a useful tool to expedite clinical decision-making, but there is no evidence relating to the impact on the nature of clinical decision-making by HCPs. Further research in this area would help us to better understand the impact of POCUS on clinical practice, leading to better understanding of practicable and acceptable aspects of clinical utility.
Footnotes
Twitter: @Shellie_Jean
Contributors: SJR is acting as the submission’s guarantor. All coauthors have approved this final version of the manuscript for submission. SJR undertook the literature searches, read and analysed the data, conducted a narrative review, wrote the manuscript and collated reviews from coauthors. GM was second author, reading and analysing the data, contributed to the narrative review of the data, whole manuscript review and final editing. CC was appointed as third reviewer, however no discrepancies occurred. PL, BS and professor ST offer expert overview and whole manuscript review.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer: This study is funded by the National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands (ARC EM). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Competing interests: SJR, BS, PL and CC—nil to declare GWM has received educational support from Abbvie, Janssen, NAPP, Takeda Pharmaceuticals, Merck Sharp & Dohme Ltd, Ferring and Dr Falk. He has received speaker honoraria from Merck Sharp & Dohme Ltd, Abbvie, Janssen, Ferring and Takeda Pharmaceuticals. He attended advisory boards for Abbvie, Celgene, Takeda Pharmaceuticals, Janssen, Medtronic, Phebra Pharmaceuticals, Servertus Associates Ltd and Dr Falk. Dr Moran is a consultant for Alimentiv. SAT is consultant to Alimentiv and has share options in Motilent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Chu TPC, Moran GW, Card TR. The pattern of underlying cause of death in patients with inflammatory bowel disease in England: a record linkage study. J Crohns Colitis 2017;11:578–85. 10.1093/ecco-jcc/jjw192 [DOI] [PubMed] [Google Scholar]
- 2. Burisch J, Vardi H, Schwartz D, et al. Health-care costs of inflammatory bowel disease in a pan-European, community-based, inception cohort during 5 years of follow-up: a population-based study. Lancet Gastroenterol Hepatol 2020;5:454–64. 10.1016/S2468-1253(20)30012-1 [DOI] [PubMed] [Google Scholar]
- 3. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International organization for the study of IBD (IOIBD): determining therapeutic goals for Treat-to-Target strategies in IBD. Gastroenterology 2021;160:1570–83. 10.1053/j.gastro.2020.12.031 [DOI] [PubMed] [Google Scholar]
- 4. Bouguen G, Levesque BG, Feagan BG, et al. Treat to target: a proposed new paradigm for the management of Crohn's disease. Clin Gastroenterol Hepatol 2015;13:1042–50. 10.1016/j.cgh.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 5. Kennedy NA, Heap GA, Green HD, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol 2019;4:341–53. 10.1016/S2468-1253(19)30012-3 [DOI] [PubMed] [Google Scholar]
- 6. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for Treat-to-Target. Am J Gastroenterol 2015;110:1324–38. 10.1038/ajg.2015.233 [DOI] [PubMed] [Google Scholar]
- 7. Allocca M, Fiorino G, Bonifacio C, et al. Comparative accuracy of bowel ultrasound versus magnetic resonance Enterography in combination with colonoscopy in assessing Crohn's disease and guiding clinical decision-making. J Crohns Colitis 2018;12:1280–7. 10.1093/ecco-jcc/jjy093 [DOI] [PubMed] [Google Scholar]
- 8. Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019;13:144–64. 10.1093/ecco-jcc/jjy113 [DOI] [PubMed] [Google Scholar]
- 9. Quaia E, Sozzi M, Gennari AG, et al. Impact of gadolinium-based contrast agent in the assessment of Crohn's disease activity: is contrast agent injection necessary? J Magn Reson Imaging 2016;43:688–97. 10.1002/jmri.25024 [DOI] [PubMed] [Google Scholar]
- 10. Allocca M, Furfaro F, Fiorino G, et al. Point-Of-Care ultrasound in inflammatory bowel disease. J Crohn’s Colitis 2021;15:143–51. 10.1093/ecco-jcc/jjaa151 [DOI] [PubMed] [Google Scholar]
- 11. Peters MDJ, Godfrey CM, Khalil H, et al. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc 2015;13:141–6. 10.1097/XEB.0000000000000050 [DOI] [PubMed] [Google Scholar]
- 12. Smart A. A multi-dimensional model of clinical utility. Int J Qual Health Care 2006;18:377-82. 10.1093/intqhc/mzl034 [DOI] [PubMed] [Google Scholar]
- 13. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 14. Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010;5:69. 10.1186/1748-5908-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–73. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 16. Girlich C, Ott C, Strauch U, et al. Clinical feature and bowel ultrasound in Crohn's disease - does additional information from magnetic resonance imaging affect therapeutic approach and when does extended diagnostic investigation make sense? Digestion 2011;83:18–23. 10.1159/000314590 [DOI] [PubMed] [Google Scholar]
- 17. Calabrese E, Kucharzik T, Maaser C, et al. Real-time interobserver agreement in bowel ultrasonography for diagnostic assessment in patients with Crohn's disease: an international multicenter study. Inflamm Bowel Dis 2018;24:2001–6. 10.1093/ibd/izy091 [DOI] [PubMed] [Google Scholar]
- 18. Maconi G, Carsana L, Fociani P, et al. Small bowel stenosis in Crohn's disease: clinical, biochemical and ultrasonographic evaluation of histological features. Aliment Pharmacol Ther 2003;18:749–56. 10.1046/j.1365-2036.2003.01673.x [DOI] [PubMed] [Google Scholar]
- 19. Calabrese E. Crohn’s disease: a comparative prospective study of transabdominal ultrasonography, small intestine contrast ultrasonography, and small bowel enema, 2005. Available: https://academic.oup.com/ibdjournal/article/11/2/139/4683858 [DOI] [PubMed]
- 20. Sturm EJC, Cobben LPJ, Meijssen MAC, et al. Detection of ileocecal Crohn's disease using ultrasound as the primary imaging modality. Eur Radiol 2004;14:778–82. 10.1007/s00330-003-2204-1 [DOI] [PubMed] [Google Scholar]
- 21. Novak KL, Wilson SR. Sonography for surveillance of patients with Crohn disease. J Ultrasound Med 2012;31:1147-52. 10.7863/jum.2012.31.8.1147 [DOI] [PubMed] [Google Scholar]
- 22. Valette PJ, Rioux M, Pilleul F, et al. Ultrasonography of chronic inflammatory bowel diseases. Eur Radiol 2001;11:1859–66. 10.1007/s003300101065 [DOI] [PubMed] [Google Scholar]
- 23. Castiglione F, Testa A, Rea M, et al. Transmural healing evaluated by bowel sonography in patients with Crohn's disease on maintenance treatment with biologics. Inflamm Bowel Dis 2013;19:1928–34. 10.1097/MIB.0b013e31829053ce [DOI] [PubMed] [Google Scholar]
- 24. Potthast S, Rieber A, Von Tirpitz C, et al. Ultrasound and magnetic resonance imaging in Crohn's disease: a comparison. Eur Radiol 2002;12:1416–22. 10.1007/s00330-001-1191-3 [DOI] [PubMed] [Google Scholar]
- 25. Novak KL, Kaplan GG, Panaccione R, et al. A simple ultrasound score for the accurate detection of inflammatory activity in Crohn's disease. Inflamm Bowel Dis 2017;23:2001–10. 10.1097/MIB.0000000000001174 [DOI] [PubMed] [Google Scholar]
- 26. Grunshaw ND. Initial experience of a rapid-access ultrasound imaging service for inflammatory bowel disease. Gastrointestinal Nurs 2019;17:42–8. 10.12968/gasn.2019.17.2.42 [DOI] [Google Scholar]
- 27. Calabrese E, Maaser C, Zorzi F, et al. Bowel ultrasonography in the management of Crohn's disease. A review with recommendations of an international panel of experts. Inflamm Bowel Dis 2016;22:1168–83. 10.1097/MIB.0000000000000706 [DOI] [PubMed] [Google Scholar]
- 28. Wilkens R, Novak KL, Lebeuf-Taylor E, et al. Impact of intestinal ultrasound on classification and management of Crohn's disease patients with inconclusive colonoscopy. Can J Gastroenterol Hepatol 2016;2016:8745972. 10.1155/2016/8745972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hollerbach S, Geissler A, Schiegl H, et al. The accuracy of abdominal ultrasound in the assessment of bowel disorders. Scand J Gastroenterol 1998;33:1201–8. 10.1080/00365529850172575 [DOI] [PubMed] [Google Scholar]
- 30. Novak K, Tanyingoh D, Petersen F, et al. Clinic-based point of care transabdominal ultrasound for monitoring Crohn's disease: impact on clinical decision making. J Crohns Colitis 2015;9:795-801. 10.1093/ecco-jcc/jjv105 [DOI] [PubMed] [Google Scholar]
- 31. Kucharzik T, Kannengiesser K, Petersen F. The use of ultrasound in inflammatory bowel disease. Ann Gastroenterol 2017;30:135–44. 10.20524/aog.2016.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Conti CB, Giunta M, Gridavilla D, et al. Role of bowel ultrasound in the diagnosis and follow-up of patients with Crohn's disease. Ultrasound Med Biol 2017;43:725-734. 10.1016/j.ultrasmedbio.2016.12.014 [DOI] [PubMed] [Google Scholar]
- 33. Bollegala N, Griller N, Bannerman H, et al. Ultrasound vs endoscopy, surgery, or pathology for the diagnosis of small bowel Crohn's disease and its complications. Inflamm Bowel Dis 2019;25:1313–38. 10.1093/ibd/izy392 [DOI] [PubMed] [Google Scholar]
- 34. Fraquelli M, Castiglione F, Calabrese E, et al. Impact of intestinal ultrasound on the management of patients with inflammatory bowel disease: how to apply scientific evidence to clinical practice. Dig Liver Dis 2020;52:9–18. 10.1016/j.dld.2019.10.004 [DOI] [PubMed] [Google Scholar]
- 35. Kucharzik T, Maaser C. Intestinal ultrasound and management of small bowel Crohn's disease. Therap Adv Gastroenterol 2018;11:175628481877136. 10.1177/1756284818771367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bryant RV, Friedman AB, Wright EK, et al. Gastrointestinal ultrasound in inflammatory bowel disease: an underused resource with potential paradigm-changing application. Gut 2018;67:973–85. 10.1136/gutjnl-2017-315655 [DOI] [PubMed] [Google Scholar]
- 37. Pascu M, Roznowski AB, Adler A, et al. Clinical relevance of transabdominal ultrasonography and magnetic resonance imaging in patients with inflammatory bowel disease of the terminal ileum and large bowel background: Ileocolonoscopy represents the diagnostic standard, 2004. Available: https://academic.oup.com/ibdjournal/article/10/4/373/4718183 [DOI] [PubMed]
- 38. Calabrese E, Zorzi F, Lolli E, et al. Positioning ultrasonography into clinical practice for the management of Crohn's disease. Gastroenterol Hepatol 2015;11:384-90. [PMC free article] [PubMed] [Google Scholar]
- 39. Taylor SA, Mallett S, Bhatnagar G, et al. Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn's disease (metric): a multicentre trial. Lancet Gastroenterol Hepatol 2018;3:548–58. 10.1016/S2468-1253(18)30161-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bhatnagar G, Quinn L, Higginson A, et al. Observer agreement for small bowel ultrasound in Crohn's disease: results from the metric trial. Abdom Radiol 2020;45:3036–45. 10.1007/s00261-020-02405-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parente F, Greco S, Molteni M, et al. Role of early ultrasound in detecting inflammatory intestinal disorders and identifying their anatomical location within the bowel. Aliment Pharmacol Ther 2003;18:1009–16. 10.1046/j.1365-2036.2003.01796.x [DOI] [PubMed] [Google Scholar]
- 42. Calabrese E, Zorzi F, Pallone F. Ultrasound of the small bowel in Crohn's disease. Int J Inflam 2012;2012:1–6. 10.1155/2012/964720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maconi G, Radice E, Greco S, et al. Bowel ultrasound in Crohn's disease. Best Pract Res Clin Gastroenterol 2006;20:93–112. 10.1016/j.bpg.2005.09.001 [DOI] [PubMed] [Google Scholar]
- 44. Parente F, Maconi G, Bollani S, et al. Bowel ultrasound in assessment of Crohn's disease and detection of related small bowel strictures: a prospective comparative study versus X ray and intraoperative findings. Gut 2002;50:490–5. 10.1136/gut.50.4.490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ripollés T, Paredes JM, Martínez-Pérez MJ, et al. Ultrasonographic changes at 12 weeks of anti-TNF drugs predict 1-year sonographic response and clinical outcome in Crohn's disease: a multicenter study. Inflamm Bowel Dis 2016;22:2465–73. 10.1097/MIB.0000000000000882 [DOI] [PubMed] [Google Scholar]
- 46. Taylor SA, Avni F, Cronin CG, et al. The first joint ESGAR/ ESPR consensus statement on the technical performance of cross-sectional small bowel and colonic imaging. Eur Radiol 2017;27:2570–82. 10.1007/s00330-016-4615-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bots S, Nylund K, Löwenberg M, et al. Ultrasound for assessing disease activity in IBD patients: a systematic review of activity scores. J Crohns Colitis 2018;12:920–9. 10.1093/ecco-jcc/jjy048 [DOI] [PubMed] [Google Scholar]
- 48. Smith R, Taylor K, Friedman A, et al. P245 inter-rater reliability of gastrointestinal ultrasound in the assessment of disease activity in patients with inflammatory bowel disease prior to commencing medical therapy. J Crohn’s Colitis 2020;14:S269–71. 10.1093/ecco-jcc/jjz203.374 [DOI] [Google Scholar]
- 49. Paredes JM, Ripollés T, Cortés X, et al. Abdominal sonographic changes after antibody to tumor necrosis factor (anti-TNF) alpha therapy in Crohn's disease. Dig Dis Sci 2010;55:404–10. 10.1007/s10620-009-0759-7 [DOI] [PubMed] [Google Scholar]
- 50. Kucharzik T, Wittig BM, Helwig U, et al. Use of intestinal ultrasound to monitor Crohn's disease activity. Clin Gastroenterol Hepatol 2017;15:535–42. 10.1016/j.cgh.2016.10.040 [DOI] [PubMed] [Google Scholar]
- 51. Panaccione R, Steinhart AH, Bressler B, et al. Canadian association of gastroenterology clinical practice guideline for the management of luminal Crohn's disease. Clin Gastroenterol Hepatol 2019;17:1680–713. 10.1016/j.cgh.2019.02.043 [DOI] [PubMed] [Google Scholar]
- 52. Lamb CA, Kennedy NA, Raine T, et al. British Society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68:s1-s106. 10.1136/gutjnl-2019-318484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol 2018;113:481–517. 10.1038/ajg.2018.27 [DOI] [PubMed] [Google Scholar]
- 54. Miles A, Bhatnagar G, Halligan S, et al. Magnetic resonance enterography, small bowel ultrasound and colonoscopy to diagnose and stage Crohn's disease: patient acceptability and perceived burden. Eur Radiol 2019;29:1083–93. 10.1007/s00330-018-5661-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Taylor SA, Mallett S, Bhatnagar G, et al. Magnetic resonance enterography compared with ultrasonography in newly diagnosed and relapsing Crohn’s disease patients: the METRIC diagnostic accuracy study. Health Technol Assess 2019;23:1–162. 10.3310/hta23420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Greenup A-J, Bressler B, Rosenfeld G. Medical imaging in small bowel crohn's disease-computer tomography enterography, magnetic resonance enterography, and ultrasound: "which one is the best for what?". Inflamm Bowel Dis 2016;22:1246–61. 10.1097/MIB.0000000000000727 [DOI] [PubMed] [Google Scholar]
- 57. RCR . Clinical radiology UK workforce census report 2020, 2020. [Google Scholar]
- 58. Hertz D, Taggart C, Waterman J, et al. Is there utility in clinical utility modeling for diagnostic technologies? Value Heal 2015;18:A52. [Google Scholar]
- 59. McCormack RT, Billings PR. Clinical utility: informing treatment decisions by changing the paradigm. NAM Perspect 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
flgastro-2021-101897supp001.pdf (52.8KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.


