Abstract
Background
To determine how clinicians with a DATA waiver to prescribe buprenorphine for opioid use disorder (OUD) adapted during the COVID-19 pandemic to emergency authorities, including use of telehealth to prescribe buprenorphine, the challenges faced by clinicians, and strategies employed by them to manage patients with OUD.
Methods
From June 23, 2020 to August 19, 2020, we conducted an electronic survey of U.S. DATA-waivered clinicians. Descriptive statistics and multivariable logistic regression were used for analysis.
Results
Among 10,238 respondents, 68 % were physicians, 25 % nursing-related providers, and 6% physician assistants; 28 % reported never prescribing or not prescribing in the 12 months prior to the survey. Among the 72 % of clinicians who reported past 12-month buprenorphine prescribing (i.e. active practitioners during the pandemic) 30 % reported their practice setting closed to in-person visits during COVID-19; 33 % reported remote prescribing to new patients without an in-person examination. The strongest predictors of remote buprenorphine prescribing to new patients were prescribing buprenorphine to larger numbers of patients in an average month in the past year and closure of the practice setting during the pandemic; previous experience with remote prescribing to established patients prior to COVID-19 also was a significant predictor. Among clinicians prescribing to new patients without an in-person examination, 5.5 % reported difficulties with buprenorphine induction, most commonly withdrawal symptoms.
Conclusions
Telehealth practices and prescribing to new patients without an in-person examination were adopted by DATA-waivered clinicians during the first six months of COVID-19. Permanent adoption of these authorities may enable expanded access to buprenorphine treatment.
Keywords: Buprenorphine, COVID-19, Opioid use disorder, Opioid overdose, Medication treatment for opioid use disorder
1. Introduction
The decades-long overdose crisis in the United States continues to worsen. Drug overdose deaths increased from 67,367 deaths in 2018 to 70,630 in 2019 (Hedegaard et al., 2020). In 2020, these increases appear to have accelerated so that the provisional predicted number of deaths in the prior 12 months through September 2020 was more than 90,000, increases driven particularly by overdoses involving synthetic opioids (Centers for Disease Control and Prevention (Centers for Disease Control and Prevention, 2021). The emergence of the coronavirus disease 2019 (COVID-19) pandemic and implementation of community mitigation measures such as stay-at-home orders to slow the spread of SARS-CoV-2, the virus that causes COVID-19, have raised serious concerns about exacerbation of the overdose crisis and increasing risk for substance use and overdose among individuals with opioid use disorder (OUD) (Alexander et al., 2020; Centers for Disease Control and Prevention, 2021; Davis and Samuels, 2020; Holland et al., 2021; Volkow, 2020).
The potential impact of the COVID-19 pandemic on people with OUD is multifaceted. Social distancing policies could increase feelings of isolation and induce economic, relationship, and other stressors which can worsen mental health and result in increased substance use (Ataiants et al., 2020; Volkow, 2020) Social distancing also may increase the likelihood of using opioids or other drugs alone, a well-documented overdose risk factor (Henry et al., 2020; Volkow, 2020). Further, access to addiction treatment and recovery support services for people with OUD may be disrupted due to clinician office closures, discontinuation of in-person treatment and peer-support groups, and delays in care seeking due to concerns about exposure to COVID-19 during medical visits (Alexander et al., 2020; Henry et al., 2020; Volkow, 2020).
Although data are still emerging, available research suggests trends continued to worsen during the COVID-19 pandemic. A recent survey found that in June 2020, 30.9 % of U.S. adults reported symptoms of anxiety or depressive disorders, 26.3 % reported symptoms of a trauma- and stressor-related disorder, and 13.3 % reported they had initiated or increased substance use (Czeisler et al., 2020). Data from a multi-state database that tracks overdose reports by law enforcement and first responders reported a 17.6 % increase in suspected overdose submissions during March 19, 2020 to May 19, 2020, when stay-at-home orders were in effect, compared to January 1, 2020 to March 18, 2020 (Alter and Yeager, 2020). Similarly, a CDC Health Alert Network Advisory published in December 2020, indicated that overdose deaths accelerated through at least May 2020 nationally (Centers for Disease Control and Prevention, 2020).
To mitigate opioid-related harms and facilitate access and continuity of care for people with OUD and other substance use disorders, multiple actions have been taken at the federal level since the declaration of the COVID-19 Public Health Emergency (PHE) on January 27, 2020 (U.S. Department of Health and Human Services, 2020a). The Centers for Medicare & Medicaid Services (CMS) expanded payment for telehealth services and provided flexibility on accepted communication technologies for clinical care (Centers for Medicare and Medicaid Services (2020)), including a waiver to allow use of audio-only communications; the Substance Abuse and Mental Health Services Administration (SAMHSA) relaxed policies related to take-home methadone and buprenorphine for OUD from opioid treatment programs (Substance Abuse and Mental Health Services Administration, 2020); the Office for Civil Rights (OCR) at the U.S Department of Health and Human Services (HHS) announced a waiver of potential penalties for “HIPAA violations against health care providers that serve patients through everyday communications technologies during the COVID-19 nationwide public health emergency” in March 2020 (U.S. Department of Health and Human Services, 2020b); and the Drug Enforcement Administration (DEA) provided an exemption to the requirement that clinicians with a Drug Addiction Treatment Act (DATA) waiver to prescribe buprenorphine for OUD treatment conduct an in-person examination prior to prescribing to a new patient with OUD, thus for the first time enabling clinicians to remotely prescribe buprenorphine to new patients evaluated via telehealth (DEA, 2020).
Although these actions hold promise to facilitate access to buprenorphine treatment and care, it remains unknown whether and to what extent providers utilize these new authorities. To address this gap, we surveyed a large sample of clinicians with a buprenorphine DATA waiver in order to assess the use of telehealth to prescribe buprenorphine to new and established patients, challenges faced by clinicians, and strategies employed by them to manage patients with OUD during the COVID-19 pandemic.
2. Materials and methods
2.1. Study sample and data collection
By using DEA files to identify clinicians with a DATA waiver, a current controlled substances registration, and a valid e-mail address, 72,519 eligible clinicians were identified. An initial e-mail with an embedded online survey weblink was sent to each eligible clinician, using REDCap, a secure web application for online surveys and research (Harris et al., 2009). Reminder e-mails were sent on weeks 2, 3, and 6 of the data collection period (June 23 to August 19, 2020). The survey was closed 8 weeks after the original e-mail distribution. This activity was determined by CDC to meet the requirements of non-research public health surveillance as defined in 45 CFR 46.102(l)(2) (U.S. Department of Health and Human Services, 2021).
Of the 72,519 eligible clinicians, 10,326 responded to the survey (14.2 % response rate). The distribution among all DATA-waived clinicians was closely mirrored by the distributions of clinician respondents by state (Supplementary Table 1) and by clinician type (Supplementary Table 2), with correlation coefficients of ≥ 0.99.
2.2. Survey design
The 40-question survey instrument (Supplementary Table 3) was developed based on review of studies examining buprenorphine prescribing, review of emergency authorities implemented and guidance provided by DEA to clinicians on patient management during the COVID-19 PHE, and expert review. Three domains were included in the survey: (1) clinician demographic and practice characteristics; (2) buprenorphine prescribing practices and barriers to prescribing, including through the use of telehealth; and (3) challenges faced and strategies employed to manage patients during the COVID-19 PHE.
2.3. Statistical analysis
Analyses were limited to the 10,238 respondents (99.1 % of respondents) that provided responses to the question “Have you ever prescribed buprenorphine for opioid use disorder treatment since obtaining your DATA 2000 waiver?” Of the variables included in the analysis, the rate of non-response was less than 1% for 27 variables, between 1 %–3 % for six variables, and between 4 %–5 % for four variables.
Descriptive statistics are reported as frequencies and percentages for clinician demographic and practice characteristics overall and by three independent groups based on buprenorphine prescribing history since obtaining a DATA waiver: 1) never prescribed; 2) prescribed but not in the past 12 months; 3) prescribed in the past 12 months. These groups were selected to distinguish changes to practices during the pandemic from those of longstanding duration (i.e. never prescribed or no prescribing in the past 12 months). Differences in demographic and practice characteristics were assessed with chi-squared tests. To account for multiple comparisons, a Bonferroni correction was used and two-sided p-values of less than 0.0025 were considered statistically significant. In addition, among clinicians who reported never prescribing or not having prescribed in the past 12 months, percentages reporting specific reasons for not prescribing buprenorphine were calculated.
Among the subset of clinicians who reported prescribing buprenorphine in the past 12 months, frequencies and percentages were calculated for questions related to buprenorphine prescribing practices, barriers to remote prescribing, challenges faced, and strategies employed to manage patients with OUD during the COVID-19 PHE. Among these clinicians, multivariable logistic regression was used to assess characteristics associated with remote prescribing of buprenorphine to new patients without an in-person examination during the COVID-19 PHE. Results are presented as adjusted odds ratios and 95 % confidence intervals (CIs). Stata version 15.1 (College Station, Texas), was used to perform all statistical analyses.
3. Results
3.1. Characteristics of the study population
Characteristics of the 10,238 clinicians responding to the survey are found in Table 1 . Respondents were predominantly physicians (68.4 %); 25.3 % were nurse practitioners (or other nursing-related providers); 6.3 % were physician assistants. Twenty percent of clinicians reported never prescribing buprenorphine for OUD treatment since obtaining a DATA waiver, 7.6 % reported no prescribing in the past 12 months, and 72.4 % reported prescribing in the past 12 months. Significant differences (alpha = 0.0025) among the three buprenorphine prescribing groups were found for all but two demographic and practice characteristics. Overall, 68.8 % reported they were aware of the DEA exemption to the in-person examination requirement for new patients issued during the COVID-19 PHE. Awareness was highest among those with past 12-month buprenorphine prescribing (76.7 %) and lower among those without past 12-month (52.0 %) or never prescribing (46.1 %). Among the multiple reasons for not prescribing buprenorphine most cited by clinicians not prescribing in the past 12 months or never prescribing were lack of patient demand, lack of access to psychosocial services, and lack of institutional support. In addition, nearly 1 in 6 clinicians who had ever prescribed buprenorphine but not in the past 12 months and 1 in 17 who had never prescribed buprenorphine reported they were required to get a DATA 2000 waiver for their job but were not interested in prescribing. (see Supplementary Figure).
Table 1.
Characteristics of the Drug Addiction Treatment Act (DATA) 2000 Clinicians Survey respondents, June-August 2020 (n = 10,238).
| Total n (%) | Never prescribed n (%) | Prescribed, not past 12 months n (%) | Prescribed, past 12 months n (%) | p-value* | |
|---|---|---|---|---|---|
| Overall | 10,238 | 2042 (20.0) | 777 (7.6) | 7419 (72.4) | |
| Gender | |||||
| Female | 5484 (54.1) | 1216 (60.1) | 393 (51.2) | 3875 (52.8) | <0.001 |
| Male | 4648 (45.9) | 809 (39.9) | 374 (48.8) | 3465 (47.2) | |
| Age | |||||
| 25−34 | 1208 (11.9) | 266 (13.1) | 54 (7.0) | 888 (12.1) | <0.001 |
| 35−44 | 2592 (25.5) | 538 (26.5) | 147 (19.1) | 1907 (25.9) | |
| 45−54 | 2413 (23.8) | 499 (24.6) | 173 (22.5) | 1741 (23.7) | |
| 55−64 | 2329 (23.0) | 437 (21.5) | 208 (27.1) | 1684 (22.9) | |
| 65 or older | 1618 (15.9) | 289 (14.2) | 187 (24.3) | 1142 (15.5) | |
| U.S. Census Region | |||||
| Northeast | 2652 (26.0) | 479 (23.6) | 200 (25.9) | 1973 (26.7) | <0.001 |
| Midwest | 1875 (18.4) | 413 (20.3) | 116 (15.1) | 1346 (18.2) | |
| South | 2774 (27.2) | 523 (25.7) | 211 (27.4) | 2040 (27.6) | |
| West | 2853 (28.0) | 596 (29.3) | 235 (30.5) | 2.022 (27.3) | |
| U.S. Territory | 49 (0.5) | 21 (1.0) | 9 (1.2) | 19 (0.3) | |
| Rural-Urban Status of Practice Location | |||||
| Urban | 4837 (47.7) | 974 (48.5) | 376 (49.5) | 3487 (47.2) | 0.007 |
| Suburban | 3093 (30.5) | 609 (30.3) | 255 (33.6) | 2229 (30.2) | |
| Rural | 2221 (21.9) | 425 (21.2) | 129 (17.0) | 1667 (22.6) | |
| Type of Provider | <0.001 | ||||
| Physician (MD/DO) | 6996 (68.4) | 1364 (67.0) | 666 (85.7) | 4966 (67.0) | |
| Nurse Practitioner or other nursing-related provider | 2588 (25.3) | 554 (27.2) | 93 (12.0) | 1941 (26.2) | |
| Physician Assistant | 643 (6.3) | 119 (5.8) | 18 (2.3) | 506 (6.8) | |
| Primary Practice Specialty | <0.001 | ||||
| Primary Care | 4991 (49.1) | 922 (45.4) | 281 (36.7) | 3788 (51.4) | |
| Addiction Medicine | 439 (4.3) | 14 (0.7) | 10 (1.3) | 415 (5.6) | |
| Psychiatry | 2711 (26.7) | 533 (26.2) | 332 (43.4) | 1846 (25.0) | |
| Pediatrician | 107 (1.1) | 45 (2.2) | 10 (.3) | 52 (0.7) | |
| Obstetrician/Gynecologist | 265 (2.6) | 75 (3.7) | 19 (2.5) | 171 (2.3) | |
| Emergency Medicine | 731 (7.2) | 202 (10.0) | 31 (4.1) | 498 (6.8) | |
| Pain Medicine/Anesthesiology | 465 (4.6) | 95 (4.7) | 37 (4.8) | 333 (4.5) | |
| Infectious Disease | 79 (0.8) | 22 (1.1) | 6 (0.8) | 51 (0.7) | |
| Other | 380 (3.7) | 123 (6.1) | 39 (5.1) | 218 (3.0) | |
| Addiction Medicine Certification | |||||
| Addiction Psychiatry (ABMS) | 401 (3.9) | 37 (1.8) | 40 (5.2) | 324 (4.4) | <0.001 |
| Addiction Medicine (ASAM/ABPM) | 1841 (18.0) | 187 (9.2) | 115 (14.8) | 1539 (20.7) | <0.001 |
| Addiction Medicine (AOA) | 170 (1.8) | 30 (1.5) | 9 (1.2) | 140 (1.9) | 0.189 |
| None | 7944 (77.6) | 1799 (88.1) | 620 (79.8) | 5525 (75.5) | <0.001 |
| Years in Practice | |||||
| Less than 5 years | 2421 (23.7) | 526 (25.9) | 92 (11.9) | 1803 (24.4) | <0.001 |
| 5−10 years | 1988 (19.5) | 393 (19.3) | 123 (15.9) | 1472 (19.9) | |
| 11−15 years | 1172 (11.5) | 246 (12.1) | 103 (13.3) | 823 (11.1) | |
| 16−19 years | 817 (8.0) | 155 (7.6) | 66 (8.5) | 596 (8.1) | |
| 20 years or more | 3814 (37.4) | 715 (35.1) | 390 (50.4) | 2709 (36.6) | |
| Primary Practice Setting | |||||
| Office-based solo practice | 1531 (15.1) | 217 (10.8) | 139 (18.3) | 1175 (15.9) | <0.001 |
| Office-based group practice | 2853 (28.1) | 537 (26.6) | 183 (24.1) | 2133 (28.9) | |
| Specialty substance use treatment facility | 524 (5.2) | 28 (1.4) | 20 (2.6) | 476 (6.5) | |
| Opioid Treatment Program | 577 (5.7) | 27 (1.3) | 22 (2.9) | 528 (7.2) | |
| Community clinic (e.g., FQHC, RHC, CCBHC) | 1816 (17.9) | 359 (17.8) | 106 (14.0) | 1351 (18.3) | |
| Emergency Department | 679 (6.7) | 200 (9.9) | 38 (5.0) | 441 (6.0) | |
| Urgent Care | 127 (1.3) | 47 (2.3) | 12 (1.6) | 68 (0.9) | |
| Criminal Justice setting | 281 (2.8) | 67 (3.3) | 31 (4.1) | 183 (2.5) | |
| In-patient/Hospital setting | 1058 (10.4) | 314 (15.6) | 103 (13.6) | 641 (8.7) | |
| Veterans Affairs/Department of Defense/Indian Health Service | 394 (3.9) | 87 (4.3) | 29 (3.8) | 278 (3.8) | |
| Other (e.g., school-based clinic, LTCF, public health) | 321 (3.2) | 135 (6.7) | 76 (10.0) | 110 (1.5) | |
| Type of Insurance Accepted | |||||
| Medicaid | 7838 (76.6) | 1573 (77.0) | 484 (62.3) | 5781 (77.9) | <0.001 |
| Medicare | 7968 (77.8) | 1574 (77.1) | 509 (65.5) | 5.885 (79.3) | <0.001 |
| Private Insurance | 8133 (79.4) | 1582 (77.5) | 521 (67.1) | 6030 (81.3) | <0.001 |
| Other | 1492 (14.6) | 287 (14.1) | 120 (15.4) | 1085 (14.6) | 0.628 |
| Do not accept any type of Insurance | 1.095 (10.7) | 231 (11.3) | 141 (18.2) | 723 (9.8) | <0.001 |
| Granted permission to be listed on SAMHSA Buprenorphine Treatment Locator | |||||
| No | 3482 (35.0) | 1153 (59.0) | 404 (54.1) | 1925 (26.6) | <0.001 |
| Yes | 6463 (65.0) | 801 (41.0) | 343 (45.9) | 5319 (73.4) | |
| DATA 2000 Patient Limit | |||||
| 30 | 4377 (44.9) | 1514 (83.8) | 344 (51.0) | 2519 (34.6) | <0.001 |
| 100 | 3543 (36.3) | 266 (14.7) | 258 (38.3) | 3019 (41.5) | |
| 275 | 1835 (18.8) | 26 (1.4) | 72 (10.7) | 1737 (23.9) | |
| Awareness of DEA exemption of in-person physical examination prior to prescribing buprenorphine for first time | |||||
| No | 3181 (31.2) | 1088 (53.9) | 371 (48.0) | 1722 (23.3) | <0.001 |
| Yes | 7006 (68.8) | 929 (46.1) | 402 (52.0) | 5675 (76.7) | |
Source: CDC-NIDA Survey of DATA 2000 Clinicians, June-August 2020.
ABMS = American Board of Medical Specialties; ASAM = American Society of Addiction Medicine; ABPM = American Board of Preventive Medicine; AOA = American Osteopathic Association.
FQHC = Federally Qualified Health Center, RHC = Rural Health Center, CCBHC = Certified Community Behavioral Health Clinic, LTCF = Long-term Care Facility.
p value threshold for statistical significance set at 0.0025 based on Bonferroni correction.
3.2. Clinical practices during the COVID-19 Public Health Emergency
Analyses of clinical practices during the COVID-19 PHE are limited to the 72.4 % (n = 7419) of clinicians who prescribed buprenorphine in the past 12 months (Table 2 ). Nearly 30 % (29.9 %) reported their practice setting had closed to in-person visits during the COVID-19 PHE. Among those that closed, 70.4 % reported closing for 6 or more weeks and 52.3 % reported their practice setting had re-opened at the time of the survey. Thirty-three percent of clinicians reported remote prescribing to a new patient without conducting an in-person examination after DEA issued its COVID-19 PHE exemption; 87.0 % of these clinicians reported patients did not encounter difficulties with buprenorphine induction. Clinicians reported they used multiple communications technologies to engage new patients, including laptop or desktop with video (54.4 %), telephone without video (54.3 %), telephone with video (46.3 %), and iPad or other tablet with video (18.8 %).
Table 2.
Clinical practices among clinicians prescribing buprenorphine for opioid use disorder in past 12 months (n = 7419)¥.
| Clinical Practices | n (%) |
|---|---|
| Practice setting closed to in-person visits since the COVID-19 PHE | |
| No | 4985 (70.1) |
| Yes | 2127 (29.9) |
| Time practice setting was closed to in-person visits?* | |
| 1−2 weeks | 107 (5.0) |
| 3−4 weeks | 235 (11.1) |
| 5−6 weeks | 288 (13.5) |
| 6 or more weeks | 1497 (70.4) |
| Currently seeing patients in person* | |
| No | 1009 (47.7) |
| Yes | 1107 (52.3) |
| Prescribed buprenorphine remotely to established patients prior to the COVID-19 PHE | |
| No | 5831 (79.3) |
| Yes | 1518 (20.7) |
| Prescribed buprenorphine to new patients without conducting in-person examination since DEA issued exemption during COVID-19 PHE | |
| No | 4921 (67.0) |
| Yes | 2429 (33.0) |
| £Telecommunications technologies used to engage new patients in the absence of conducting in-person examination?** | |
| Laptop or desktop with video | 1321 (54.4) |
| Telephone without video | 1320 (54.3) |
| Telephone with video | 1125 (46.3) |
| iPad or other tablet with video | 456 (18.8) |
| Other | 79 (3.3) |
| New patients prescribed buprenorphine in the absence of in-person examination encountered difficulties with buprenorphine induction** | |
| No | 2104 (87.0) |
| Yes | 133 (5.5) |
| Unknown | 182 (7.5) |
| £Induction-related problems experienced by patients*** | |
| Significant withdrawal symptoms | 69 (51.9) |
| Over-sedation | 11 (8.3) |
| Allergic reaction | 2 (1.5) |
| Other (e.g., nausea/vomiting, difficulty getting medication) | 55 (41.4) |
| £Barriers preventing clinicians from prescribing buprenorphine to new patients without conducting an in-person examination**** | |
| Prefer to see patients in person | 1702 (34.6) |
| No new patients | 1671 (34.0) |
| Practice remains open for in-person visits | 1650 (33.5) |
| Concern about buprenorphine diversion | 612 (12.4) |
| Concern about home induction | 546 (11.1) |
| Inadequate remote technology for patients | 519 (10.6) |
| Inadequate reimbursement for telemedicine services | 186 (3.8) |
| Security or privacy concerns | 184 (3.7) |
| Inadequate broadband/internet in practice | 167 (3.4) |
| At DATA 2000 patient limit | 44 (0.9) |
| Other | 764 (15.5) |
| £Telecommunications technologies used to engage existing patients since COVID-19 PHE | |
| Telephone without video | 4717 (63.6) |
| Laptop or desktop with video | 2746 (50.5) |
| Telephone with video | 3507 (47.3) |
| iPad or other tablet with video | 1543 (20.8) |
| Other | 618 (8.3) |
| £App(s) used during remote patient encounters since COVID-19 PHE | |
| Zoom | 2690 (36.3) |
| FaceTime | 1107 (14.9) |
| Skype | 407 (5.5) |
| WebEx | 353 (4.8) |
| Microsoft Teams | 338 (4.6) |
| GoToMeeting | 190 (2.6) |
| VSee | 159 (2.1) |
| Other (e.g., Ring Central, Doximity, Epic Integrated) | 3974 (53.6) |
| Change in access to treatment as a result of DEA exemption to the in-person examination requirement | |
| Decreased access to treatment | 617 (8.8) |
| No change in access to treatment | 3742 (53.1) |
| Increased access to treatment | 2690 (38.2) |
| Patient demand for opioid use disorder treatment during the COVID-19 PHE | |
| Decreased demand for treatment | 769 (10.8) |
| No change in demand for treatment | 4287 (60.0) |
| Increased demand for treatment | 2095 (29.3) |
| Change in the number of days of buprenorphine prescribed per prescription since COVID-19 PHE | |
| Fewer days per prescription | 336 (4.7) |
| No change | 5120 (71.6) |
| More days per prescription | 1695 (23.7) |
| Likelihood of using long-acting or extended-release buprenorphine formulations compared to sublingual or buccal buprenorphine formulations since COVID-19 PHE | |
| Less likely to use long-acting or extended-release buprenorphine | 341 (4.8) |
| No change | 5249 (73.4) |
| More likely to use long-acting or extended release buprenorphine | 281 (3.9) |
| Long-acting or extended-release buprenorphine products are not available in my practice | 1278 (17.9) |
| Likelihood of using extended-release injectable naltrexone compared to sublingual or buccal buprenorphine formulations since COVID-19 PHE | |
| Less likely to use extended-release naltrexone | 478 (6.7) |
| No change | 5246 (75.2) |
| More likely to use extended-release naltrexone | 639 (9.0) |
| Extended-release naltrexone is not available in my practice | 649 (9.1) |
| Likelihood of prescribing naloxone to patient with opioid use disorder since COVID-19 PHE | |
| Less likely to prescribe naloxone | 180 (2.5) |
| No change | 5661 (79.2) |
| More likely to prescribe naloxone | 1308 (18.3) |
| £Strategies used to engage and monitor patients since the COVID-19 PHE | |
| In-Person Strategies | |
| In-person urine drug testing | 4208 (56.7) |
| Individual in-person counseling | 2990 (40.3) |
| Group in-person counseling | 1258 (17.0) |
| In-person pill/film checks | 1622 (21.9) |
| Remote Strategies | |
| Individual remote tele-health counseling with video | 3797 (51.2) |
| Individual remote tele-health counseling without video | 3494 (47.1) |
| Group remote tele-health counseling with video | 918 (12.4) |
| Group remote tele-health counseling without video | 478 (6.4) |
| Other Strategies | |
| Check state Prescription Drug Monitoring Program | 5160 (69.6) |
| Prescribe naloxone for overdose prevention | 3709 (50.0) |
| Remote pill/film checks with video | 639 (8.6) |
| Other (e.g., video and at-home urine drug screens) | 273 (3.7) |
| £Changes made to in-person patient encounters since the COVID-19 PHE | |
| Require providers to wear Personal Protective Equipment (e.g., surgical masks, face shields, N95 respirators) | 5678 (76.5) |
| Require patients to wear PPE or cloth face masks | 5501 (74.2) |
| Require staff to wear PPE | 5466 (73.7) |
| Temperature checks of patients | 4718 (63.6) |
| Temperature checks of providers | 4353 (58.7) |
| Temperature checks of staff | 4349 (58.6) |
| Limit number of patients in waiting room to maintain social distancing | 5613 (75.7) |
| Limit number of patients in group counseling sessions to maintain social distancing | 2127 (28.7) |
| Curbside prescription pick-up | 1261 (17.0) |
Source: CDC-NIDA Survey of DATA 2000 Clinicians, June-August 2020.
PHE = Public Health Emergency.
Among the 7419 respondents who reported prescribing buprenorphine in the past 12 months.
Respondents could choose multiple options.
Among clinicians that reported their practice setting had closed to in-person visits during COVID-19 (survey conducted June 23 to August 19, 2020) (n = 2127).
Among clinicians that prescribed buprenorphine to a new patient without conducting an in-person examination (n = 2429).
Among clinicians that reported their patient encountered a difficulty during at home induction (n = 133).
Among clinicians that did not prescribe buprenorphine to a new patient without conducting an in-person examination (n = 4921).
Among the 67.0 % of clinicians who reported no remote buprenorphine prescribing to a new patient without conducting an in-person examination, the most commonly cited barriers to remote prescribing were preferring to see patients in person (34.6 %), having no new patients (34.0 %), and having their practice remain open for in-person visits (33.5 %) (Table 2).
As with new patients, clinicians used a variety of telecommunication technologies to engage established patients during the COVID-19 PHE, including telephone without video (63.6 %), laptop or desktop with video (50.5 %), telephone with video (47.3 %), and iPad or other tablet with video (20.8 %). Clinicians reported using multiple applications such as Zoom (36.3 %) and FaceTime (14.9 %) during remote patient encounters.
Strategies reported to engage and monitor patients included in-person services (individual counseling [40.3 %]; group counseling [17.0 %]; urine drug testing [56.7 %]; pill/film checks [21.9 %]), and remote services (individual tele-health counseling without video [47.1 %] and with video [51.2 %]; group tele-health counseling without video [6.4 %] and with video [12.4 %]; video pill/film checks [8.6 %]; and state prescription drug monitoring program checks [69.6 %]). Prescribing naloxone for overdose prevention was reported by 50.0 %.
Changes to facilitate in-person patient encounters since the COVID-19 PHE included requiring clinicians to wear personal protective equipment (PPE) such as surgical masks, face shields or N95 respirators (76.5 %), requiring staff to wear PPE (73.7 %), and requiring patients to wear PPE or cloth face masks (74.2 %); conducting temperature checks for providers (58.7 %), staff (58.6 %), and patients (63.6 %); limiting numbers of patients both in the waiting room (75.7 %) and in group counseling sessions (28.7 %); and offering curbside prescription pick-up (17.0 %).
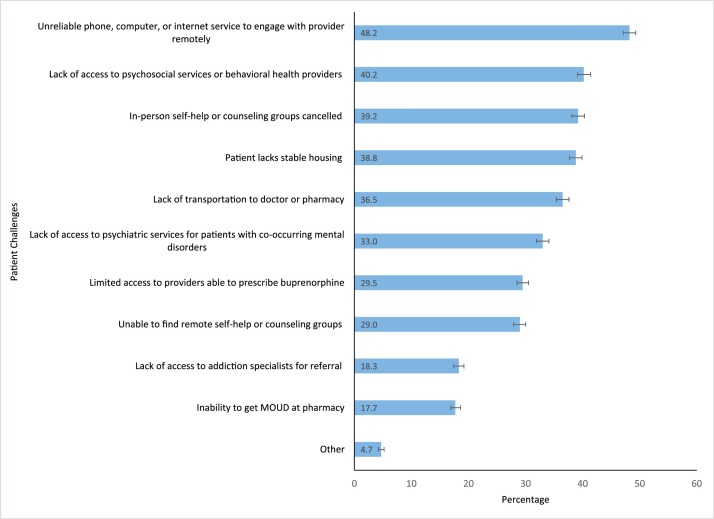
The most common patient challenges related to OUD treatment reported by clinicians (Fig. 1 ) were patients experiencing unreliable telecommunications (i.e. phone, computer, or internet service) to engage with provider remotely (48.2 %); lack of access to psychosocial services or behavioral health providers (40.2 %); cancellation of in-person self-help or counseling groups (39.2 %); unstable housing (38.8 %); lack of transportation to the doctor or pharmacy (36.5 %); lack of access to psychiatric services for patients with co-occurring mental disorders (33.0 %); limited access to providers able to prescribe buprenorphine (29.5 %); and patients unable to find remote self-help or counseling groups (29.0 %).
Fig. 1.
Clinician-reported challenges patients have experienced regarding opioid use disorder treatment during the COVID-19 Public Health Emergency¥ (n = 7419).
Figure Footnotes.
¥Among clinicians prescribing buprenorphine for opioid use disorder treatment in the past 12 months.
Error bars represent 95 % confidence intervals.
Source: CDC-NIDA Survey of DATA 2000 Clinicians, June-August 2020.
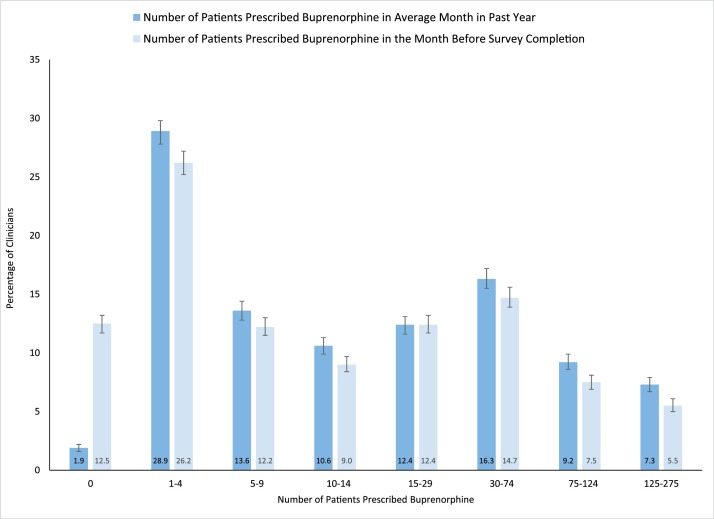
Significantly more clinicians reported not prescribing buprenorphine to any patients in the month prior to survey completion compared to an average month in the past 12 months (12.5 % versus 1.9 %; p < 0.001). Shifts among the other categories of number of patients prescribed buprenorphine were more modest but almost all were trending downward. (Fig. 2 ).
Fig. 2.
Number of patients prescribed buprenorphine by clinicians that prescribed buprenorphine for opioid use disorder treatment in the past 12 months¥ (n = 7365).
Figure Footnote.
¥Among clinicians prescribing buprenorphine for opioid use disorder treatment in the past 12 months.
Error bars represent 95 % confidence intervals.
Source: CDC-NIDA Survey of DATA 2000 Clinicians, June-August 2020.
3.3. Clinician characteristics associated with remote prescribing of buprenorphine
In the adjusted model (Table 3 ), clinician characteristics positively associated with remote prescribing of buprenorphine to new patients without conducting an in-person examination during the COVID-19 PHE were: younger clinicians compared to those aged 65 years or older (aged 25−34 years, odds ratio, 1.95; 95 % CI, 1.44−2.64; aged 35−44 years, odds ratio, 1.59; 95 % CI, 1.23−2.05); emergency medicine specialty compared to primary care specialty (odds ratio, 1.67; 95 % CI, 1.02−2.73); office-based group practice (odds ratio, 1.56; 95 % CI, 1.30−1.87), specialty substance use treatment facility (odds ratio, 2.06; 95 % CI, 1.56-2.67), opioid treatment program (odds ratio, 1.99; 95 % CI, 1.55−2.56), community clinic (odds ratio, 2.15; 95 % CI, 1.75−2.64), Veterans Affairs, Department of Defense, or Indian Health Service setting (odds ratio, 2.28; 95 % CI, 1.65−3.15), or other practice setting (odds ratio, 1.82; 95 % CI, 1.05−3.14) compared to office-based solo practice; prescribing buprenorphine to more than 4 patients in an average month (odds ratios increased with number of patients treated in an average month from 2.27; 95 % CI, 1.86−2.78 for 5−9 patients to 9.95; 95 % CI, 7.72−12.82 for 125−275 patients) compared to 1−4 patients in an average month; reporting practice setting closed during the COVID-19 PHE (odds ratio, 2.85; 95 % CI, 2.52−3.23) compared to clinicians whose practice setting did not close; and previously prescribing buprenorphine remotely to established patients prior to the COVID-19 PHE (odds ratio, 1.17; 95 % CI, 1.02−1.33) compared to those who had not engaged in remote prescribing to established patients prior to the COVID-19 PHE.
Table 3.
Clinician characteristics associated with remote prescribing to a new patient without conducting an in-person examination during the COVID-19 Public Health Emergency¥ (n = 6734).
| Clinician Characteristic | Adjusted Odds Ratio (95 % Confidence Interval) |
|---|---|
| Gender | |
| Female | Ref |
| Male | 0.83 (0.73−0.95) |
| Age Group | |
| 25−34 | 1.95 (1.44−2.64) |
| 35−44 | 1.59 (1.23−2.05) |
| 45−54 | 1.17 (0.94−1.45) |
| 55−64 | 1.10 (0.90−1.33) |
| 65 or older | Ref |
| U.S. Census Region | |
| Northeast | Ref |
| Midwest | 0.74 (0.62−0.88) |
| South | 0.68 (0.58−0.80) |
| West | 0.95 (0.82−1.12) |
| U.S. Territory | 0.52 (0.13−2.02) |
| Rural-Urban Status of Practice Location | |
| Urban | Ref |
| Suburban | 0.80 (0.70−0.91) |
| Rural | 0.67 (0.57−0.77) |
| Type of Provider | |
| Physician (MD/DO) | Ref |
| Nurse Practitioner or other nursing-related provider | 0.86 (0.74−1.00) |
| Physician Assistant | 0.52 (0.41−0.67) |
| Primary Practice Specialty | |
| Primary Care | Ref |
| Addiction Medicine | 1.24 (0.97−1.59) |
| Psychiatry | 0.94 (0.82−1.09) |
| Pediatrician | 0.69 (0.33−1.45) |
| Obstetrician/Gynecologist | 0.67 (0.44−1.02) |
| Emergency Medicine | 1.67 (1.02−2.73) |
| Pain Medicine/Anesthesiology | 0.82 (0.61−1.09) |
| Infectious Disease | 1.14 (0.60−2.18) |
| Other | 1.07 (0.76−1.52) |
| Addiction Medicine Board Certification | |
| No | Ref |
| Yes | 1.06 (0.93−1.21) |
| Years in Practice | |
| Less than 5 years | Ref |
| 5−10 years | 0.98 (0.82−1.17) |
| 11−15 years | 0.96 (0.77−1.21) |
| 16−19 years | 0.88 (0.67−1.15) |
| 20 years or more | 0.83 (0.66−1.05) |
| Primary Practice Setting | |
| Office-based solo practice | Ref |
| Office-based group practice | 1.56 (1.30−1.87) |
| Specialty substance use treatment facility | 2.06 (1.56−2.67) |
| Opioid Treatment Program | 1.99 (1.55−2.56) |
| Community clinic (e.g., FQHC, RHC, CCBHC) | 2.15 (1.75−2.64) |
| Emergency Department | 0.52 (0.29−0.94) |
| Urgent Care | 0.77 (0.39−1.51) |
| Criminal Justice setting | 1.36 (0.87−2.13) |
| In-patient/Hospital setting | 1.01 (0.76−1.35) |
| Veterans Affairs/Department of Defense/Indian Health Service | 2.28 (1.65−3.15) |
| Other (e.g., school-based clinic, LTCF, public health) | 1.82 (1.05−3.14) |
| Number of patients treated with buprenorphine in average month in past 12 months | |
| None | 0.17 (0.05−0.54) |
| 1−4 | Ref |
| 5−9 | 2.27 (1.86−2.78) |
| 10−14 | 3.01 (2.43−3.72) |
| 15−29 | 4.01 (3.28−4.91) |
| 30−74 | 5.91 (4.87−7.17) |
| 75−124 | 6.11 (4.86−7.70) |
| 125−275 | 9.95 (7.72−12.82) |
| Practice setting closed during COVID-19 Public Health Emergency | |
| No | Ref |
| Yes | 2.85 (2.52−3.23) |
| Prescribed buprenorphine remotely to established patients prior to the COVID-19 Public Health Emergency | |
| No | Ref |
| Yes | 1.17 (1.02−1.33) |
| Listed on the SAMHSA Buprenorphine Provider Locator | |
| No | Ref |
| Yes | 1.11 (0.96−1.28) |
Source: CDC-NIDA Survey of DATA 2000 Clinicians, June-August 2020.
Bold text indicates statistically significant findings.
PHE = Public Health Emergency.
FQHC = Federally Qualified Health Center, RHC = Rural Health Center, CCBHC = Certified Community Behavioral Health Clinic, LTCF = Long-term Care Facility.
Model adjusted for all variables in the table.
Among clinicians prescribing buprenorphine for opioid use disorder treatment in the past 12 months.
Clinician characteristics negatively associated with remote prescribing of buprenorphine to new patients without conducting an in-person examination during the COVID-19 PHE included: male gender (odds ratio, 0.83; 95 % CI, 0.73−0.95) compared to female gender; practicing in the Midwest (odds ratio, 0.74; 95 % CI, 0.62−0.88) or South (odds ratio, 0.68; 95 % CI, 0.58−0.80) compared to the Northeast; practicing in suburban (odds ratio, 0.80; 95 % CI, 0.70−0.91) or rural (odds ratio, 0.67; 95 % CI, 0.57−0.77) locations compared to urban locations; physician assistants (odds ratio, 0.52; 95 % CI, 0.41−0.67) compared to physicians; practicing in an emergency department (odds ratio, 0.52; 95 % CI, 0.29−0.94) compared to an office-based solo practice; and prescribing buprenorphine to no patients in an average month (odds ratio, 0.17; 95 % CI, 0.05−0.54) compared to 1−4 patients in an average month.
4. Discussion
Understanding buprenorphine practice patterns during the COVID-19 pandemic can inform short and long-term improvements in the treatment of persons with OUD. First, more than 1 in 4 clinicians waivered to prescribe buprenorphine reported they have never prescribed buprenorphine or have not prescribed in the past 12 months. In addition, 31 % of the entire sample of DATA-waivered clinicians (and 23.3 % of those who prescribed in the past 12-months) were unaware of the DEA exemption for in-person examination of new patients during the COVID-19 PHE. Further, nearly 1 in 6 clinicians who had prescribed buprenorphine at one time but not in the past 12 months reported they were required to get a DATA waiver for their job but were not actually interested in prescribing. Taken together, these findings suggest untapped potential to provide care for substantially more persons with OUD, during and after the pandemic. Thus, our findings are consistent with other studies that report a large reservoir of providers who could provide care because they have passed the first threshold (getting the waiver) but are not engaging in the practice due to a variety of barriers such as payment and reimbursement issues, clinician stigma, lack of access to mental health and addiction specialists, and lack of patient demand (Andrilla et al., 2017, 2020; Duncan et al., 2020; Hutchinson et al., 2014; Jones and McCance-Katz, 2019; Stone et al., 2021; Walley et al., 2008). In addition, many clinicians reported lack of ability to provide services during the COVID-19 PHE. Practice closures, complications with use of technology to support remote care, and lack of access to needed ancillary services for complex patients, among others, all speak to areas in need of practice improvements to bring quality care to persons with OUD during and beyond the COVID-19 PHE. Promising approaches to addressing this gap include use of evidence-based digital psychotherapies and other remote services for patients that might be implemented at a distance.
Despite these practice constraints, 20.7 % of past-12 month buprenorphine prescribers reported they had remotely prescribed buprenorphine to established patients prior to the COVID-19 PHE whereas 33.0 % reported prescribing buprenorphine remotely to new patients without an in-person examination under the DEA exemption issued during the COVID-19 PHE. Few (5.5 %) of those initiating patients remotely reported patient difficulties with this new practice, consistent with available research (Lee et al., 2014). A majority (54.3 %) of buprenorphine prescribers also reported use of telephone without video to provide telehealth services. These findings suggest that emergency changes to regulations enabling remote buprenorphine prescribing to new patients using various communications technologies, including phone without video, is positively impacting access to care during the pandemic. Future research may be needed to determine the geographic characteristics of these clinical encounters as restrictions on cross-state provision of services have been lifted by the emergency authorities and understanding how often these cross-state interventions have occurred could be informative for future policy decisions.
Characteristics associated with greater likelihood of remote buprenorphine prescribing to new patients included younger age and female gender, certain practice settings, prescribing buprenorphine to larger numbers of patients, on average, and previous experience with remote prescribing to established patients. These characteristics indicate that educational and outreach efforts might target specific subgroups of clinicians in order to increase ability to prescribe remotely, particularly those in smaller practices. Finding lower rates of remote buprenorphine prescribing for clinicians in rural locations suggests that patients in locations where transportation may be particularly difficult may not be receiving the benefits of remote prescribing. It is also possible that technology difficulties, reported for both clinicians and patients in this study, as well as financial means or other barriers to computers, internet service, and cell phone access may impede the ability to implement remote buprenorphine prescribing to new patients in these locations. Urban practice locations may also have been disproportionately impacted by COVID-19, motivating more clinicians in these locations, compared to those in rural or suburban locations, to use their new authority to initiate buprenorphine remotely. Although not assessed in this study, prior research has documented disparities in access to buprenorphine-based MOUD, with white persons significantly more likely to receive buprenorphine compared to black persons (Lagisetty et al., 2019), disparities that have likely worsened during the COVID-19 pandemic (Jordan and Tiako, 2021). Coupled with the finding of variations in characteristics of clinicians associated with remote buprenorphine prescribing to new patients, it is clear that opportunities and targets for practice and systems-level interventions that advance equitable access to buprenorphine across racial and ethnic groups and geographic areas and leverage improvements in access to remote technologies exist.
An additional finding that warrants further discussion is that of increased adjusted odds of remote prescribing of buprenorphine to new patients among emergency medicine specialty physicians and the finding of lower adjusted odds among clinicians practicing in the emergency department setting. One potential explanation for this counterintuitive finding is the increasing adoption of ED-initiated buprenorphine and innovative ED bridge models that provide buprenorphine as part of in-person, ED-based care and then directly bridge patients into ongoing substance use disorder treatment (Martin et al., 2020). Use of these care models, especially during the pandemic when other outpatient and office-based practices were closed, may have contributed to this finding in our study. Another potential explanation is that some clinicians who are trained in emergency medicine are practicing in settings other than EDs, as approximately 40 % of the emergency medicine specialty physicians in our survey who prescribed buprenorphine remotely to new patients during the COVID-19 PHE reported practicing in a non-ED setting. Future research should further explore these practice patterns.
This study is subject to limitations. First, given the cross-sectional nature of the survey, we cannot draw causal inferences from our findings. Second, our survey included a range of barriers to prescribing buprenorphine and clinician strategies to manage patients during the COVID-19 PHE; however, other important barriers or strategies we did not assess may exist. Third, while our survey was largely drawn from prior surveys of DATA-waived clinicians and expert review, some respondents may not have sufficiently comprehended survey questions. Finally, although our response sample was large and incorporated variation with regard to demographic and clinical characteristics, the response rate was 14.2 %; thus, findings are at risk for non-response bias. Clinicians who are more actively engaged in providing care for patients with OUD may be over-represented. Despite these limitations, our study has significant strengths. This survey represents the largest survey to date of DATA-waived clinicians and includes clinicians from all 50 states, the District of Columbia, and two U.S. territories. Further, survey respondents were highly similar to all DATA-waived clinicians with regard to state and clinician type, the two variables available for comparison, with correlation coefficients of ≥ 0.99. The demographic and clinical practice characteristics of respondents also were generally consistent with prior surveys of DATA-waived clinicians (Andrilla et al., 2017, 2020; Duncan et al., 2020; Jones and McCance-Katz, 2019; Walley et al., 2008). Finally, given the lack of data on clinical practices related to management of OUD during COVID-19, the timeliness of this study provides new, actionable insights to inform policy and clinical practice initiatives.
5. Conclusions
In the first nation-wide survey during the COVID-19 pandemic of clinicians with a DATA waiver, we found that many clinicians have adapted their practices and used new emergency authorities such as telehealth-based buprenorphine prescribing and telehealth via phones without video to provide care for patients with OUD during the COVID-19 PHE. However, we also identified important barriers, including barriers unique to the COVID-19 environment, as well as long-standing clinical and systems-level barriers that constrain prescribing of buprenorphine. Efforts focused on clinician training and education, systems-level changes to support innovative service delivery and payment reforms, consideration of permanent adoption of these emergency authorities, and public awareness about medications to treat OUD and where treatment can be obtained are urgently needed.
Disclaimer
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the National Institute on Drug Abuse, the National Institutes of Health, the Centers for Disease Control and Prevention or the U.S. Department of Health and Human Services.
Contributors
Drs. Jones and Compton conceived of the study and designed the survey instrument. Drs. Diallo, Schier, and Eisenstat provided input on the survey instrument. Dr. Jones conducted the data analysis and drafted the manuscript. Drs. Compton, Diallo, Vythilingam, Schier, and Eisenstat provided critical review and revisions to the manuscript.
Declaration of Competing Interest
Dr. Compton reports long-term holdings in General Electric Company, 3 M Companies and Pfizer, Incorporated, unrelated to the present work.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.drugalcdep.2021.108783.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Alexander G.C., Stoller K.B., Haffajee R.L., Saloner B. An epidemic in the midst of a pandemic: opioid use disorder and COVID-19. Ann. Intern. Med. 2020;173(1):57–58. doi: 10.7326/M20-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter A., Yeager C. 2020. COVID-19 Impact on U.S. National Overdose Crisis. Available at: http://odmap.org/Content/docs/news/2020/ODMAP-Report-June-2020.pdf Accessed on January 6, 2021. [Google Scholar]
- Andrilla C.H.A., Coulthard C., Larson E.H. Barriers rural physicians face prescribing buprenorphine for opioid use disorder. Ann. Fam. Med. 2017;15(4):359–362. doi: 10.1370/afm.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrilla C.H.A., Jones K.C., Patterson D.G. Prescribing practices of nurse practitioners and physician assistants waivered to prescribe buprenorphine and the barriers they experience prescribing buprenorphine. J. Rural Health. 2020;36(2):187–195. doi: 10.1111/jrh.12404. [DOI] [PubMed] [Google Scholar]
- Ataiants J., Roth A.M., Mazzella S., Lankenau S.E. Circumstances of overdose among street-involved, opioid-injecting women: drug, set, and setting. Int. J. Drug Policy. 2020;78:102691. doi: 10.1016/j.drugpo.2020.102691. Epub 2020 Feb 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2020. Health Alert Network Advisory. December. Available at: https://emergency.cdc.gov/han/2020/han00438.asp Accessed on February 8, 2021. [Google Scholar]
- Centers for Disease Control and Prevention . 2021. National Center for Health Statistics Monthly Provision Overdose Mortality. Available at: https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm Accessed on February12. [Google Scholar]
- Centers for Medicare and Medicaid Services . 2020. Centers for Medicare & Medicaid Services (CMS) and Substance Abuse and Mental Health Services Administration (SAMHSA): Leveraging Existing Health and Disease Management Programs to Provide Mental Health and Substance Use Disorder Resources During the COVID-19 Public Health Emergency (PHE) Available at: https://www.cms.gov/CCIIO/Programs-and-Initiatives/Health-Insurance-Marketplaces/Downloads/Mental-Health-Substance-Use-Disorder-Resources-COVID-19.pdf Accessed on January 9, 2021. [Google Scholar]
- Czeisler M.E., Lane R.I., Petrosky E., et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic – United States, June 24-30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69(32):1049–1057. doi: 10.15585/mmwr.mm6932a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C.S., Samuels E.A. Continuing increased access to buprenorphine in the United States via telemedicine after COVID-19. Int. J. Drug Policy. 2020;15(August):102905. doi: 10.1016/jdrugpo.2020.102905. online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan A., Anderman J., Deseran T., Reynolds I., Stein Bd. Monthly patient volumes of buprenorphine-waivered clinicians in the U.S. JAMA Network Open. 2020;3(8):e2014045. doi: 10.1001/jamanetworkopen.2020.14045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap) — a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H., Miniño A.M., Warner M. National Center for Health Statistics; MD: 2020. Drug Overdose Deaths in the United States, 1999–2019. NCHS Data Brief, No 394. Hyattsville. [Google Scholar]
- Henry B.F., Mandavia A.D., Paschen-Wolff M.M., et al. COVID-19, mental health, and opioid use disorder: old and new public health crises intertwine. Psychol. Trauma. 2020;12(S1):S111–S112. doi: 10.1037/tra0000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland K.M., Jones C., Vivolo-Kantor A.M., et al. Trends in U.S. Emergency department visits for mental health, overdose, and violence outcomes before and during the COVID-19 pandemic. JAMA Psychiatry. 2021;3(Febuary):e204402. doi: 10.1001/jamapsychiatry.2020.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson E., Catlin M., Andrilla C.H.A., Baldwin L.M., Rosenblatt R.A. Barriers to primary care physicians prescribing buprenorphine. Ann. Fam. Med. 2014;12:128–133. doi: 10.1370/afm.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.M., McCance-Katz E.F. Characteristics and prescribing practices of clinicians recently waivered to prescribe buprenorphine for the treatment of opioid use disorder. Addiction. 2019;111(3):471–482. doi: 10.1111/add.14436. [DOI] [PubMed] [Google Scholar]
- Jordan M., Tiako N. Addressing racial & socioeconomic disparities in access to medications for opioid use disorder amid COVID-19. J. Subst. Abuse Treat. 2021;122(March):108214. doi: 10.1016/j.jsat.2020.108214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagisetty P.A., Ross R., Bohnert A., Clay M., Maust D.T. Buprenorphine treatment divide by race/ethnicity and payment. JAMA Psychiatry. 2019;76(9):979–981. doi: 10.1001/jamapsychiatry.2019.0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.D., Vocci F., Fiellin D.A. Unobserved “home” induction onto buprenorphine. J. Addict. Med. 2014:299–308. doi: 10.1097/ADM.0000000000000059. [DOI] [PubMed] [Google Scholar]
- Martin A., Butler K., Chavez T., Herring A., Wakeman S., Hayes B.D., Raja A. Beyond buprenorphine: models of follow-up care for opioid use disorder in the Emergency Department. West. J. Emerg. Med. 2020;21(6):257–263. doi: 10.5811/westjem.2020.7.46079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone E.M., Kennedy-Hendricks A., Barry C.L., Bachhuber M.A., McGinty E.E. The role of stigma in U.S. Primary care physicians’ treatment of opioid use disorder. Drug Alcohol Depend. 2021;221(April):108627. doi: 10.1016/j.drugalcdep.2021.108627. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . 2020. Opioid Treatment Program (OTP): Guidance (March 16, 2020) Available at: https://www.samhsa.gov/sites/default/files/otp-guidance-20200316.pdf Accessed on December 17, 2020. [Google Scholar]
- U.S. Department of Health and Human Services . 2020. Public Health Emergency Determination for 2019 Novel Coronavirus. Availabe at: https://www.phe.gov/emergency/news/healthactions/phe/Pages/2019-nCoV.aspx Accessed on January 6, 2021. [Google Scholar]
- U.S. Department of Health and Human Services . 2020. Office of Civil Rights: Notification of Enforcement Discretion for Telehealth Remote Communications during the COVID-19 Nationwide Public Health Emergency. Available at: https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html Accessed February 10, 2021. [Google Scholar]
- U.S. Department of Health and Human Services . 2021. Title 45 Code of Federal Regulations 46, Protection of Human Subjects. [Google Scholar]
- Volkow N.D. Collision of the COVID-19 and addiction epidemics. Ann. Intern. Med. 2020;173(1):61–62. doi: 10.7326/M20-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walley A.Y., Alperen J.K., Cheng D.M., et al. Office-based management of opioid dependence with buprenorphine: clinical practices and barriers. J. Gen. Intern. Med. 2008;23(9):1393–1398. doi: 10.1007/s11606-008-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.