Abstract
Anaerobic enrichments with acetate as the electron donor and Fe(III) as the terminal electron acceptor were obtained from sediments of Salt Pond, a coastal marine basin near Woods Hole, Mass. A pure culture of a facultatively anaerobic Fe(III) reducer was isolated, and 16S rRNA analysis demonstrated that this organism was most closely related to Pantoea (formerly Enterobacter) agglomerans, a member of the family Enterobacteriaceae within the gamma subdivision of the Proteobacteria. This organism, designated strain SP1, can grow by coupling the oxidation of acetate or H2 to the reduction of a variety of electron acceptors, including Fe(III), Mn(IV), Cr(VI), and the humic substance analog 2,6-anthraquinone disulfonate, but not sulfate. To our knowledge, this is the first mesophilic facultative anaerobe reported to couple acetate oxidation to dissimilatory metal reduction.
The decomposition of complex organic matter coupled to dissimilatory metal reduction is becoming increasingly recognized as an important process in anoxic sedimentary environments (22, 24, 31), and acetate is generally considered to be one of the key organic intermediates driving these processes (21, 23). Although many microorganisms have been shown to have the capacity to anaerobically reduce Fe(III) (20, 24), only a limited number of organisms are known to couple acetate oxidation to Fe(III) reduction. The majority of these are members of the family Geobacteriaceae, with Geobacter species predominating in freshwater sediments and Desulfuromonas species predominating in marine environments (9, 10). To date, the only other mesophilic organisms known to possess this acetate-oxidizing, Fe(III)-reducing capacity are Geothrix fermentens (20), Geovibrio ferrireducens (5), and the recently described organism Ferribacterium limneticum (11), all of which are obligate anaerobes.
The best-characterized group of facultatively anaerobic Fe(III) reducers are within the genus Shewanella. These organisms are capable of using a wide variety of electron acceptors, including oxygen, but their ability to utilize electron donors is somewhat limited, in that they are unable to use acetate anaerobically. Recently, facultative organisms within the genus Aeromonas (16), as well as the species Sulfurospirillum (formerly Geospirillum) barnesii (19, 35) and Ferrimonas balaerica (34), have also been shown to utilize Fe(III) as an anaerobic electron acceptor, but, like Shewanella species, they are unable to use acetate as an electron donor.
The well-studied Fe(III) reducers Shewanella alga and Geobacter metallireducens can also substitute humic substances and the humic substance analog 2,6-anthraquinone disulfonate (AQDS) for Fe(III) as the terminal electron acceptor (26). The capacity to transfer electrons to humic acids and AQDS is of importance for metal cycling because, once reduced, these compounds can catalyze the rapid chemical reduction of both iron and manganese oxides (27, 37, 38). To date, all of the acetate-oxidizing AQDS reducers recovered from sediments have been members of the family Geobacteriaceae (8).
The objective of this study was to enrich for and isolate microorganisms capable of coupling acetate oxidation to Fe(III) reduction. In doing so, we discovered a facultative anaerobe, Pantoea agglomerans strain SP1, which has extensive metabolic capabilities under anaerobic conditions. It is capable of growing via the dissimilatory reduction of Fe(III), Mn(IV), AQDS, and the toxic metal Cr(VI). The ability to utilize diverse electron acceptors under anaerobic conditions may be more common than previously recognized in suboxic sedimentary environments.
MATERIALS AND METHODS
Source of organisms.
Grab samples of nearshore surficial sediments were collected from Salt Pond, a coastal pond near Woods Hole, Mass. These sediments served as inocula for enrichment cultures of Fe(III)-reducing bacteria.
Cultivation techniques.
Cells were cultivated in serum bottles or Balch tubes capped with black butyl rubber stoppers and aluminum crimp seals under an N2 atmosphere (2). A bicarbonate-buffered anaerobic medium (42) supplemented with 10 mM acetate and 40 mM solid Fe(OH)3 was used for initial enrichment cultures. Single colonies were obtained using agar shakes (42) with acetate and soluble Fe(III)-nitrilotriacetic acid [Fe(III)-NTA] or Fe(III)-citrate as electron acceptors. Pure cultures of facultative anaerobes were obtained using aerobic plating techniques. Colonies were transferred from agar into 25-ml Balch tubes filled with 10 ml of anaerobic medium (pH 7.2 to 7.4) and incubated at 30°C. The composition of basal freshwater medium N1 was identical to that described by Widdel and Bak (42) for sulfate-reducing bacteria, except that sulfate and yeast extract were omitted. In experiments with acetate as the electron donor, a small amount of yeast extract (0.001%) was added to the medium to stimulate growth.
Alternative electron acceptors and donors.
Growth on alternative electron acceptors was tested in N1 medium supplemented with 10 mM acetate and one of the following as the sole electron acceptor: Na2SO4 (20 mM), trimethylamine N-oxide (5 mM), NaNO3 (10 mM), fumarate (10 mM), Mn(IV) as δMnO2 (0.3 mM), Co(III)-EDTA (10 mM), AQDS (5 mM) (Sigma), Cr(VI) as Na2CrO4 (0.1 mM), or U(VI) as uranyl acetate (1 mM), unless otherwise noted. Elemental sulfur was provided as sublimed flowers (∼1%) (Fisher Scientific). Amorphous Fe(OH)3 was synthesized by titrating a solution of FeCl3 · 6H2O with 10% NaOH to pH 9.0. Synthetic MnO2, Fe(III)-NTA, and Fe(III)-citrate were prepared as previously described (17). Co(III)-EDTA was synthesized from CoCl2 · 6H2O by peroxide oxidation as previously described (12). With Fe(III)-pyrophosphate (10 mM) as the electron acceptor, the following substrates were tested as electron donors: propionate (10 mM), butyrate (10 mM), valerate (10 mM), citrate (10 mM), lactate (20 mM), succinate (10 mM), peptone (1%), yeast extract (1%), and hydrogen. When growth was observed with a given electron acceptor, this was confirmed by transferring the culture to medium with the same electron acceptor (and the corresponding electron donor) and observing growth in three successive transfers.
Analytical methods.
Cell numbers were determined either by direct counting using 4′,6-diamidino-2-phenylindole (DAPI) and epifluorescence microscopy (32) or by using a Petroff-Hauser counting chamber with phase-contrast microscopy. Fe(III) reduction was measured by the ferrozine technique (36). Mn(IV) was measured using the Leucoberbelin blue colorimetric assay (18). Co(III)-EDTA reduction was measured visually or by measuring the absorbance at 535 nm as previously described (5). Cr(VI) was measured colorimetrically using diphenylcarbazide (29). The reduction of AQDS was measured by the increase of absorbance at 450 nm (26). Sulfide was measured colorimetrically by the methylene blue method (7). Acetate was measured using a Beckman high-pressure liquid chromatography (HPLC) system equipped with an Aminex HPX-87U column (7.8 by 300 mm) and UV detection at 210 nm.
Phylogenetic analysis.
Preliminary phenotypic characterization and identification of aerobic isolates were performed using the BIOLOG GN system as recommended by the manufacturer (BIOLOG Inc., Hayward, Calif.). For genotypic analysis, DNA was extracted from colonies by boiling lysis and from cultures by using the QIAamp Tissue kit (Qiagen Inc., Chatsworth, Calif.). The eubacterial primer 27F and the universal primer 1492R were used to PCR amplify 16S rRNA genes (41), which were subsequently cloned into the TA cloning vector pCR2.1-TOPO (Invitrogen, San Diego, Calif.). Both strands were sequenced by automated dye dideoxy terminator sequencing using an Applied Biosystems 373A automated sequencer. Phylogenetic placement was determined using the BLAST program (1) and the Ribosomal Database Project (30) web site.
Nucleotide sequence accession number.
The 16S rRNA gene sequence of strain SP1 has been deposited in GenBank under accession number AF199029.
RESULTS
Enrichment cultures and isolation.
Active enrichment cultures were obtained from Salt Pond sediments with solid Fe(III) as the electron acceptor and acetate as the electron donor in anaerobic medium. The reduction of iron in these enrichments was accompanied by the production of a black magnetic precipitate, presumably magnetite. After three successive transfers in liquid medium with acetate and Fe(OH)3, agar shakes were prepared with soluble Fe(III)-NTA and acetate. From the 10−5 dilution, single colonies were transferred to liquid medium containing acetate and either soluble Fe(III)-citrate or Fe(III)-pyrophosphate. The purity of the cultures was assessed by colony morphology and microscopy.
Phylogenetic and phenotypic characterization.
To determine the phylogenetic identity of the anaerobic acetate-oxidizing, Fe(III)-reducing culture, DNA was extracted and 16S rRNA genes were PCR amplified, cloned, and sequenced. This analysis revealed that the organism was most closely related (99.6%; 1,449 nucleotide positions considered) to the facultative anaerobe P. agglomerans JCM (Japan Collection of Microorganisms) 1236 (accession no. AB004691), a member of the family Enterobacteriaceae within the gamma subdivision of the Proteobacteria. This organism was isolated aerobically on Luria-Bertani plates, where it formed yellow-pigmented colonies, one of the characteristics that distinguishes certain P. agglomerans strains from other closely related species. Microscopic examination revealed highly motile, gram-negative, straight rods. BIOLOG analysis confirmed the identification of this organism as P. agglomerans and it was designated P. agglomerans strain SP1. Growth of strain SP1 occurred over a wide range of conditions, including temperature (5 to 40°C), pH (6.0 to 8.5), and NaCl concentration (0 to 5%); optimal growth occurred at 30°C, pH 6 to 7.2, and 0.5% NaCl.
Fe(III) and Mn(IV) reduction.
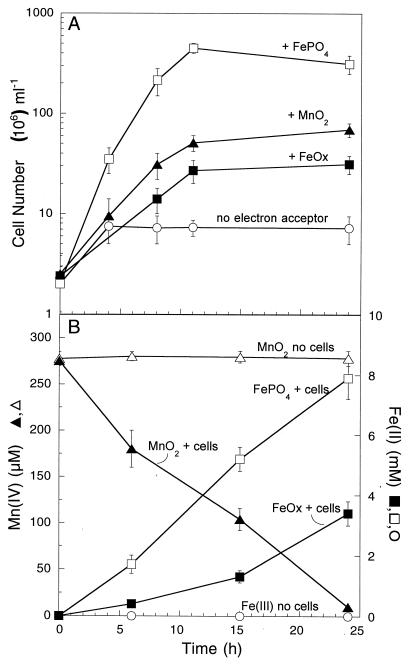
Strain SP1 was capable of using lactate, acetate, and H2 as electron donors for dissimilatory metal reduction, and the latter two substrates were chosen for more detailed experiments. Hydrogen consistently yielded the most rapid growth coupled to metal reduction, with the fastest growth (doubling time, ∼3 h) occurring in the presence of H2 and soluble Fe(III)-pyrophosphate (Fig. 1). In contrast, growth with insoluble Fe(III), as well as Mn(IV), yielded much lower growth rates (doubling times, ∼9 h). Mn(IV) was completely reduced during growth, although a higher yield may have been reached if a higher Mn(IV) concentration (>0.3 mM) was provided. During growth on poorly crystalline Fe(III), only 15 to 20% of the Fe(III) was reduced.
FIG. 1.
Anaerobic growth of (A) and metal reduction by (B) strain SP1 with H2 as the electron donor and Fe(III)-pyrophosphate (FePO4), Fe(III) hydroxide (FeOx), or MnO2 as the electron acceptor. The results are means and SDs from duplicate cultures.
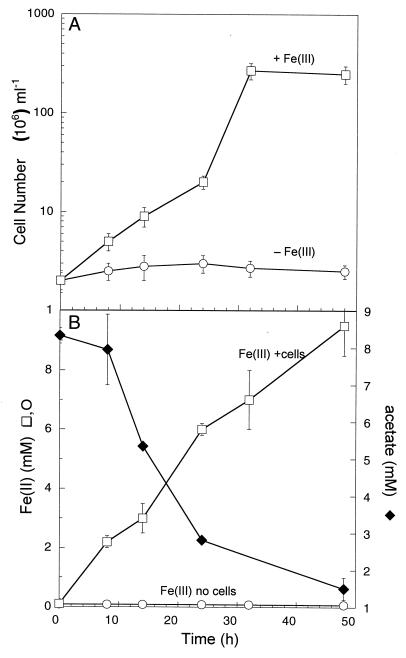
Acetate is generally considered to be the primary electron donor driving anaerobic respiration in many anoxic environments (21, 23), but until now there have been no reports of mesophilic facultative anaerobes coupling the oxidation of acetate to Fe(III) reduction. Strain SP1 was able to couple acetate oxidation to the reduction of several forms of Fe(III), including three soluble forms [Fe(III)-NTA, Fe(III)-citrate, and Fe(III)-pyrophosphate] as well as poorly crystalline Fe(OH)3. Soluble Fe(III)-pyrophosphate was used as the electron acceptor in time course experiments. Fe(III) reduction was always accompanied by an increase in cell numbers (Fig. 2A) as well as acetate consumption (Fig. 2B). Although the doubling time with soluble Fe(III) and acetate was considerably longer than that with soluble Fe(III) and H2 (9 h versus 3 h), acetate oxidation yielded a significant increase in cell numbers. In these experiments, no growth was ever observed in the absence of Fe(III) or an electron donor. With acetate as the electron donor, no Mn(IV) reduction was observed but some (slow) reduction of Fe(OH)3 did occur (data not shown). Although a black magnetic precipitate was formed in the original crude Fe(III)-reducing enrichment cultures from Salt Pond sediments, the pure culture of strain SP1 alone did not produce detectable magnetic material during growth on solid amorphous Fe(OH)3 with H2 or acetate under our experimental conditions.
FIG. 2.
(A) Anaerobic growth of strain SP1 with acetate as the electron donor and Fe(III)-pyrophosphate as the electron acceptor. (B) Fe(II) production and acetate consumption during this experiment. The results are means and SDs from duplicate cultures.
To examine the stoichiometry of acetate oxidation coupled to Fe(III) reduction, Fe(II) production was measured during growth of SP1 with Fe(III)-pyrophosphate and a limiting concentration of acetate (5 mM) (data not shown). The ratio of Fe(II) produced to acetate consumed was approximately 6.0 ± 0.1 (mean ± standard deviation [SD]; n = 3). This ratio is similar to those observed with other acetate-oxidizing Fe(III) reducers (11) and to the theoretical ratio of 8:1 (Fe to acetate) predicted from the stoichiometry of the reaction (28).
Cr(VI) reduction.
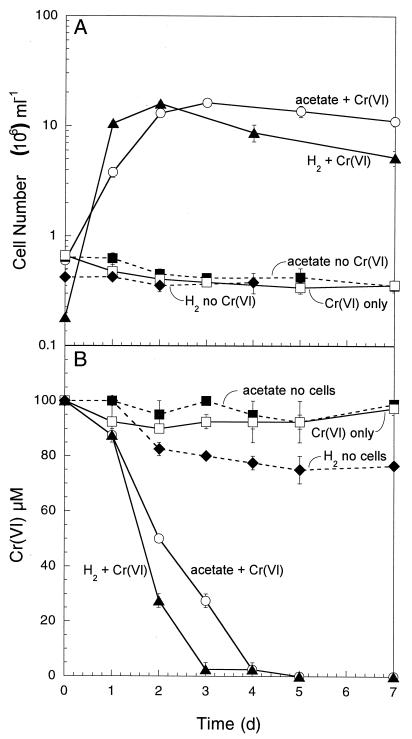
The capacity to couple anaerobic growth to the reduction of chromium(VI) was investigated because, to date, very few organisms have been definitively shown to use this toxic metal as an electron acceptor. SP1 was able to grow by coupling the oxidation of lactate (data not shown), acetate, and hydrogen to the reduction of Cr(VI) (Fig. 3A). An initial Cr(VI) concentration of 0.1 mM was used in these experiments because growth inhibition started to occur at Cr(VI) concentrations higher than this (data not shown). Nevertheless, 0.1 mM Cr(VI) was almost completely reduced over a period of 5 days (Fig. 3B), resulting in the formation of a fine gray precipitate, presumably Cr(III). No significant growth was observed in the absence of Cr(VI) with any of the electron donors, and no growth was observed with Cr(VI) without an electron donor. As was the case with Fe(III) and Mn(IV) reduction, the most rapid growth and Cr(VI) reduction occurred with H2 as the electron donor, followed by acetate and lactate.
FIG. 3.
Anaerobic growth of (A) and Cr(VI) reduction by (B) strain SP1 with acetate or H2 as the electron donor and Cr(VI) as the electron acceptor. The results are means and SDs from duplicate cultures.
Other electron acceptors and donors.
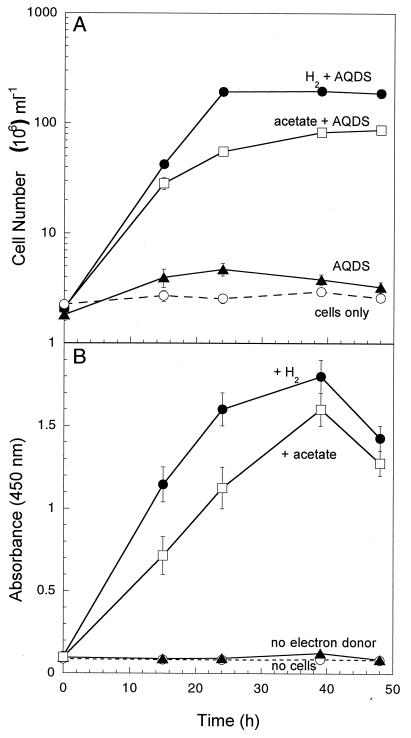
After it was established that SP1 had the capacity for dissimilatory metal reduction, the humic substance analog AQDS was tested as a potential electron acceptor. It has recently been shown that many Fe(III)-reducing organisms are also capable of reduction of humic substances and AQDS (8, 26). SP1 grew by coupling both acetate and hydrogen oxidation to the reduction of AQDS (Fig. 4), with hydrogen yielding the most rapid and greatest overall AQDS reduction. In the absence of an electron donor, no significant growth or AQDS reduction occurred.
FIG. 4.
Anaerobic growth of (A) and AQDS reduction by (B) strain SP1 with acetate or H2 as the electron donor and AQDS as the electron acceptor. Reduction of AQDS was quantified by the absorbance of anthrahydroquinone disulfonate produced at 450 nm. The results are means and SDs from duplicate cultures.
SP1 was also able to grow using several other alternative electron acceptors with acetate as the electron donor, including nitrate, Co(III)-EDTA, fumarate, trimethylamine N-oxide, and elemental sulfur. Sulfate and U(VI) were not used as electron acceptors by SP1. Electron donors that did not support growth coupled to Fe(III) reduction were propionate, butyrate, valerate, succinate, peptone, yeast extract, and citrate. Citrate was tested as a potential electron donor because commercial Fe(III)-pyrophosphate may also contain citrate as a chelating agent (4). No fermentative growth was observed with peptone or yeast extract under anaerobic conditions.
DISCUSSION
Strain SP1 has the ability to couple the oxidation of both acetate and hydrogen to the dissimilatory reduction of Fe(III) as well as many other electron acceptors. Acetate and H2 have been suggested to be the two major extracellular intermediates in the oxidation of fermentable organics in Fe(III)-reducing sediments (10, 21, 23, 25). In addition, the capacity to utilize acetate may provide a competitive advantage over H2 oxidizers like Shewanella and Desulfovibrio species (10, 21, 23). To our knowledge, SP1 is the first mesophilic facultative anaerobe reported to couple acetate oxidation to Fe(III) reduction. Although a small amount of yeast extract was used to stimulate growth of SP1 with acetate as the electron donor, yeast extract alone could not be used as a growth substrate or electron donor.
Depending on the electron donor as well as the Fe(III) source (soluble or solid), significant differences in anaerobic growth were observed. As has been reported previously for other Fe(III) reducers (21), growth was considerably faster with soluble forms of Fe(III). However, unlike some Fe(III) reducers (8, 10, 20), this organism was able to use all three soluble Fe(III) sources [Fe(III)-NTA, Fe(III)-citrate, and Fe(III)-pyrophosphate]. Although growth of SP1 was slower with poorly crystalline Fe(III) hydroxide, the capacity to utilize solid forms of Fe(III) is important because these are the forms most often encountered in sediments (22, 31, 33). The fact that the original enrichment cultures with acetate and Fe(OH)3 produced magnetic material, while SP1 alone did not, suggests that another organism or a combination of organisms may have been responsible for the formation of this material. Alternatively, it is also possible that the chemical conditions within the enrichment cultures (e.g., pH) favored the formation of magnetic material (3), relative to the conditions within the pure culture alone. The findings that growth with solid Fe(OH)3 was most rapid with H2 as the electron donor and that solid MnO2 reduction occurred only with H2 as the electron donor indicate that H2 is the most effective electron donor for the reduction of solid forms of metals by SP1. This may be due to the greater amount of energy (lower E0′) available with H2, relative to acetate, as the electron donor, or perhaps acetate uptake is less efficient than H2 uptake.
In contrast to Fe(III) and Mn(IV), Cr(VI) is a soluble and highly toxic metal which can be reduced to the more insoluble and less toxic Cr(III) form. Although a variety of organisms have been shown to reduce Cr(VI), both aerobically and anaerobically (6, 22), few studies have clearly demonstrated the capacity to couple anaerobic growth to this process. Enterobacter cloacae HO1, which is also a member of the family Enterobacteriaceae, has been shown to reduce Cr(VI) under anaerobic conditions (40). However, it is unclear whether this organism actually uses Cr(VI) as an electron acceptor for growth, because it can grow anaerobically in the absence of added Cr(VI) with several of the potential electron donors and no evidence for Cr(VI)-dependent growth was presented (22). Recently, the sulfate-reducing organism Desulfotomaculum reducens MI-1 has been shown to have the unique capacity to couple anaerobic growth to the reduction of a variety of metals, including Cr(VI), although the mechanism has not yet been elucidated (39).
In the present study, P. agglomerans SP1 was shown to couple anaerobic growth to the reduction of Cr(VI) with acetate, hydrogen, and lactate as electron donors. Growth appeared to be Cr(VI) dependent, since no growth was observed with electron donors alone, even with the fermentable substrate lactate. In addition, the decrease in cell numbers after the Cr(VI) was completely reduced suggests that growth was dependent on the availability of this electron acceptor. The capacity to reductively precipitate Cr(VI) during anaerobic respiration may be an important mechanism for the natural attenuation of Cr(VI) toxicity in contaminated sediments.
SP1 was assayed for the capacity to use the humic substance analog AQDS as an electron acceptor because, to date, all of the AQDS reducers isolated from sediments have been members of the family Geobacteriaceae (8). All of those organisms were also capable of reducing both humic acids and Fe(III)-citrate. In the case of SP1, hydrogen and acetate both yielded rapid growth coupled to AQDS reduction, which suggests that SP1 may be the one of the first organisms cultivated from sediments, outside the family Geobacteriaceae, reported to grow via AQDS reduction. The capacity to utilize actual humic substances as electron acceptors was not explored, but like the strictly anaerobic humic substance reducers isolated by Coates et al. (8), SP1 has the capacity to couple growth to the reduction of AQDS as well as Fe(III).
P. agglomerans is a member of the family Enterobacteriaceae, which, in addition to the coliform bacteria of the genera Escherichia, Salmonella, and Shigella, includes the closely related genera Erwinia, Serratia, Klebsiella, Enterobacter, and Pantoea, which generally occupy different ecological niches (15). While coliform bacteria inhabit the intestinal tracts of humans or other vertebrates, members of the latter group of bacteria are found primarily in soil, water, plants, insects, and occasionally humans.
P. agglomerans is considered ubiquitous in the environment (14), perhaps most frequently found associated with soils, waters, and plants (13). Although these organisms are facultative anaerobes, known to grow anaerobically by fermentation, they have not been studied extensively in terms of other potential modes of anaerobic growth. Our results suggest that SP1-like organisms have a wide range of anaerobic capabilities, including the ability to grow via the dissimilatory reduction of a variety of electron acceptors, which could provide a selective advantage in suboxic sedimentary environments. This study not only adds to the growing list of organisms involved in metal reduction (and acetate oxidation) but also suggests that a closer examination of the anaerobic metabolic diversity of previously characterized groups of organisms may be necessary.
ACKNOWLEDGMENTS
We thank Irene Davidova for the acetate analysis.
This project was initiated as part of the Microbial Diversity summer course at the Marine Biological Laboratory, Woods Hole, Mass. C.A.F. was supported by a STAR Graduate Fellowship from the U.S. Environmental Protection Agency. This research was supported in part by Office of Naval Research grant N00014-99-1-0107 and the University of California Toxic Substances Research and Teaching Program.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Balch W E, Fox G E, Margrum L J, Woese R S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979;43:260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell P E, Mills A L, Herman J S. Biogeochemical conditions favoring magnetite formation during anaerobic iron reduction. Appl Environ Microbiol. 1987;53:2610–2616. doi: 10.1128/aem.53.11.2610-2616.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caccavo F, Jr, Lonergan D J, Lovley D R, Davis M, Stolz J F, McInerney M J. Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl Environ Microbiol. 1994;60:3752–3759. doi: 10.1128/aem.60.10.3752-3759.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caccavo F, Jr, Coates J D, Rosello-Mora R A, Ludwig W, Schleifer K-H, Lovley D R, McInerney M J. Geovibrio ferrireducens, a phylogenetically distinct dissimilatory Fe(III)-reducing bacterium. Arch Microbiol. 1996;165:370–376. doi: 10.1007/s002030050340. [DOI] [PubMed] [Google Scholar]
- 6.Chen J M, Hao O J. Microbial chromium(VI) reduction. Crit Rev Environ Sci Technol. 1998;28:219–251. [Google Scholar]
- 7.Cline E. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr. 1969;14:454–458. [Google Scholar]
- 8.Coates J D, Ellis D J, Blunt-Harris E L, Gaw C V, Roden E E, Lovley D R. Recovery of humic-reducing bacteria from a diversity of environments. Appl Environ Microbiol. 1998;64:1504–1509. doi: 10.1128/aem.64.4.1504-1509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coates J D, Lonergan D J, Lovley D R. Desulfuromonas palmitatis sp. nov., a long-chain fatty acid oxidizing Fe(III) reducer from marine sediments. Arch Microbiol. 1995;164:406–413. [PubMed] [Google Scholar]
- 10.Coates J D, Phillips E J P, Lonergan D J, Jenter H, Lovley D R. Isolation of Geobacter species from diverse sedimentary environments. Appl Environ Microbiol. 1996;62:1531–1536. doi: 10.1128/aem.62.5.1531-1536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummings D E, Caccavo F, Jr, Spring S, Rosenzweig R F. Ferribacterium limneticum, gen. nov., sp. nov., an Fe(III)-reducing microorganism isolated from mining-impacted freshwater lake sediments. Arch Microbiol. 1999;171:183–188. [Google Scholar]
- 12.Dwyer F P, Gyarfas E C, Mellor D P. The resolution and racemization of potassium ethylenediaminetetraacetatocobaltate(III) J Phys Chem. 1955;59:296–297. [Google Scholar]
- 13.Gavini F, Mergaert J, Beji A, Mielcarek C, Izard D, Kersters K, De Ley J. Transfer of Enterobacter agglomerans (Bjeijerinck 1888) Ewing and Fife 1972 to Pantoea gen. nov. as Pantoea agglomerans comb. nov. and description of Pantoea dispersa sp. nov. Int J Syst Bacteriol. 1989;39:337–345. [Google Scholar]
- 14.Grimont F, Grimont P A D. The genus Enterobacter. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 2797–2815. [Google Scholar]
- 15.Harada H, Ishikawa H. Phylogenetical relationship based on groE genes among phenotypically related Enterobacter, Pantoea, Klebsiella, Serratia, and Erwinia species. J Gen Appl Microbiol. 1997;43:355–361. doi: 10.2323/jgam.43.355. [DOI] [PubMed] [Google Scholar]
- 16.Knight V, Blakemore R. Reduction of diverse electron acceptors by Aeromonas hydrophila. Arch Microbiol. 1998;169:239–248. doi: 10.1007/s002030050567. [DOI] [PubMed] [Google Scholar]
- 17.Kostka J, Nealson K H. Isolation, cultivation and characterization of iron- and manganese-reducing bacteria. In: Burlage R S, et al., editors. Techniques in microbial ecology. New York, N.Y: Oxford University Press; 1998. pp. 58–78. [Google Scholar]
- 18.Krumbein W E, Altmann H J. A new method for the detection and enumeration of manganese oxidizing and reducing microorganisms. Helgoland Wiss Meeresunters. 1973;25:347–356. [Google Scholar]
- 19.Laverman A M, Switzer Blum J, Schaefer J K, Phillips E J, Lovley D R, Oremland R S. Growth of strain SES-3 with arsenate and other diverse electron acceptors. Appl Environ Microbiol. 1995;61:3556–3561. doi: 10.1128/aem.61.10.3556-3561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lonergan D J, Jenter H L, Coates J D, Phillips E J P, Schmidt T M, Lovley D R. Phylogenetic analysis of dissimilatory Fe(III)-reducing bacteria. J Bacteriol. 1996;178:2402–2408. doi: 10.1128/jb.178.8.2402-2408.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovley D R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991;55:259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovley D R. Dissimilatory metal reduction. Annu Rev Microbiol. 1993;47:263–290. doi: 10.1146/annurev.mi.47.100193.001403. [DOI] [PubMed] [Google Scholar]
- 23.Lovley D R. Microbial reduction of iron, manganese, and other metals. Adv Agron. 1995;54:175–231. [Google Scholar]
- 24.Lovley D R. Microbial Fe(III) reduction in subsurface environments. FEMS Microbiol Rev. 1997;20:305–313. [Google Scholar]
- 25.Lovley D R, Caccavo F, Phillips E J P. Acetate oxidation by dissimilatory Fe(III) reducers. Appl Environ Microbiol. 1992;58:3205–3206. doi: 10.1128/aem.58.9.3205-3208.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovley D R, Coates J D, Blunt-Harris E L, Phillips E J P, Woodward J C. Humic substances as electron acceptors for microbial respiration. Nature. 1996;382:445–448. [Google Scholar]
- 27.Lovley D R, Fraga J L, Blunt-Harris E L, Hayes L A, Phillips E J P, Coates J D. Humic substances as a mediator for microbially catalyzed metal reduction. Acta Hydrochim Hydrobiol. 1998;26:152–157. [Google Scholar]
- 28.Lovley D R, Phillips E J P. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 1988;54:11472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovley D R, Phillips E J P. Reduction of chromate by Desulfovibrio vulgaris and its C3 cytocrome. Appl Environ Microbiol. 1994;60:726–728. doi: 10.1128/aem.60.2.726-728.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maidak B, Larsen N, McCaughey M, Overbeek R, Olsen G, Fogel K, Blandy J, Woese C. The ribosomal database project. Nucleic Acids Res. 1994;22:3485–3487. doi: 10.1093/nar/22.17.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nealson K H, Saffarini D. Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Annu Rev Microbiol. 1994;48:311–343. doi: 10.1146/annurev.mi.48.100194.001523. [DOI] [PubMed] [Google Scholar]
- 32.Porter K G, Feig Y S. The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr. 1980;25:943–948. [Google Scholar]
- 33.Roden E E, Zachara J M. Microbial reduction of crystalline iron(III) oxides: influence of oxide surface area and potential for cell growth. Environ Sci Technol. 1996;30:1618–1628. [Google Scholar]
- 34.Rosello-Mora R A, Ludwig W, Kampfer P, Amann R, Schleifer K H. Ferrimonas balaerica gen. nov., spec. nov., a new marine facultative Fe(III)-reducing bacterium. Syst Appl Microbiol. 1995;18:196–202. [Google Scholar]
- 35.Stolz J F, Ellis D J, Switzer Blum J, Ahmann D, Oremland R S, Lovley D R. Sulfurospirillum barnesii sp. nov. and Sulfurospirillum arsenophilus sp. nov., new members of the Sulfurospirillum clade of the ɛ-Proteobacteria. Int J Syst Bacteriol. 1999;49:1177–1180. doi: 10.1099/00207713-49-3-1177. [DOI] [PubMed] [Google Scholar]
- 36.Stookey L L. Ferrozine—a new spectrophotometric reagent for iron. Anal Chem. 1970;42:779–781. [Google Scholar]
- 37.Sunda W G, Kieber D J. Oxidation of humic substances by manganese oxides yields low-molecular-weight organic substances. Nature. 1994;367:62–64. [Google Scholar]
- 38.Szilagyi M. Reduction of Fe3+ ion by humic acid preparations. Soil Sci. 1971;111:233–235. [Google Scholar]
- 39.Tebo B M, Obraztsova A Y. Sulfate-reducing bacterium grows with Cr(VI), U(VI), Mn(IV), and Fe(III) as electron acceptors. FEMS Microbiol Lett. 1998;162:193–198. [Google Scholar]
- 40.Wang P, Mori T, Komori K, Sasatsu M, Toda K, Ohtake H. Isolation and characterization of an Enterobacter cloacae strain that reduces hexavalent chromium under anaerobic conditions. Appl Environ Microbiol. 1989;55:1665–1669. doi: 10.1128/aem.55.7.1665-1669.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weisburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Widdel F, Bak F. Gram-negative mesophilic sulfate-reducing bacteria. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 3352–3378. [Google Scholar]