Abstract
Background
Diabetic nephropathy (DN) is a chronic microvascular complication caused by long-term hyperglycemia in patients with diabetes and an important cause of end-stage renal disease. Although some studies have shown that soluble Klotho(sKlotho) levels of patients with DN are lower than those without DN, in the early stage of patients with DN with normal renal function and albuminuria, the change in sKlotho is still controversial.
Aim
This meta-analysis was conducted to statistically evaluate sKlotho levels in patients with DN.
Methods
We searched the following electronic databases: Web of Science, Embase, PubMed, Google Scholar, and China National Knowledge Infrastructure (CNKI). The following search terms were used for the title or abstract: “diabetic kidney disease”, “diabetic nephropathy”, OR “DN” in combination with “Klotho”. The meta-analysis results were presented as standardized mean differences (SMDs) with corresponding 95% confidence intervals (CIs).
Results
Fourteen articles were included in the meta-analysis. In our meta-analysis, we found that the sKlotho level in patients with DN was significantly lower than that in patients without DN (SMD: -1.52, 95% CI [-2.24, -0.80]), and it was also significantly lower in the early stage of DN (SMD: -1.65, 95% CI [-2.60, -0.70]).
Conclusions
This systematic review was the first to evaluate the relationship between sKlotho levels and DN. The sKlotho level was significantly lower in the early stages of DN, indicating that sKlotho might be a new biomarker of DN in the future.
Keywords: Klotho, diabetic nephropathy, DN, meta- analyses, Systematic (Literature) Review
Introduction
Diabetic nephropathy (DN) is a chronic microvascular complication caused by long-term hyperglycemia in patients with diabetes and an important cause of chronic kidney disease (CKD). The pathogenesis of DN is complex. It is associated with hyperglycemia and insulin resistance in patients with diabetes. It has also been associated with abnormal lipid metabolism, inflammation, and oxidative stress (1, 2). The onset of DN is insidious, and there are often no obvious clinical manifestations in its early stages. Once it enters the clinical stage of nephropathy, renal lesions will be irreversible, which will delay the treatment of the disease. Therefore, early diagnosis and treatment are effective for treating DN (3). Microalbuminuria is considered to be the most common early manifestation of DN. However, microalbuminuria is often intermittent and fluctuating, and the early stage of nephropathy is often not accompanied by obvious symptoms, which can easily be ignored and missed (4). Therefore, new biomarkers are urgently required to assist in the early diagnosis of DN.
Klotho is a protein with anti-aging activity discovered by Japanese scientists in spontaneously hypertensive rats in 1997 (5). The Klotho gene is about 50 kb long, and two mRNA transcripts can arise through alternative splicing: one generates the type I transmembrane protein (130 kDa), the other is assumed to generate a secreted protein (70 kDa). Transmembrane Klotho protein is expressed mainly in choroid plexus epithelial cells of the brain and the distal convoluted tubules of the kidney. The extracellular region of transmembrane Klotho protein can be cleaved by α- and β-secretases, and eventually finds its way into blood, urine and cerebrospinal fluid. This cleaved Klotho protein is commonly known as the soluble Klotho(sKlotho) (6, 7). Previous studies have mainly focused on anti-aging functions, such as cell survival, proliferation, and apoptosis (8). In recent years, it has also been found to be involved in the regulation of energy metabolism. As the concentration of sKlotho increased, it exerted a protective effect on renal endothelial cells (9, 10). However, in the early stage of DN in patients with normal renal function, the change in sKlotho remains controversial (11–15). Therefore, this study aimed to investigate the relationship between the sKlotho levels and DN, especially its relationship with the early stage of DN to guide clinical treatment and prognosis.
Methods
Search
We searched the following electronic databases: Web of Science, Embase, PubMed, Google Scholar, and China National Knowledge Infrastructure (CNKI). The following search terms were used for the title or abstract: “diabetic kidney disease” OR “diabetic nephropathy” in combination with the term “Klotho.” The retrieval time was limited to 1980–2020, and the language was limited to English and Chinese. We also checked the references of the retrieved articles to avoid missing additional eligible studies. We did not search for any unpublished studies. The registration number for the systematic review and meta-analysis was CRD 42022309103 in PROSPERO. A complete list of the preferred reporting items for systematic reviews and meta-analyses is provided in the Supplementary Data (S1) .
Inclusion Criteria
The studies included in this meta-analysis met the following criteria: (1) detailed data about the sKlotho levels in patients with diabetes and DN; (2) patients with different stages of DN; and (3) multiple studies of the same author and department; only the study with the largest sample size was selected.
The degree of DN was based on the urine albumin-creatinine ratio (UACR) and chronic kidney disease-Kidney Disease Outcomes Quality Initiative (CKD-KDOQI) criteria. Patients with diabetes were categorized into three groups according to the UACR: UACR < 30 mg/g creatinine (normoalbuminuria), UACR 30–299 mg/g creatinine (microalbuminuria), and UACR ≥ 300 mg/g creatinine (macroalbuminuria). The early stage of DN was defined as microalbuminuria or CKD stage 1–2.
Data Extraction and Risk of Bias
Two reviewers independently searched according to the search strategy and independently read the title and abstract according to the search results for preliminary screening to exclude the study that did not meet the inclusion criteria. The full text of the papers were analyzed to determine whether they meet the inclusion criteria. Two reviewers can contact and crosscheck the author if the information is incomplete. If the conclusions of the two evaluators were not consistent, the differences were resolved through discussion. If the discussion failed to resolve the differences, it was judged and arbitrated by a third researcher. The Newcastle-Ottawa Scale (NOS) is a risk of a bias assessment tool for observational studies recommended by the Cochrane Collaboration (16, 17). The quality of the included studies was evaluated according to the NOS. The NOS includes three aspects: the selection method of the case and control groups, comparability of the case and control groups, and evaluation method of exposure. The NOS ranged from zero to nine stars, and quality was based on star scores.
Statistical Analysis
The data were expressed as standardized mean differences (SMD) and 95% confidence intervals (CIs). Heterogeneity among the included studies was assessed using the I2 statistic. If I2 was < 50%, the heterogeneity among studies was low or moderate, and the fixed-effect model was adopted. Otherwise, if I2 was > 50%, the random-effects model was used for analysis. Sensitivity analysis was performed to judge the stability of the results. The Begg’s and Egger’s tests were used to identify publication bias. Statistical significance was set at P < 0.05. Data were analyzed using Stata version 12.0 (College Station, TX, USA).
Results
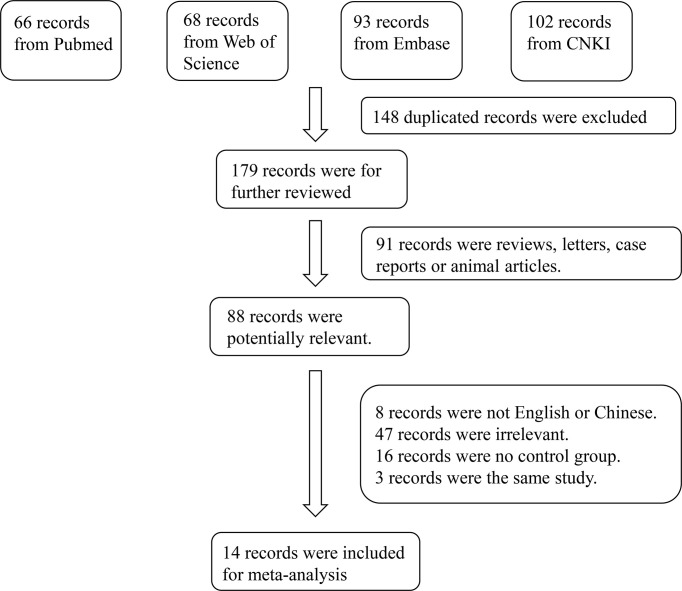
A total of 329 studies were retrieved from the databases. After screening, 14 articles were selected (11–14, 18–27). The flow diagram of the article selection process is shown in Figure 1 . The characteristics of the selected studies are summarized in Table 1 .
Figure 1.
Flowchart of the detailed procedure for the inclusion or exclusion of selected studies.
Table 1.
Study characteristics of the published studies included in the meta-analysis.
| Author | Publication Year | Study Period | Region | Study Design | Study Sample | Sex | Age | Details | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Kacso IM (11) | 2012 | – | Romania | Case-control study | 146 patients with type 2 diabetes and 43 healthy controls | patients with type 2 diabetes: 87 males; healthy controls: 20 males. | patients with type 2 diabetes: mean age 63.38 ± 12.20 years; healthy controls: mean age 61.07 ± 11.47 years. | The diabetic patients were categorized into 3 groups according to UACR. The mean duration of diabetes was 8.8 ± 5.6 years. Ninety-two of the diabetic patients were on insulin treatment, 43 were treated with metformin, and 44 with sulfonylureas. | 5 |
| Lee EY (18) | 2014 | February 2010 - February 2012 | Korea | Case-control study | 147 patients with type 2 diabetes and 43 healthy controls | patients with type 2 diabetes: 63 males; healthy controls: 14 males. | patients with type 2 diabetes: mean age 56.6 ± 10.6 years; healthy controls: mean age 50.9 ± 7.6 years. | The diabetic patients were categorized into 3 groups according to UACR. The mean duration of diabetes was 7.8 ± 5.9 years. | 6 |
| Wu C (12) | 2014 | April 2010 - July 2013 | China | Case-control study | 462 patients with type 2 diabetes and 160 healthy controls | patients with type 2 diabetes: 214 males; healthy controls: 78 males. | patients with type 2 diabetes: mean age 52.9 ± 6.3 years; healthy controls: mean age 51.8 ± 6.5 years. | The diabetic patients were categorized into 3 groups according to UACR. The mean duration of diabetes was 7.7 ± 4.2 years. | 7 |
| Dogan B (13) | 2016 | February 2012 - March 2013 | Turkey | Case-control study | 147 patients with type 1 diabetes and 76 healthy controls | patients with type 1 diabetes: 65 males; healthy controls: 33 males. | patients with type 1 diabetes: mean age 34.1 ± 9.2 years; healthy controls: mean age 33.9 ± 9.1 years. | – | 7 |
| Inci A (14) | 2016 | January 2014 - June 2014 | Turkey | Case-control study | 109 patients with type 2 diabetes and 32 healthy controls | patients with type 2 diabetes: 62 males; healthy controls: 12 males. | patients with type 2 diabetes: mean age 61.63 ± 9.77 years; healthy controls: mean age 49.53 ± 7.32 years. | The diabetic patients were categorized into 3 groups according to UACR. | 7 |
| Maltese G (19) | 2017 | – | London | Observational study | 78 patients with type 1 diabetes | patients with type 1 diabetes: 43 males | patients with microalbuminuria: mean age 54.4 ± 11.6 years; controls: mean age 43.3 ± 9.6 years. | The diabetic patients were categorized into 2 groups according to UACR. The mean duration of diabetes was 30.9 ± 10.0 years | 5 |
| Nie F (20) | 2017 | 2014 - 2016 | China | Observational study | 96 patients with type 2 diabetes, 90 patients with diabetic nephropathy and 106 healthy controls | patients with type 2 diabetes: 42 males; patients with diabetic nephropathy: 43 males; healthy controls: 52 males | patients with type 2 diabetes: 59.5(53-71) years; patients with diabetic nephropathy: 62(57.3-73.5) years; healthy controls: 57(47-63) years. | The diabetic patients were categorized into 3 groups according to UACR. | 6 |
| Ye M (21) | 2017 | June 2016 - January 2017 | China | Case-control study | 160 patients with type 2 diabetes and 80 healthy controls | patients with type 2 diabetes: 101 males; healthy controls: 47 males. | patients with type 2 diabetes: mean age 52.85 ± 11.15 years; healthy controls: mean age 52.47 ± 12.15 years. | All the patients were newly diagnosed and categorized into 2 groups according to UACR. | 6 |
| Fountoulakis N (22) | 2018 | 2004-2006 | London | Observational study | 101 patients with type 2 diabetes | patients with type 2 diabetes: 60 males | mean age (range) was 60 (40-82) years. | The mean duration of diabetes was 9.8 ± 6.6 years, with a mean eGFR of 90.4 ± 20.0 ml/min. The diabetic patients were categorized into 2 groups according to UACR. | 8 |
| Zhang L (23) | 2018 | January 2016 - January 2018 | China | Case-control study | 127 patients with type 2 diabetes, 106 patients with diabetic nephropathy and 140 healthy controls | patients with type 2 diabetes: 59 males; patients with diabetic nephropathy: 55 males; healthy controls: 77 males | patients with type 2 diabetes: 52.5 (41–71) years; patients with diabetic nephropathy: 54.6 (42–77) years; healthy controls: 52.9 (39–71) years. | – | 7 |
| Li Q (24) | 2019 | February 2017 - September 2017 | China | Case-control study | 68 patients with type 2 diabetes and 22 healthy controls | patients with type 2 diabetes: 39 males; healthy controls: 12 males. | patients with type 2 diabetes: mean age 48.1 ± 6.3 years; healthy controls: mean age 45.5 ± 4.2 years. | The diabetic patients were categorized into 3 groups according to UACR. | 6 |
| Chen J (25) | 2020 | February 2015 - August 2018 | China | Case-control study | 160 patients with type 2 diabetes and 60 healthy controls | patients with type 2 diabetes: 70 males; healthy controls: 20 males. | patients with type 2 diabetes: mean age 57.8 ± 5.6 years; healthy controls mean age 50.2 ± 6.9 years. | The diabetic patients were categorized into 3 groups according to UACR. | 7 |
| Tian Q (26) | 2020 | November 2016 - November 2018 | China | Case-control study | 174 patients with type 2 diabetes and 55 healthy controls | patients with type 2 diabetes: 90 males; healthy controls: 26 males. | patients with type 2 diabetes: mean age 58.0 ± 7.9 years; healthy controls: mean age 56.1 ± 7.4 years. | The mean duration of diabetes was 10.9 ± 3.7 years. The diabetic patients were categorized into 3 groups according to UACR. | 6 |
| Nie L (27) | 2021 | February 2017 - May 2018 | China | Case-control study | 80 patients with type 2 diabetes and 50 healthy controls | patients with type 2 diabetes: 36 males; healthy controls: 23 males. | patients with type 2 diabetes: mean age 58.6 ± 5.58 years; healthy controls: mean age 58.27 ± 5.35 years. | The mean duration of diabetes was 6.3 ± 0.7 years. Diabetic nephropathy was diagnosed by biopsy. | 5 |
NOS, Newcastle-Ottawa Scale; UACR, urine albumin-creatinine ratio.
Results of the Meta-Analysis
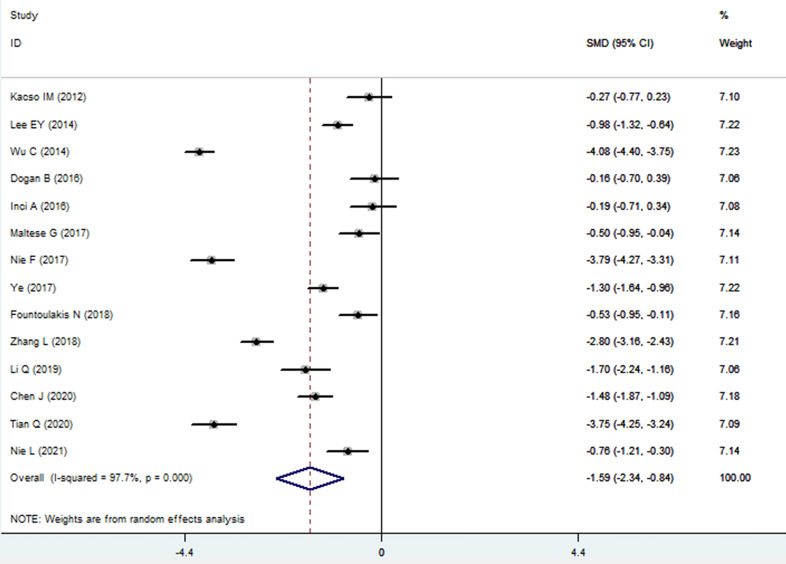
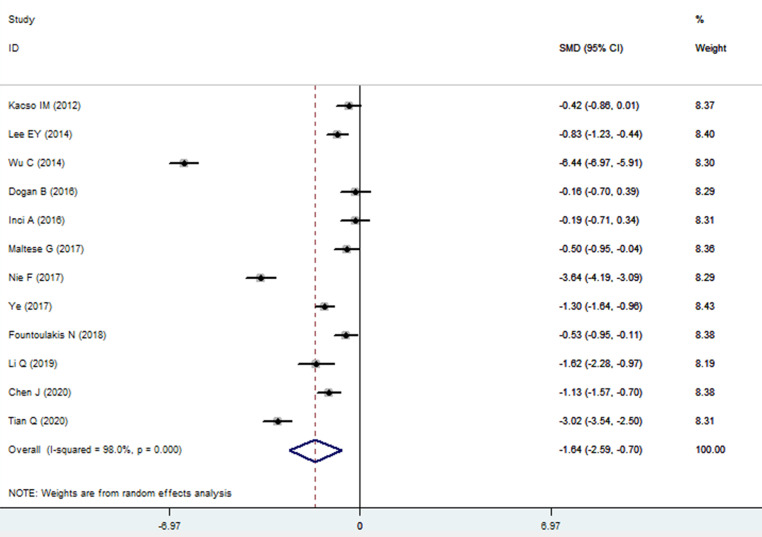
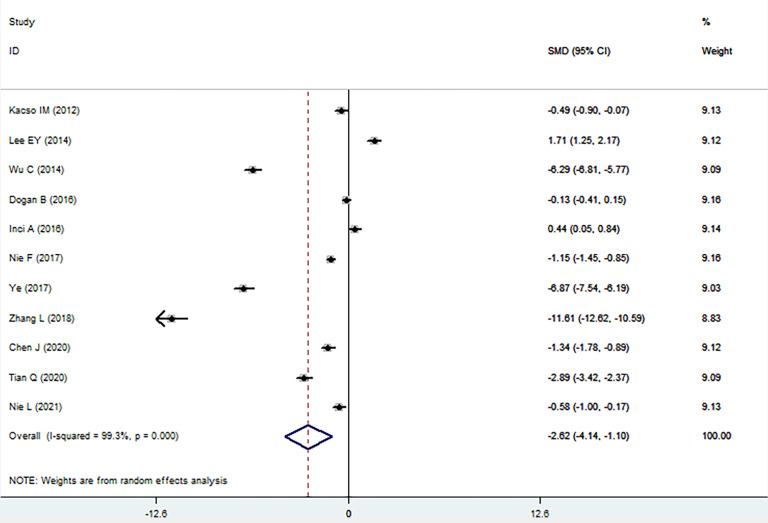
The sKlotho level in patients with DN was significantly lower than that in patients with diabetes (SMD: -1.52, 95% CI [-2.24, -0.80]). Forest plots of the sKlotho levels in patients with DN compared to those with diabetes are presented in Figure 2 . Moreover, there was a significant difference in the sKlotho levels between patients with diabetes without DN and those with early stage of DN (SMD: -1.65, 95% CI [-2.60, -0.70]). Forest plots of the sKlotho levels are shown in Figure 3 . Compared with the controls, the sKlotho level was also significantly lower in patients with diabetes (SMD: -2.12, 95% CI [-4.14, -1.10], Figure 4 ).
Figure 2.
Forest plots and funnel plots of sKlotho level in patients with diabetic nephropathy compared to diabetes. Diamond represents the pooled SMDs at 95% CI. SMD, standardized mean difference; CI, confidence interval.
Figure 3.
Forest plots and funnel plots of sKlotho level in patients with early stage of diabetic nephropathy compared to diabetes without diabetic nephropathy. Diamond represents the pooled SMDs at 95% CI. SMD, standardized mean difference; CI, confidence interval.
Figure 4.
Forest plots and funnel plots of sKlotho level in patients with diabetes compared to the controls. Diamond represents the pooled SMDs at 95% CI. SMD, standardized mean difference; CI, confidence interval.
Sensitivity Analysis and Publication Bias
Sensitivity analysis was performed to examine the influence of each study. We found no significant difference between the sensitivity analysis results and our previous estimates, indicating that the data of a single study had a little overall impact. Thus, it can be inferred that the results of this meta-analysis were stable. ( Figures S1 - S3 ). A careful and comprehensive search was performed for articles obtained from the database. In addition, the Begg’s and Egger’s tests were performed to determine whether there was a potential publication bias in the reviewed study. The results (P > 0.05) indicated no publication bias.
Discussion
This systematic review was the first to evaluate the relationship between the sKlotho levels and DN in patients with diabetes. Although some studies have shown that the sKlotho levels of patients with DN are lower than those without DN, in the early stage of patients with DN with normal renal function and albuminuria, the change in sKlotho is still controversial. Fourteen independent studies were included in the meta-analysis. We concluded that the sKlotho level in patients with DN was significantly lower than that in patients without DN (SMD: -1.52, 95% CI [-2.24, -0.80]), and it was also significantly lower in the early stages of DN (SMD: -1.65, 95% CI [-2.60, -0.70]).
Diabetic nephropathy is characterized by changes in the structure and function of the glomeruli. The disease is reversible at an early stage and irreversible in patients with persistent proteinuria. The International Diabetes Federation predicts that by 2030, the total number of patients with type 2 diabetes will reach 439 million worldwide (28). The incidence of diabetes-related complications is also increasing with an increase in the number of patients with diabetes. Identifying serum biomarkers for the early diagnosis of DN is of great significance for improving the prognosis of patients with diabetes. Determination of renal function is an important means of diagnosing DN (29). However, there are still some changes in renal function indices in some patients. In recent years, the role of Klotho in diabetes and kidney disease has attracted increasing attention (30).
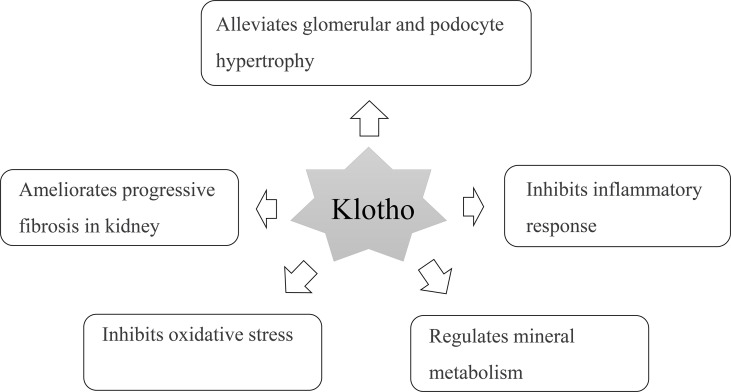
Klotho protein is a transmembrane protein and mainly expressed in the kidney, heart, and brain and can inhibit the inflammatory response (31). It protects islet β-cell function and can promote insulin secretion, thereby reducing blood glucose and delaying the process of renal disease ( Figure 5 ) (32). Supplementing exogenous α-Klotho can reduce hyperglycemia injury by promoting glycogen storage, inhibiting gluconeogenesis, improving insulin sensitivity in type 2 diabetes, anti-inflammatory, antioxidant stress, and inhibiting fibrosis; thus, protecting the kidney (33–35). Klotho attenuates diabetic nephropathy in db/db mice and ameliorates high glucose-induced injury of human renal glomerular endothelial cells (36). In addition, Klotho can inhibit the renin-angiotensin-aldosterone system, and the nuclear factor kappa B (NF-κB) signaling pathway inhibits renal fibrosis caused by the inflammatory response. Therefore, the consumption of Klotho in patients with DN is relatively high, resulting in a decrease in the Klotho levels (37, 38). Animal experiments have shown that mice lacking the Klotho gene show significant changes, such as endothelial cell injury and abnormal energy metabolism. After sKlotho supplementation, the expression of nitric oxide in vascular endothelial cells increases, reverses vascular inflammatory reactions, and protects renal function (39). Exogenous Klotho attenuates high glucose (HG)-induced profibrotic genes, TGF-β signaling and cell hypertrophy in rat renal interstitial fibroblasts (NRK-49F) cells. Moreover, Klotho attenuates HG-induced fibronectin expression and cell hypertrophy via the ERK1/2 and p38 kinase-dependent pathways (40). Klotho protein overexpression attenuates renal hypertrophy and glomerular injury in this mouse model of diabetic nephropathy. Klotho overexpression attenuated renal hypertrophy, albuminuria, glomerular mesangial expansion, and endothelial glycocalyx loss in the AKITA mice (41). In our meta-analysis, we found that even in the early stages of DN, the sKlotho level was significantly lower in patients with diabetes without DN. We hope that sKlotho is a more sensitive biomarker during the early stages of DN.
Figure 5.
Multifactorial effects of Klotho in the pathology of diabetic nephropathy.
This meta-analysis aimed to statistically evaluate the sKlotho levels in patients with DN. However, this study had some limitations. In different studies, the duration of diabetes and severity of the disease were variant. Meanwhile, the overall quality of the studies was not high. All of these factors may have affected the results; therefore, the findings of this meta-analysis should be interpreted cautiously, as further research is needed.
Conclusion
This systematic review was the first to evaluate the relationship between the sKlotho levels and DN. The sKlotho level was significantly lower in the early stages of DN, indicating that sKlotho might be a new biomarker of DN in the future.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
TG designed the study. CX searched databases and collected the data. XS and ZL assessed the quality of the study. XS performed the analysis. TG and CX wrote the manuscript. All authors contributed to this systematic review and meta-analysis. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.902765/full#supplementary-material
Preferred reporting items for systematic review and meta-analyses (PRISMA) checklist.
The sensitivity analysis results of sKlotho level in patients with diabetic nephropathy compared to diabetes.
The sensitivity analysis results of sKlotho level in patients with early stage of diabetic nephropathy compared to diabetes without diabetic nephropathy.
The sensitivity analysis results of sKlotho level in patients with diabetes compared to the controls.
References
- 1. Sagoo MK, Gnudi L. Diabetic Nephropathy: An Overview. Methods Mol Biol (2020) 2067:3–7. doi: 10.1007/978-1-4939-9841-8_1 [DOI] [PubMed] [Google Scholar]
- 2. Guo J, Zheng HJ, Zhang W, Lou W, Xia C, Han XT, et al. Accelerated Kidney Aging in Diabetes Mellitus. Oxid Med Cell Longev (2020), 1234059. doi: 10.1155/2020/1234059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Viigimaa M, Sachinidis A, Toumpourleka M, Koutsampasopoulos K, Alliksoo S, Titma T, et al. Macrovascular Complications of Type 2 Diabetes Mellitus. Curr Vasc Pharmacol (2020) 18(2):110–16. doi: 10.2174/1570161117666190405165151 [DOI] [PubMed] [Google Scholar]
- 4. Fineberg D, Jandeleit-Dahm KA, Cooper ME. Diabetic Nephropathy: Diagnosis and Treatment. Nat Rev Endocrinol (2013) 9(12):713–23. doi: 10.1038/nrendo.2013.184 [DOI] [PubMed] [Google Scholar]
- 5. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the Mouse Klotho Gene Leads to a Syndrome Resembling Ageing. Nature (1997) 390(6655):45–51. doi: 10.1038/36285 [DOI] [PubMed] [Google Scholar]
- 6. Xu Y, Sun Z. Molecular Basis of Klotho: From Gene to Function in Aging. Endocr Rev (2015) 36(2):174–93. doi: 10.1210/er.2013-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dalton GD, Xie J, An SW, Huang CL. New Insights Into the Mechanism of Action of Klotho. Front Endocrinol (2017) 8:1e10. doi: 10.3389/fendo.2017.00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuro-O M. Klotho in Health and Disease. Curr Opin Nephrol Hypertens (2012) 21:362.e368. doi: 10.1097/MNH.0b013e32835422ad [DOI] [PubMed] [Google Scholar]
- 9. Kim HR, Nam BY, Kim DW, Kang MW, Han JH, Lee MJ, et al. Circulating α-Klotho Levels in CKD and Relationship to Progression. Am J Kidney Dis (2013) 61:899.e909. doi: 10.1053/j.ajkd.2013.01.024 [DOI] [PubMed] [Google Scholar]
- 10. Marlena T, Agnieszka P. Antiinflammatory Actions of Klotho: Implications for Therapy of Diabetic Nephropathy. Int J Mol Sci (2021) 22(2):956. doi: 10.3390/ijms22020956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kacso IM, Bondor CI, Kacso G. Soluble Serum Klotho in Diabetic Nephropathy: Relationship to VEGF-A. Clin Biochem (2012) 45(16-17):1415–20. doi: 10.1016/j.clinbiochem.2012.07.098 [DOI] [PubMed] [Google Scholar]
- 12. Wu C, Wang Q, Lv C, Qin N, Lei S, Yuan Q, et al. The Changes of Serum Sklotho and NGAL Levels and Their Correlation in Type 2 Diabetes Mellitus Patients With Different Stages of Urinary Albumin. Diabetes Res Clin Pr (2014) 106(2):343–50. doi: 10.1016/j.diabres.2014.08.026 [DOI] [PubMed] [Google Scholar]
- 13. Dogan B, Arikan IH, Guler D, Keles N, Isbilen B, Isman F, et al. Fibroblast Growth Factor-23 But Not Sklotho Levels Are Related to Diastolic Dysfunction in Type 1 Diabetic Patients With Early Diabetic Nephropathy. Int Urol Nephrol (2016) 48(3):399–407. doi: 10.1007/s11255-015-1190-y [DOI] [PubMed] [Google Scholar]
- 14. Inci A, Sari F, Coban M, Olmaz R, Dolu S, Sarıkaya M, et al. Soluble Klotho and Fibroblast Growth Factor 23 Levels in Diabetic Nephropathy With Different Stages of Albuminuria. J Invest Med (2016) 64(6):1128–33. doi: 10.1136/jim-2016-000142 [DOI] [PubMed] [Google Scholar]
- 15. Farías-Basulto A, Martínez-Ramírez HR, Gómez-García EF, Cueto-Manzano AM, Cortés-Sanabria L, Hernández-Ramos LE, et al. Circulating Levels of Soluble Klotho and Fibroblast Growth Factor 23 in Diabetic Patients and Its Association With Early Nephropathy. Arch Med Res (2018) 49(7):451–5. doi: 10.1016/j.arcmed.2019.01.008 [DOI] [PubMed] [Google Scholar]
- 16. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non Randomised Studies in Meta-Analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed 2014 Aug 5).
- 17. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Available at: http://www.cochrane-handbook.org (Accessed 2014 Aug).
- 18. Lee EY, Kim SS, Lee JS, Kim IJ, Song SH, Cha SK, et al. Soluble Alpha-Klotho as a Novel Biomarker in the Early Stage of Nephropathy in Patients With Type 2 Diabetes. PLoS One (2014) 9(8):e102984. doi: 10.1371/journal.pone.0102984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maltese G, Fountoulakis N, Siow RC, Gnudi L, Karalliedde J. Perturbations of the Anti-Ageing Hormone Klotho in Patients With Type 1 Diabetes and Microalbuminuria. Diabetologia (2017) 60(5):911–4. doi: 10.1007/s00125-017-4219-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nie F, Wu D, Du H, Yang X, Yang M, Pang X, et al. Serum Klotho Protein Levels and Their Correlations With the Progression of Type 2 Diabetes Mellitus. J Diabetes Complications (2017) 31(3):594–8. doi: 10.1016/j.jdiacomp.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 21. Ye M, Yang Y, Cao J. The Relationships of Serum Klotho Protein With Vascular Endothelial Growth Factor and Urinary Albumin Creatinine Ratio in Patients With Early Diabetic Nephropathy. J Med Theor Prac (2017) 30(18):2673–5. doi: 10.19381/j.issn.1001-7585.2017.18.005 [DOI] [Google Scholar]
- 22. Fountoulakis N, Maltese G, Gnudi L, Karalliedde J. Reduced Levels of Anti-Ageing Hormone Klotho Predict Renal Function Decline in Type 2 Diabetes. J Clin Endocrinol Metab (2018) 103(5):2026–32. doi: 10.1210/jc.2018-00004 [DOI] [PubMed] [Google Scholar]
- 23. Zhang L, Liu T. Clinical Implication of Alterations in Serum Klotho Levels in Patients With Type 2 Diabetes Mellitus and Its Associated Complications. J Diabetes Complicat (2018) 32(10):922–30. doi: 10.1016/j.jdiacomp.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 24. Li Q, Wang Z, Chu Y. Relationship Between Proteinuria, Serum TGF-β1, and Klotho Protein in Patients With Type 2 Diabetic Nephropathy. J Hubei Minzu Univ (Medical Edition) (2019) 36(01):25–7. doi: 10.13501/j.cnki.42-1590/r.2019.01.007 [DOI] [Google Scholar]
- 25. Chen J. Correlation Between Cystatin C, α-Klotho Protein, sICAM-1 and Diabetic Nephropathy. Lab Med (2020) 35(10):1032–5. doi: 10.3969/j.issn.1673-8640.2020.10.015 [DOI] [Google Scholar]
- 26. Tian Q, Li B, Li N, Guo S, Zhang M, Li J, et al. Changes and Significance of Serum Pentraxin 3 and α-Klotho Protein Levels in Patients With Type 2 Diabetic Kidney Disease. Anhui Med J (2020) 41(08):900–6. doi; 10.3969/j.issn.1000-0399.2020.08.010 [DOI] [Google Scholar]
- 27. Nie L, Zang J, Zhou L, Peng L, Maimaitiming K, Zhai Y., et al. Correlation Analysis of Serum GDF-15, sVCAM-1, YKL-40, α-Klotho Protein Levels With Glucose and Lipid Metabolism, Insulin Resistance and Renal Function in Patients With Type 2 Diabetic Nephropathy. Prog Modern Biomedicine (2021) 21(14):2703–7. doi: 10.13241/j.cnki.pmb.2021.14.022 [DOI] [Google Scholar]
- 28. Shaw JE, Sicree RA, Zimmet PZ. Global Estimates of the Prevalence of Diabetes for 2010 and 2030. Diabetes Res Clin PR (2010) 87(1):4–14. doi: 10.1016/j.diabres.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 29. Samsu N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. BioMed Res Int (2021) 2021:1497449. doi: 10.1155/2021/1497449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang CL. Regulation of Ion Channels by Secreted Klotho: Mechanisms and Implications. Kidney Int (2010) 77:855.e60. doi: 10.1038/ki.2010.73 [DOI] [PubMed] [Google Scholar]
- 31. Drueke TB, Massy ZA. Circulating Klotho Levels: Clinical Relevance and Relationship With Tissue Klotho Expression. Kidney Int (2013) 83:13e5. doi: 10.1038/ki.2012.370 [DOI] [PubMed] [Google Scholar]
- 32. Kim JH, Hwang KH, Park KS, Kong ID, Cha SK. Biological Role of Anti-Aging Protein Klotho. J Lifestyle Med (2015) 5:1–6. doi: 10.15280/jlm.2015.5.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuro-O M. The Klotho Proteins in Health and Disease. Nature Reviews. Nephrology (2019) 15(1):27–44. doi: 10.1038/s41581-018-0078-3 [DOI] [PubMed] [Google Scholar]
- 34. Neyra JA, Hu MC. α-Klotho and Chronic Kidney Disease. Vitam Horm (2016) 101:270–310. doi: 10.1016/bs.vh.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Silva AP, Mendes F, Carias E, Goncalves RB, Fragoso A, Dias C, et al. Plasmatic Klotho and FGF23 Levels as Biomarkers of CKD-Associated Cardiac Disease in Type 2 Diabetic Patients. Int J Mol Sci (2019) 20(7):1536. doi: 10.3390/ijms20071536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang Q, Ren D, Li Y, Xu G. Klotho Attenuates Diabetic Nephropathy in Db/Db Mice and Ameliorates High Glucose-Induced Injury of Human Renal Glomerular Endothelial Cells. Cell Cycle (2019) 18(6-7):696–707. doi: 10.1080/15384101.2019.1580495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao Y, Banerjee S, Dey N, LeJeune WS, Sarkar PS, Brobey R, et al. Klotho Depletion Contributes to Increased Inflammation in Kidney of the Db/Db Mouse Model of Diabetes via RelA (Serine)536 Phosphorylation. Diabetes (2011) 60(7):1907–16. doi: 10.2337/db10-1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Su XM, Yang W. α-Klotho Is an Acute Phase Protein and Altered by Restraint Stress in Mice. Int J Clin Exp Pathol (2014) 7(9):5922–592. [PMC free article] [PubMed] [Google Scholar]
- 39. Lin Y, Kuro-O M, Sun Z. Genetic Deficiency of Anti-Aging Gene Klotho Exacerbates Early Nephropathy in STZ-Induced Diabetes in Male Mice. Endocrinology (2013) 154(10):3855–63. doi: 10.1210/en.2013-1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang JS, Chuang CT, Liu MH, Lin SH, Guh JY, Chuang LY, et al. Klotho Attenuates High Glucose-Induced Fibronectin and Cell Hypertrophy via the ERK1/2-P38 Kinase Signaling Pathway in Renal Interstitial Fibroblasts. Mol Cell Endocrinol (2014) 390(1-2):45–53. doi: 10.1016/j.mce.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 41. Kadoya H, Satoh M, Haruna Y, Sasaki T, Kashihara N. Klotho Attenuates Renal Hypertrophy and Glomerular Injury in Ins2Akita Diabetic Mice. Clin Exp Nephrol (2015) 20(5):1–8. doi: 10.1007/s10157-015-1202-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preferred reporting items for systematic review and meta-analyses (PRISMA) checklist.
The sensitivity analysis results of sKlotho level in patients with diabetic nephropathy compared to diabetes.
The sensitivity analysis results of sKlotho level in patients with early stage of diabetic nephropathy compared to diabetes without diabetic nephropathy.
The sensitivity analysis results of sKlotho level in patients with diabetes compared to the controls.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.