Abstract
Following a request from the European Commission, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) was asked to deliver an opinion on zinc l‐carnosine as a novel food (NF) pursuant to Regulation (EU) 2015/2283 and as a source of zinc for use in food supplements. The NF is produced by chemical synthesis and is proposed to be used in food supplements as a source of zinc. The target population proposed by the applicant is individuals above the age of 12, excluding pregnant and lactating women. The NF which is the subject of the application is a chelate‐complex, formed between Zn2+ and l‐carnosine and is present as a mixture of a monomer and a dimer. The material is a powder with particulate nature and is insoluble in water at neutral pH. No relevant data using an existing zinc source as comparator have been made available by the applicant and the actual bioavailability of the zinc provided by the NF at the proposed use levels remains uncharacterised. Owing to the lack of a correct characterisation of the fraction of small particles, including nanoparticles of the NF, the Panel is not in the position to evaluate specification limits for the size of the constituent particles in the NF. Owing to the lack of information on the size distribution and the physico‐chemical properties of the particles constituting the NF, the Panel is not in the position to confirm whether the ADME studies and the toxicological studies provided by the applicant are appropriate to assess the safety of the NF. The Panel concludes that the NF is absorbed and provides zinc, but as it is in an insufficiently characterised particulate form, its safety has not been established and the bioavailability has not been determined.
Keywords: Novel Foods, food supplement, nutrient source, zinc, zinc l‐carnosine, safety, bioavailability
1. Introduction
1.1. Background and Terms of Reference as provided by the European Commission
On 4 December 2019, the company Hamari Chemicals, Ltd. submitted a request to the Commission in accordance with Article 10 of Regulation (EU) No 2015/2283 to place on the EU market Zinc l‐carnosine.
Zinc l‐carnosine is intended to be used in food supplements. In addition, as Zinc l‐carnosine is also a new source of zinc, the opinion should also address the bioavailability of zinc from this source in the context of Directive 2002/46/EC of the European Parliament and of the Council laying down requirements for food supplements.
In accordance with Article 10(3) of Regulation (EU) 2015/2283, the European Commission asks the European Food Safety Authority to provide a scientific opinion on Zinc l‐carnosine. In addition, as Zinc l‐carnosine is also a new source of zinc, the opinion should also address the bioavailability of zinc from this source in the context of Directive 2002/46/EC on food supplements.1
1.2. Information on existing evaluations and authorisations
Zinc is a very ubiquitous nutrient found in different food sources. In 2014, the NDA Panel published an opinion on dietary reference values for zinc (EFSA NDA Panel, 2014). Dietary zinc intake from the background diet were calculated by EFSA in 2014 using the EFSA Comprehensive Food Consumption Database (EFSA, 2011) and the EFSA Food Composition Database. The main dietary groups contributing to zinc intake as calculated by EFSA in 2014 were meat and meat products, grains and grain‐based products, and milk and dairy (EFSA NDA Panel, 2014).
In 2003, the Scientific Committee on Food (SCF) published an opinion on the tolerable upper intake level (UL) for zinc (SCF, 2003). An UL for zinc of 25 mg/day was established for adults, including pregnant and lactating women based on studies of zinc supplementation for up to 14 weeks. In the absence of data on adverse effects of zinc on children and adolescents, the UL established for those population groups was extrapolated from the UL for adults (25 mg/day) using body weight to the power of 0.75 and reference body weights for European children (SCF, 1993). Therefore, the established ULs for zinc in children and adolescents were 7 mg/day for 1‐3 years, 10 mg/day for 4–6 years, 13 mg/day for 7–10 years, 18 mg/day for 11–14 years and 22 mg/day for 15–17 years (SCF, 2003).
Currently, Directive 2002/46/EC1 on food supplements includes the following mineral substances which may be used in the manufacture of food supplements as a source of zinc: zinc acetate, zinc l‐ascorbate, zinc l‐aspartate, zinc bisglycinate, zinc chloride, zinc citrate, zinc gluconate, zinc lactate, zinc l‐lysinate, zinc malate, zinc mono‐l‐methionine sulfate, zinc oxide, zinc carbonate, zinc l‐pidolate, zinc picolinate and zinc sulfate.
2. Data and methodologies
2.1. Data
The safety assessment of this NF is based on data supplied in the application and information submitted by the applicant following EFSA’s requests for supplementary information and information provided by the EFSA Working Group on nanomaterials.
Administrative and scientific requirements for NF applications referred to in Article 10 of Regulation (EU) 2015/2283 are listed in the Commission Implementing Regulation (EU) 2017/24692.
A common and structured format on the presentation of NF applications is described in the EFSA guidance on the preparation and presentation of an NF application (EFSA NDA Panel, 2016). As indicated in this guidance, it is the duty of the applicant to provide all of the available (proprietary, confidential and published) scientific data (including both data in favour and not in favour) that are pertinent to the safety of the NF.
This NF application does not include a request for the protection of proprietary data.
2.2. Methodologies
The assessment follows the methodology set out in the EFSA guidance on NF applications (EFSA NDA Panel, 2016) and the principles described in the relevant existing guidance documents from the EFSA Scientific Committee. The legal provisions for the assessment are laid down in Article 11 of Regulation (EU) 2015/2283 and in Article 7 of the Commission Implementing Regulation (EU) 2017/2469.
This assessment concerns only the risks that might be associated with consumption of the NF under the proposed conditions of use, and is not an assessment of the efficacy of the NF with regard to any claimed benefit.
The evaluation of bioavailability of the nutrient (zinc) from the source (zinc l‐carnosine) was conducted in line with the principles contained in the ‘Guidance on safety evaluation of sources of nutrients and bioavailability of nutrient from the sources’ (EFSA ANS Panel, 2018).
The assessment of small particles in the NF was conducted in line with the principles of the ‘Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles’ (EFSA Scientific Committee, 2021).
3. Assessment
3.1. Introduction
The NF which is the subject of the application is zinc l‐carnosine. The NF is produced by chemical synthesis and is proposed to be used in food supplements as a source of zinc. The target population is individuals above 12 years of age, excluding pregnant and lactating women. The NF falls under the following categories, as defined in Art. 3 of Regulation (EU) 2015/2283: (i) food with a new or intentionally modified molecular structure, where that structure was not used as, or in, a food within the Union before 15 May 1997; (ix) vitamins, minerals and other substances used in accordance with Directive 2002/46/EC, Regulation (EC) No 1925/2006 or Regulation (EU) No 609/2013.
3.2. Identity of the NF
The NF is zinc l‐carnosine, a chelate‐complex, formed between Zn2+ and l‐carnosine, which is a naturally occurring dipeptide consisting of l‐histidine and the non‐proteinogenic amino acid ß‐alanine.
Zinc l‐carnosine is registered with CAS number 107667‐60‐7. According to the applicant, the NF can be described with two IUPAC names, namely one designating the monomer and one designating the dimer, i.e. as a mixture of the monomer {zinc(2+)‐μ‐[β‐alanyl‐l‐histidinato(2‐)‐κN, κNα, κO:κNτ]} and the dimer di{zinc(2+)‐μ‐[β‐alanyl‐l‐histidinato(2‐)‐κN, κNα, κO:κNτ]}.
Synonyms include β‐alanyl‐l‐histidinato zinc, polaprezinc, Z‐103, zinc carnosine, zinc l‐carnosine complex, zinc N‐(3‐aminopropionyl)histidine.
Zinc l‐carnosine is described in the monograph of the JP (Japanese Pharmacopoeia) under the synonym ‘polaprezinc’.
According to the applicant, the molecular formula of zinc l‐carnosine is (C9H12N4O3Zn)n, and the molecular weight is (289.61)n Da, where n = 1–2. Accordingly, the molecular weight varies between 289.61 and 579.22 g/mol.
The molecular structure of the NF is reported in Figure 1.
Figure 1.
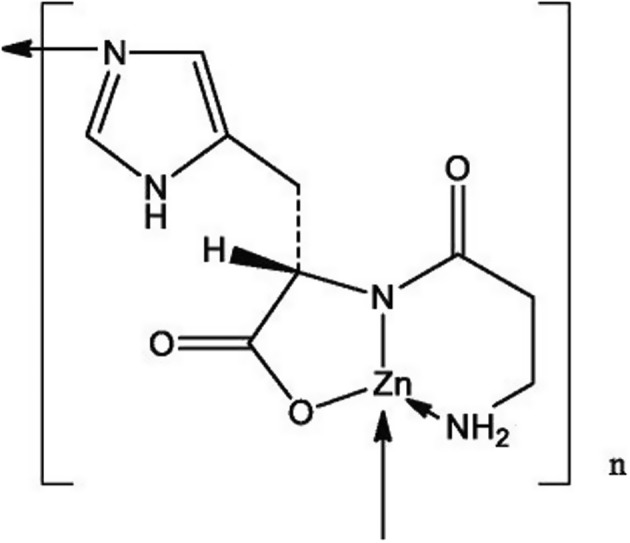
Molecular structure of chelate formed by zinc l‐carnosine (as provided by the applicant)
To confirm the identity of the NF, the applicant provided: analyses by Fourier transform infrared spectroscopy (FTIR), solid‐state 13C NMR and 15N‐1H cross‐polarisation magic angle spinning (CP‐MAS‐NMR) nuclear magnetic resonance spectroscopy and X‐ray photoelectron spectroscopy (XPS). To support the identity of the NF the applicant provided also X‐ray powder diffraction (XRPD) and time of flight‐mass spectrometry (TOF‐MS). All data indicate the presence of a Zn‐carnosine complex, but evidence demonstrating that the NF is a mixture of a monomer (M) and a dimer (D), and in which ratio (M:D), is lacking.
3.2.1. Particle size and solubility of the NF
The NF is a solid powdered material, with a particulate nature. Upon EFSA’s request on particle size, the applicant provided data on the particle size distribution, measured by laser diffraction, of five independently manufactured lots of zinc L‐carnosine, showing a d10 diameter in the range of 1,260–1,464 nm. The Panel notes that laser diffraction is not a valid technique complying with the relevant EFSA guidance (EFSA Scientific Committee, 2021), as it does not have enough sensitivity to detect nanoparticles and does not provide number‐based size distributions. Therefore, the particle size distribution of the NF remains uncharacterised.
The NF is insoluble in water and shows some solubility in diluted hydrochloric acid. The applicant provided a dissolution test at pH 2.0 at ambient temperature on five independently manufactured lots of the NF. In the test, zinc l‐carnosine (29 mg) was added to a 1,000 mL hydrochloric acid solution (pH 2.0) and stirred for 10 min. Then, the suspension was filtered through a 0.2‐µm membrane filter; the use of such pore size was justified by the applicant by the observation that at pH 7 the same filter retained all zinc l‐carnosine particles. Zinc l‐carnosine was indirectly determined by quantifying l‐carnosine in the filtrate by HPLC and quantitative recovery was obtained. The Panel notes that this experiment does not provide evidence of dissolution according to the relevant guidance (EFSA Scientific Committee, 2021) since the necessary filtration using a membrane filter with pore size in the range 3–10 kDa was not performed. In addition to the lack of this filtration step, the test is not a valid dissolution test according to Section 2.3.2. of the EFSA Guidance (EFSA Scientific Committee, 2021) since (i) the medium was not water with 85 mmol/L NaHCO3 and 40 mmol/L NaCl, (ii) the test concentration was not correctly chosen, (iii) if degradation/dissolution is pH dependent, the dissolution test should have to be carried out at different points covering the pH range of physiological relevance with pH = 3 as the lowest one and (iv) the (mass‐based) dissolution rate of the material was not determined. From the experiment, it can be concluded that at pH 2, the particle size may be reduced so that many particles may be sized < 200 nm and pass through the filter.
The Panel notes that the NF has a particulate nature and is insoluble in water at pH 7. The particle size distribution of the NF remains uncharacterised.
3.3. Production process
According to the applicant, the NF is produced according to good manufacturing practice (GMP) principles.
The NF is produced by chemical synthesis in two steps. The first step is the chemical reaction of zinc acetate dissolved in methanol with a dispersion of l‐carnosine, and sodium methylate in methanol. The formed complex is insoluble under these conditions and is filtered. According to the applicant, excess methanol is removed by centrifugation. The second step consists of purification by washing and further processing including drying under vacuum, milling and packaging.
The Panel considers that the production process is sufficiently described.
3.4. Compositional data
The NF is a complex of zinc and L‐carnosine. In order to confirm that the manufacturing process is reproducible and adequate to produce on a commercial scale a product with certain required characteristics, the applicant provided analytical information on five batches of the NF (Tables 1 and 2).
Table 1.
Batch to batch analysis of the NF
| Parameter (unit) | Batch number | Method of analysis | |||||
|---|---|---|---|---|---|---|---|
|
#1 |
#2 |
#3 |
#4 |
#5 |
|||
| Appearance | White to pale yellow‐white crystalline powder | White to pale yellow‐white crystalline powder | White to pale yellow‐white crystalline powder | White to pale yellow‐white crystalline powder | White to pale yellow‐white crystalline powder | Visual | |
| Identification | Positive | Positive | Positive | Positive | Positive | ||
| Optical rotation [α] D (c 2 in 3N HCl) | + 9° | + 9° | + 9° | + 9° | + 9° |
JP 2.49 polarimetry |
|
|
Related substances (%) |
l‐Histidine | 0.13% | 0.11% | 0.11% | 0.17% | 0.13% | HPLC‐UV |
| Other impurities | 0.03% | 0.02% | < 0.02% | 0.02% | 0.02% | ||
| Total | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | ||
|
Moisture content (%) |
1.6% | 1.6% | 1.7% | 1.8% | 1.7% | JP 2.48 | |
| Zinc | 21.9% | 22.2% | 22.0% | 22.0% | 22.0% | In house method‐ titration | |
HPLC‐UV: High‐Performance Liquid Chromatography‐Ultraviolet, JP: Japanese Pharmacopoeia.
Table 2.
Batch to batch analysis of the NF
|
Parameter (unit) |
Batch number | Method of analysis | ||||
|---|---|---|---|---|---|---|
|
#6 |
#7 |
#8 |
#9 |
#10 |
||
|
Assay Zinc l‐carnosine (%) |
101.0 | 101.1 | 100.3 | 100.8 | 101.3 | HPLC‐UV |
| Moisture (%) | 1.6 | 1.5 | 1.5 | 1.3 | 1.7 | Gravimetry |
| Water activity | 0.26 | 0.24 | 0.28 | 0.23 | 0.44 | Hygrometry |
| Lead (µg/g) | 1.1 | 1.1 | 1.1 | 1.3 | 0.2 |
ICP‐MS |
|
Arsenic (µg/g) |
< 0.15 | < 0.15 | < 0.15 | < 0.15 | < 0.15 | |
|
Cadmium (µg/g) |
0.1 | 0.1 | 0.1 | 0.1 | < 0.05 | |
|
Mercury (µg/g) |
< 0.3 | < 0.3 | < 0.3 | < 0.3 | < 0.3 | |
HPLC‐UV: High‐Performance Liquid Chromatography‐Ultraviolet; ICP‐MS: inductively coupled plasma‐mass spectrometry.
Upon an EFSA request for information, the applicant clarified that there is less than 0.1% w/w of free l‐carnosine in the NF.
Zinc‐l‐carnosine is not directly determined. The method, reported in the Japanese Pharmacopoeia, assumes one equivalent of zinc and one equivalent of l‐carnosine in one mole of zinc l‐carnosine. A molecular weight conversion factor of 1.292 is used to convert the l‐carnosine content to zinc l‐carnosine content. The conversion factor is derived by dividing the molecular weight of zinc l‐carnosine (289.61 g/mol, C9H12N4O3Zn) by the molecular weight of the l‐carnosine fragment of zinc l‐carnosine (224.22 g/mol, C9H12N4O3).
The applicant also provided information on microbiological parameters in the NF (Table 3) and developed TAMC and TYMC test methods based on the harmonised EP/USP/JP test methods.
Table 3.
Batch to batch analysis of the NF
| Parameter (unit) | Batch number | Method of analysis | ||||
|---|---|---|---|---|---|---|
| #11 | #12 | #13 | #14 | #15 | ||
|
TAMC (CFU/g) |
≤ 1,000 | ≤ 1,000 | ≤ 1,000 | ≤ 1,000 | ≤ 1,000 |
In‐house method Based on Plate‐count method (surface spread) in Soybean‐Casein Digest Agar (SCDA). |
|
TYMC (CFU/g) |
≤ 100 | ≤ 100 | ≤ 100 | ≤ 100 | ≤ 100 |
In‐house method. Based on Plate‐count method (surface spread) in Sabouraud Dextrose Agar. |
TAMC: total aerobic microbial count; TYMC: total yeast and mould count; CFU: colony forming units.
The applicant provided analytical data on methanol analysed for 18 independently produced lots of the NF manufactured between 2013 and 2020 using GC‐flame ionisation detector (FID). The minimum and maximum methanol levels were 7 mg/kg and 71 mg/kg, respectively. The control for residual methanol is indicated to be not more than 300 mg/kg (0.03% w/w) based on ICH Q3C (R6)’s guideline.3
3.4.1. Stability
The applicant indicated to have performed stability tests on three batches of the NF. The tests were carried out under accelerated conditions at 40 ± 2°C and 75 ± 5% relative humidity (RH) for a period of 6 months and under normal conditions at 25 ± 2°C, 60 ± 5%RH for a period of 18 and 60 months.
The batches were analysed for appearance, identification, optical rotation, related substances (l‐histidine), moisture content, zinc and l‐carnosine measured according to the JP Pharmacopoeia’s method.
3.5. Specifications
The specifications of the NF are indicated in Table 4.
Table 4.
Specifications of the NF
| Parameter | Specification |
|---|---|
| Appearance | White to pale yellow‐white crystalline powder |
| Chemical composition | |
| Zinc l‐Carnosine (DM)( a ) | 98.0–102.0% |
| Zinc (DM)( a ) |
21.5–23.0% |
| Water | ≤ 5% |
| Optical rotation [α]D (c 2, 3N HCl) (DM) | +8° to +9° |
| l‐Histidine | ≤ 2% |
| Other impurities | ≤ 0.1% |
| Total other impurities | ≤ 1% |
| Microbiological | |
| Total aerobic microbial count | ≤ 1,000 CFU/g |
| Total yeast/mould count | ≤ 100 CFU/g |
DM: Dry matter (calculated as dry matter).
The Panel notes that the NF has a particulate nature and is insoluble in water at pH 7.
The Panel considers that the information provided on the specifications of the NF is sufficient and does not raise safety concerns, with exception of the particle size distribution. Owing to the lack of a correct characterisation of the fraction of small particles, including nanoparticles, the Panel is not in the position to establish proper specification limits for the size of the constituent particles of the NF.
3.6. History of use of the NF
According to the applicant, the NF is used in Japan and South Korea as a drug for gastric ulcers. The applicant also provided information that zinc L‐carnosine is registered in the U.S. as ‘New Dietary Ingredient’, in Canada as a ‘Natural Health Product’, in Australia as ‘Type 2 Simple Complementary Medicine Substance’.
3.7. Proposed uses and use levels and anticipated intake
3.7.1. Target population
The target population proposed by the applicant is individuals above the age of 12, and according to the applicant, the NF ‘is not intended for use in pregnant or lactating women, adults with reduced liver functions, and poorly nourished copper deficient adults’. The justification for the target population proposed by the applicant is based on the monograph for zinc l‐carnosine as a drug in Japan.
3.7.2. Proposed uses and use levels
The applicant intends to market the NF for use in food supplements, at a maximum use level of 112.5 mg per day.
3.7.3. Anticipated intake of the NF
The applicant proposes that the NF should be consumed up to a maximum of 112.5 mg per day. Specifically, the applicant proposes the NF as a source of zinc in dietary supplements at a maximum use level of 112.5 mg (3 × 37.5 mg) per day in a solid dosage form, corresponding to approximately 25 mg of zinc and 87 mg of l‐carnosine.
3.8. Absorption, distribution, metabolism and excretion (ADME)
The applicant provided three studies on the ADME of zinc l‐carnosine in rats. In the first study, a single dose of either 14C‐ or 65Zn‐labelled compound suspended in aqueous 0.5% sodium carboxymethylcellulose was administered by gavage (Sano et al., 1991). The 14C‐radioactivity showed a dose‐dependent increase of Cmax and AUC values in the dose range from 13.1 to 100 mg/kg body weight (bw) per day and was detected for longer time in the blood than the 65Zn‐radioactivity. A non‐linear increase of AUC was observed with 65Zn‐radioactivity administered at doses in the range 3–100 mg/kg bw, suggesting decreasing proportion of absorption at higher doses, especially > 50 mg/kg bw. At the dose of 50 mg/kg bw, 85.0% of the administered dose was excreted into the faeces and 10.5% of the dose remained in the carcass. The zinc absorption was estimated to be approximately 11%.
In the second study, zinc L‐carnosine suspended in 0.5% aqueous sodium carboxymethylcellulose was administered by gavage to rats for 21 days at doses of 50 mg/kg bw per day (Toyama et al., 1991). Faecal zinc excretion increased significantly compared to control rats and returned to the control level at 48 h after the last administration. Urinary zinc excretion increased non‐significantly compared to untreated rats. The total amount of zinc in the carcass also increased non‐significantly compared to untreated rats.
In the third study, zinc l‐carnosine suspended in 0.5% aqueous sodium carboxymethylcellulose was administered by gavage to rats for 13 weeks at doses of 150–1,200 mg/kg bw per day or for 52 weeks at 75 or 150 mg/kg bw per day (Yamaguchi et al., 1996). The substance was also administered by the same route to dogs for 13 weeks at doses of 50–300 mg/kg bw per day or for 52 weeks in the range 8–50 mg/kg bw per day. In the 13‐week study in rats, zinc concentrations in blood and tissues (liver, kidney, spleen, brain, heart, lungs, testes, prostate, adrenals) increased dose‐dependently. In the 52‐week study in rats, a slight increase in zinc levels at the highest dose in blood, liver and kidney was detected. In the 13‐week study in dogs, zinc levels in plasma and in most tissues increased at the highest dose. In the 52‐week study in dogs, a transient increase in zinc serum levels (peaking at week 13) and a slight increase in liver, spleen, kidney, ileum and colon were detected at the highest dose.
The Panel notes that in these studies, zinc l‐carnosine was administered at high levels, that insufficient information on the kinetic behaviour or the material was obtained and that the material was not tested against an authorised source of zinc according to Directive 2002/46/EC (EFSA ANS Panel, 2018). Due to the limitations of the studies and the lack of a zinc source as comparator, no information on the bioavailability of the NF can be obtained.
The Panel considers that the actual bioavailability of the zinc form provided by the NF at the proposed use levels remains uncharacterised.
3.9. Nutritional information
The NF is proposed to be used as a source of zinc. Zinc is an essential element with a wide array of vital physiological functions. In the diet, divalent zinc is present in chemical species in which it is bound predominantly to organic ligands, particularly protein thiols and nitrogen ligands (EFSA NDA Panel, 2014). Zn2+ is likely to be released from these ligands to enter a common pool in the acidic environment of the stomach and, subsequently, in the distal duodenum, to be bound to a variety of other organic ligands, including phytate. The majority of zinc is absorbed in the upper small intestine and the luminal contents of the duodenum and jejunum, especially the phytate content, can have a major impact on the percentage of zinc available for absorption. With diets low in phytate and low in zinc, e.g. less than 4 mg/day, the fraction of zinc absorbed may be as high as 60% or more. The fraction of absorbed zinc then decreases progressively with increasing dietary zinc; the uptake of zinc and its transfer into the body by the enterocyte is regulated in response to the quantity of bioavailable zinc ingested (EFSA NDA Panel, 2014).
As described in Section 3.2 ‘Identity of the NF’, the NF has a particulate nature and is insoluble in water at neutral pH. Zinc in the NF is present in a different chemical form from that of naturally occurring, water‐soluble chemical species in the diet. As shown in Section 3.8, the bioavailability of the zinc contained in the NF has not been established. Considering the above, there is insufficient evidence that the NF provides zinc in a form than can be utilised (i.e. enter the functional zinc body pool to fulfil physiological functions) and to what extent this may happen. Therefore, the nutritional value of the NF cannot be established.
With regard to the carnosine (β‐alanyl‐l‐histidine) moiety in the NF, the Panel considers the contribution resulting from consumption of the NF is negligible in relation to the overall amino acid intake and hence is of no nutritional relevance.
The Panel considers that, taking into account the composition of the NF and the proposed conditions of use, it cannot be established whether or not the consumption of the NF is nutritionally disadvantageous.
3.10. Toxicological information
Owing to the lack of a correct characterisation of the fraction of small particles, including nanoparticles, of the NF (EFSA Scientific Committee, 2021), the Panel is not in the position to confirm whether the toxicological testing strategy proposed by the applicant is appropriate to assess the safety of the NF.
The applicant did not provide studies with the NF but refers to studies available in literature for the assessment of zinc L‐carnosine used as a drug for gastric ulcers. These studies are summarised in Table 5.
Table 5.
List of toxicological studies with the NF
| Reference | Type of study | Test system | Treatment/Dose | |
|---|---|---|---|---|
| Shibata et al. (1991a) | Genotoxicity | Bacterial reverse mutation test | Escherichia coli WP2 urvA, Salmonella Typhimurium SD100 and TM677 | Up to 5,000 µg/plate (absence and presence of S9 mix) |
| Chromosomal aberration test | Chinese hamster lung cells (CHL) |
6 h + 18 h recovery +/− S9 24 h and 48 h – S9 Range from 1 × 10–3–3.3 × 10−6 mol/L |
||
|
In vivo mammalian erythrocyte micronucleus test |
ddY male mice | 100–200 and 400 mg/kg | ||
|
Study number T‐G584 |
In vitro micronucleus test | Human lymphoblast TK6 cells |
4 h + 20 h recovery absence and presence of S9 24 h in absence of S9 Range from 0 to.50 ug/L |
|
| Matsuda et al. (1991a) | Acute toxicity | Acute single‐dose toxicity study | ICR mice and Sprague‐Dawley rats |
566–2,500 mg/kg in mice 4,823–10,000 mg/kg in rats |
| Matsuda et al. (1991b) | Subchronic toxicity | 90‐day repeated dose oral study | Crj:CD(SD) rats | 0, 37.5, 75, 150, 300, 600 and 1,200 mg/kg bw per day |
| 52‐week repeat‐dose toxicity | Crj:CD(SD) rats | 0, 18.75, 37.5, 75 and 150 mg/kg bw per day | ||
| Matsuda et al. (1995) | 13‐week repeated‐dose toxicity | Beagle dogs | 0, 50, 120 and 300 mg/kg bw per day | |
| 13‐week repeated‐dose toxicity | Beagle dogs | 0, 8 and 20 mg/kg bw per day | ||
| 52‐week repeated‐dose toxicity | Beagle dogs | 0, 20 and 50 mg/kg bw per day | ||
|
Yamaguchi et al. (1996) These studies are the same as Matsuda 1991b and 1995 where zinc, iron and copper contents in tissues are measured |
90‐day repeated‐dose oral study | Crj:CD(SD) rats | 0, 150, 300, 600 and 1,200 mg/kg bw per day | |
| 52‐week repeat‐dose toxicity | Crj:CD(SD) rats | 0, 75 and 150 mg/kg bw per day | ||
| 13‐week repeated‐dose toxicity | Beagle dogs | 0, 50, 120 and 300 mg/kg bw per day | ||
| 52‐week repeated‐dose toxicity | Beagle dogs | 0, 20 and 50 mg/kg bw per day | ||
| Matsuda et al. (1991c) |
Reproductive and evelopmental toxicity |
Males: 9 weeks prior and during mating Females: 2 weeks prior to mating through day 7 of gestation |
Crj:CD(SD) rats | 0, 300, 600 and 1,200 mg/kg bw per day |
| Females: days 7–17 of gestation | Crj:CD(SD) rats | 0, 150, 300 and 600 mg/kg bw per day | ||
| Females: from days 17 of gestation through day 20 post‐partum | Crj:CD(SD) rats | 0, 100, 250 and 600 mg/kg bw per day |
The Panel notes that the genotoxicity testing strategy did not comply with the EFSA Guidance which requests a tiered approach to address genotoxicity in a first step with a bacterial reverse mutation test and an in vitro micronucleus test (EFSA Scientific Committee, 2011). Upon an EFSA request, the applicant provided an in vitro micronucleus test (Unpublished, 2021)‐ Study number T‐G584).
The Panel notes that there is no indication whether the studies were conducted in compliance with Organisation for Economic Co‐operation and Development (OECD) principles of GLP (OECD, 1998). The applicant stated that genotoxicity, toxicological and reproductive and developmental studies were performed according to the respective OECD test guidelines. However, no documentation on OECD guideline compliance was provided by the applicant.
3.10.1. Human data
The applicant has provided a number of publications involving human subjects and using the NF as a test substance in combination with therapy for a number of diseases (Kashimura et al., 1999; Tan et al., 2017; Mahmood et al., 2007; Fukushima et al., 2009; Nagamine et al., 2000; Takagi et al., 2001; Sakae and Yanagisawa, 2014; Itagaki et al., 2014; Watanabe et al., 2010).
The Panel notes that the human studies provided by the applicant were primarily designed to investigate the efficacy of the drug ‘Polaprezinc’ and considers that these studies are of no relevance for the safety assessment of the substance as an NF.
3.11. Allergenicity
The NF is a complex of zinc and l‐carnosine. The antigenicity of the zinc l‐carnosine was evaluated by Shibata et al. (1991b) using the following tests: active systemic anaphylaxis test (sensitised guinea pigs), passive cutaneous anaphylaxis test (naïve guinea pigs), delayed type skin reactions (guinea pig maximisation test), passive cutaneous anaphylaxis test (naïve rats) and a passive haemagglutination test (serum from rabbits). The authors concluded that the NF has no antigenicity under the conditions applied in the studies. The Panel notes that these tests are not applicable to humans as diagnostic tests for the detection of allergenicity.
The applicant also referred to the Japanese drug monograph reporting that ‘hypersensitivity reactions rarely occur’.
The Panel considers that the likelihood of adverse allergenic reactions to the NF in the target population under the proposed conditions of use is low.
4. Discussion
The NF which is the subject of the application is a chelate‐complex, formed between Zn2+ and l‐carnosine and is present as a mixture of a monomer and a dimer. The material is a powder, has a particulate nature and is insoluble in water at neutral pH.
Zinc in the NF is present in a different chemical form from that of naturally occurring dietary zinc, with different physico‐chemical properties, as shown by the particulate nature and the water insolubility at neutral pH. For this reason, it is not possible to consider zinc in the NF as equivalent to the zinc in the diet and no combined intake can be meaningfully estimated.
No relevant data using an existing zinc source as comparator have been made available by the applicant and the actual bioavailability of the zinc provided by the NF at the proposed use levels remains uncharacterised.
The applicant did not provide the full study reports for any of the published toxicity studies with the NF and no documentation on OECD guideline compliance was provided.
Owing to the lack of a correct characterisation of the fraction of small particles, including nanoparticles, of the NF, the Panel is not in the position to evaluate specification limits for the size of the constituent particles in the NF. In addition, owing to the lack of information on the size distribution and the physico‐chemical properties of the particles constituting the NF, the Panel is not in the position to confirm whether the ADME studies and the toxicological studies provided by the applicant are appropriate to assess the safety of the NF.
5. Conclusions
The Panel concludes that the NF is absorbed and provides zinc, but as it is in an insufficiently characterised particulate form, its safety has not been established and the bioavailability has not been determined.
6. Steps taken by EFSA
On 27/07/2020 EFSA received a letter from the European Commission with the request for a scientific opinion on the safety of Zinc l‐carnosine and bioavailability of zinc from this source. Ref. Ares(2020)3942753.
On 27/07/2020, a valid application on Zinc l‐carnosine, which was submitted Hamari Chemicals, Ltd., was made available to EFSA by the European Commission through the Commission e‐submission portal (NF 2019/1090) and the scientific evaluation procedure was initiated.
On 16/10/2020 and 15/02/2021 EFSA requested the applicant to provide additional information to accompany the application and the scientific evaluation was suspended.
On 14/12/2020 and 09/02/2022 additional information was provided by the applicant through the Commission e‐submission portal and the scientific evaluation was restarted.
During its meeting on 29/04/2022, the NDA Panel, having evaluated the data, adopted a scientific opinion on the safety of Zinc l‐carnosine as a NF pursuant to Regulation (EU) 2015/2283 and the bioavailability of zinc from this source in the context of Directive 2002/46/EC on food supplements.
Abbreviations
- ADME
Absorption, distribution, metabolism and excretion
- ANS
EFSA ANS Panel on Food Additives and Nutrient Sources added to Food, now Panel on Food Additives and Flavourings (FAF)
- AR
Average requirement
- AUC
area under the curve
- bw
body weight
- CAS
Chemical Abstracts Service
- CFU
Colony forming unit
- Cmax
maximum concentration
- CP‐MAS‐NMR
15N‐1Hcross‐polarization magic angle spinning nuclear magnetic resonance spectroscopy
- D
Dimer
- DM
Dry matter
- EP
European Pharmacopoeia
- FTIR
Fourier transform infrared spectroscopy
- GC‐FID
Gas chromatography‐flame‐ionization detector
- GLP
Good Laboratory Practice
- GMP
Good Manufacturing Practice
- HACCP
Hazard Analysis Critical Control Points
- HPLC‐UV
High‐Performance Liquid Chromatography‐Ultraviolet
- ICH
International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use
- ICP‐MS
inductively coupled plasma‐mass spectrometry
- IUPAC
International Union of Pure and Applied Chemistry
- JP
Japanese Pharmacopoeia
- M
Monomer
- NDA
EFSA Panel on Nutrition, Novel Foods and Food Allergens, formerly Panel on Dietetic Products, Nutrition and Allergies
- NOAEL
no observed adverse effect level
- NF
novel food
- NMR
nuclear magnetic resonance
- OECD
Organisation for Economic Co‐operation and Development
- PRI
population reference intake
- RH
relative humidity
- SCDA
Plate‐count method (surface spread) in Soybean‐Casein Digest Agar
- SCF
Scientific Committee on Food
- TAMC
total aerobic microbial count
- TOF‐MS
time of flight‐mass spectrometry
- TYMC
total yeast and mould count
- UL
Tolerable Upper Intake level
- USP
United States Pharmacopeia
- w/w
weight per weight
- XPS
X‐ray photoelectron spectroscopy
- XRPD
X‐ray powder diffraction
- Zn
Zinc
Suggested citation: EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , Turck D, Bohn T, Castenmiller J, De Henauw S, Hirsch‐Ernst KI, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Cubadda F, Frenzel T, Heinonen M, Marchelli R, Neuhäuser‐Berthold M, Poulsen M, Prieto Maradona M, Schlatter JR, van Loveren H, Roldán‐Torres R and Knutsen HK, 2022. Scientific Opinion on the safety of zinc l‐carnosine as a Novel food pursuant to Regulation (EU) 2015/2283 and the bioavailability of zinc from this source in the context of Directive 2002/46/EC on food supplements. EFSA Journal 2022;20(6):7332, 15 pp. 10.2903/j.efsa.2022.7332
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00263
Panel members: Dominique Turck, Torsten Bohn, Jacqueline Castenmiller, Stefaan De Henauw, Karen Ildico Hirsch‐Ernst, Helle Katrine Knutsen, Alexandre Maciuk, Inge Mangelsdorf, Harry J McArdle, Androniki Naska, Carmen Pelaez, Kristina Pentieva, Alfonso Siani, Frank Thies, Sophia Tsabouri and Marco Vinceti.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: The EFSA NDA Panel wishes to thank the members of the EFSA Cross‐cutting WG nanotechnologies and the EFSA staff members Annamaria Rossi, Paolo Colombo and Reinhard Ackerl for the support provided to this scientific output.
Adopted: 29 April 2022
Notes
Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements.
Commission Implementing Regulation (EU) 2017/2469 of 20 December 2017 laying down administrative and scientific requirements for applications referred to in Article 10 of Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. OJ L 351, 30.12.2017, pp. 64‐71.
References
- EFSA (European Food Safety Authority) , 2011. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA ANS Panel (Panel on Food Additives and Nutrient Sources added to Food) , Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipič M, Frutos MJ, Galtier P, Gundert‐Remy U, Kuhnle GG, Lambre C, Leblanc J‐C, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Di Domenico A, Fairweather‐Tait S, McArdle H, Smeraldi C and Gott D, 2018. Guidance on safety evaluation of sources of nutrients and bioavailability of nutrient from the sources. EFSA Journal 2018;16(6):5294, 35 pp. 10.2903/j.efsa.2018.5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) , 2014. Scientific Opinion on Dietary Reference Values for zinc. EFSA Journal 2014;12(10):3844, 76 pp. 10.2903/j.efsa.2014.3844 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) , 2016. Guidance on the preparation and presentation of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA Journal 2016;14(11):4594, 24 pp. 10.2903/j.efsa.2016.4594 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2011. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9( 9):2379, 69 pp. 10.2903/j.efsa.2011.2379 [DOI]
- EFSA Scientific Committee , More S, Bampidis V, Benford D, Bragard C, Halldorsson T, Hernández‐Jerez A, Bennekou SH, Koutsoumanis K, Lambré C, Machera K, Naegeli H, Nielsen S, Schlatter J, Schrenk D, Silano (deceased) V, Turck D, Younes M, Castenmiller J, Chaudhry Q, Cubadda F, Franz R, Gott D, Mast J, Mortensen A, Oomen AG, Weigel S, Barthelemy E, Rincon A, Tarazona J and Schoonjans R, 2021. Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles. EFSA Journal 2021;19(8):6769, 48 pp. 10.2903/j.efsa.2021.6769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima H, Horike S, Fujiki S, Kitada TS and Kashihara N, 2009. Zinc deficiency anaemia and effects of zinc therapy in maintenance haemodialysis patients. Therapeutic Apheresis and Dialysis, 13, 213–219. [DOI] [PubMed] [Google Scholar]
- Itagaki M, Saruta M, Saijo H, Mitobe J, Arihiro S, Matsuoka M, Kato T, Ikegami M and Tajiri H, 2014. Efficacy of zinc‐carnosine chelate compound, Polaprezinc, enemas in patients with ulcerative colitis. Scandinavian Journal of Gastroenterology, 49, 164–172. [DOI] [PubMed] [Google Scholar]
- Kashimura K, Suzuki M, Hassan K, Ikezawa T, Sawahata T, Watanabe A, Nakahara HM and Tanaka N, 1999. Polaprezinc, a mucosal protective agent, in combination with lansoprazole, amoxicillin and clarithromycin increases the cure rate of Helicobacter pylori infection. Alimentary Pharmacology & Therapeutics, 13, 483–487. [DOI] [PubMed] [Google Scholar]
- Mahmood A, FitzGerald AJ, Marchbank T, Ntatsaki E, Murray D, Ghosh S and Playford RJ, 2007. Zinc carnosine, a health food supplement that stabilises small bowel integrity and stimulates gut repair processes. Gut, 56, 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Mera Y, Wada H, Aruga H, Saik Y and Taniguchi Y, 1991b. Repeated dose toxicity studies on catena‐(S)‐[mu‐[N alpha‐(3‐aminopropionyl) histidinato(2‐)‐N1, N2, O: N tau]‐zinc] in rats. Arzneimittel‐Forschung, 41, 1036–1041. [PubMed] [Google Scholar]
- Matsuda K, Nishi N, Hiramatsu Y, Shimizu M, Ohta T and Kato M, 1991c. Reproductive and developmental toxicity studies on catena‐(S)‐[mu‐[N alpha‐(3‐aminopropionyl) histidinato(2‐)‐N1, N2, O: N tau]‐zinc]. Arzneimittel‐Forschung, 41, 1042–1048. [PubMed] [Google Scholar]
- Matsuda K, Shibata K, Morikami H, Kato T and Aruga F, 1991a. Single dose toxicity study on catena‐(S)‐[mu‐[N alpha‐(3‐aminopropionyl) histidinato(2‐)‐N1, N2, O: N tau]‐zinc] in mice and rats. Arzneimittel‐Forschung, 41, 1033–1035. [PubMed] [Google Scholar]
- Matsuda K, Yamaguchi I and Wada H, 1995. Toxicity of the novel anti‐peptic ulcer agent polaprezinc in beagle dogs. Arzneimittel‐Forschung, 45, 52–60. [PubMed] [Google Scholar]
- Nagamine T, Takagi H, Takayama H, Kojima A, Kakizaki S, Mori M and Nakajima K, 2000. Preliminary study of combination therapy with interferon‐α and zinc in chronic hepatitis C patients with genotype 1b. Biological Trace Element Research, 75, 53–63. [DOI] [PubMed] [Google Scholar]
- OECD (Organization for Economic Co‐operation and Development) , 1998. Principles of Good Laboratory Practice (as revised in 1997). ENV/MC/CHEM(98)17, No. 1, Environment Directorate, Paris (1998).
- Sakae K and Yanagisawa H, 2014. Oral treatment of pressure ulcers with Polaprezinc (zinc Lcarnosine complex): 8‐week open label trial. Biological Trace Element Research, 158, 280–288. [DOI] [PubMed] [Google Scholar]
- Sano H, Furuta S, Toyama S, Miwa M, Ikeda Y, Suzuki M, Sato H and Matsuda K, 1991. Study on the metabolic fate of catena‐(S)‐[mu‐[N alpha‐(3‐ aminopropionyl)histidinato(2‐)‐N1, N2, O: N tau]‐zinc]. 1st communication: absorption, distribution, metabolism and excretion after single administration to rats. Arzneimittel‐Forschung, 41, 965–975. [PubMed] [Google Scholar]
- SCF (Scientific Committee for Food) , 1993. Nutrient and energy intakes for the European Community. Reports of the Scientific Committee for Food, 31st series, Food ‐ Science and Techniques. Scientific Committee for Food, Luxembourg, European Commission. 248 pp. [Google Scholar]
- SCF (Scientific Committee on Food) , 2003. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of zinc (expressed on 5 March 2003). SCF/CS/NUT/UPPLEV/62 Final, 18 pp.
- Shibata K, Matsuda K, Ohta T, Sasaki Y and Sutou S, 1991a. Mutagenicity studies on catena‐(S)‐[mu‐[N alpha‐(3‐aminopropionyl)histidinato(2‐)‐N1, N2, O: N tau]‐zinc]. Arzneimittel‐Forschung, 41, 1053–1057. [PubMed] [Google Scholar]
- Shibata K, Matsuda K, Wada H, Aruga F and Yamada Y, 1991b. Antigenicity studies on catena‐(S)‐[mu‐[N alpha‐(3‐aminopropionyl)histidinato(2‐)‐N1, N2, O: N tau]‐zinc]. Arzneimittel‐Forschung, 41, 1048–1052. [PubMed] [Google Scholar]
- Takagi H, Nagamine T, Abe T, Takayama H, Sato K, Otsuka T, Kakizaki S, Hashimoto Y, Matsumoto T, Kojima A, Takezawa J, Suzuki K, Sato S and Mori M, 2001. Zinc supplementation enhances the response to interferon therapy in patients with chronic hepatitis C. Journal of Viral Hepatitis, 8, 367–371. [DOI] [PubMed] [Google Scholar]
- Tan B, Luo H‐Q, Xu H, Lv N‐H, Shi R‐H, Luo H‐S, Li J‐S, Ren J‐L, Zou Y‐Y, Li Y‐Q, Ji F, Fang J‐Y and Qian J‐M, 2017. Polaprezinc combined with clarithromycin‐based triple therapy for Helicobacter pyloriassociated gastritis: a prospective, multicenter, randomized clinical trial. PLoS One, 12, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama S, Furuta S, Miwa M, Suzuki M, Sano H and Matsuda K, 1991. Study on the metabolic fate of catena‐(S)‐[mu‐[N alpha‐(3‐ aminopropionyl)histidinato(2‐)‐N1, N2, O: N tau]‐zinc]. 2nd communication: absorption, distribution, metabolism and excretion after repeated administration to rats. Arzneimittel‐Forschung, 41, 976–983. [PubMed] [Google Scholar]
- Unpublished , 2021. Study number T‐G584. In vitro micronucleus test. Document “Hamari, CZL, Genotoxicity, Confidential, Att1”.
- Watanabe T, Ishihara M, Matsuura K, Mizuta K and Itoh Y, 2010. Polaprezinc prevents oral mucositis associated with radiochemotherapy in patients with head and neck cancer. International Journal of Cancer, 127, 1984–1990. [DOI] [PubMed] [Google Scholar]
- Yamaguchi I, Shibata K, Takei M and Matsuda K, 1996. Changes in tissue contents of zinc, copper and iron in rats and beagle dogs treated with polaprezinc. Journal of Toxicological Sciences, 21, 177–187. 10.2131/jts.21.3_177 [DOI] [PubMed] [Google Scholar]


