Abstract
Objective
Neonatal encephalopathy (NE) is an important cause of mortality and disability worldwide. Therapeutic hypothermia (HT) is an effective therapy, however not all babies benefit. Novel agents are urgently needed to improve outcomes. Melatonin in preclinical studies has promising neuroprotective properties. This meta‐analysis assessed the efficacy of melatonin in term animal models of NE on cerebral infarct size, neurobehavioural tests and cell death.
Methods
A literature search was carried out using Embase, MEDLINE and Web of Science (31 May 2021). We identified 14 studies and performed a meta‐analysis with a random effects model using standardised mean difference (SMD) as the effect size. The risk of bias was assessed using the Systematic Review Centre for Laboratory animal Experimentation tool and publication bias was assessed with funnel plots, and adjusted using trim and fill analysis. Subgroup and meta‐regression analyses were performed to assess the effects of study design variables.
Results
We observed significant reduction in brain infarct size (SMD −2.05, 95% CI [−2.93, −1.16]), improved neurobehavioural outcomes (SMD −0.86, 95% CI [−1.23, −0.53]) and reduction in cell death (SMD −0.60, 95% CI [−1.06, −0.14]) favouring treatment with melatonin. Neuroprotection was evident as a single therapy and combined with HT. Subgroup analysis showed greater efficacy with melatonin given before or immediately after injury and with ethanol excipients. The overall effect size remained robust even after adjustment for publication bias.
Interpretation
These studies demonstrate a significant neuroprotective efficacy of melatonin in term neonatal models of hypoxia‐ischaemia, and suggest melatonin is a strong candidate for translation to clinical trials in babies with moderate–severe NE.
Introduction
Neonatal encephalopathy (NE) is a leading cause of mortality and morbidity in newborns across the world. The regional incidence varies from 2.4 to 2.6 per 1,000 live births in England 1 to estimates of 14.9 per 1,000 live births in Sub‐Saharan Africa. 2 This highlights the disproportionately higher burden of disease in low resource settings. 2
Over several decades, scientific attention has focussed on the development of novel neuroprotective therapies to improve outcomes for infants with NE. Preclinical studies have provided key information advancing our understanding of the evolution of brain injury following hypoxia‐ischaemia (HI) 3 and have supported the translation of therapeutic hypothermia (HT) into large randomised clinical trials (RCTs) for infants with moderate to severe NE. 4 While HT is now an integral aspect of neonatal neurocritical care in high resource settings, children still suffer long‐term complications. The rate of cerebral palsy remains static at 14–19% and no significant improvement in IQ or disability was observed at 6–7‐year follow‐up in children who received HT. 5 The current HT protocol targeting 33.5°C for 72 h is optimal, as confirmed by the studies of deeper and longer cooling. 6 , 7 In low‐ and middle‐income countries (LMICs), a recent large RCT suggests no benefit of HT in infants with NE. 8 Taken together, there is now an urgent need to translate the most promising neuroprotective therapies from preclinical studies to early phase RCTs.
There is compelling preclinical evidence of the safety and efficacy of melatonin as a neuroprotective therapy. 9 , 10 Melatonin is an indolamine hormone and a potent free radical scavenger, which removes toxic reactive oxygen species generated following HI. 11 , 12 , 13 Melatonin also exhibits anti‐apoptotic 14 , 15 and anti‐inflammatory 16 actions. Clinical trials of melatonin have so far been limited to small, underpowered studies, lacking consistent neurodevelopmental outcomes. 17 While preclinical studies provide vital safety and efficacy data to support clinical trials, promising neuroprotective agents in animals have not always translated into a positive biological effect in RCTs. 18 Several groups have supported the use of preclinical systematic reviews and meta‐analyses to improve the transparency and accessibility to animal data. 19 Conducting systematic reviews not only provides the ability to synthesise the overall effect size, but also allows scrutiny in the validity of the preclinical evidence. Data from meta‐analyses may highlight gaps in knowledge, and thereby reducing unnecessary duplication leading to improved adherence to the 3Rs (Replacement, Refinement, Reduction).
We carried out a systematic review and meta‐analysis to assess the neuroprotective efficacy of melatonin, with and without HT, in term neonatal animal models of NE. The primary outcome measures of neuroprotective efficacy were gross cerebral infarct size, neurobehavioural outcomes and cell death assessed using histology. These outcomes were chosen as they are commonly reported, robust biomarkers of neuroprotection and neuropathology in animal studies. MacLeod et al. previously reported a 42.8% improvement in outcome with melatonin in adult stroke models 20 however, the efficacy in neonatal models is unknown. It is critical to assess neuroprotective agents for newborn brain injury in animals of equivalent gestation due to the vulnerability of the yet fully developed brain.
Materials and Methods
This study was reported in accordance with the updated Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA 2020). The review was conducted using the standard Systematic Review Centre for Laboratory animal Experimentation (SYRCLE) 21 template prior to data collection. While this study was not preregistered on the PROSPERO database, we included the full protocol as a supplementary document. No retrospective alterations to the protocol were made.
Eligibility criteria
The selection criteria for inclusion in the systematic review were; (1) animal models of near‐term gestation (rodents at ≥ postnatal day 7 [P7], sheep at ≥126 gestational days, newborn piglets) (2) subjected to brain injury with both a hypoxic and ischaemic component, (3) received melatonin (4) compared with normothermic or HT controls (5) with ≥1 of the three predefined outcome measures: (i) overall infarct size, (ii) neurobehavioural outcome and (iii) histological assessment of cell death. Fully peer‐reviewed published and in‐press articles were included. Conference abstracts, preterm models and in‐vitro studies were excluded.
Search criteria
A literature search was carried out on 31 May 2021 using Ovid Embase, MEDLINE and Clarivate Web of Science (Core Collection) databases using the following search terms; Melatonin AND brain injury OR neonatal encephalopathy OR hypoxia OR hypoxia‐ischaemia OR foetal hypoxia OR perinatal asphyxia OR asphyxia neonatorum OR asphyxia. Please see supplementary document for full search terms. We elected not to search the preprint servers. A previously published 22 search filter of animal studies for Embase was utilised. No other filters or limits were applied to the search strategy to allow maximal retrieval of results.
Screening and data extraction
Publication titles and abstracts were screened independently by two reviewers (R. P. and H. J. H.) using the Systemic Review Facility (SyRF) platform (RRID:SCR_018907). 23 Discrepancies were resolved by a third reviewer (C. M.). Studies meeting the eligibility criteria were subjected to full‐text review. Animal characteristics (age, sex, weight, temperature control), brain injury methodology and quantitative outcome data (mean and error values, number of animals) were extracted. Where the error value was given but the error type not stated, SEM was used as a conservative approach to ensure the effect size was not over‐estimated. For neurobehavioural outcomes, all tests performed were collected. Where the same test was repeated over several intervals, the final time point was used. For cell death markers, values were recorded for each available brain region and total cell counts were excluded to avoid repetition. The melatonin dose over the first 24 h (mg/kg), time of first dose, dosing regimens, route of administration and excipients used were extracted. The dose of melatonin was allometrically scaled to human equivalent doses (HED) for a 3‐kg infant using the simple dose by factor method 24 to allow comparisons between the species. Data between the reviewers (R. P. and H. J. H.) were compared to ensure consistency and discrepancies were resolved through review of full‐text and discussion until consensus reached. Where data was given in graphical form, the first and last authors were approached.
Assessment of bias
The risk of bias was assessed for each study by two reviewers (R. P., H. J. H.) independently using the SYRCLE Risk of Bias (RoB) tool. Studies were assessed for selection, performance, detection, attrition and reporting bias and categorised as high or low risk. Where authors did not provide sufficient detail in the manuscript to assess the risk adequately, the risk was categorised as unclear. A final consensus was reached between the two reviewers through a consensus‐orientated discussion.
Statistical analysis
Statistical analysis was performed using JMP (Version 15, SAS, Marlow, Buckinghamshire, UK), Review Manager (Version 5.4, The Cochrane Collaboration, London, UK) and STATA (version 17, StataCorp, College Station, Texas, USA). Hedge's standardised mean difference (SMD) was used to summarise the effect size, which adjusts for small sample size and accounts for the difference in the scale of measurement. For outcomes with multiple data values available, data were combined into a single, ‘nested outcome’ as described by Vesterinen. 25 All available primary outcome measures for each study were combined using the same method to allow subgroup analysis.
Subgroup analyses were performed to provide evidence to inform future clinical neuroprotection studies. We used stratified subgroup meta‐analysis for categorical variables and meta‐regression analysis for continuous variables. We firstly assessed the efficacy of melatonin as a monotherapy and as an adjunct agent to HT. Subgroup stratification provided an intuitive method to assess the effect size separately, which is important to support future studies relevant to the low or high resource setting, respectively. Subgroup stratification by melatonin dose (scaled to HED), time of first dose, excipient used in the melatonin formulation were also assessed to inform future studies. The influence of preclinical study design factors including sex, animal species, anaesthetic agent and type of HI brain injury were assessed in the same manner. Significance testing of subgroup difference was performed using the Borenstein method implemented on RevMan. Meta‐regression analysis fitted to a random effects (restricted maximum‐likelihood) model was performed to explore the effect of dose and time of melatonin administration. Similarly, the influence of the study quality on the pooled effect estimate was assessed using the same model with the RoB score as the explanatory variable.
The meta‐analyses were performed to obtain a pooled effect size estimate (95% CI) using the random effects model (DerSimmonian and Laird method) and presented as forest plots, ordered by effect size. This model is commonly used in preclinical studies to account for the expected heterogeneity between animal studies. 25 Heterogeneity was assessed using chi‐squared test with degrees of freedom and I 2. A higher significance level of p < 0.10 was used to compensate for the low power of the test. 26 Missing data were assessed by subgroup and meta‐regression analysis. Publication bias of the three outcome measures was explored using a funnel plot of SMD versus SE and adjusted using trim and fill analysis. Forest plot using a sample‐based precision approach (SMD vs. 1/√n) was also explored given the susceptibility of standard SMD versus SE funnel plots to overestimate publication bias. 27 To inform sample size estimates for future neuroprotection studies, power calculations were performed on the cerebral infarct size using the observed 25th, 50th and 75th percentile SMD values with the median SD at 80% power and alpha value of 0.05.
Results
A total of 1686 records were identified and after removal of duplicate records (n = 565), 844 titles and abstracts were screened for eligibility. The PRISMA flow diagram is shown in Figure 1. Following exclusion of irrelevant records, 31 full‐text articles were retrieved and assessed. Six articles were excluded as they were inappropriate animal models (preterm n = 5, intrauterine growth restriction n = 1) and six further articles were excluded as the primary outcome measures were not assessed. A further five articles were excluded as primary outcome data were available in graphical form only and authors did not respond to data request. The study characteristics of the remaining 14 studies, 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 which met the inclusion criteria and reported in this meta‐analysis are shown in Table 1. This included six studies with quantitative data made available following data request from corresponding authors. 28 , 29 , 30 , 31 , 36 , 41
Figure 1.
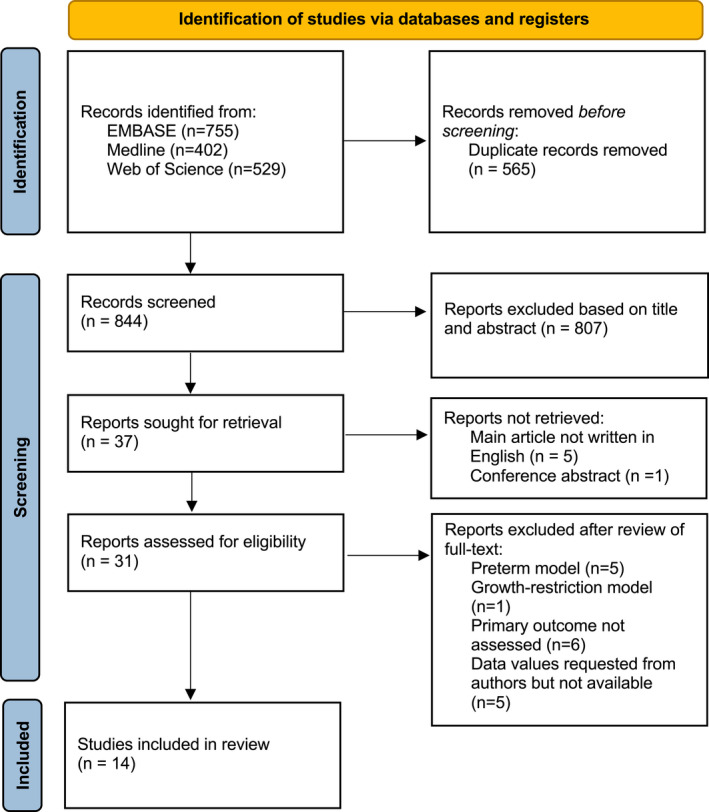
PRISMA flow diagram of the literature search.
Table 1.
Summary of study characteristics.
| Publication | Species, age, sex | HT | Anaesthesia | Brain injury | Dosing regime | Melatonin Dose mg/kg (HED) | Excipient | Melatonin levels (Cmax) mg/L | Duration of experiment (days) | Infarct size | Neurobehavioural tests | Histological assessment of cell death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carloni (2008) | Rat, P7, NS | No | Isoflurane | Carotid artery ligation, ↓FiO2 | Intraperitoneal: Pre‐HI, 5 min after HI, 24 h, 48 h | 15 (2.6) | 5% DMSO | Not reported | 60 | % ipsilateral injury using toluidine blue staining | Circular maze test | No response to data request |
| Cetinkaya (2011) | Rat, P7, mixed | No | Isoflurane | Carotid artery ligation ↓FiO2 | Intraperitoneal: Pre‐HI, immediate after HI, 24 h | 40 (7) | 10% ethanol | Not reported | 3 | % infarct volume with TTC | NS | No response to data request |
| Alonso‐Alconada (2012) | Rat, P7, NS | No | Isoflurane | Carotid artery ligation ↓FiO2 | Intraperitoneal: 5 mins after HI, 24 h, 48 h | 15 (2.6) | 5% DMSO | Not reported | 7 | Mean left:right hemisphere area ratio using cresyl violet staining | NS | qualitative only |
| Ozyener (2012) | Rat, P7, mixed | No | Isoflurane | Carotid artery ligation ↓FiO2 | Intraperitoneal: Pre HI, immediate after HI, 24 h | 40 (7) | 10% ethanol | Not reported | 3 | % infarct volume with TTC | NS | No response to data request |
| Yawno (2012) | Lamb, 126 days, mixed | No | Halothane | Umbilical cord occlusion | intravenous: 2 h before HI | 0.75 (0.82) | 1% ethanol | Not reported | 2 | NS | NS | CC3 |
| Robertson (2013) | Piglet, <48 h old, Male | Yes | Isoflurane | Carotid artery ligation ↓FiO2 | Intravenous Infusion over 6 h: Immediately after HI, 24 h | 30 (26.2) | 2.5% ethanol | 21 | 2 | NS | NS | TUNEL |
| Revuelta (2017) | Rat, P7, NS | No | Isoflurane | Carotid artery ligation ↓FiO2 | Intraperitoneal: after HI | 15 (2.6) | 5% DMSO | Not reported | 7 | NS | NS | NeuN |
| Aridas (2018) | Lamb, 139–141 l days, mixed | No | Sodium thiopentone | Umbilical cord occlusion | Intravenous boluses of 5 mg every 2 h, from 30 min to 24.5 h after HI | 15 (16.5) | 3% ethanol | <1 | 3 | NS | Feeding, suckle, tone, standing | CC3 |
| Berger (2019) | Rat, P7, mixed | No | Isoflurane | Carotid artery ligation ↓FiO2 | Intraperitoneal: After HI, 6 h, 25 h | 20 (3.5) | 5% DMSO | Not reported | 43 | NS | Cylinder test, novel object recognition | H&E |
| Robertson (2019) | Piglet, <48 h old, male | Yes | Isoflurane | Carotid artery ligation ↓FiO2 | Intravenous 2 h infusion: 1 h after HI, 26 h | 15 (13.1) | Ehanol‐free | 16.8 | 2 | NS | NS | TUNEL |
| Robertson (2020) | Piglet, <48 h old, male | Yes | Isoflurane | Carotid artery ligation ↓FiO2 | Intravenous 6 h infusion: 1 h after HI, 24 h | 18 (15.7) | 2.5% ethanol | 18.8 | 2 | NS | NS | TUNEL |
| Aridas (2021) | Lamb, 139–141 days, mixed | Both | Sodium Thiopentone | Umbilical cord occlusion | Intravenous boluses of 5 mg every 2 h, 30 min to 24.5 h after HI | 15 (16.5) | 5% ethanol | <1 | 3 | NS | Standing, feeding | CC3 |
| Pang (2021) | Piglet, <48 h old, male | Yes | Isoflurane | Carotid artery ligation ↓FiO2 | Intravenous 2 h infusion: 1 h after HI, 24 h, 48 h | 20 (17.5) | Ehanol‐free | 27.8 | 3 | NS | NS | TUNEL |
| Sun (2021) | Mouse, P10, mixed | No | Diethyl ether | Carotid artery ligation ↓FiO2 | Intravenous boluses of 5 mg every 2 h, 30 min to 24.5 h after HI: Immediately after HI, repeated every 24 h for 28 days | 10 (1.2) | 3% tween | NS | 28 | % infarct volume with TTC | Negative geotaxis, cliff avoidance, Forelimb suspension, surface righting, novel object recognition, open field, step‐through, foot fault, cylinder tests | NS |
CC3, cleaved‐caspase‐3; DMSO, diethyl sulfoxide; H&E, haematoxylin and eosin; HT, hypothermia; HED, human equivalent dose; NeuN, neuronal nuclei; TTC, triphenyltetrazolium chloride; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labelling; NS, not stated.
Study characteristics
Included in this meta‐analysis were seven small animal (P7 rats n = 6, P10 mice n = 1), and seven large animal (lamb n = 3, piglets n = 4) studies. Four studies assessed the efficacy of melatonin in combination with HT whereas nine studies were in normothermic animals. One study assessed the efficacy of melatonin in both HT and normothermic animals, so was subdivided into two comparative studies.
Melatonin dosing regimens varied across the studies (Table 1): four studies administered the first dose before HI, five studies immediately after HI, and five studies delayed by 30 min–2 h after HI. Melatonin HED ranged from 0.8 to 26.2 mg/kg. Most studies used ethanol as the excipient (n = 7), four studies used 5% dimethyl sulfoxide (DMSO), two studies used an ethanol‐free excipient developed by Chiesi and one study used 3% Tween. Pharmacokinetics studies were reported in six studies, with peak melatonin levels of <1 mg/L in two lamb studies, 15–20 mg/L in two piglet studies levels of >20 mg/L in two piglet studies.
Five studies reported an overall cerebral infarct size, five studies reported neurobehavioural outcomes and nine reported histological assessment of cell death. For histology, four studies reported terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL), three studies reported cleaved‐caspase 3, one study reported a histology injury score based on neuronal loss from haematoxylin‐eosin staining and one study reported NeuN+ cell counts. Histology data from three studies were not included in this review as the authors did not respond to data request.
Overall effect size
Meta‐analysis was performed on brain infarct size, neurobehavioural outcomes and histological assessment of cell death (Fig. 2A). In melatonin treated animals, we observed significant reduction in brain infarct size (pooled SMD estimate −2.05, 95% CI [−2.93 to −1.16], p < 0.001, n = 110 animals), improved neurobehavioural outcomes (SMD −0.86, 95% CI [−1.23 to −0.50], p < 0.001, n = 141 animals) and reduction in cell death on histology (SMD −0.60, 95% CI [−1.06 to −0.14], p = 0.01, n = 207 animals) compared to untreated controls. The interstudy heterogeneity was low for brain infarct size (χ 2 = 5.99, p = 0.2, I 2 = 33%) and neurobehavioural outcomes (χ 2 = 4.82, p = 0.44, I 2 = 0%) and moderate for histology (χ 2 = 21.61, p = 0.01, I 2 = 58%). The pooled estimate of effect size remained significant when all three outcomes were combined (SMD −0.92, 95% CI [−1.26 to −0.58], p = 0.02) with moderate heterogeneity (χ 2 = 46.13, df = 20, p < 0.001, I 2 = 57%).
Figure 2.
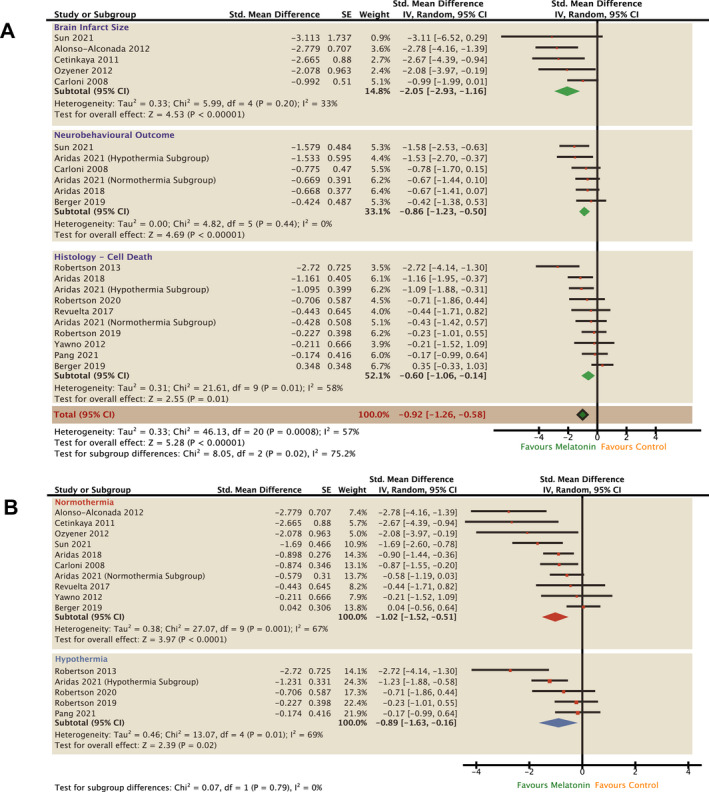
Effect of melatonin on gross cerebral infarct size, neurobehavioural outcome and cell death (A). Further stratified subgroup analysis to assess the efficacy of melatonin as a single therapy or as an adjunct agent with HT on combined neurological outcome (B). Forest plots of SMD with 95% CI ordered by effect size. Pooled estimate of effect size calculated using random effects model. SMD, standardised mean difference; HT, hypothermia.
Subgroup analysis was performed to assess the efficacy of melatonin as a single agent in normothermic animals and as an adjunct to HT (Fig. 2B). As a single agent, melatonin was associated with a significant improvement in combined outcomes (SMD −1.02, 95% CI [−1.52 to −0.51], p < 0.001, I 2 = 67%). Melatonin in combination with HT was also associated with a significant improvement in outcomes (SMD −0.89, 95% [−1.63 to −0.16], p = 0.02, I 2 = 69%) compared to HT alone. The test for subgroup differences was not significant (p = 0.79).
Melatonin regimens
Melatonin administration regimens (Table 1) were compared using subgroup analysis (Fig. 3). Melatonin formulations containing ethanol were most protective (SMD −1.14, 95% CI [−1.64 to −0.65], p < 0.001, I 2 = 52%) whereas we observed no significant efficacy in studies using the non‐ethanol excipient (SMD −0.2, 95% CI [−0.77 to 0.36], p = 0.93, I 2 = 0%) or DMSO (−0.89, 95% CI [−1.88 to 0.09], p = 0.002, I 2 = 79%) (Fig. 3A). Neuroprotection was also observed in melatonin dissolved in Tween (SMD −1.69, 95% CI [−2.60 to −0.78], p < 0.001) from one study.
Figure 3.
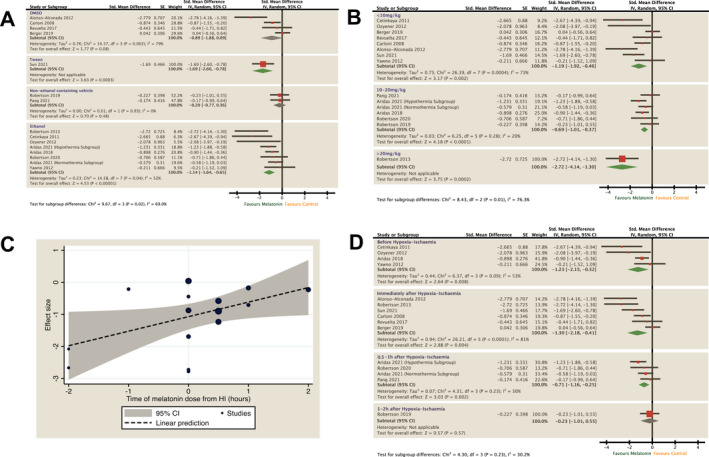
Subgroup analysis to assess the effect of excipients (A), time of melatonin administration (B and C) and allometrically scaled HED of melatonin (D) on combined neurological outcome. Forest plots of SMD with 95% CI ordered by effect size (A and D) or dose (B). Association between time to dose of melatonin from HI and effect size shown as bubble plot (C) with trend line and 95% CI after meta‐regression analysis. HED, human equivalent doses; SMD, standardised mean difference; HI, hypoxia‐ischaemia.
Early melatonin administration was associated with a greater degree of improvement in pooled outcomes (Fig. 3D). We observed the greatest efficacy in animals who received melatonin before HI (SMD −1.23, 95% CI −2.15 to −0.32, I 2 = 53%) and immediately (5–30 min) after HI (SMD – 1.3, 95% CI −2.18 to −0.41, I 2 = 81%). Efficacy reduced when melatonin was given after 1 h (−0.71, 95% CI −1.16 to −0.25, I 2 = 30%) and no significant effect was observed in one study where melatonin was given after a delay of 2 h (SMD −0.23 [95% CI −1.01 to 0.55]). Meta‐regression analysis showed a significant positive correlation between time of melatonin administration from HI and reduction in pooled effect size (co‐efficient 0.45, 95% CI [0.15–0.89], p = 0.043, r 2 = 0.252) (Fig. 3C). We observed improved outcomes in all melatonin doses and meta‐regression analysis showed no significant correlation between melatonin dose and effect size (r = −0.01, 95% CI [−0.07 to 0.05], r 2 = 0.00) (Fig. 3B).
Study characteristics
Stratified subgroup analysis was performed to assess the influence of study design on outcomes (Fig. 4). No significant differences were observed between subgroups stratified by sex, animal species, insult type and anaesthetic agent used (p > 0.10).
Figure 4.
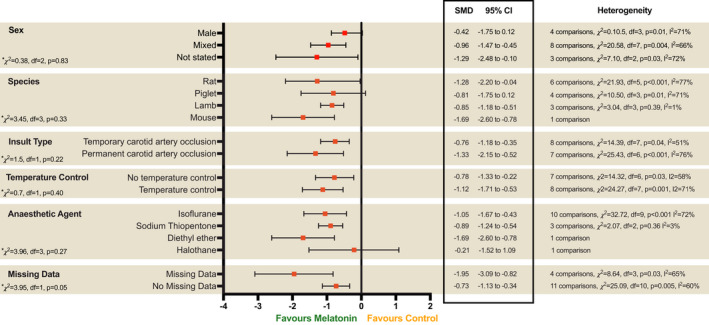
Subgroup analysis to assess the influence of study characteristics. Forest plots of SMD with 95% CI. Test of subgroup differences where p > 0.1 represents statistical significance.
Quality of studies
The results from the risk of bias assessment are shown in Figure 5A and B. No studies were judged as low risk across all 10 domains. The median score was three (IQR 2–4.75). Of note, few studies reported the use of random sequence generation for group assignment (n = 2, 14.3%), allocation concealment (n = 1, 7.1%) or random selection for outcome assessment (n = 1, 7.1%). No studies reported blinding to intervention group and 12 studies (85.7%) at high risk of performance bias. Meta‐regression analysis showed no correlation between risk of bias score and effect size (r = 0.05, 95% CI [−0.28 to 0.37], p = 0.78, I 2 = 0.72) (Fig. 5C). Three studies provided a statement of adherence to ARRIVE guidelines. Seven of 14 studies reported a statement of temperature control but no significant differences in effect size was observed (p = 0.40) (Fig. 4). For the meta‐analysis, histology data were missing for four studies leading to high risk of attrition bias. In three studies, authors did not respond to data request and in one study 28 only qualitative data from micrographs were available. Subgroup analysis of studies with missing data (SMD −1.95, 95% [CI −3.09 to −0.82], p = 0.03, I 2 = 65%) was associated with large effect size estimate compared to studies with no missing data (SMD −0.73, 95% CI [−1.13 to −0.34], p = 0.005, I 2 = 60%) (Fig. 4). The meta‐regression analysis also showed significant differences between studies with and without missing data (p = 0.03). The presence of publication bias was detected by an observed asymmetry in the funnel plot using the standard error (Fig. 5D) and a sample size‐based (1/√n) approach (Fig. 5E). A trim and fill analysis were performed with one imputed study resulting in an adjusted overall effect size of SMD −0.90, 95% CI (−1.23 to −0.57) from the observed (SMD −0.92, 95% CI [−1.26 to −0.58]).
Figure 5.
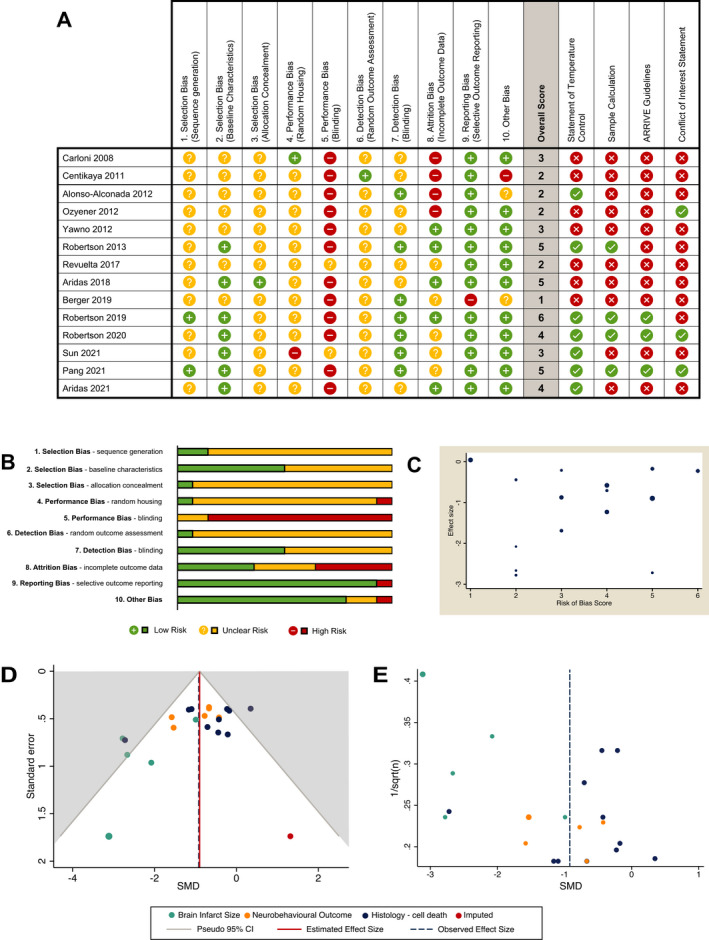
Quality of studies assessed SYRCLE RoB tool with 10 items summarised as an overall score (A and B). Influence between RoB total score and effect size shown as bubble plot (C) after meta‐regression analysis. Publication bias assess by funnel plot using SMD versus SE (D) and SMD versus 1/√n (E). For SMD versus SE, a trim and fill analysis was performed, with the observed pooled effect size estimate shown as a dashed line compared with the adjusted effect size (solid line) (D). SMD, standardised mean difference; SE, standard error.
Sample size
Four studies reported sample size calculation. These were in piglet studies powered to magnetic resonance spectroscopy lactate/N‐acetyl Aspartate peak area ratio (Lac/NAA) 38 , 39 , 40 , 42 (Fig. 5).
To inform future rodent neuroprotection studies, a post hoc sample size calculation was performed for the cerebral infarct size outcome measure. The sample size required to obtain 80% power at a significance level of 0.05 for the median (2.37), 25th (1.26) and 75th centile (2.75) SMD in cerebral infarct size using the median pooled SD of 4.27 was 104 (80–362) animals.
Discussion
This preclinical meta‐analysis demonstrates evidence of significant neuroprotection of melatonin in preclinical, term newborn animal models of perinatal HI. A key strength of the study is the strict inclusion criteria of term and near‐term animal models only. Combining 14 preclinical studies, we observed significant differences in the pooled effect size estimate, favouring melatonin in all three primary outcomes of gross cerebral infarct size, neurobehavioural outcomes and histological measure of cell death. Combined, melatonin was associated with a SMD reduction in brain injury of 0.92 (95% CI −1.26 to −0.58), which remained similar after adjusting for publication bias (adjusted SMD −0.90, 95% CI −1.23 to −0.57). This contrasts with the estimated efficacy of 0.428 (SMD) for melatonin in preclinical adult stroke models reported by McLeod et al (2005). 43 This data are supported by several preclinical studies, which report the antioxidant 11 and anti‐inflammatory 16 properties of melatonin.
Importantly, the neuroprotective efficacy of melatonin remained robust in stratified subgroup analysis as a single agent and as an adjunct therapy to HT. This suggests that melatonin has the potential to be therapeutic in high and low resource settings, with and without HT. RCTs to assess novel therapies in combination with HT to improve outcomes are already underway however trials assessing other neuroprotective agents are still necessary as combined therapies with several complimentary agents targeting different aspects of the neurotoxic cascade are best placed to optimise outcomes. 44 This meta‐analysis provides robust evidence of melatonin's potential to augment HT neuroprotection and supports the need to assess melatonin in early phase clinical trials. Although HT has been rigorously assessed in high resource settings, there is concern that HT in LMICs is not protective. 8 The observation that melatonin is protective as a single therapy in subgroup analysis of 10 studies in this review is important and indicates potential for melatonin as a neuroprotective agent in LMICs. Infection and inflammation are significant perinatal risk factors for NE in sub‐Saharan Africa 45 and in animal models, 46 , 47 inflammation‐sensitisation is known to exacerbate brain injury. Further assessment in preclinical, inflammation‐sensitisation models is also needed prior to its translation to LMICs.
Currently, there are no parenteral melatonin formulations available for intravenous use. Preclinical studies have used several excipients to enhance the solubility of melatonin. Stratified subgroup analysis highlighted improved neuroprotection in animals treated with melatonin in ethanol compared to non‐ethanol containing formulations (DMSO and a non‐ethanol based excipient). Neuroprotective effects of ethanol have been reported in newborn 39 and adult 48 animal models with possible mechanisms including anti‐apoptotic effects, 49 increased HIF‐1alpha, 48 attenuation of hyperglycolysis 50 and free radical scavenging. 51 While the combination of melatonin with ethanol shows promise, the translation to human trials require adherence to safe blood ethanol levels stipulated by medicines regulatory authorities. Meta‐regression analysis also showed a weak correlation between time of melatonin administration and effect size, demonstrating that outcomes improved with earlier administration of melatonin following HI. The antioxidant properties of melatonin are likely to be a key factor. Melatonin and its metabolites remove toxic reactive oxygen species formed at the time of HI; this important early effect of melatonin was elegantly shown in real‐time in newborn lambs where the cerebral efflux of free radicals was ameliorated following umbilical cord occlusion when treated with melatonin. 13 While early melatonin administration is preferred for optimal protection, no studies administered melatonin after 2 h, therefore further studies are needed to delineate the window of opportunity. Similarly, we were unable to assess the correlation between serum melatonin levels and effect size as too few studies carried out pharmacokinetic studies. While we observed protection across all HED of melatonin, simple allometric scaling is not without its pitfalls 52 ; variation in drug metabolism and genetic polymorphisms (such as CYP450 isoenzymes) between species are not considered, and varying maturity in renal and hepatic physiology in newborn models are likely to affect the accuracy of allometric scaling. Therefore, while we observed no dose effect based on HED, we are not certain whether the limitations of allometric scale may have confounded these findings. In vitro, 15 a dose‐dependent reduction in cell death in organotypic hippocampal slices with ~0.23–22.8 mg/L of melatonin was observed, which concurred with our piglet data; serum melatonin levels of 15–30 mg/L within the first hours after HI was necessary for optimal protection. 37 , 38 , 39 , 40 Translating to clinical trials, the pharmacokinetic profile for intravenous melatonin in term newborns receiving HT requires assessment in dose‐escalating phase I studies. PK studies are only reported in preterm infants 53 , 54 and with enteral administration. 53 , 55 Extrapolating from the piglet to term infants, where body weight and the half‐life of melatonin are similar (~20 h 38 , 55 ), intravenous doses of 20 mg/kg/24 h are likely to be required.
Although this meta‐analysis reports the neuroprotective efficacy of melatonin, some caution is needed given some concerns around the quality of the studies. No studies reported low risk of bias in all domains. Few studies report evidence of sequence generation, allocation concealment and only half reported baseline characteristics. These domains have been identified as study characteristics that may exaggerate the treatment effect in clinical trials. 56 No studies reported blinding of intervention during the experiment, which may introduce bias to the neurocritical care management of animals and therefore neurological outcomes. While blinding may not be feasible in all interventions, researchers should, where possible, design animal studies to minimise these confounding factors. It is important to note that the study quality scores reflect inadequate reporting as clear evidence in the publication was required for a domain to be classified as low risk of bias. Despite the publication of the ARRIVE guidelines in 2010 to improve reporting of preclinical studies, statement of adherence was only observed in three publications since its inception. Of note, only four studies (limited to the piglet model) reported sample calculations to ensure studies were adequately powered. These studies used Lac/NAA to power the study. The use of sample size calculations in rodent studies is uncommon, therefore we performed a sample size estimation on the most frequently used outcome marker, cerebral infarct size to inform future study design. This showed that over 100 rodents are needed for the median SMD. We also attempted to estimate the sample size required for neurobehavioural outcomes however this was not feasible as the studies used different neurobehavioural assessment methods.
There are limitations to this meta‐analysis. First, the histology data were aggregated into a single nested outcome to allow for subgroup analysis. While this is a conservative approach to avoid inflation of the effect size estimate, we acknowledge that regional neuroprotective effects may be concealed. In the piglet model, we observed no difference in TUNEL‐positive cell counts overall, however there was significant regional protection in the sensorimotor cortex, a vulnerable region of high metabolism. 37 , 40 We also acknowledge that histological data were missing for three studies where authors did not respond to data request. This was associated with a larger SMD in subgroup analysis therefore we cannot exclude that data attrition may contribute to an overestimation of the effect size. However, the effect size remained significant, albeit smaller in studies with no missing data. In the presence of small sample size, the use of SMD may introduce measurement error, but this was unavoidable given the heterogeneous nature of preclinical studies. The assessment of publication bias when using SMD is also complex, with reports of overestimation in publication bias in when the sample size is small using SMD versus SE funnel plots. 27 We used a sample size‐based precision estimate (1/√n) approach as recommended by Zwetsloot et al, 27 to confirm the presence of asymmetry and publication bias, which highlights the need to interpret the overall pooled SMD with caution. Our meta‐analysis was also limited to histopathological and animal neurobehavioural outcomes, which are inherently heterogeneous in nature and therefore difficult to combine. Further sources of heterogeneity include variability across study design, drug administration protocols and the effect of animal species. We overcame this by using a random effects model, which estimates the mean distribution of effects. We also observed no significant influences of sex, species, insult type and anaesthetic agents although some subgroups were moderately heterogeneous so should be cautiously interpreted. For example, we cannot exclude the influence of species on the larger effect size in cerebral infarct (exclusive rodents) compared to cell death (mixed large and small animals). Nevertheless, despite these limitations, the study still highlighted a positive pooled outcome in all subgroup analyses.
We suggest the need for a more collaborative approach to standardise outcome measurement for preclinical neuroprotection studies. Translation biomarkers such as proton magnetic resonance spectroscopy Lac/NAA peak area ratio and amplitude‐integrated electroencephalography have been increasingly used in animal neuroprotection studies, 29 , 37 , 38 , 39 , 40 validated in the clinical setting 57 to expedite early assessment of the biological effect. We have not included this surrogate biomarker in the meta‐analysis to avoid publication bias as these were largely limited to studies from our group (n = 4) and one other study. 29 Nonetheless, Lac/NAA holds great promise as a standard primary outcome measure for preclinical neuroprotection studies and may be used to estimate the sample size. A standardised approach would reduce heterogeneity, improve the quality of future preclinical meta‐analyses, and improve the translational relevance of animal studies.
In conclusion, this meta‐analysis provides strong preclinical evidence of the neuroprotective efficacy in melatonin as a postnatal treatment for HI in term newborn animals, supporting the translation into clinical trials for babies with NE. Melatonin was effective at augmenting HT neuroprotection and as a single therapy after HI, making it relevant to high‐ and low‐income settings respectively. The neuroprotective benefit of melatonin appears to be time critical, and the use of ethanol excipients augments neuroprotection, however we did not observe a dose response based on simple allometric scaling. Early treatment with melatonin provided better protection, which resembles the findings with HT. Early cooling <3 h after birth provided better protection compared to delayed treatment at 3–6 h. 58 It is likely that melatonin needs to be initiated as early as possible in clinical trials before the onset of secondary energy failure. The ease of administration of intravenous melatonin will enable out‐born babies to receive treatment as soon as possible after birth while awaiting transfer to specialist cooling centres for HT. Preclinical studies defining the therapeutic window for melatonin therapy are needed and the potential benefit of melatonin in inflammation sensitised models is unclear, although likely. 59 Finally, these data demonstrate that closer collaboration between translational researchers is needed to standardise outcomes to reduce heterogeneity between studies, improve the quality of preclinical meta‐analysis and links with clinical researchers are important to ensure the design of clinical trials is optimal.
Conflict of Interest
None declared.
Author Contributions
Raymand Pang and Nicola J. Robertson conceptualised and designed the study. Raymand Pang and Hyun Jee Han carried out the literature search, data extraction and quality assessment of the studies. Christopher Meehan acted as the third reviewer to resolve discrepancies during screening. Raymand Pang carried out the statistical analysis, organised and wrote the first draft of the manuscript. Nicola J. Robertson secured funding, supervised and assisted in the interpretation and write up of the data. Suzanne L. Miller contributed to the data and together with Xavier Golay, provided valuable input to analysis of the data and the manuscript. All authors reviewed and approval the final version of the manuscript and agreed to be accountable for all aspects of the work.
Supporting information
Data S1. Systematic review protocol (based on the SYRCLE template), and the full search terms used in the literature search
Acknowledgements
We thank the authors of the studies included in this meta‐analysis including the first authors: J. Aridas, S. Carloni, M. Cetinkaya, D. Alonso‐Alconada, F. Ozyener, M. Revuelta, H. Berger and Y. Sun, T. Yawno. We would also like to thank David Rose for providing statistical support. Our research receives a proportion of funding from the United Kingdom Department of Health's National Institute for Health Research Biomedical Research Centres Funding Scheme. This work was supported by funding from the Wellbeing of Women (RG2222) and The Bill and Melinda Gates Foundation (INV‐002322). Open Access funding enabled and organized by Projekt DEAL.
Funding Information
Our research receives a proportion of funding from the United Kingdom Department of Health's National Institute for Health Research Biomedical Research Centres Funding Scheme. This work was supported by funding from the Wellbeing of Women (RG2222) and The Bill and Melinda Gates Foundation (INV‐002322).
Funding Statement
This work was funded by Bill and Melinda Gates Foundation grant INV‐002322; Wellbeing of Women grant RG2222; United Kingdom Department of Health's National Institute for Health Research Biomedical Research Centres Funding Scheme; UNIVERSITAET OSNABRUCK.
References
- 1. Gale C, Statnikov Y, Jawad S, Uthaya SN, Modi N; Brain Injuries Expert Working G . Neonatal brain injuries in England: population‐based incidence derived from routinely recorded clinical data held in the National Neonatal Research Database. Arch Dis Child Fetal Neonatal ed. 2018;103(4):F301‐F306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee AC, Kozuki N, Blencowe H, et al. Intrapartum‐related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res. 2013;74 Suppl 1(suppl 1):50‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gunn AJ, Laptook AR, Robertson NJ, et al. Therapeutic hypothermia translates from ancient history in to practice. Pediatr Res. 2017;81(1–2):202‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jacobs SE, Berg M, Hunt R, Tarnow‐Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;2013(1):CD003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shankaran S, Pappas A, McDonald SA, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366(22):2085‐2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shankaran S, Laptook AR, Pappas A, et al. Effect of depth and duration of cooling on death or disability at age 18 months among neonates with hypoxic‐ischemic encephalopathy: a randomized clinical trial. JAMA. 2017;318(1):57‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davidson JO, Yuill CA, Zhang FG, Wassink G, Bennet L, Gunn AJ. Extending the duration of hypothermia does not further improve white matter protection after ischemia in term‐equivalent fetal sheep. Sci Rep. 2016;6:25178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thayyil S, Pant S, Montaldo P, et al. Hypothermia for moderate or severe neonatal encephalopathy in low‐income and middle‐income countries (HELIX): a randomised controlled trial in India, Sri Lanka, and Bangladesh. Lancet Glob Health. 2021;9(9):e1273‐e1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pang R, Advic‐Belltheus A, Meehan C, Fullen DJ, Golay X, Robertson NJ. Melatonin for neonatal encephalopathy: from bench to bedside. Int J Mol Sci. 2021;22(11):1–24. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8196955/pdf/ijms‐22‐05481.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D'Angelo G, Cannavo L, Reiter RJ, Gitto E. Melatonin administration from 2000 to 2020 to human newborns with hypoxic‐ischemic encephalopathy. Am J Perinatol. 2020. https://www‐thieme‐connect‐de.libproxy.ucl.ac.uk/products/ejournals/abstract/10.1055/s‐0040‐1719151 [DOI] [PubMed] [Google Scholar]
- 11. Signorini C, Ciccoli L, Leoncini S, et al. Free iron, total F‐isoprostanes and total F‐neuroprostanes in a model of neonatal hypoxic‐ischemic encephalopathy: neuroprotective effect of melatonin. J Pineal Res. 2009;46(2):148‐154. [DOI] [PubMed] [Google Scholar]
- 12. Balduini W, Carloni S, Perrone S, et al. The use of melatonin in hypoxic‐ischemic brain damage: an experimental study. J Matern Fetal Neonatal Med. 2012;25(suppl 1):119‐124. [DOI] [PubMed] [Google Scholar]
- 13. Miller SL, Yan EB, Castillo‐Melendez M, Jenkin G, Walker DW. Melatonin provides neuroprotection in the late‐gestation fetal sheep brain in response to umbilical cord occlusion. Dev Neurosci. 2005;27(2–4):200‐210. [DOI] [PubMed] [Google Scholar]
- 14. Carloni S, Carnevali A, Cimino M, Balduini W. Extended role of necrotic cell death after hypoxia‐ischemia‐induced neurodegeneration in the neonatal rat. Neurobiol Dis. 2007;27(3):354‐361. [DOI] [PubMed] [Google Scholar]
- 15. Carloni S, Facchinetti F, Pelizzi N, Buonocore G, Balduini W. Melatonin acts in synergy with hypothermia to reduce oxygen‐glucose deprivation‐induced cell death in rat hippocampus organotypic slice cultures. Neonatology. 2018;114(4):364‐371. [DOI] [PubMed] [Google Scholar]
- 16. Carloni S, Favrais G, Saliba E, et al. Melatonin modulates neonatal brain inflammation through endoplasmic reticulum stress, autophagy, and miR‐34a/silent information regulator 1 pathway. J Pineal Res. 2016;61(3):370‐380. [DOI] [PubMed] [Google Scholar]
- 17. Ahmed J, Pullattayil SA, Robertson NJ, More K. Melatonin for neuroprotection in neonatal encephalopathy: a systematic review & meta‐analysis of clinical trials. Eur J Paediatr Neurol. 2021;31:38‐45. [DOI] [PubMed] [Google Scholar]
- 18. Azzopardi D, Chew AT, Deierl A, et al. Prospective qualification of early cerebral biomarkers in a randomised trial of treatment with xenon combined with moderate hypothermia after birth asphyxia. EBioMedicine. 2019;47:484‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pound P, Ritskes‐Hoitinga M. Can prospective systematic reviews of animal studies improve clinical translation? J Transl Med. 2020;18(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Macleod MR, O'Collins T, Horky LL, Howells DW, Donnan GA. Systematic review and meta‐analysis of the efficacy of melatonin in experimental stroke. J Pineal Res. 2005;38(1):35‐41. [DOI] [PubMed] [Google Scholar]
- 21. de Vries RBM, Hooijmans CR, Langendam MW, et al. A protocol format for the preparation, registration and publication of systematic reviews of animal intervention studies. Evid Based Preclin Med. 2015;2(1:1‐9. https://onlinelibrary.wiley.com/doi/epdf/10.1002/ebm2.7 [Google Scholar]
- 22. de Vries RB, Hooijmans CR, Tillema A, Leenaars M, Ritskes‐Hoitinga M. Updated version of the Embase search filter for animal studies. Lab Anim. 2014;48(1):88. [DOI] [PubMed] [Google Scholar]
- 23. Bahor Z, Liao J, Currie G, et al. Development and uptake of an online systematic review platform: the early years of the CAMARADES systematic review facility (SyRF). BMJ Open Sci. 2021;5(1):e100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vesterinen HM, Sena ES, Egan KJ, et al. Meta‐analysis of data from animal studies: a practical guide. J Neurosci Methods. 2014;221:92‐102. [DOI] [PubMed] [Google Scholar]
- 26. Higgins JPT; Cochrane Collaboration . Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Wiley‐Blackwell; 2020. [Google Scholar]
- 27. Zwetsloot PP, Van Der Naald M, Sena ES, et al. Standardized mean differences cause funnel plot distortion in publication bias assessments. Elife. 2017;6. https://elifesciences.org/articles/24260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alonso‐Alconada D, Alvarez A, Lacalle J, Hilario E. Histological study of the protective effect of melatonin on neural cells after neonatal hypoxia‐ischemia. Histol Histopathol. 2012;27(6):771‐783. [DOI] [PubMed] [Google Scholar]
- 29. Aridas JDS, Yawno T, Sutherland AE, et al. Systemic and transdermal melatonin administration prevents neuropathology in response to perinatal asphyxia in newborn lambs. J Pineal Res. 2018;64(4):e12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aridas JD, Yawno T, Sutherland AE, et al. Melatonin augments the neuroprotective effects of hypothermia in lambs following perinatal asphyxia. J Pineal Res. 2021;71:e12744. [DOI] [PubMed] [Google Scholar]
- 31. Berger HR, Nyman AKG, Morken TS, Wideroe M. Transient effect of melatonin treatment after neonatal hypoxic‐ischemic brain injury in rats. PLoS One. 2019;14(12):e0225788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carloni S, Perrone S, Buonocore G, Longini M, Proietti F, Balduini W. Melatonin protects from the long‐term consequences of a neonatal hypoxic‐ischemic brain injury in rats. J Pineal Res. 2008;44(2):157‐164. [DOI] [PubMed] [Google Scholar]
- 33. Cetinkaya M, Alkan T, Ozyener F, Kafa IM, Kurt MA, Koksal N. Possible neuroprotective effects of magnesium sulfate and melatonin as both pre‐ and post‐treatment in a neonatal hypoxic‐ischemic rat model. Neonatology. 2011;99(4):302‐310. [DOI] [PubMed] [Google Scholar]
- 34. Ozyener F, Cetinkaya M, Alkan T, et al. Neuroprotective effects of melatonin administered alone or in combination with topiramate in neonatal hypoxic‐ischemic rat model. Restor Neurol Neurosci. 2012;30(5):435‐444. [DOI] [PubMed] [Google Scholar]
- 35. Sun Y, Ma L, Jin M, Zheng Y, Wang D, Ni H. Effects of melatonin on neurobehavior and cognition in a cerebral palsy model of plppr5−/− mice. Front Endocrinol (Lausanne). 2021;12:598788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yawno T, Castillo‐Melendez M, Jenkin G, Wallace EM, Walker DW, Miller SL. Mechanisms of melatonin‐induced protection in the brain of late gestation fetal sheep in response to hypoxia. Dev Neurosci. 2012;34(6):543‐551. [DOI] [PubMed] [Google Scholar]
- 37. Pang R, Avdic‐Belltheus A, Meehan C, et al. Melatonin and/or erythropoietin combined with hypothermia in a piglet model of perinatal asphyxia. Brain Commun. 2021;3(1). https://academic.oup.com/braincomms/article/3/1/fcaa211/6015127?login=true [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Robertson NJ, Faulkner S, Fleiss B, et al. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain. 2013;136(Pt 1):90‐105. [DOI] [PubMed] [Google Scholar]
- 39. Robertson NJ, Lingam I, Meehan C, et al. High‐dose melatonin and ethanol excipient combined with therapeutic hypothermia in a newborn piglet asphyxia model. Sci Rep. 2020;10(1):3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Robertson NJ, Martinello K, Lingam I, et al. Melatonin as an adjunct to therapeutic hypothermia in a piglet model of neonatal encephalopathy: a translational study. Neurobiol Dis. 2019;121:240‐251. [DOI] [PubMed] [Google Scholar]
- 41. Revuelta M, Arteaga O, Alvarez A, Martinez‐Ibarguen A, Hilario E. Characterization of gene expression in the rat brainstem after neonatal hypoxic‐ischemic injury and antioxidant treatment. Mol Neurobiol. 2017;54(2):1129‐1143. [DOI] [PubMed] [Google Scholar]
- 42. Bubenik GA, Pang SF. The role of serotonin and melatonin in gastrointestinal physiology: ontogeny, regulation of food intake, and mutual serotonin‐melatonin feedback. J Pineal Res. 1994;16(2):91‐99. [DOI] [PubMed] [Google Scholar]
- 43. Macleod, MR , O'Collins, T , Horky, LL , Howells, DW , Donnan, GA : (Systematic review and meta‐analysis of the efficacy of melatonin in experimental stroke. J Pineal Res. 2005;38(1), 35‐41). 10.1111/j.1600-079X.2004.00172.x [DOI] [PubMed] [Google Scholar]
- 44. Chakkarapani AA, Aly H, Benders M, et al. Therapies for neonatal encephalopathy: targeting the latent, secondary and tertiary phases of evolving brain injury. Semin Fetal Neonatal Med. 2021;26(5):101256. [DOI] [PubMed] [Google Scholar]
- 45. Tann CJ, Nakakeeto M, Willey BA, et al. Perinatal risk factors for neonatal encephalopathy: an unmatched case‐control study. Arch Dis Child Fetal Neonatal ed. 2018;103(3):F250‐F256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martinello KA, Meehan C, Avdic‐Belltheus A, et al. Acute LPS sensitization and continuous infusion exacerbates hypoxic brain injury in a piglet model of neonatal encephalopathy. Sci Rep. 2019;9(1):10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eklind S, Mallard C, Arvidsson P, Hagberg H. Lipopolysaccharide induces both a primary and a secondary phase of sensitization in the developing rat brain. Pediatr Res. 2005;58(1):112‐116. [DOI] [PubMed] [Google Scholar]
- 48. Wang F, Wang Y, Geng X, et al. Neuroprotective effect of acute ethanol administration in a rat with transient cerebral ischemia. Stroke. 2012;43(1):205‐210. [DOI] [PubMed] [Google Scholar]
- 49. Yuan Y, Peng C, Li K, et al. Ethanol reduces expression of apoptotic proteins after hypoxia/reoxygenation in a brain slice model. Neurol Res. 2012;34(4):373‐378. [DOI] [PubMed] [Google Scholar]
- 50. Kochanski R, Peng C, Higashida T, et al. Neuroprotection conferred by post‐ischemia ethanol therapy in experimental stroke: an inhibitory effect on hyperglycolysis and NADPH oxidase activation. J Neurochem. 2013;126(1):113‐121. [DOI] [PubMed] [Google Scholar]
- 51. Phillis JW, Estevez AY, O'Regan MH. Protective effects of the free radical scavengers, dimethyl sulfoxide and ethanol, in cerebral ischemia in gerbils. Neurosci Lett. 1998;244(2):109‐111. [DOI] [PubMed] [Google Scholar]
- 52. Huang Q, Riviere JE. The application of allometric scaling principles to predict pharmacokinetic parameters across species. Expert Opin Drug Metab Toxicol. 2014;10(9):1241‐1253. [DOI] [PubMed] [Google Scholar]
- 53. Carloni S, Proietti F, Rocchi M, et al. Melatonin pharmacokinetics following oral administration in preterm neonates. Molecules. 2017;22(12):1‐12. https://www.mdpi.com/1420‐3049/22/12/2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Merchant N, Azzopardi D. Early predictors of outcome in infants treated with hypothermia for hypoxic‐ischaemic encephalopathy. Dev Med Child Neurol. 2015;57Suppl 3(S3):8‐16. [DOI] [PubMed] [Google Scholar]
- 55. Balduini W, Weiss MD, Carloni S, et al. Melatonin pharmacokinetics and dose extrapolation after enteral infusion in neonates subjected to hypothermia. J Pineal Res. 2019;66(4):e12565. [DOI] [PubMed] [Google Scholar]
- 56. Page MJ, Higgins JP, Clayton G, Sterne JA, Hrobjartsson A, Savovic J. Empirical evidence of study design biases in randomized trials: systematic review of meta‐epidemiological studies. PLoS One. 2016;11(7):e0159267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mitra S, Kendall GS, Bainbridge A, et al. Proton magnetic resonance spectroscopy lactate/N‐acetylaspartate within 2 weeks of birth accurately predicts 2‐year motor, cognitive and language outcomes in neonatal encephalopathy after therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2019;104(4):F424‐F432. [DOI] [PubMed] [Google Scholar]
- 58. Thoresen M, Tooley J, Liu X, et al. Time is brain: starting therapeutic hypothermia within three hours after birth improves motor outcome in asphyxiated newborns. Neonatology. 2013;104(3):228‐233. [DOI] [PubMed] [Google Scholar]
- 59. Pang R, Meehan C, Advic‐Belltheus A, et al., eds. 1180506: melatonin for neuroprotection in the newborn inflammation‐sensitised hypoxia‐ischaemia piglet model. Pediatric Academic Societies (PAS) Meeting; 2022 April 25, 2022; Denver, CO.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Systematic review protocol (based on the SYRCLE template), and the full search terms used in the literature search


