Non-alcoholic fatty liver disease (NAFLD) is an umbrella term, describing a range of chronic liver disorders associated with fat deposition in hepatocytes, defined as steatosis, and it is mostly emerged in the presence of obesity, hyperlipidemia and insulin resistance. NAFLD has been classified as the most frequent liver condition worldwide with more than 10% of NAFLD patients progressing to the inflammatory and fibrotic disorder of non-alcoholic steatohepatitis (NASH), which in turn can lead to end stage liver disease including hepatocellular carcinoma (HCC); the most frequent malignant liver tumor (1). During HCC development the immune system interacts with the tumour microenvironment and it can stimulate its growth by maintaining a chronic inflammatory milieu and the production of factors that favor tumor growth, survival, and angiogenesis. Similar phenomena may also take place and have significant implications in the transition from NASH to HCC (2). Although, it is well established that chronic inflammation within the hepatic microenvironment is of great significance in HCC development, the exact mechanisms leading to the transition from NASH to HCC are yet to be elucidated.
Regulatory T cells (Tregs) is an immunosuppressive subset of CD4+ T cells which co-express the IL-2 receptor-α chain, CD25, together with the expression of the master transcription factor forkhead box protein P3 (FOXP3). Tregs normally prevent autoreactivity toward self-antigens and suppress the production and proliferation of effector T cells during infection-induced immune responses and inflammation (3). Recently, there has been an increase of literature aiming to investigate the role of CD4+ and especially Tregs in the advancement of cancer and in suppressing tumor-specific immunity. In vivo work has showed an increase in spontaneous as well as in carcinogen-induced tumors in immunocompromised mice (e.g., IFN-γ, IFN-γ-R, perforin, and Rag-2 gene knockouts), thus confirming the importance of immunosurveillance in cancer initiation and advancement. Specifically, animal studies have showed that CD4+ T cells are of high importance for cancer immunosurveillance, either by directly eliminating cancer cells or indirectly controlling the tumor microenvironment (4). In the context of NASH-associated HCC (NASH-HCC), reduction of CD4+ T cells stimulates carcinogenesis, and at the same time the IFN-γ-secreting T helper-1 (Th1) CD4+ T cells seem to control immunosurveillance over precancerous hepatocytes (2). Although NAFLD, and its progression to NASH, is strongly linked to the activation of innate immune response, thus likely predisposing to the emergence of NASH-HCC, the role of Tregs within the whole spectrum of the disease, up to HCC, is not fully understood. Indeed, previous studies utilizing several approaches, including genetic depletion as well as adoptive transfer of Tregs display controversial data on their beneficial or detrimental role to the emergence of steatosis and to subsequent progression to fibrosis and NASH (5-7). During HCC, Tregs seem to suppress the anti-tumour immune response and thus favour tumor progression. Accordingly, previous studies have reported increased numbers of Tregs in the blood of HCC patients as compared to healthy individuals, as well as in the tumor, compared to the adjacent healthy tissue (2,8). On the other hand, the number of CD4+ effector cells (Teffs) are decreased during NASH-HCC, thereby explaining the reduction of the total CD4+ population. Indeed, findings from the Stelic Animal Model (STAM) NASH-HCC model have shown increased hepatic levels of IL-10, a crucial suppressive cytokine reducing the proliferation rates of Teffs (2,9,10). Intrahepatic Tregs obtained from NASH mice displayed an increased suppressive effect toward Teffs. Interestingly, although both Tregs and Teffs derive from naive CD4+ cells, inflammatory Teffs depend on high glycolytic rates while Tregs are oxidative and rely on mitochondrial electron transport to assist their differentiation and activity (11). Nevertheless, important mechanistic information is still missing on the role of adaptive immunity, and especially Tregs, during the special case of NASH-associated HCC, including the transition from NASH to HCC.
From the side of the innate immunity, neutrophils are the largest population of granulocytes and the main innate immune cells remaining active during hepatic inflammation and injury. Evidence that neutrophils are capable of releasing net-like structures known as neutrophil extracellular traps (NETs) has raised growing interest as it has shown to be essential in chronic inflammatory conditions and cancer development (12,13). NETs consist of extracellular DNA fibers, histones and cytoplasmic granule proteins, which were initially observed in response to microbial invasions. The formation of NETs in the liver has been reported to take place within the whole spectrum of NAFLD. In the early stages of NASH, the formation of NETs depends on S1P receptor 2 signaling and promotes inflammation, further perpetuating the progress of the disease (14). In the later stage of the disease, NETs has been shown to be implicated in NASH to HCC transition, and likely also to HCC metastases (12,15).
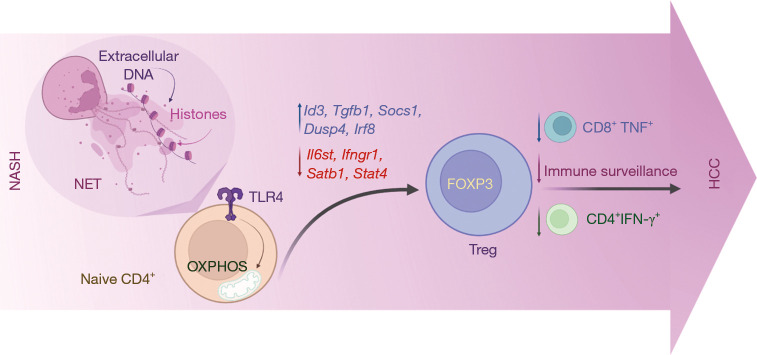
Although NASH to HCC transition is thought to take place under conditions of Treg-related disturbed immunosurveillance, while other studies associate NETs with the protumorigenic inflammatory setting in NASH, a mechanistic link between these two major players of adaptive and innate immunity in NASH-HCC was not so far studied. In a recent paper by Wang et al. in the Journal of Hepatology, findings from a STAM and a Choline-Deficient, High-Fat Diet + Diethylnitrosamine (DEN-HFCD) model showed that neutrophils increase significantly in comparison to other cell types in the NASH liver (10). Infiltration of macrophages, dendritic cells and B cells has also appeared elevated. On the other hand, CD8+ T lymphocytes Kupffer cells, NK cells, and NK T cells did not appear to change in the NASH liver. Importantly, although the total number of CD4+ lymphocytes was found to be decreased in NASH livers as compared to control ones, a tremendous increase in the numbers of Tregs was observed. By utilizing both genetic and anti-CD25-mediated depletion of Tregs, the authors also showed that this population was associated with increased burden of NASH-HCC and that their ablation resulted to an increase of both CD4+IFN-γ+ Th1 cells and TNF-secreting CD8+ cells. Of note, NETs appeared to impact the differentiation of Tregs from naïve CD4+ cells, as well as their suppressive function in the NASH liver microenvironment. Wang et al. also used transcriptome analysis to demonstrate that NETs can alter the equilibrium between regulatory and effective gene profiles in naïve CD4+ T cells, favouring a more anti-inflammatory (Treg-related) expression program (10). Specifically, Id3, a gene responsible for activating Foxp3 gene transcription in TGF-β-induced iTreg differentiation and sustaining Treg suppressive activity, was upregulated following NETs stimulation, as opposed to other genes influencing T-effector cells activity, such as Il6st, Ifngr1, Satb1, and Stat4, which were down-regulated following NETs stimulation. Importantly, RNA-seq data suggested that NETs stimulate Treg differentiation from naïve CD4+ T cells during NASH through enhancing mitochondrial oxidative phosphorylation (OXPHOS) in a Toll-like receptor 4 (TLR4)-dependent manner (Figure 1). These findings were confirmed by inhibiting NET formation in NASH livers in vivo by using PAD4-/- mice or DNase I treatment and resulting to decreased Treg proliferation (10).
Figure 1.
During Nonalcoholic steatohepatitis (NASH), neutrophil extracellular traps (NETs) enable the crosstalk between innate and adaptive immunity by promoting T regulatory cell (Treg) proliferation and activity. As a result, the Treg subpopulation selectively increases in the course of NASH and continues to do so during the transition to Hepatocellular carcinoma (HCC). As a result, immune surveillance is impaired along with a decrease in both CD4+IFN-γ+ and TNF-secreting CD8+ cells.
Taken together, the importance of Tregs in HCC initiation and advancement through the interaction with NETs is unquestionable. The novel work conducted by Wang et al. associates the activity of Tregs and NETs in NASH-induced HCC and their findings further support the theory that Tregs should be classified as an opponent when it comes to NASH-HCC prevention. Metabolic pathways controlling Treg development have indeed emerged as potential therapeutic targets for HCC; such as in this case the metabolic reprogramming of naïve CD4+ T cells towards OXPHOS through TLR4 signalling, which facilitates Treg differentiation (10,11). However, the parts of the NET structure that are able to activate TLR4 on CD4+ T cells, the exact downstream pathways that are activated and how the latter affect the suppression competence of Tregs in vivo are still to be identified.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Provenance and Peer Review: This article was commissioned by the editorial office, Hepatobiliary Surgery and Nutrition. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-557/coif). The authors have no conflicts of interest to declare.
References
- 1.Katsarou A, Moustakas II, Pyrina I, et al. Metabolic inflammation as an instigator of fibrosis during non-alcoholic fatty liver disease. World J Gastroenterol 2020;26:1993-2011. 10.3748/wjg.v26.i17.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ringelhan M, Pfister D, O'Connor T, et al. The immunology of hepatocellular carcinoma. Nat Immunol 2018;19:222-32. 10.1038/s41590-018-0044-z [DOI] [PubMed] [Google Scholar]
- 3.Van Herck MA, Weyler J, Kwanten WJ, et al. The Differential Roles of T Cells in Non-alcoholic Fatty Liver Disease and Obesity. Front Immunol 2019;10:82. 10.3389/fimmu.2019.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borst J, Ahrends T, Bąbała N, et al. CD4+ T cell help in cancer immunology and immunotherapy. Nat Rev Immunol 2018;18:635-47. 10.1038/s41577-018-0044-0 [DOI] [PubMed] [Google Scholar]
- 5.Dywicki J, Buitrago-Molina LE, Noyan F, et al. The Detrimental Role of Regulatory T Cells in Nonalcoholic Steatohepatitis. Hepatol Commun 2022;6:320-33. 10.1002/hep4.1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma X, Hua J, Mohamood AR, et al. A high-fat diet and regulatory T cells influence susceptibility to endotoxin-induced liver injury. Hepatology 2007;46:1519-29. 10.1002/hep.21823 [DOI] [PubMed] [Google Scholar]
- 7.Chatzigeorgiou A, Chung KJ, Garcia-Martin R, et al. Dual role of B7 costimulation in obesity-related nonalcoholic steatohepatitis and metabolic dysregulation. Hepatology 2014;60:1196-210. 10.1002/hep.27233 [DOI] [PubMed] [Google Scholar]
- 8.Zhou SL, Zhou ZJ, Hu ZQ, et al. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology 2016;150:1646-1658.e17. 10.1053/j.gastro.2016.02.040 [DOI] [PubMed] [Google Scholar]
- 9.Fukushima H, Kono H, Hirayama K, et al. Changes in Function and Dynamics in Hepatic and Splenic Macrophages in Non-Alcoholic Fatty Liver Disease. Clin Exp Gastroenterol 2020;13:305-14. 10.2147/CEG.S248635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Zhang H, Wang Y, et al. Regulatory T-cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J Hepatol 2021;75:1271-83. 10.1016/j.jhep.2021.07.032 [DOI] [PubMed] [Google Scholar]
- 11.Gerriets VA, Kishton RJ, Nichols AG, et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest 2015;125:194-207. 10.1172/JCI76012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan X, Lu Y, Zhu H, et al. The Crosstalk Between Cancer Cells and Neutrophils Enhances Hepatocellular Carcinoma Metastasis via Neutrophil Extracellular Traps-Associated Cathepsin G Component: A Potential Therapeutic Target. J Hepatocell Carcinoma 2021;8:451-65. 10.2147/JHC.S303588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatzigeorgiou A, Mitroulis I, Chrysanthopoulou A, et al. Increased Neutrophil Extracellular Traps Related to Smoking Intensity and Subclinical Atherosclerosis in Patients with Type 2 Diabetes. Thromb Haemost 2020;120:1587-9. 10.1055/s-0040-1714371 [DOI] [PubMed] [Google Scholar]
- 14.Zhao X, Yang L, Chang N, et al. Neutrophils undergo switch of apoptosis to NETosis during murine fatty liver injury via S1P receptor 2 signaling. Cell Death Dis 2020;11:379. 10.1038/s41419-020-2582-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Windt DJ, Sud V, Zhang H, et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology 2018;68:1347-60. 10.1002/hep.29914 [DOI] [PMC free article] [PubMed] [Google Scholar]