Abstract
Background
The effect of empagliflozin on the cardiovascular outcome is consistent in heart failure with reduced ejection fraction (HFrEF) patients regardless of the presence or absence of diabetes. More evidence is needed regarding the cost-effectiveness of empagliflozin in HFrEF patients. This study sought to evaluate the economic outcomes of adding empagliflozin to the standard treatment for HFrEF patients from the perspective of the Chinese healthcare system, and thus to provide information for decision makers.
Methods
Based on the EMPEROR-Reduced clinical trial and other published literature data, the direct medical costs and quality-adjusted life years (QALYs) of patients with HFrEF over a 15-year study period were simulated by a Markov model, and the incremental cost-effectiveness ratio (ICER) was calculated. The price of empagliflozin referred to the data released by Menet, the hospitalization expenses and utility values were derived from published studies in China. A one-way sensitivity analysis and probabilistic sensitivity analysis were conducted to evaluate the robustness of the model.
Results
The results of the cost-effectiveness analysis showed that the cost of the combination arm was $5,220.98, with a utility of 4.86 QALYs, and the cost of the standard arm was $4,873.96, with a utility of 4.68 QALYs, which equated to an ICER of $1,893.59 per QALY gained. The subgroup analysis showed that patients with HFrEF and diabetes in empagliflozin group had a higher QALY (4.62 vs. 4.35) and a lower cost ($5,213.28 vs. $5,958.60) than standard group. The corresponding ICER for non-diabetic patients was $2,568.15 per QALY. Deterministic sensitivity analysis showed robust results. At the willingness-to-pay threshold of 3 times gross domestic product (GDP) per capita ($31,510.57), almost all of the scattered points in three scenarios were below the threshold line.
Conclusions
At a willingness-to-pay threshold of $31,510.57, adding empagliflozin to standard treatment is a very cost-effective option for HFrEF patients with or without diabetes in China.
Keywords: Empagliflozin, cost-effectiveness analysis (CEA), quality-adjusted life years (QALYs), heart failure (HF)
Introduction
Heart failure (HF) is a clinical syndrome that occurs in the terminal stage of various cardiovascular (CV) diseases, has a grave prognosis, and has become a serious global public health problem (1). With the aging population, the prevalence of HF is increasing worldwide. The latest epidemiological data from China show that the prevalence of HF in individuals aged ≥35 years is 1.3% (i.e., about 8.9 million patients), and the prevalence of HF has increased by 44% in the past 15 years (2). In addition to the poor quality of life of HF patients, HF places a heavy financial burden on patients and their families. According to research data from 2013, the national economic burden of hospitalization for HF had increased to about 168.940 billion yuan, which represent an increase of 87% in 10 years (3).
Currently, drug therapy is still the main treatment for heart failure and reduced ejection fraction (HFrEF), and includes renin-angiotensin system inhibitors, β-receptor blockers, mineralocorticoid receptor antagonists, angiotensin receptor enkephalinase inhibitors, and diuretics (4). However, high admission and fatality rates still cause a bottleneck in HFrEF treatment. Thus, it is necessary to develop new and effective treatment strategies. Sodium-glucose cotransporter-2 inhibitors (SGLT-2i) are a new type of hypoglycemic drug that mainly play a hypoglycemic role by competing with glucose affinity for SGLT-2 (5). Over the years, SGLT-2i, in the form of empagliflozin and dapagliflozin, has shown great application prospects in the field of HF treatment. Clinical research suggests that adding SGLT-2i to routine regimens not only improves patients’ quality of life, but also significantly reduces the CV mortality and hospitalization rates of patients with HFrEF, regardless of whether or not they also have diabetes (6,7). The EMPEROR-Reduced study (8) has provided high-quality evidence of the safety and efficacy of the treatment of HFrEF combined with empagliflozin, but there has been no report on its economy.
Cost-effectiveness analysis (CEA) is one of the most widely used pharmacoeconomic evaluation methods. By comparing the cost consumed per unit of health output (physiological parameters, functional status, disability-adjusted life years, etc.) under different interventions, decision-makers could select the optimal intervention strategy from a variety of alternatives. Quality-adjusted life years (QALYs) is the most recommended health outcome index of CEA. However, it has to be noted that the use of different measurement methods, tools or utility value integration systems will have a considerable impact on the results of QALYs. In that way, it is generally recommended to use utility value data based on the local population, and to describe relevant measurement methods or tools. The results of pharmacoeconomic evaluation from the perspective of healthcare can provide valuable information for decision makers of national or local health systems. Thus, we conducted a CEA to examine the economics of adding empagliflozin to the standard treatment for HFrEF patients from the perspective of the Chinese healthcare system to provide a reference for health policymakers. We present the following article in accordance with the CHEERS reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-463/rc).
Methods
Patients and therapy
The modelled population was the same as that of the participants with HFrEF at the baseline of the EMPEROR-Reduced trial (NCT03057977), which was a randomized, double-blind, placebo-controlled, event-driven, multicenter study. In brief, to be eligible to participate in the study, participants had to meet the following criteria: be a man or woman aged ≥18 years, have been diagnosed with functional class II–IV HF under the New York Heart Association (NYHA) classification system, and have a left ventricular ejection fraction ≤40%. The key exclusion criteria included a history of adverse reactions to any SGLT-2i, and an estimated glomerular filtration rate <30 mL per minute per 1.73 m2 body surface area. The hypothetical population that entered the model had a mean age of 65 years, and 49.8% of the population had type II diabetes. Detailed characteristics are outlined elsewhere (8).
In this multicenter study, researchers randomly assigned 3,730 patients with HFrEF to receive empagliflozin (10 mg once daily) or placebo, in addition to recommended therapy. The primary outcome (a composite of CV death or hospitalization for HF) was observed during a median of 16 months. Among patients receiving recommended therapy for HF, those in the empagliflozin group had a lower risk of CV death or hospitalization for HF than those in the placebo group regardless of the presence or absence of diabetes.
In accordance with the local legislation and institutional requirements, ethical review and approval was not required for this study on human participants. In accordance with the national legislation and the institutional requirements, written informed consent for participation was not required for this study.
Model structure
We used King’s Markov model to perform a decision analysis comparing the following 2 treatment strategies for patients with HFrEF (9): (I) empagliflozin plus standard treatment (the empagliflozin group); and (II) standard treatment (the control group). A total of 5 mutually exclusive Markov states, including the NYHA classes I to IV and deaths, were defined in the model. Except for death, patients could stay in the original state or move to other NYHA classes and enter the next cycle at the end of each cycle. Events included HF hospitalization, CV death and non-CV death. As HF patients experienced a vulnerable period (within 2 months of discharge), the risk of readmission during this period was substantially higher than that in the stable period, and thus we set a secondary event of HF readmission for patients who experienced a HF hospitalization under our model.
The model creation and analyses were carried out by TreeAge Pro Suite 2011 (TreeAge Software, Williamstown, Massachusetts, USA). Considering the mean age (65 years in the EMPEROR-Reduced trial) and the life expectancy of the participants (10), the model employed a 15-year time horizon, with a trimonthly cycle length, consistent with previous HF economic models. The probability of beginning the 1st cycle in a given NYHA class was determined based on the NYHA class distribution in the empagliflozin group of the EMPEROR-Reduced trial at the time of randomization (75.1% NYHA II, 24.4% NYHA III, and 0.5% NYHA IV). According to “The Guidelines of Pharmacoeconomic Evaluations of China (2020)”, the usual discount rate of 5% per year was adopted to eliminate the effects of inflation on future costs and QALYs, and we applied a range of discount rates from 0 to 8% in sensitivity analysis (11). A half‐cycle correction was applied to prevent the overestimate of expected survival.
Clinical data inputs
We assumed that the probabilities of HF-related hospitalization and CV death in both groups were fixed over time. Based on the 16-month follow-up data of the EMPEROR-Reduced trial, the CV mortality rate was 10.04% in the empagliflozin group and 10.82% in the control group (we converted these to 3‐month probabilities of 1.96% and 2.12%, respectively). Of the participants, 13.20% of participants in the empagliflozin group and 11.5% of participants in the control group were hospitalized for reasons associated with HF (we converted these to 3-month probabilities of 1.644% and 1.996%, respectively). Given that non-CV mortality was not the primary endpoint of the EMPEROR-Reduced trial, we derived the non-CV mortality rate based on data released by the Chinese Center for Disease Control and Prevention (12). To a certain extent, the use of the local data was more advisable for the practical scenarios of the Chinese population. The HF readmission rates were obtained from published articles, and we assumed that the probabilities of HF readmission and non-CV death were the same for both groups. Detailed parameters are set out in Table 1.
Table 1. Clinical probability parameters of the Markov model.
| Parameter | Value | Range | Distribution | Source |
|---|---|---|---|---|
| CV mortality | ||||
| Empagliflozin group | 1.96 | 1.87–2.06 | Beta | EMPEROR-Reduced (8) |
| Control group | 2.12 | 2.02–2.23 | Beta | EMPEROR-Reduced (8) |
| With diabetes | ||||
| Empagliflozin group | 2.20 | 2.09–2.31 | Beta | EMPEROR-Reduced (8) |
| Control group | 2.41 | 2.29–2.53 | Beta | EMPEROR-Reduced (8) |
| Without diabetes | ||||
| Empagliflozin group | 1.73 | 1.64–1.82 | Beta | EMPEROR-Reduced (8) |
| Control group | 1.85 | 1.76–1.94 | Beta | EMPEROR-Reduced (8) |
| Non-CV mortality (age) | China CDC (12) | |||
| 65~ years | 0.24 | – | – | – |
| 70~ years | 0.31 | – | – | – |
| 75~ years | 0.45 | – | – | – |
| HF hospitalization | ||||
| Empagliflozin group | 2.62 | 2.49–2.75 | Beta | EMPEROR-Reduced (8) |
| Control group | 3.51 | 3.33–3.69 | Beta | EMPEROR-Reduced (8) |
| With diabetes | ||||
| Empagliflozin group | 3.02 | 2.87–3.17 | Beta | EMPEROR-Reduced (8) |
| Control group | 5.90 | 5.61–6.20 | Beta | EMPEROR-Reduced (8) |
| Without diabetes | ||||
| Empagliflozin group | 2.22 | 2.11–2.33 | Beta | EMPEROR-Reduced (8) |
| Control group | 3.00 | 2.85–3.15 | Beta | EMPEROR-Reduced (8) |
| HF readmission | 16.23 | 15.42–17.04 | Beta | Huang et al. (2) |
Values are percentages. All inputs are based on a 3-month cycle length. CDC, Center for Disease Control and Prevention; CV, cardiovascular; HF, heart failure.
EMPEROR-Reduced trial has shown that adding empagliflozin to standard treatment improves the classification of cardiac function in HFrEF patients and reduces the risk of deterioration; however, the specific transition probabilities for movement between NYHA classes under the treatment of empagliflozin remains unclear. Thus, we derived the NYHA class specific transition probabilities from the SENIORS (Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure) trial (13). The detailed transition matrix was reported in several CEA studies (9,14,15).
Costs and utilities
All inputs of costs were converted to United States (US) dollars based on the annual average exchange rate of 2020 (1 Chinese Renminbi =0.14493 US dollars). As our study was conducted from the perspective of the healthcare system, only direct medical costs were considered, including the drug costs, hospitalization costs, and standard treatment costs of HFrEF. The price of empagliflozin was obtained from the Menet Network (https://www.menet.com.cn/), a drug information database, and we tested a range of drug costs from the lowest to the highest bid-winning prices across the different provinces of China in the sensitivity analysis. Other costs were derived from previously published research (2). All the cost inputs were inflated to reflect those of 2020 by the usual discount rate of 5% per year, as recommended by the relevant guidelines (11).
The health utility values under different NYHA functional classifications were derived from a real-world survey in China (16). The researchers used the European Five-dimension Health Scale (EQ-5D-5L) (https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/), Chinese version, to obtain the health utility of 150 in-patients with HFrEF from 8 tertiary hospitals of 4 representative cities of China. Our study hypothesized that hospitalization led to worse health outcomes; thus, a 1-time disutility of –0.1 was applied to the 3-month cycle for any cycle in which a HF hospitalization occurred (9,17). The cost and utility values are shown in Table 2.
Table 2. Cost and utility values.
| Parameter | Value | Range | Distribution | Source | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Cycle cost ($) | |||||
| Empagliflozin | 28.42 | 8.50 | 83.58 | Gamma | Menet |
| Standard treatment | 110.55 | 88.44 | 132.66 | Gamma | Huang et al. (2) |
| HF hospitalization | 2,016.51 | 1,613.21 | 2,419.81 | Gamma | Huang et al. (2) |
| HF readmission | 1,407.05 | 1,125.64 | 1,688.46 | Gamma | Huang et al. (2) |
| Utility | |||||
| NYHA II | 0.780 | 0.741 | 0.819 | Beta | Xuan et al. (16) |
| NYHA III | 0.715 | 0.679 | 0.751 | Beta | Xuan et al. (16) |
| NYHA IV | 0.660 | 0.627 | 0.693 | Beta | Xuan et al. (16) |
| Disutility | |||||
| Hospitalization/readmission | –0.1 | –0.13 | –0.08 | Beta | King et al. (9), Yao et al. (17) |
HF, heart failure; NYHA, New York Heart Association.
Health outcomes and incremental analysis
The QALY index, which took into account both survival time and quality of life, can be used as a unified “metric” to compare the health outcomes of different interventions, and thus to help make decisions between different diseases or interventions. It has become the most recommended health outcome index in pharmacoeconomic evaluation studies. We also conducted an incremental analysis comparing the empagliflozin group and the control group. In the incremental analysis, we compared two dimensions (cost and output) between the intervention and control groups. If the intervention group had a lower cost and higher output than the control group, the intervention was the dominant strategy. Conversely, if the intervention group had a higher cost but less output than the control group, then the intervention was the absolutely inferior scheme. If the intervention group had a higher cost and a higher output than the control group, the incremental cost-effectiveness ratio (ICER) between the two schemes was calculated. When the ICER was < or equal to the threshold, the intervention was considered economic. If the ICER exceeded the threshold, the intervention was considered not economic.
Sensitivity analysis
The stability of the results was verified by a one-way sensitivity analysis and a probabilistic sensitivity analysis (PSA). The tornado diagram is shown in Figure 1. The PSA consisted of a 2nd-order Monte Carlo simulation with 10,000 iterations. All input parameters, uncertainty levels, and distributions for costs and effects are detailed in Tables 1,2.
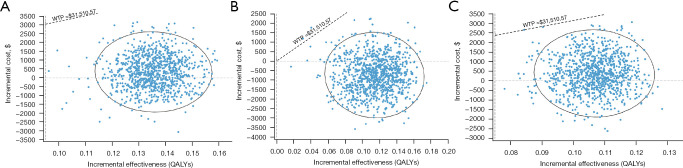
Figure 1.
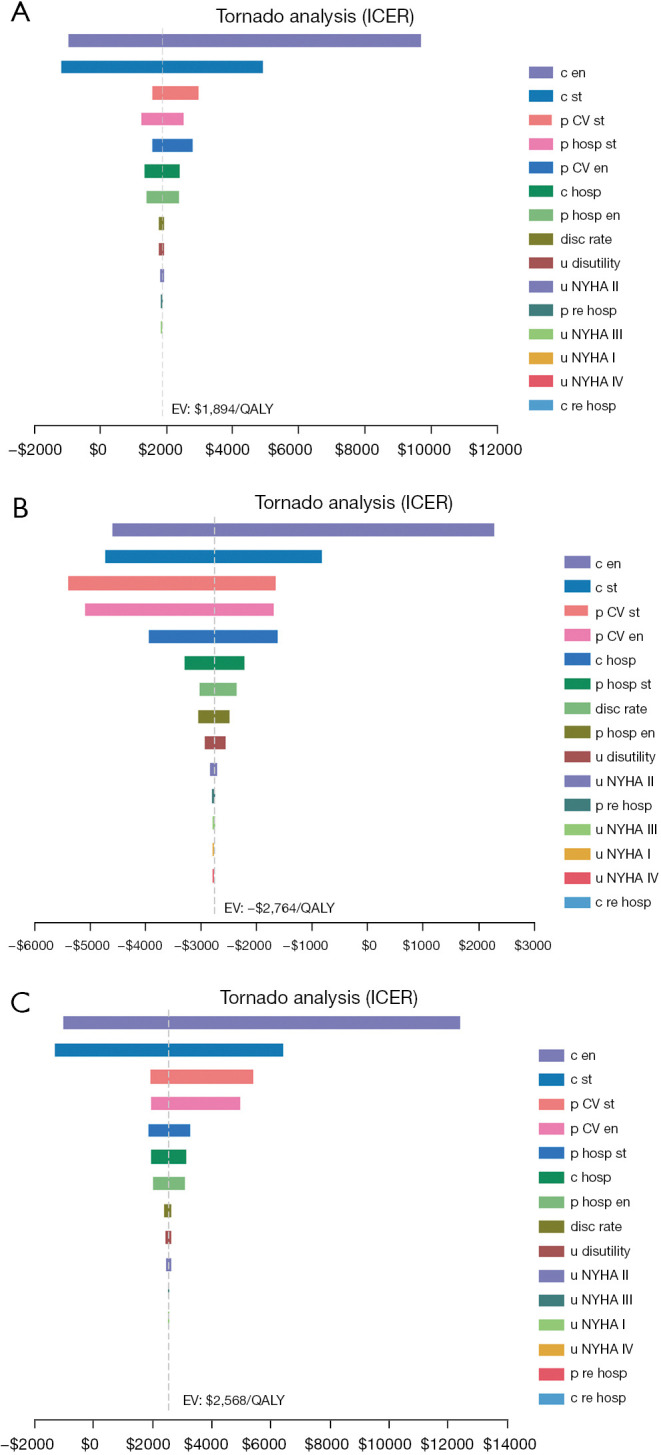
Tornado diagrams of the univariable sensitivity analysis. The results of the ICER of the empagliflozin group vs. the control group for different populations: (A) total population; (B) those with diabetes; (C) those without diabetes. CV, cardiovascular; EV, expected value; QALYs, quality-adjusted life years; ICER, incremental cost-effectiveness ratio; c en, cost of empagliflozin; c st, cost of standard treatment; p CV st, probability of cardiovascular mortality for control group; p hosp st, probability of hospitalization for control group; p CV en, probability of cardiovascular mortality for empagliflozin group; c hosp, cost of hospitalization; p hosp en, probability of hospitalization for empagliflozin group; disc rate, discount rate; u disutility, value of disutility; u NYHA II, utility for New York Heart Association class II; p re hosp, probability of rehospitalization; u NYHA III, utility for New York Heart Association class III; u NYHA I, utility for New York Heart Association class I; u NYHA IV, utility for New York Heart Association class IV; c re hosp, cost of rehospitalization.
Due to the lack of acceptable thresholds for the Chinese population, the recommendations of the World Health Organization were adopted. An intervention was considered “very cost-effective” if the ICER value was < the per capita gross domestic product (GDP), “cost-effective” if the ICER value fell between 1–3 times that of the capita national GDP, and “not cost-effective” if the ICER value was >3 times that of the GDP per capita (11). Thus, the willingness to pay (WTP) threshold was set at 3 times the GDP per capita of China in 2020 ($31,510.57).
Results
Base case analysis
The primary outcomes of our model included the total cost and QALYs. According to the results of the Markov model, the mean total costs for patients in the empagliflozin group and the control group were $5,220.98 and $4,873.96 over a 15‐year time horizon, respectively. The empagliflozin group yielded 4.86 QALYs while the control group yielded 4.68 QALYs. Thus, the ICER was $1,893.59 per QALY, which did not exceed our established threshold of $31,510.57/QALY. Based on these results, we consider empagliflozin plus standard of care (SoC) to be a cost-effective option for HFrEF patients.
The ICER values were estimated for the subgroup populations grouped according to the different states of diabetes. The subgroup analyses indicated that the use of empagliflozin plus the standard treatment appeared to be a more cost-effective alternative than the use of the standard treatment alone among HFrEF patients with diabetes, as the ICER was lower in those without diabetes. The results of the subgroup analyses are presented in Table 3. Notably, empagliflozin plus the standard treatment was found to be a cost-effective alternative on all occasions.
Table 3. Base case results over a 15-year time horizon.
| Parameter | Empagliflozin group | Control group | Incremental analysis |
|---|---|---|---|
| Mean total cost ($) | 5,220.98 | 4,873.96 | 347.02 |
| Mean QALYs | 4.86 | 4.68 | 0.18 |
| ICER | – | – | 1,893.59 |
| Diabetes status | |||
| With diabetes | |||
| Mean total cost ($) | 5,213.28 | 5,958.60 | −745.31 |
| Mean QALYs | 4.62 | 4.35 | 0.27 |
| ICER | – | – | Dominant |
| Without diabetes | |||
| Mean total cost ($) | 5,221.50 | 4,830.90 | 390.60 |
| Mean QALYs | 5.12 | 4.97 | 0.15 |
| ICER | – | – | 2,568.15 |
QALYs, quality-adjusted life years; ICER, incremental cost-effectiveness ratio.
Sensitivity analysis
One-way sensitivity analysis
The results of the one-way sensitive analysis for the main parameters are set out in Figure 1. The results of the tornado diagrams varied slightly among different subgroups and the total population; however, the major factors affecting the ICER were the cost of empagliflozin, the cost of the standard treatment, the CV mortality rate in the standard group, the admission rate in the standard group, the CV mortality rate in the empagliflozin group, and the admission rate in the empagliflozin group. However, fluctuations in all the above-mentioned factors within a certain range did not cause the reversal of the economic results.
PSA
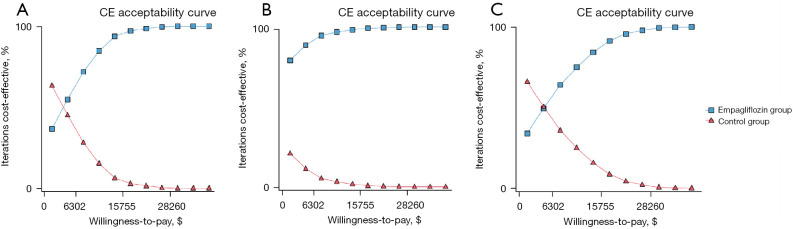
ICER scatter diagrams were generated based on the results of Monte Carlo simulations with 10,000 iterations. The results are presented in Figure 2. At the threshold level of 3 times GDP per capita ($31,510.57), almost all of the scattered points fell below the threshold line, regardless of whether or not the HFrEF patients had diabetes. The cost-effectiveness acceptability curves of the 2 treatment strategies are shown in Figure 3. The acceptance rates for empagliflozin plus standard treatment on all occasions (for HFrEF patients with or without diabetes) increased as WTP increased and exceeded that of the control group before the threshold of acceptability was reached.
Figure 2.
Probabilistic sensitivity analysis of the empagliflozin group compared to the control group for different populations: (A) total population; (B) those with diabetes; (C) those without diabetes. WTP, willingness to pay; QALYs, quality-adjusted life years.
Figure 3.
Cost-effectiveness acceptability curves of the empagliflozin group compared to the control group for different populations: (A) total population; (B) those with diabetes; (C) those without diabetes. CE, cost-effectiveness.
Discussion
SGLT-2is have been shown to improve HF outcomes in early clinical trials among patients with diabetes (18). On this basis, the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) (19) and EMPEROR-Reduced trials (8) were successively conducted to further explore the unique performance of SGLT-2is in HFrEF patients. The evidence suggests that SGLT-2is significantly reduce the risk of CV death and HF-associated hospitalization in HFrEF patients regardless of whether or not they had diabetes mellitus. To some extent, these clinical studies indicated that the CV benefits of SGLT-2is may not be related to their hypoglycemic effects; however, the specific mechanism of action remains unclear. It has been speculated that SGLT-2is improve the prognosis of HF patients by affecting myocardial energy metabolism and regulating the expression of adipokine and anti-arteriosclerosis (20).
Previously, a number of studies have shown that adding dapagliflozin to the standard treatment of HFrEF generates advantages in cost-effectiveness (17,21-23), but there are few reports on the economic evaluation of empagliflozin in the field of HF. Reifsnider et al. (24). conducted an economic evaluation of empagliflozin combined with basic treatment for patients with chronic HF from the perspectives of the healthcare systems in the US and the United Kingdom, respectively. However, the clinical parameters referred to in that study came from subgroup data from the EMPA-REG trial, which included type 2 diabetes patients with established CV diseases. Thus, the research might not fully reflect the economic advantages of empagliflozin in non-diabetic patients given that such subjects were not targeted. Additionally, the results of the pharmacoeconomic evaluation are limited in transferability and extrapolation due to the diversities of medical costs or native cultures among different countries. To be more referential for decision making in China, an evaluation needed to be conducted using research based on the scenario of the Chinese healthcare system.
In our study, we assessed the cost-effectiveness of empagliflozin in addition to standard treatment compared to standard treatment alone among patients with HFrEF from the perspective of the Chinese healthcare system. Our results showed that the cumulative direct medical costs of the empagliflozin group were higher than those of the control group within the 15-year study period, and the empagliflozin group also had more QALYs. The ICER of the 2 groups was $1,893.59 per QALY, which was far lower than the threshold of 1 times GDP per capita ($10,503.52 in 2020) in China. Thus, the increased costs of adding empagliflozin to the standard treatment was completely worthwhile for HFrEF patients. In a further subgroup analysis, we observed that for HFrEF patients with diabetes, the empagliflozin group not only yielded higher cumulative QALYs, but also had lower costs than the control group, which might be associated with the higher hospitalization rate and CV mortality rate in HFrEF patients with diabetes. As for the non-diabetic patients, the empagliflozin group also showed a significant cost-effectiveness advantage, and the results of our study provide economic data support for the application of empagliflozin in HFrEF patients without diabetes. Additionally, the uncertainty analysis of the model parameters also suggested that the model structures were robust.
Our research had some limitations. First, the subjects of the EMPEROR-Reduced clinical trial were multicenter sources, and only a small portion of the included population was Chinese. Thus, population bias might be present in our study. Additionally, clinical outcomes may differ greatly between trial conditions and real-world circumstances. Deriving the model parameters from a rigorous randomized controlled trial would have a considerable effect on the extrapolation validity of the evaluation results in real-world applications. Second, our model assumed that the CV mortality and HF hospitalization/rehospitalization rates of the HFrEF patients were fixed throughout the simulation period; however, in reality, the incidences of these clinical events increase with age. Additionally, clinical study has suggested that empagliflozin improves the grading of cardiac function in HFrEF patients (25), but the specific effect is still not understood; thus, the use of the same transition probability in both groups resulted in the economic benefits of the empagliflozin group being underestimated. Finally, the societal perspective is considered the optimal choice for pharmacoeconomic evaluations according to the social welfare standpoint. Correspondingly, all direct medical costs, direct non-medical costs, and indirect costs should be included in cost accounting (10). In our research, as other types of cost data were difficult to obtain, we only collected direct medical costs from the perspective of the healthcare system and failed to conduct a CEA of empagliflozin combined with the standard treatment for HFrEF patients from the perspective of all society.
Conclusions
The results of this study indicate that adding empagliflozin to the standard treatment is a very cost-effective treatment option for HFrEF patients regardless of whether or not they have diabetes. The findings may serve as a reference for rational drug use and health decision making, but further cost-effectiveness analyses based on real-world studies of Chinese populations need to be conducted.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: The project was supported by the Natural Science Foundation of Fujian Province, China (grant No. 2020J011341) and the Health Youth Scientific Research Project of Fujian Province, China (grant No. 2021QNA075).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. In accordance with the local legislation and institutional requirements, ethical review and approval was not required for this study on human participants. In accordance with the national legislation and the institutional requirements, written informed consent for participation was not required for this study.
Reporting Checklist: The authors have completed the CHEERS reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-463/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-463/coif). All authors report the project was supported by the Natural Science Foundation of Fujian Province, China (grant No. 2020J011341) and the Health Youth Scientific Research Project of Fujian Province, China (grant No. 2021QNA075). The authors have no other conflicts of interest to declare.
(English Language Editor: L. Huleatt)
References
- 1.Tomasoni D, Adamo M, Anker MS, et al. Heart failure in the last year: progress and perspective. ESC Heart Fail 2020;7:3505-30. 10.1002/ehf2.13124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang J, Yin H, Zhang M, et al. Understanding the economic burden of heart failure in China: impact on disease management and resource utilization. J Med Econ 2017;20:549-53. 10.1080/13696998.2017.1297309 [DOI] [PubMed] [Google Scholar]
- 3.Hu S, He J, Sun T, et al. Budget effect analysis of ivabradine in the treatment of heart failure on medical insurance fund in China. China Pharmacy 2019,30:1094-9. [Google Scholar]
- 4.Wang H, Liang Y. Guidelines for diagnosis and treatment of heart failure in China: 2018. Chinese Journal of Cardiology 2018,46:760-89.30369168 [Google Scholar]
- 5.Piperidou A, Loutradis C, Sarafidis P. SGLT-2 inhibitors and nephroprotection: current evidence and future perspectives. J Hum Hypertens 2021;35:12-25. 10.1038/s41371-020-00393-4 [DOI] [PubMed] [Google Scholar]
- 6.Dahal R, Acharya Y, Mukherjee D. Sodium-Glucose Cotransporter Inhibitors in Non- Diabetic Heart Failure: A Narrative Review. Cardiovasc Hematol Disord Drug Targets 2021;21:1-6. 10.2174/1871529X20999201231205504 [DOI] [PubMed] [Google Scholar]
- 7.Singh AK, Singh R. Cardiovascular Outcomes with SGLT-2 inhibitors in patients with heart failure with or without type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr 2021;15:351-9. 10.1016/j.dsx.2021.01.006 [DOI] [PubMed] [Google Scholar]
- 8.Packer M, Anker SD, Butler J, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med 2020;383:1413-24. 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 9.King JB, Shah RU, Bress AP, et al. Cost-Effectiveness of Sacubitril-Valsartan Combination Therapy Compared With Enalapril for the Treatment of Heart Failure With Reduced Ejection Fraction. JACC Heart Fail 2016;4:392-402. 10.1016/j.jchf.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 10.Wan D. Building a moderately prosperous society in all respects from the perspective of social development [EB/OL]. [2021-03-04]. State Statistics Bureau. Available online: http://www.stats.gov.cn/tjsj/sjjd/202008/t20200804_1780439.html
- 11.Liu G. The guideline of pharmacoeconomic evaluation of China: 2020. 1st edition. Peking: China Market Press, 2020:27. [Google Scholar]
- 12.Chinese Center for Disease Control and Prevention. In China mortality surveillance dataset. 1st edition. Peking: China Science and Technology Press, 2018:17-82. [Google Scholar]
- 13.Flather MD, Shibata MC, Coats AJS, et al. Randomised trial to determine the effect of nebivolol on mortality and hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 2005;26:215-25. 10.1093/eurheartj/ehi115 [DOI] [PubMed] [Google Scholar]
- 14.Yao G, Freemantle N, Flather M, et al. Long-term cost-effectiveness analysis of nebivolol compared with standard care in elderly patients with heart failure: an individual patient-based simulation model. Pharmacoeconomics 2008;26:879-89. 10.2165/00019053-200826100-00007 [DOI] [PubMed] [Google Scholar]
- 15.Ford E, Adams J, Graves N. Development of an economic model to assess the cost-effectiveness of hawthorn extract as an adjunct treatment for heart failure in Australia. BMJ Open 2012;2:e001094. 10.1136/bmjopen-2012-001094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xuan J, Tao L, Zhu S, et al. Real world survey of non-direct medical cost and quality of life for heart failure patients of China. China Health Insurance 2017;3:61-4. [Google Scholar]
- 17.Yao Y, Zhang R, An T, et al. Cost-effectiveness of adding dapagliflozin to standard treatment for heart failure with reduced ejection fraction patients in China. ESC Heart Fail 2020;7:3582-92. 10.1002/ehf2.12844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pancholia AK. Sodium-glucose cotransporter-2 inhibition for the reduction of cardiovascular events in high-risk patients with diabetes mellitus. Indian Heart J 2018;70:915-21. 10.1016/j.ihj.2018.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 2019;381:1995-2008. 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 20.Santos-Ferreira D, Gonçalves-Teixeira P, Fontes-Carvalho R. SGLT-2 Inhibitors in Heart Failure and Type-2 Diabetes: Hitting Two Birds with One Stone? Cardiology 2020;145:311-20. 10.1159/000504694 [DOI] [PubMed] [Google Scholar]
- 21.Krittayaphong R, Permsuwan U. Cost-utility analysis of add-on dapagliflozin treatment in heart failure with reduced ejection fraction. Int J Cardiol 2021;322:183-90. 10.1016/j.ijcard.2020.08.017 [DOI] [PubMed] [Google Scholar]
- 22.Savira F, Wang BH, Kompa AR, et al. Cost-effectiveness of dapagliflozin in chronic heart failure: an analysis from the Australian healthcare perspective. Eur J Prev Cardiol 2021;28:975-82. 10.1177/2047487320938272 [DOI] [PubMed] [Google Scholar]
- 23.McEwan P, Darlington O, McMurray JJV, et al. Cost-effectiveness of dapagliflozin as a treatment for heart failure with reduced ejection fraction: a multinational health-economic analysis of DAPA-HF. Eur J Heart Fail 2020;22:2147-56. 10.1002/ejhf.1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reifsnider OS, Kansal AR, Franke J, et al. Cost-effectiveness of empagliflozin in the UK in an EMPA-REG OUTCOME subgroup with type 2 diabetes and heart failure. ESC Heart Fail 2020;7:3910-8. 10.1002/ehf2.12985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Packer M, Anker SD, Butler J, et al. Effect of Empagliflozin on the Clinical Stability of Patients With Heart Failure and a Reduced Ejection Fraction: The EMPEROR-Reduced Trial. Circulation 2021;143:326-36. 10.1161/CIRCULATIONAHA.120.051783 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as