Abstract
Background
Recently, circulating tumor-cell-associated white blood cell (CTC-WBC) clusters have been reported to have prognostic value in some cancers. The prognostic role of CTC-WBC clusters in lung cancer has not yet been elucidated. Very little information is available about the biological characteristics of CTC-WBC clusters.
Methods
A total of 82 patients with non-small cell lung cancer (NSCLC) were included in this study, and 61 patients with advanced-stage disease were closely followed-up. All patients had blood drawn prior to treatment. Subtraction enrichment and immunostaining-fluorescence in situ hybridization (SE-iFISH) platform was used to isolate and identify CTCs and CTC-WBC clusters. Kaplan-Meier survival analysis and Cox regression analysis were applied to assess patient progression-free survival (PFS). Further, qualitative and quantitative analyses the size and ploidy characteristics of CTC-WBC clusters.
Results
Firstly, CTC‐WBC clusters appeared more in the advanced (stage III and IV) stage (P=0.043) than in the early stage. Furthermore, the multivariable analysis (Cox proportional hazards model) revealed that the high‐CTC (≥7/6 mL) group and CTC‐WBC clusters (≥1/6 mL) positive group both had significantly worse PFS, with a hazard ratio (HR) of 2.89 [95% confidence interval (CI): 1.36–6.17, P=0.006] and 2.18 (95% CI: 1.07–4.43, P=0.031), respectively. In the conjoint analysis, compared to patients with <7 CTCs/6 mL without CTC-WBC clusters, patients with ≥7 CTCs/6 mL with CTC-WBC clusters had the highest risk of progression (HR =7.13, 95% CI: 2.51–20.23, P<0.001). In addition, the presence of ≥3-cell CTC-WBC clusters in patients may indicate a shorter PFS (P<0.05) and a higher risk of progression (HR =2.90, 95% CI: 1.06–7.89, P=0.037). Furthermore, compared with the characteristics of the total CTCs, almost all of the CTCs that could recruit WBCs were large cells (≥5 µm) and exhibited polyploidy (≥ tetraploid) (both P<0.01).
Conclusions
The presence of CTC-WBC clusters was an independent prognostic factor for advanced NSCLC. The joint analysis of CTCs and CTC-WBC clusters could provide additional prognostic value to the enumeration of CTCs alone. Besides, most of the CTCs in CTC‐WBC clusters were large polyploid cells.
Keywords: Circulating tumor cell (CTC), circulating tumor-cell-associated white blood cell cluster (CTC-WBC cluster), non-small cell lung cancer (NSCLC), progression-free survival (PFS)
Introduction
Lung cancer is the leading cause of cancer deaths worldwide. According to global cancer statistics 2020, lung cancer accounted for ~1.8 million deaths worldwide in 2020 (1). Roughly 90% percent of cancer deaths are caused by the metastatic spread of primary tumors (2). For patients with advanced lung cancer, it is important to identify real-time and effective biological markers to monitor disease progression, provide guidance for treatment measures, and improve the prognosis of patients. In addition to stage of the disease, mutated genes such as EGFR, KRAS have been validated as prognostic biomarkers in non-small cell lung cancer (NSCLC) (3,4). But obtaining such genetic information requires invasive procedures, and high sequencing costs. Currently, liquid biopsy provides a new method for predicting the prognosis of NSCLC, especially in advanced stage.
Circulating tumor cells (CTCs) are rare cancer cells that generally escape from primary or metastatic tumor lesions and enter the circulation (5), and are regarded as the “seeds” for tumor metastasis. In reality, the majority of CTCs perish within hours, primarily owing to the shear force exerted by the blood flow, immune attack, oxidative stress, and anoikis (6,7). Only very few CTCs survive, which eventually become involved in the development of relapses or metastases. Clinically, it is critical to focus on the subgroup of CTCs that are characterized by strong survival and metastatic potential. CTCs can not only exist as single cells, but also form heterogeneous clusters with white blood cells (WBCs) (8). Recent studies have revealed that WBCs play a crucial role in the promotion of tumor progression and metastasis, both in the bloodstream and tumor microenvironment (9,10). However, the role of circulating tumor-cell-associated white blood cell (CTC-WBC) clusters has been overlooked so far. The prognostic value of CTC-WBC cluster, a combination of “seed” and “soil”, has not been investigated in NSCLC. And information about the biological characteristics of CTC-WBC cluster is scarce.
The present study aimed to assess the prognostic clinical value of CTC-WBC clusters in patients with advanced NSCLC, and progression-free survival (PFS) was selected as the study endpoint. We also assessed the biological characteristics of the subgroup of CTCs that can recruit WBCs. We present the following article in accordance with the REMARK reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-423/rc).
Methods
Study subjects
This study was approved by the Ethics Committee of Huadong Hospital Affiliated to Fudan University (No. 2018K099). Patients with newly diagnosed NSCLC with no history of other malignant tumors were deemed eligible for inclusion in this study. A total of 82 patients with NSCLC were enrolled between October 2020 and June 2021. Among them, 21 patients were in early-stage (stages I and II), and 61 patients were in advanced stage (stages III and IV). All advanced patients included in the study were inoperable and were followed up. The endpoint analyzed in this study was the PFS based on the RECIST (Response Evaluation Criteria in Solid Tumors) criteria (11). PFS was calculated from the time of collection of the blood samples until the date of tumor progression or death. Individuals’ age, gender, smoking history, pathology type stage and therapy elements were included as covariates to the regression analysis. Blood was drawn before the start of surgery or systemic therapies. Follow-up was conducted via clinic visits and telephone interviews. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and all patients provided informed consent.
Enrichment of CTCs from whole blood
CTCs were isolated via the subtraction enrichment according to the manufacturer’s instructions (Cytelligen, San Diego, CA, USA) (12). For each patient, 6 mL of the antecubital blood was drawn into a tube containing acid citrate dextrose (ACD) anticoagulant before treatment. In most cases, the samples were processed within 24 h, with a maximum processing time of 48 h. At room temperature, the blood samples were centrifuged at 200 ×g for 15 min, the supernatant was discarded, and 3 mL of CTC buffer was added to re-suspend the blood cells. Next, the sample was gently added to the non-hematopoietic cell separation matrix in a 50-mL centrifuge tube and centrifuged at room temperature for 6 min (350 ×g). The buffy coat was pipetted and transferred to a new 50-mL centrifuge tube and incubated with 300 µL magnetic beads coated with the anti-Cluster Differentiation45 monoclonal antibody for 20 min at room temperature. The centrifuge tube was then placed on the magnetic frame (Promega, Madison, WI, USA) to remove the WBC-bound magnetic beads. The retained supernatant was subsequently centrifuged and cleaned to obtain approximately 100 µL of CTCs and the WBC suspension for subsequent experimental procedures.
Immunostaining-fluorescence in situ hybridization (i-FISH)
The i-FISH was operated according to the method described in the instruction manual (Cytelligen) (13). First, immunofluorescence staining was performed on the CTCs, and the WBC suspension was obtained through subtraction enrichment. Next, 2 µL of the citrate buffer solution was added into the cell suspension, mixed, and stood for 10 min. The staining solution was prepared by mixing 1 µL of the CD45 staining solution and 1 µL of the Vimentin staining solution into a 200-uL blood cell analysis diluent. The staining solution was then thoroughly mixed with the cell suspension and incubated for 20 min at room temperature away from light. Subsequently, 5 mL of CRC was added to wash the cells and prepare 100 µL of the cell suspension. Next, 100 µL of the Cytelligen Fixative was added to the stained cell sample for cell fixation. The sample was then spread on Cytelligen format slides and dried overnight at 30 ℃, followed by subjecting the samples to FISH. In the next step, 10 µL of chromosome 8 (Chr8) centromeric probe (CEP8) was added to the glass slide, denatured for 10 min at 76 ℃, and hybridized at 37 ℃ for 3 h. After washing, the slides were dried with a hairdryer, dropped into 10 µL of the 4,6-diamino-2-phenyl indole dye solution on the specimen frame, and covered with a cover slide.
CTCs and CTC-WBC clusters image scanning and enumeration
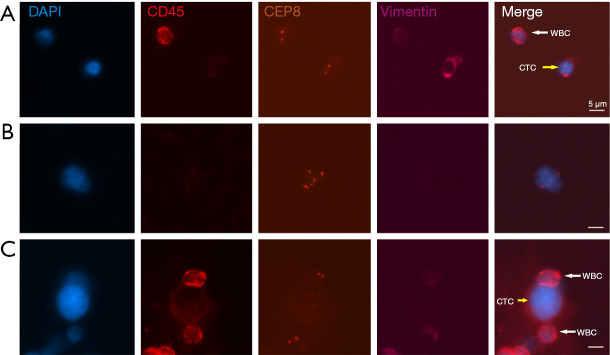
Slides in the Cytelligen format were scanned to obtain and analyze images using the metafer-Ifish® Automated Scanning and Image Analysis system (Carl Zeiss, MetaSystems, Cytelligen). Automatic X-Y plane scanning and the Z section were performed at 1-mm depth intervals to obtain complete fluorescence signals for each channel. The system automatically classifies and analyzes the CTCs and WBCs; CTC was defined as DAPI +/CD45−/Vim +/− with aneuploid Chr8 or DAPI +/CD45−/Vim+ with diploid Chr8; WBC was defined as DAPI+/CD45+ with diploid Chr8; and CTC-WBC cluster was defined as ≥1 CTC was adhered to by ≥1 WBC (Figure 1). The ploidy of Chr8 was precisely counted in all CTCs, and all samples were independently reviewed by two skilled researchers.
Figure 1.
Examples of CTC (including diploid CTC and aneuploid CTC), WBC, and CTC-WBC cluster detected by the Subtraction Enrichment-iFISH platform. (A) Diploid CTCs are DAPI+/CD45−/Vimentin+ cells with diploid Chr8 (yellow arrow); WBCs are DAPI+/CD45+ cells with diploid Chr8 (white arrow). (B) Aneuploid CTCs are DAPI+/CD45− cells with aneuploid Chr8. (C) CTC-WBC cluster (the yellow arrow indicates CTC, and the white arrows indicate WBCs adhered to a CTC). The scale bars are 5 µm. DAPI, 4,6-diamino-2-phenyl indole; WBC, white blood cell; CTC, circulating tumor cell; CTC-WBC cluster, circulating tumor-cell-associated white blood cell cluster; iFISH, immunostaining-fluorescence in situ hybridization.
Statistical analyses
Categorical variables were expressed as counts (percentages) and compared using Pearson’s Chi-squared tests or Yates’ continuity correction analysis. The distribution of continuous variables was tested for normality via the Shapiro-Wilk test. Then, continuous variables were presented as median (first, third quartile) and compared using the Mann-Whitney U-test (two-sided). The survival curve was obtained by the Kaplan-Meier method and compared with the log-rank test. Associations between the presence of CTC-WBC cluster with PFS were presented as hazard ratios (HRs) and 95% confidence intervals (CI), calculated by univariate and multivariate Cox proportional hazards models. Multivariable analyses were adjusted by including clinical factors with P<0.2 in univariate analysis. The α level for significance was set to 0.05. SPSS 22.0 (IBM Corporation, Chicago, USA) and GraphPad Prism 8.0.1 (GraphPad Corporation, San Diego, USA) were used for data analysis. P<0.05 was considered statistically significant.
Results
The presence of CTC-WBC clusters tended to be late events in NSCLC
The present study included 82 patients with NSCLC. Among the 21 patients with early-stage cancer, 17 patients had adenocarcinomas and four patients had squamous cell carcinomas. Patients with advanced-stage cancer included 34 cases of adenocarcinomas and 27 cases of squamous cell carcinomas. Other clinical characteristics of the included patients are presented in Table 1. CTCs were detected in 78 of 82 (95.12%) patients, and CTC-WBC clusters were detected in 24 of 82 (29.27%) patients. The number of CTCs and CTC-WBC clusters in each patient ranged from 0–78/6 mL and 0–17/6 mL, respectively. The results of the correlation analysis between the CTCs/CTC-WBC clusters and the clinical characteristics of the patients revealed that the total number of CTCs and CTC-WBC cluster events were only correlated with the patient’s pathological stage (P=0.022 and 0.043, respectively) (Table 1).
Table 1. Correlation analysis between CTCs/CTC-WBC clusters and the patients’ clinical characteristics.
| Variables | Total, n (%) | CTC count, median (IQR) | P | ≥1 CTC-WBC cluster (%) | P |
|---|---|---|---|---|---|
| Age (years) | |||||
| ≤60 | 29 (35.37) | 5.00 (2.50–10.00) | 8 (27.59) | ||
| >60 | 53 (64.63) | 7.00 (3.00–13.00) | 0.321 | 16 (30.19) | 0.804 |
| Gender | |||||
| Male | 45 (54.88) | 6.00 (3.00–12.00) | 11 (24.44) | ||
| Female | 37 (45.12) | 6.00 (2.50–11.00) | 0.914 | 13 (35.14) | 0.290 |
| Smoking history | |||||
| Yes | 33 (39.51) | 6.00 (3.00–14.00) | 9 (27.27) | ||
| No | 49 (60.49) | 6.00 (2.50–11.00) | 0.729 | 15 (30.61) | 0.744 |
| Pathologic type | |||||
| Adenocarcinoma | 51 (62.20) | 5.00 (3.00–11.00) | 16 (31.37) | ||
| Squamous cell carcinoma | 31 (37.80) | 7.00 (2.00–11.00) | 0.901 | 8 (25.81) | 0.591 |
| Stage | |||||
| I&II | 21 (25.61) | 3.00 (1.50–10.00) | 2 (9.52) | ||
| III&IV | 61 (74.39) | 7.00 (3.00–14.00) | 0.022 | 22 (36.07) | 0.043 |
| Main therapy (III&IV) | |||||
| Targeted therapy | 18 (29.51) | 6.50 (4.75–22.25) | 6 (33.33) | ||
| Non-targeted therapy | 43 (70.49) | 7.00 (3.00–11.00) | 0.330 | 16 (37.21) | 0.774 |
CTC, circulating tumor cell; CTC-WBC cluster, circulating tumor-cell-associated white blood cell cluster; IQR, inter-quartile range.
CTC counts and the presence of CTC clusters had prognostic predictive value
A total of 61 patients with advanced-stage cancer were followed up until December 24, 2021. The median follow-up time for the patients was 255 days (range, 63–441 days). Furthermore, the late-stage patients were divided into two groups based on the median number of CTCs: a high-CTC (≥7 CTCs/6 mL) group and a low-CTC (<7 CTCs/6 mL) group. There were 32 cases in the high-CTC group and 29 cases in the low-CTC group. CTC-WBC clusters were detected in 22 patients (CTC-WBC cluster positive group) and were not detected in 39 patients (CTC-WBC cluster negative group).
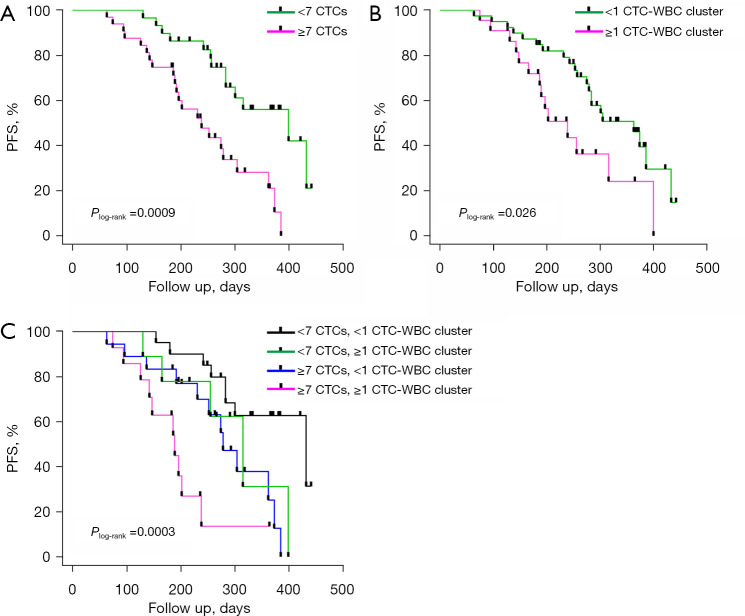
The PFS of patients with advanced cancer in the high-CTC group and CTC-WBC cluster positive group were significantly shorter compared to those in the low-CTC (P=0.0009) (Figure 2A) and CTC-WBC cluster negative (P=0.026) groups (Figure 2B). Univariate Cox regression analysis revealed an increased risk of progression in the high-CTC group (HR =3.25, 95% CI: 1.56–6.78, P=0.002) and CTC-WBC cluster positive group (HR =2.17, 95% CI: 1.08–4.36, P=0.030). Both these observations were markedly different in the multivariate Cox regression analysis, wherein other clinical variables were also included (described in Table 2).
Figure 2.
Analysis of CTC counts and CTC-WBC clusters with patient PFS. (A) Kaplan-Meier curves showing the difference in PFS between the high-CTC (≥7 CTCs/6 mL) and low-CTC (<7 CTCs/6 mL) groups. (B) PFS analysis between CTC-WBC-cluster positive (≥1) and negative (<1) groups. (C) Combined analysis of CTCs and CTC-WBC clusters with patient PFS. Kaplan-Meier curves displaying the PFS of patients with <7 CTCs without CTC-WBC clusters, patients with <7 CTCs with CTC-WBC clusters, patients with ≥7 CTCs without CTC-WBC clusters, and patients with ≥7 CTCs with CTC-WBC clusters. CTC, circulating tumor cell; CTC-WBC cluster, circulating tumor-cell-associated white blood cell cluster; PFS, progression-free survival.
Table 2. Univariate and multivariate analyses of NSCLC patients with PFS.
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI)* | P* | ||
| Gender | |||||
| Male vs. female | 0.71 (0.36–1.38) | 0.306 | |||
| Age | |||||
| ≤60 vs. >60 | 0.83 (0.39–1.80) | 0.643 | |||
| Smoking history | |||||
| Yes vs. no | 1.17 (0.59–2.31) | 0.661 | |||
| Stage | |||||
| III vs. IV | 0.73 (0.34–1.57) | 0.416 | |||
| Pathologic type | |||||
| Adenocarcinoma | |||||
| Squamous cell carcinoma | 0.52 (0.26–1.06) | 0.073 | 0.62 (0.27–1.43) | 0.262 | |
| Main therapy (III&IV) | |||||
| Targeted therapy | |||||
| Non-targeted therapy | 0.50 (0.21–1.17) | 0.108 | 0.89 (0.33–2.39) | 0.811 | |
| CTCs | |||||
| ≥7 vs. <7 | 3.25 (1.56–6.78) | 0.002 | 2.89 (1.36–6.17) | 0.006 | |
| CTC-WBC clusters | |||||
| ≥1 vs. <1 | 2.17 (1.08–4.36) | 0.030 | 2.18 (1.07–4.43) | 0.031 | |
*, adjusted for pathologic type, main therapy, number of CTCs, and CTC-WBC clusters. HR, hazard ratio; CI, confidence interval; NSCLC, non-small cell lung cancer; CTC, circulating tumor cell; CTC-WBC cluster, circulating tumor-cell-associated white blood cell cluster.
Combined analysis of CTCs and CTC-WBC clusters provided additional prognostic value
To further analyze the combined effect of CTCs and CTC-WBC clusters, patients with advanced cancer were divided into four risk groups: (A) patients with <7 CTCs/6 mL and without CTC-WBC clusters; (B) patients with <7 CTCs/6 mL and with CTC-WBC clusters; (C) patients with ≥7 CTCs/6 mL and without CTC-WBC clusters; and (D) patients with ≥7 CTCs/6 mL and with CTC-WBC clusters. The median PFS of patients in groups A, B, C, and D were 432, 315, 278, and 189 days, respectively. Compared to patients in group A, patients in groups C and D showed shorter PFS (Plog-rank <0.05, respectively) (Figure 2C), and had an increased risk of progression (HRgroup C =3.09, 95% CI: 1.15–8.36, P=0.026; HRgroup D =7.13, 95% CI: 2.51–20.23, P<0.001) (Table 3). Data from this cohort indicated that patients with ≥7 CTCs and CTC-WBC clusters had the highest risk of progression, and was identified as an independent prognostic factor of PFS, which could provide additional prognostic value for CTC count alone.
Table 3. Combined analysis of CTCs and CTC-WBC clusters with patient PFS.
| Variables | n (%) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI)* | P* | |||
| <7 CTCs, <1CTC-WBC cluster | 20 (32.79) | 1 | 1 | |||
| <7 CTCs, ≥1CTC-WBC cluster | 9 (14.75) | 2.43 (0.77–7.71) | 0.130 | 2.77 (0.80–9.67) | 0.110 | |
| ≥7 CTCs, <1CTC-WBC cluster | 18 (29.51) | 3.25 (1.26–8.42) | 0.015 | 3.09 (1.15–8.36) | 0.026 | |
| ≥7 CTCs, ≥1CTC-WBC cluster | 14 (22.95) | 8.03 (2.85–22.61) | <0.001 | 7.13 (2.51–20.23) | <0.001 | |
*, adjusted for pathologic type, and main therapy. CTC, circulating tumor cell; CTC-WBC cluster, circulating tumor-cell-associated white blood cell cluster; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval.
Prognostic value of CTC-WBC cluster count and cluster size analysis
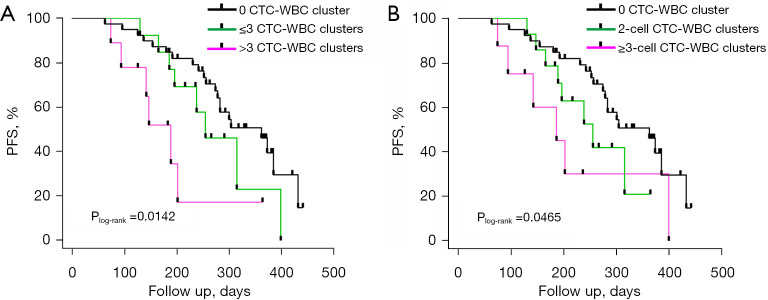
In order to further assess the relationship between the number of CTC-WBC clusters and patient prognosis, patients with CTC-WBC clusters were stratified according to the median number of the CTC-WBC clusters. Thirteen patients with ≤3 CTC-WBC clusters and nine patients with >3 CTC-WBC clusters were recorded. Patients without CTC-WBC clusters served as references. Patients with >3 CTC-WBC clusters showed shorter PFS (Plog-rank<0.05) (Figure 3A). However, this difference disappeared after adjusting for additional clinical risk factors. No significant difference in the risk of progression was observed between patients with ≤3 CTC-WBC clusters and those without CTC-WBC clusters (Table 4).
Figure 3.
Prognostic value of CTC-WBC cluster count and cluster size analysis. PFS Kaplan-Meier curves for patients without CTC-WBC clusters, patients with ≤3 CTC-WBC clusters and patients with >3 CTC-WBC clusters (A); and PFS Kaplan-Meier curves for patients without CTC-WBC clusters, patients with two-cell CTC-WBC clusters and patients with ≥ three-cell CTC-WBC clusters (B). PFS, progression-free survival; CTC-WBC cluster, circulating tumor-cell-associated white blood cell cluster.
Table 4. Associations between the CTC-WBC cluster count and the PFS of NSCLC patients.
| Variables | n (%) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI)* | P* | |||
| 0 CTC-WBC cluster | 39 (63.93) | 1 | 1 | |||
| ≤3 CTC-WBC clusters | 13 (21.31) | 1.67 (0.73–3.83) | 0.226 | 2.18 (0.91–5.22) | 0.079 | |
| >3 CTC-WBC clusters | 9 (14.75) | 3.72 (1.44–9.59) | 0.007 | 2.18 (0.83–5.77) | 0.116 | |
*, adjusted for pathologic type, main therapy, and number of CTCs. CTC-WBC cluster, circulating tumor-cell-associated white blood cell cluster; PFS, progression-free survival; NSCLC, non-small cell lung cancer; HR, hazard ratio; CI, confidence interval.
We further evaluated the prognostic value of the CTC-WBC cluster size. In this study, the total number of CTCs plus WBCs in the CTC clusters was between two and five. Compared to patients without CTC-WBC clusters, patients with ≥ three-cell CTC-WBC clusters had a shorter PFS (P<0.05) (Figure 3B), and the multivariate Cox regression analysis showed an increased risk of progression (HR =2.90, 95% CI: 1.06–7.89, P=0.037) (Table 5). Patients with two-cell CTC-WBC clusters had a HR of 1.87 (95% CI: 0.81–4.34, P=0.142).
Table 5. Associations between the CTC-WBC cluster size and the PFS of NSCLC patients.
| Variables | n (%) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI)* | P* | |||
| 0 CTC-WBC cluster | 39 (69.93) | 1 | 1 | |||
| 2-cell CTC-WBC clusters | 14 (22.95) | 1.82 (0.79–4.21) | 0.163 | 1.87 (0.81–4.34) | 0.142 | |
| ≥3-cell CTC-WBC clusters | 8 (13.11) | 2.91 (1.14–7.43) | 0.026 | 2.90 (1.06–7.89) | 0.037 | |
*, adjusted for pathologic type, main therapy, and number of CTCs. CTC-WBC cluster, circulating tumor-cell-associated white blood cell cluster; PFS, progression-free survival; NSCLC, non-small cell lung cancer; HR, hazard ratio; CI, confidence interval.
Characteristics analysis of CTCs in CTC-WBC clusters
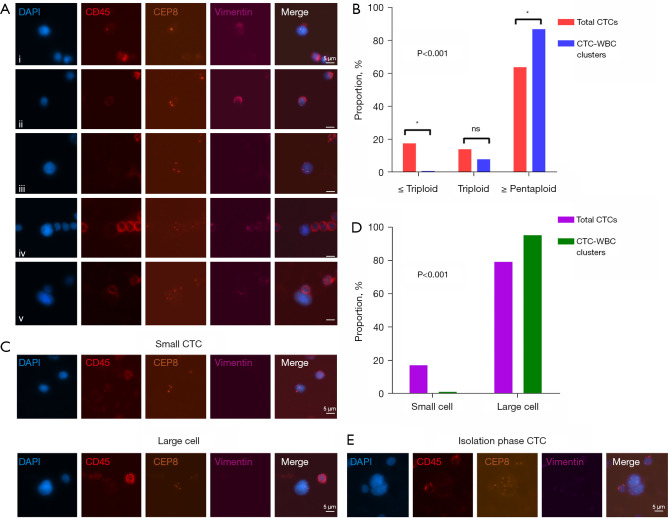
Given the clinical significance of ploidy of CTC chromosomes, CTCs were divided into three types (≤ triploid, tetraploid, and ≥ pentaploid), according to the ploidy situation. The typical chromosome ploidy for CTCs is shown in Figure 4A. In our study, a total of 863 CTCs were captured from 82 patients. Among these CTCs, 165 CTCs (19.12%) were found to be ≤ triploid type, 134 (15.53%) were tetraploid type, and 564 (65.35%) were ≥ pentaploid type. As for the CTC-WBC clusters, 85 CTCs were recorded in 82 CTC-WBC cluster events: two were triploid (2.35%) type; eight were tetraploid (9.41%) type; and 75 were ≥ pentaploid (88.24%) type. A significant difference was recorded when the distribution of ploidy numbers was compared between the two groups (P<0.001) (Figure 4B). This difference was mainly caused by more ≥ pentaploid (P<0.05) and less ≤ triploid (P<0.05) CTCs in the CTC-WBC clusters. In other words, more than 97% of the CTCs in the CTC-WBC clusters were recorded as polyploid in nature (tetraploid or higher ploidy).
Figure 4.
Characteristics analysis of CTCs in CTC-WBC clusters. (A) Schematic diagram of CTC chromosome ploidy detected by Subtraction Enrichment-iFISH platform. i–v representCTCs with haploid, diploid, triploid, tetraploid, and ≥ pentaploid Chr8, respectively. (B) Histogram of CTC ploidy distribution difference in the total CTCs and CTC-WBC clusters (*, denotes P<0.05; ns, non-significant). (C) A typical image of small (<5 µm) and large (≥5 µm) CTCs detected by Subtraction Enrichment-iFISH platform. (D) Histogram of the ratio of large and small CTCs to the total CTCs and CTC-WBC clusters. (E) A CTC in a CTC-WBC cluster displays a dividing nucleus with an intact cell membrane. The scale bars are 5 µm. DAPI, 4,6-diamino-2-phenyl indole; CTC, circulating tumor cell; CTC-WBC cluster, circulating tumor-cell-associated white blood cell cluster; iFISH, immunostaining-fluorescence in situ hybridization.
CTCs are known to vary greatly in terms of size (14). Large CTCs are defined as having a maximum diameter of ≥5 µm, while small CTCs are defined as have a maximum diameter of <5 µm (9). Representative images are shown in Figure 4C. In total CTCs, 158 (18.31%) small CTCs and 705 (81.69%) large CTCs were recorded in this study. In the CTC-WBC cluster populations, only two (2.35%) small CTCs and 83 (97.65%) large CTCs were observed (P<0.001) (Figure 4D).
Generally, it is expected that the vast majority of CTCs would undergo rapid apoptosis or are cleared by immune cells. However, in the present study, we speculated that some CTCs in the CTC-WBC clusters would be non‐senescent or apoptotic cells, whereas a group of cells would exhibit active proliferation. As shown in Figure 4E, a CTC in a CTC-WBC cluster was observed to be in the state of cell division.
Discussion
CTCs are rare cancer cells that are known to escape from primary lesions to enter the circulatory system. Generally, CTCs are considered to be seeds of metastasis in cancers (6,15). Several previous studies reported that the number of CTCs was related to the prognosis of multiple cancer types, including lung (16), breast (17), colon (18), and prostate (19) cancers. In the present study, it was also demonstrated that NSCLC patients with a high number of CTCs exhibited a shorter PFS.
Nevertheless, CTCs encounter a harsh environment once within the circulation and they adopt multiple strategies to ensure their survival (20). Previous studies have demonstrated that CTCs can combine with stromal cells, such as immune cells (21), fibroblasts (22), and platelets (23). The present study focused on the prognostic value of CTC‐WBC clusters and their biological characteristics in patients with advanced NSCLC. These heterogeneous CTC clusters could promote the survival of CTCs, and enhance the metastatic potential via different mechanisms. In particular, neutrophils, which account for the majority of WBCs, play a significant part in tumor growth and metastasis (24,25). Recent advances in research have indicated that neutrophils exhibit a longer life cycle and are characterized by the ability to secrete active biomolecules that promote tumor progression. Neutrophils are primarily anchored by the endothelial glycocalyx via autocrine Interleukin 8 and tumor‐derived C-X-C motif chemokine ligand-1, which are known to disrupt the endothelial barrier and promote the extravasation of attached tumor cells (26).
Currently, only a few clinical studies are available on CTC-WBC clusters. To the best of our knowledge, no relevant clinical research has been previously conducted in the field of lung cancer. In our study, it was observed that CTC-WBC cluster events were more likely to occur in advanced stages of NSCLC. Moreover, the presence of CTC-WBC clusters was identified as an independent prognostic factor for PFS. These results could help clinicians assess patient outcomes and develop treatment strategies. Similar findings have been demonstrated for advanced renal cell carcinoma (27,28), liver cancer (29), breast cancer, and gastric cancer (30,31). In addition, according to the combined analysis, patients with ≥7 CTCs and with CTC-WBC clusters had the highest risk of progression. Considering that the number of CTC does not always act as a reliable predictor of prognosis, the additional predictive value provided by the CTC-WBC clusters is needed as it reinforces the clinical value of CTC enumeration without any additional testing.
In the analysis of the relationship between the number of CTC clusters and prognosis, >3 CTC clusters exhibited a shorter PFS (Plog-rank<0.05), but this statistically significant difference disappeared after adjustment for other clinical factors, which may be related to the number of CTCs included in the multiple factors. In addition, another new finding of this study is the prognostic value of the size of CTC-WBC clusters. Specifically, patients with ≥ three-cell CTC-WBC clusters had a higher risk of progression (HR =2.90, P=0.037). Such heterogeneous clusters containing multiple cells may confer proliferative and metastatic advantages to CTCs (32). However, the number of patients with ≥ three-cell CTC-WBC clusters was small, and thus, further evaluation of this conclusion is needed.
At present, no general understanding exists regarding the frequency of CTC-WBC clusters in various cancer types. Previous studies have reported the frequency of patients positive for CTC-WBC clusters to be in the range of 3.4–41.6% (27,29,30). Meanwhile, in the present study, the frequency of these patients was 29.27%, which might be related to the tumor types and enrichment methods. Thus, more data is required from clinical studies in order to obtain a more comprehensive understanding of CTC-WBC clusters.
The present study analyzed the characteristics of CTCs adhered to by WBCs, and we observed that most of these CTCs were large cells that exhibited polyploidy (tetraploid or higher ploidy). Polyploid tumor cells often represent an increase in genetic material and produce more mutations, which could contribute to tumor progression and drug resistance, further promoting malignant behavior (33-36). Furthermore, the recruitment of WBCs by CTCs might be related to the induction of specific mutations. In a previous study, Szczerba et al. reported that a group of CTCs exhibited high-impact mutations in genes, such as TLE1, which further facilitated neutrophil recruitment to CTCs (37). Moreover, WBCs might also be involved in promoting the proliferation of CTCs (37). This study provided direct evidence that CTCs in CTC clusters possess splitting ability.
The present study had some limitations that should be noted. Firstly, this study involved a small sample size from a single-center, which may limit the ability of statistical analysis. Our conclusions will be more reliable if they are subsequently verified by large multi-center clinical studies. In addition, the follow-up period in this study was inadequate, and overall survival data was not included as an endpoint of follow-up. More patients will be included and follow-up will be extended in future research.
Conclusions
In conclusion, the presence of CTC-WBC clusters could be used as an independent prognostic factor for advanced NSCLC. The combined analysis results revealed that the presence of CTC-WBC clusters could provide additional prognostic value to the enumeration of CTCs alone. Furthermore, a larger-size CTC-WBC cluster represented a higher risk of shorter PFS. CTCs that can recruit WBCs are likely to be a specific subtype of tumor cells with proliferative ability. Therefore, attention should be paid to the phenomenon of CTC-WBC clusters both in clinical practice and basic research.
Supplementary
The article’s supplementary files as
Acknowledgments
We sincerely appreciate the patients for the provision of blood samples for research. We are also grateful to colleagues from Department of Radiology and Medical Oncology, Huadong Hospital Affiliated to Fudan University for assistance with patient recruitment.
Funding: This research was supported by Clinical Science and Technology Innovation Project of Shanghai Hospital Development Center (SHDC12018113); and Shanghai Hospital Clinical Research Cultivation Project (SHDC12019X11).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Huadong Hospital Affiliated to Fudan University (No. 2018K099). All patients provided informed consent.
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-423/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-423/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-423/coif). ZL, LF, YW, YN, XZ, BW, YY, CC, NQ, DT, WG report funding from Clinical Science and Technology Innovation Project of Shanghai Hospital Development Center (SHDC12018113), and Shanghai Hospital Clinical Research Cultivation Project (SHDC12019X11). DDW and PPL are from Cytelligen, San Diego and report that this research has received technical support from Cytelligen, San Diego, CA, USA. The authors have no other conflicts of interest to declare.
(English Language Editor: A. Kassem)
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science 2011;331:1559-64. 10.1126/science.1203543 [DOI] [PubMed] [Google Scholar]
- 3.Hotta K, Kiura K, Toyooka S, et al. Clinical significance of epidermal growth factor receptor gene mutations on treatment outcome after first-line cytotoxic chemotherapy in Japanese patients with non-small cell lung cancer. J Thorac Oncol 2007;2:632-7. 10.1097/JTO.0b013e318074bc0d [DOI] [PubMed] [Google Scholar]
- 4.Guin S, Ru Y, Wynes MW, et al. Contributions of KRAS and RAL in non-small-cell lung cancer growth and progression. J Thorac Oncol 2013;8:1492-501. 10.1097/JTO.0000000000000007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohme M, Riethdorf S, Pantel K. Circulating and disseminated tumour cells - mechanisms of immune surveillance and escape. Nat Rev Clin Oncol 2017;14:155-67. 10.1038/nrclinonc.2016.144 [DOI] [PubMed] [Google Scholar]
- 6.Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature 2016;529:298-306. 10.1038/nature17038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao X, Choudhury AD, Yamanaka YJ, et al. Functional analysis of single cells identifies a rare subset of circulating tumor cells with malignant traits. Integr Biol (Camb) 2014;6:388-98. 10.1039/c3ib40264a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giuliano M, Shaikh A, Lo HC, et al. Perspective on Circulating Tumor Cell Clusters: Why It Takes a Village to Metastasize. Cancer Res 2018;78:845-52. 10.1158/0008-5472.CAN-17-2748 [DOI] [PubMed] [Google Scholar]
- 9.Heeke S, Mograbi B, Alix-Panabières C, et al. Never Travel Alone: The Crosstalk of Circulating Tumor Cells and the Blood Microenvironment. Cells 2019;8:714. 10.3390/cells8070714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrido-Navas C, de Miguel-Perez D, Exposito-Hernandez J, et al. Cooperative and Escaping Mechanisms between Circulating Tumor Cells and Blood Constituents. Cells 2019;8:1382. 10.3390/cells8111382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Liu Y, Zhang L, et al. Vimentin expression in circulating tumor cells (CTCs) associated with liver metastases predicts poor progression-free survival in patients with advanced lung cancer. J Cancer Res Clin Oncol 2019;145:2911-20. 10.1007/s00432-019-03040-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin PP, Gires O, Wang DD, et al. Comprehensive in situ co-detection of aneuploid circulating endothelial and tumor cells. Sci Rep 2017;7:9789. 10.1038/s41598-017-10763-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozkumur E, Shah AM, Ciciliano JC, et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci Transl Med 2013;5:179ra47. 10.1126/scitranslmed.3005616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer 2003;3:453-8. 10.1038/nrc1098 [DOI] [PubMed] [Google Scholar]
- 16.Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011;29:1556-63. 10.1200/JCO.2010.28.7045 [DOI] [PubMed] [Google Scholar]
- 17.Eroglu Z, Fielder O, Somlo G. Analysis of circulating tumor cells in breast cancer. J Natl Compr Canc Netw 2013;11:977-85. 10.6004/jnccn.2013.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardingham JE, Grover P, Winter M, et al. Detection and Clinical Significance of Circulating Tumor Cells in Colorectal Cancer--20 Years of Progress. Mol Med 2015;21 Suppl 1:S25-31. 10.2119/molmed.2015.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008;14:6302-9. 10.1158/1078-0432.CCR-08-0872 [DOI] [PubMed] [Google Scholar]
- 20.El-Kenawi A, Hänggi K, Ruffell B. The Immune Microenvironment and Cancer Metastasis. Cold Spring Harb Perspect Med 2020;10:a037424. 10.1101/cshperspect.a037424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leone K, Poggiana C, Zamarchi R. The Interplay between Circulating Tumor Cells and the Immune System: From Immune Escape to Cancer Immunotherapy. Diagnostics (Basel) 2018;8:59. 10.3390/diagnostics8030059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duda DG, Duyverman AM, Kohno M, et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A 2010;107:21677-82. 10.1073/pnas.1016234107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci U S A 2014;111:E3053-61. 10.1073/pnas.1411082111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukaida N, Sasaki SI, Baba T. Two-Faced Roles of Tumor-Associated Neutrophils in Cancer Development and Progression. Int J Mol Sci 2020;21:3457. 10.3390/ijms21103457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu M, Ma M, Tan Z, et al. Neutrophil: A New Player in Metastatic Cancers. Front Immunol 2020;11:565165. 10.3389/fimmu.2020.565165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen MB, Hajal C, Benjamin DC, et al. Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promote tumor cell extravasation. Proc Natl Acad Sci U S A 2018;115:7022-7. 10.1073/pnas.1715932115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan Y, Xu F, Tian J, et al. The prognostic value of circulating tumour cells (CTCs) and CTC white blood cell clusters in patients with renal cell carcinoma. BMC Cancer 2021;21:826. 10.1186/s12885-021-08463-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Zhang P, Chong Y, et al. Perioperative Circulating Tumor Cells (CTCs), MCTCs, and CTC-White Blood Cells Detected by a Size-Based Platform Predict Prognosis in Renal Cell Carcinoma. Dis Markers 2021;2021:9956142. 10.1155/2021/9956142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo Q, Wang C, Peng B, et al. Circulating Tumor-Cell-Associated White Blood Cell Clusters in Peripheral Blood Indicate Poor Prognosis in Patients With Hepatocellular Carcinoma. Front Oncol 2020;10:1758. 10.3389/fonc.2020.01758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansson S, Bendahl PO, Larsson AM, et al. Prognostic impact of circulating tumor cell apoptosis and clusters in serial blood samples from patients with metastatic breast cancer in a prospective observational cohort. BMC Cancer 2016;16:433. 10.1186/s12885-016-2406-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu Y, Zhang X, Deng X, et al. Circulating tumor cell-associated white blood cell cluster is associated with poor survival of patients with gastric cancer following radical gastrectomy. Eur J Surg Oncol 2022;48:1039-45. 10.1016/j.ejso.2021.11.115 [DOI] [PubMed] [Google Scholar]
- 32.Aceto N. Bring along your friends: Homotypic and heterotypic circulating tumor cell clustering to accelerate metastasis. Biomed J 2020;43:18-23. 10.1016/j.bj.2019.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stopsack KH, Whittaker CA, Gerke TA, et al. Aneuploidy drives lethal progression in prostate cancer. Proc Natl Acad Sci U S A 2019;116:11390-5. 10.1073/pnas.1902645116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Shi H, Jiang T, et al. Circulating tumor cells with karyotyping as a novel biomarker for diagnosis and treatment of nasopharyngeal carcinoma. BMC Cancer 2018;18:1133. 10.1186/s12885-018-5034-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Zhang X, Liu Y, et al. PD-L1+ aneuploid circulating tumor endothelial cells (CTECs) exhibit resistance to the checkpoint blockade immunotherapy in advanced NSCLC patients. Cancer Lett 2020;469:355-66. 10.1016/j.canlet.2019.10.041 [DOI] [PubMed] [Google Scholar]
- 36.Chunduri NK, Storchová Z. The diverse consequences of aneuploidy. Nat Cell Biol 2019;21:54-62. 10.1038/s41556-018-0243-8 [DOI] [PubMed] [Google Scholar]
- 37.Szczerba BM, Castro-Giner F, Vetter M, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019;566:553-7. 10.1038/s41586-019-0915-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as