Abstract
Purpose
Serum magnesium is the most frequently used laboratory test for evaluating clinical magnesium status. Hypomagnesemia (low magnesium status), which is associated with many chronic diseases, is diagnosed using the serum magnesium reference range. Currently, no international consensus for a magnesemia normal range exists. Two independent groups designated 0.85 mmol/L (2.07 mg/dL; 1.7 mEq/L) as the low cut-off point defining hypomagnesemia. MaGNet discussions revealed differences in serum magnesium reference ranges used by members’ hospitals and laboratories, presenting an urgent need for standardization.
Methods
We gathered and compared serum magnesium reference range values from our institutions, hospitals, and colleagues worldwide.
Results
Serum magnesium levels designating “hypomagnesemia” differ widely. Of 43 collected values, only 2 met 0.85 mmol/L as the low cut-off point to define hypomagnesemia. The remainder had lower cut-off values, which may underestimate hypomagnesemia diagnosis in hospital, clinical, and research assessments. Current serum magnesium reference ranges stem from “normal” populations, which unknowingly include persons with chronic latent magnesium deficit (CLMD). Serum magnesium levels of patients with CLMD fall within widely used “normal” ranges, but their magnesium status is too low for long-term health. The lower serum magnesium reference (0.85 mmol/L) proposed specifically prevents the inclusion of patients with CLMD.
Conclusions
Widely varying serum magnesium reference ranges render our use of this important medical tool imprecise, minimizing impacts of low magnesium status or hypomagnesemia as a marker of disease risk. To appropriately diagnose, increase awareness of, and manage magnesium status, it is critical to standardize lower reference values for serum magnesium at 0.85 mmol/L (2.07 mg/dL; 1.7 mEq/L).
Keywords: Serum magnesium, Serum magnesium reference range, Chronic latent magnesium deficit, CLMD, Hypomagnesemia
Introduction
Magnesium is essential for life. Although its homeostasis at the cellular, tissue, and organism levels seems to be well buffered, there is a widely distributed tendency for low magnesium status to be associated with the most common chronic diseases [1]. In the absence of a more selective, reliable, and easily testable biomarker, serum magnesium is the most frequently used laboratory test for evaluating clinical magnesium status. Researchers use serum magnesium reference ranges to designate hypo-, normo-, or hypermagnesemic status, whereas hospitals and primary care physicians use serum magnesium values in deciding whether to administer magnesium therapy. Consequently, the lower cutoff for serum magnesium reference value of a hospital or clinical laboratory determines the number of patients diagnosed as “hypomagnesemic.”
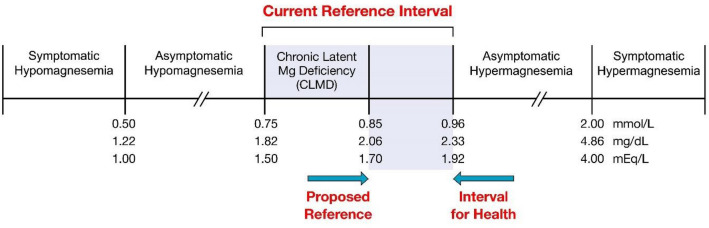
Hypomagnesemia has several clinical manifestations that vary from asymptomatic to severe. Overt symptoms present at ≤ 0.6 mmol/L [2] (Fig. 1). These clinical manifestations include metabolic issues (hypokalemia and hypocalcemia), neuromuscular-central nervous system symptoms (hyperexcitability, muscle weakness, tremors, seizures, tetany, headaches, and fatigue), cardiovascular abnormalities (tachycardia, arrhythmias such as torsade de pointes, ventricular fibrillation, mitral valve prolapse, cardiac ischemia, myocardial infarct, and hypertension), and endocrine abnormalities (insulin resistance and type 2 diabetes) [2, 3] (Table 1). Reported severe, overt symptoms of hypomagnesemia also include the mimic of acute stroke [4], life-threatening arrhythmias [3], metabolic acidosis [5], and new-onset diabetes following heart transplantation [6]. In intensive-care units, hypomagnesemia is associated with higher mortality, the need for mechanical ventilation, and increased length of stay [7].
Fig. 1.
Total serum magnesium concentration for assessment of magnesium status. Conversion factor: for mg/dL to mmol/L, multiply by 0.411; for mmol/L to mg/dL, multiply by 2.43; and for mmol/L to mEq/L, divide by 0.5.
Reproduced from Costello and Rosanoff [14], which was adapted from Costello et al. [12]
Table 1.
Clinical manifestations of hypomagnesemia.
Adapted from Ehrenpreis et al. [2] and Ahmed et al. [3] with permission
| System | Clinical manifestation |
|---|---|
| Neuromuscular/central nervous system | Positive Chvostek’s and Trousseau’s signs, tremor, fasciculations, tetany, headaches, seizures, fatigue, generalized fatigue, asthenia |
| Hyperexcitability, weakness, dysphagia, vertical nystagmus, apathy, delirium, coma | |
| Cardiovascular | Atherosclerotic vascular disease/coronary artery disease |
| Arrhythmias (Torsades de pointes, PR prolongation, progressive QRS widening, and diminution of T-waves) | |
| Hypertension | |
| Congestive heart failure | |
| Mitral valve prolapse, tachycardia, cardiac ischemia, myocardial infarct | |
| Endocrine | Altered glucose homeostasis/diabetic complications |
| Osteoporosis | |
| Insulin resistance and type 2 diabetes | |
| Biochemical/other | Hypokalemia |
| Hypocalcemia | |
| Asthma | |
| Nephrolithiasis |
In addition to hypomagnesemia presenting with overt disease states, patients can present with asymptomatic hypomagnesemia or chronic latent magnesium deficit (CLMD) at serum Mg levels well above 0.6 mmol/L (Fig. 1). CLMD has been defined as a subclinical condition that renders individuals more susceptible to disease. CLMD occurs with a small chronic negative magnesium balance, which may be attributed to decreased dietary intake, decreased gastrointestinal absorption, and/or increased renal loss [8]. The most common cause of CLMD worldwide is decreased magnesium dietary intake, since processed food and fast food tend to have low magnesium content [9, 10]. However, illness and drug use must also be taken into account. Over time, this negative magnesium balance causes the serum magnesium concentration to decrease. Since this is a subtle chronic process, some magnesium is depleted in bone to support the circulating serum magnesium pool [11]. Thus, patients with CLMD appear to have “normal” magnesium status, because their serum magnesium value falls within the traditionally normal reference ranges, but these patients are not in sufficient magnesium status for long-term health. The actual magnesium deficiency of the patient is latent—a fallacy of the reference interval as serum magnesium reference intervals have been established with “healthy” individuals, including many who unknowingly have CLMD [11]. This is the basis for updating the lower limit reference interval for the serum magnesium concentration.
Two groups of magnesium researchers, one in the United States [12] and one in Germany [13], have independently agreed on a serum magnesium value of 0.85 mmol/L as the low cut-off point to define hypomagnesemia, since serum magnesium values < 0.85 mmol/L (2.07 mg/dL or 1.7 mEq/L) have been associated with an increased risk of various diseases (e.g., cardiovascular disease [CVD], metabolic), obesity, and aging.
In response to the COVID-19 pandemic, our international group of magnesium researchers (Magnesium Global Network [MaGNet]) began meeting in September 2020 to discuss the research. In these meetings, we discussed the serum magnesium reference ranges used by our institutions’ hospitals and laboratory service providers, and we discovered that they were far from uniform. We decided to gather these values to compare them with the suggested evidence-based serum magnesium reference for hypomagnesemia of 0.85 mmol/L [12,13], and we present those findings here.
Materials and methods
We collected and evaluated the various serum magnesium reference range values of our institutions, including the laboratory methodology used to obtain those values when available. To expand our indicative database, we also gathered serum magnesium reference range values from colleagues around the world. Institutions were coded by country, and all contributed values were calculated to express commonly used alternate units of serum magnesium—milligrams per deciliter (mg/dL), millimoles per liter (mmol/L), and milliequivalents per liter (mEq/L)—for each reported value. GraphPad Prism version 9 was used to tabulate data and create Fig. 2. The independently suggested evidence-based serum magnesium reference range of 0.85–0.95 mmol/L (2.07–2.3 mg/dL or 1.7–1.9 mEq/L) was added to Figure 2 for comparison.
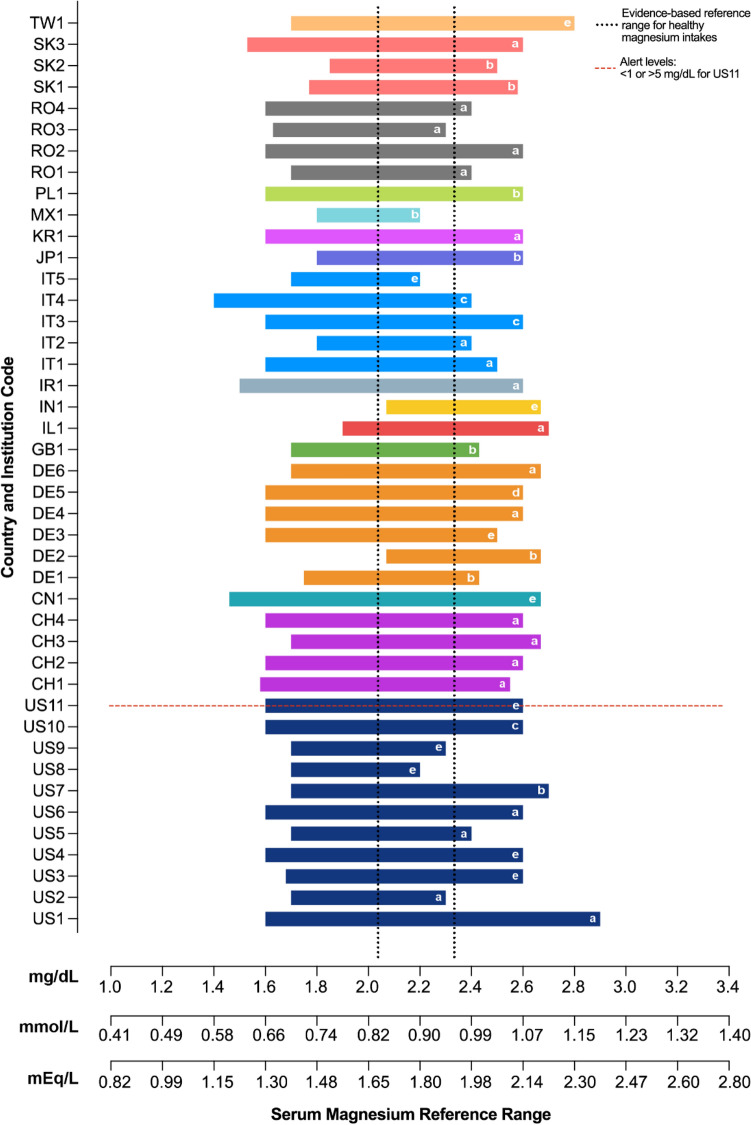
Fig. 2.
Serum magnesium reference ranges from several institutions and laboratory service providers, gathered by magnesium researchers for the MaGNet Global Magnesium Project. Assessment methods are indicated with lowercase letters as follows: colorimetric (a), colorimetric/xylidyl blue (b), enzymatic assay (c), atomic absorption spectroscopy (d), and not reported (e). Colorimetric, photometric, and spectrophometric designations of methodology are all classified under colorimetry. See Table 2 for full details
Results
As shown in Fig. 2 and Table 2, the level of serum magnesium designating a patient as hypomagnesemic differs worldwide. Forty-three values were gathered from institutions in 16 different countries, including China, Germany, India, Iran, Israel, Italy, Japan, Korea, Mexico, Poland, Romania, Slovakia, Switzerland, Taiwan, the United Kingdom, and the United States. Of those 43 values, only 2 (5%) designated a low serum magnesium reference range cut-off value of 0.85 mmol/L. Forty-one values of the 43 institutions (95%) designated their low cutoff for definition of hypomagnesemia below and even well below this suggested standard, from 0.58 mmol/L for IT4 to 0.78 mmol/L for IL1. Most of the collected values (29 of 43; 67%) used colorimetric methodology, with 9 specifying xylidyl blue and 1 indicating calmagite as the colorimetric agent. Of the 43 values, 1 (2%) used atomic absorption spectroscopy and 3 (7%) used enzymatic methodology. Ten values (21%) did not report methodology. Figure 1 summarizes the definition of low and high magnesemia as proposed by previous works on magnesium reference range [14] and highlights how CLMD is currently included in the normal serum magnesium range (see Discussion).
Table 2.
Working table of serum magnesium reference ranges for various hospitals and institutions
| Institution code | Country | Institution | Serum magnesium reference rangea | Methodb | Researcher | ||
|---|---|---|---|---|---|---|---|
| mg/dL | mmol/L | mEq/L | |||||
| CH1 | Switzerland | Kantonsspital Aarau | 1.58–2.55 | 0.65–1.05 | 1.3–2.1 | Photometric | Anton Kraus |
| CH2 | Switzerland | University Hospital, Zurich | 1.6–2.6 |
0.66–1.07 (age dependent) |
1.3–2.1 | Photometric | Anton Kraus |
| CH3 | Switzerland | Analytica Medizinische Lab, Zurich | 1.7–2.67 | 0.7–1.10 | 1.4–2.2 | Photometric | Anton Kraus |
| CH4 | Switzerland | Laboratory of Dr. Risch | 1.6–2.6 |
0.66–1.07 (age dependent) |
1.3–2.1 | Photometric | Anton Kraus |
| CN1 | China | Zhanghou Affiliated Hospital of Fujian Medical University, Zhangzhou | 1.46–2.67 | 0.6–1.1 | 1.2–2.2 | NR | Andrea Rosanoff [18] |
| DE1 | Germany | Dr. Schottdorf Augsburg Laboratory | 1.75–2.43 | 0.75–1.0 | 1.5–2.0 | Photometric, colorimetric, xylidyl blue | Bodo von Ehrlich |
| DE2 | Germany | Medical Office | 2.07–2.67 | 0.85–1.1 | 1.7–2.2 | Photometric, colorimetric, xylidyl blue | Bodo von Ehrlich |
| DE3 | Germany | St. Elisabeth Hospitals Herne | 1.6–2.5 | 0.66–1.03 | 1.3–2.06 | NR | Klaus Kisters |
| DE4 | Germany | Laboratory Enders, Stuttgart (https://www.labor-enders.de/analysenverzeichnis) | 1.6–2.6 | 0.66–1.07 | 1.3–2.1 | Colorimetric | Anton Kraus |
| DE5 | Germany | Laboratory Amedes Hosding, Hamburg (https://www.amedes-group.com) | 1.6–2.6 | 0.66–1.07 | 1.3–2.1 | Atomic absorption spectroscopy | Anton Kraus |
| DE6 | Germany | Franziskus Hospital Bielefeld | 1.7–2.67 | 0.7–1.1 | 1.4–2.2 | Photometric | Oliver Micke |
| GB1 | UK | UK Hospitals – National Health Service | 1.7–2.43 | 0.7–1.00 | 1.4–2.0 |
Colorimetric, xylidyl blue |
Rhian Touyz and Yee Pang Teoh |
| IL1 | Israel | Chaim Sheba Medical Center | 1.9–2.7 | 0.78–1.1 | 1.6–2.2 | Photometric color test | Michael Shechter |
| IN1 | India | Nirogyam Pathology Laboratory | 2.07–2.67 | 0.85–1.1 | 1.7–2.2 | NR | Oliver Micke |
| IR1 | Iran | National Research Institute of Tuberculosis and Lung Diseases, Iran | 1.5–2.6 | 0.62–1.07 | 1.2–2.1 | Colorimetric | Guitti Pourdowlat and Shadi Baniasadi |
| IT1 | Italy | University Hospital of Palermo, Italy | 1.6–2.5 | 0.66–1.03 | 1.3–2.06 | Colorimetric | Mario Barbagallo |
| IT2 | Italy | Policlinico Gemelli, Rome | 1.8–2.4 | 0.74–0.99 | 1.48–2.0 | Colorimetric | Federica Wolf |
| IT3 | Italy | Campus Biomedico, Rome | 1.6–2.6 | 0.66–1.07 | 1.3–2.1 | Enzymatic assay | Federica Wolf |
| IT4 | Italy | Clinical Pathology Laboratory at Sacco Hospital | 1.4–2.4 | 0.58–0.99 | 1.15–2.0 | Enzymatic assay (isocitrate dehydrogenase) | Jeanette Maier |
| IT5 | Italy | Used UCSF (USA) ref range for study at Reggio Emilia Hospital | 1.7–2.2 | 0.70–0.905c | 1.4–1.8 | NR | Stefano Iotti and Lucia Merolle |
| JP1 | Japan | Jikei University, Japan | 1.8–2.6 | 0.74–1.07 | 1.48–2.14 | Colorimetric, xylidyl blue | Ka Kahe and Kuninobu Yokota |
| KR1 | Korea | Ajou University School of Medicine, South Korea | 1.6–2.6 | 0.66–1.07 | 1.3–2.1 | Colorimetric | Ka Kahe and Dae Jung Kim |
| MX1 | Mexico | 1.8–2.2 | 0.74–0.905 | 1.48–1.8 | Colorimetric, xylidyl blue | Claudia Gamboa | |
| PL1 | Poland | Diagnostic Medical Laboratory "Synevo" |
1.60–2.60 (age dependent) |
0.66–1.07 | 1.3–2.1 | Colorimetric, xylidyl blue; fasting |
Jeanette Maier and Magdalena Maj-Zurawska |
| RO1 | Romania | Fundeni Clinical Hospital Bucuresti | 1.7–2.4 (adult patients) | 0.70–0.99 | 1.40–1.98 | Spectrophometric | Mihai Nechifor |
| RO2 | Romania | Iasi Recovery Clinical Hospital | 1.6–2.6 (> 12 y) | 0.66–1.07 | 1.36–2.14 | Spectrophometric | Mihai Nechifor |
| BIOCLINICA | 1.6–2.6 (> 14 y) | ||||||
| St. Spiridon County Clinical Hospital Iasi | 1.6–2.6 (no age specified) | Colorimetric | |||||
| RO3 | Romania | Timis Country Emergency Clinical Hospital, Timisoara | 1.6–2.3 (no age specified) | 0.66–0.95 | 1.36–1.90 | Spectrophometric | Mihai Nechifor |
| RO4 | Romania | Synevo network of private labs | 1.6–2.4 (> 20 y) | 0.66–0.99 | 1.36–1.98 | Colorimetric | Mihai Nechifor |
| SK1 | Slovakia | ICB, University Hospital Martin, Slovakia | 1.77–2.58 |
0.73–1.06 (adult male) |
1.46–2.1 | Colorimetric, xylidyl blue | Martin Kolisek |
| SK2 | Slovakia | ICB, University Hospital, Martin, Slovakia | 1.87–2.5 |
0.77–1.03 (adult female) |
1.5–2.06 | Colorimetric, xylidyl blue | Martin Kolisek |
| SK3 | Slovakia | Alpha Medical, Unilabs Group, Slovakia | 1.53–2.6 | 0.63–1.07 | 1.26–2.14 | Colorimetric | Martin Kolisek |
| TW1 | Taiwan | Taichung Veterans General Hospital, Taichung, Taiwan | 1.7–2.8 | 0.70–1.15 | 1.4–2.3 | NR | Fu-Chou Cheng |
| US1 | USA | Indiana University Hospital Pathology Laboratory | 1.6–2.9 | 0.66–1.19 | 1.3–2.4 | Colorimetric | Nana Gletsu-Miller and Taylor Wallace |
| US2 | USA | University of Louisville, Louisville, KY | 1.7–2.3 | 0.70–0.95 | 1.4–1.9 | Colorimetric, calmagite | Ron Elin |
| US3 | USA | Dartmouth | 1.68–2.60 | 0.69–1.07 | 1.4–2.1 | NR | Emily Campbell |
| US4 | USA | Medical University of South Carolina | 1.6–2.6 | 0.66–1.07 | 1.3–2.1 | NR | Emily Campbell |
| US5 | USA | Clinical laboratories, Hawaii | 1.7–2.4 | 0.70–0.99 | 1.4–1.98 | Colorimetric | Andrea Rosanoff |
| US6 | USA | Diagnostic laboratories, Hawaii | 1.6–2.6 | 0.66–1.07 | 1.3–2.1 | Colorimetric | Andrea Rosanoff |
| US7 | USA | Clinical Laboratory, Indiana University School of Medicine Diabetes Center Translation Core | 1.70–2.70 | 0.70–1.1 | 1.4–2.2 | Colorimetric (xylidyl blue) | Yiqing Song |
| US8 | USA | UCSF (https://www.ucsfhealth.org/medical-tests/magnesium-blood-test) | 1.7–2.2c | 0.70–0.905c | 1.4–1.8c | NR | Stefano Iotti |
| US9 | USA | Mayo Clinical Laboratories (age > 17 y) |
1.7–2.3 (age dependent) |
0.70–0.95 | 1.4–1.9 | NR | Stefano Iotti |
| US10 | USA | National Institutes of Health Clinical Center | 1.6–2.6 | 0.66–1.07 | 1.3–2.1 | Enzymatic, assayed on Abbott Architect. Alert levels: < 1.0 or > 5.0 mg/dL | Rebecca Costello |
| US11 | USA | Columbia University Presbyterian Hospital, New York | 1.6–2.6 | 0.66–1.07 | 1.3–2.1 | NR | Ka KaHe |
These data were gathered by MaGNet for the Global Magnesium Project, 2020–2021
NR, not reported; UCSF, University of California, San Francisco
aBolded values are those provided by the researchers. Nonbolded values are the respective conversions (conversion factor: for mg/dL to mmol/L, multiply by
0.4114; for mmol/L to mg/dL, multiply by 2.43; and for mmol/L to mEq/L, divide by 0.5)
bIn Fig. 2, colorimetric, photometric, and spectrophometric designations of methodology are all classified under colorimetry
cThe published UCSF serum magnesium reference range reports 1.7–2.2 mg/dL (shown in bold here) converting to 0.85–1.1 mmol/L on their webpage. However, the correct conversion for 1.7–2.2 mg/dL is 0.70–0.905 mmol/L, not 0.85–1.1 mmol/L; possibly their reported value of 1.7–2.2 is mEq/L (rather than mg/dL), which converts to 0.85–1.1 mmol/L
Discussion
Such different serum magnesium reference range values render our current use of this medical tool imprecise, causing many hypomagnesemic patients to be deemed normomagnesemic and potentially minimizing the effects of low magnesium status in research.
When different cut-off values for hypomagnesemia are used, substantially different results can ensue. For instance, in one study at a Warsaw hospital, when a lower cut-off reference value of 0.65 mmol/L was used, 7% of 20,483 patients were deemed hypomagnesemic [15]. With the lower cut-off reference value of 0.75 mmol/L, 25% of this same patient cohort showed hypomagnesemia. Finally, with a lower cut-off reference value of 0.85 mmol/L, 60% were diagnosed as hypomagnesemic [15].
Clearly, the serum magnesium reference range used by a hospital, clinical, or research laboratory is crucial in the designation of low magnesium status. The present observational study’s wide spectrum of serum reference interval values used in hospital and clinical laboratories around the world documents an urgent need for a consensus reference interval for serum magnesium concentration, specifically on the lower cut-off limit, since low magnesium status is currently a common condition worldwide [16]. The evidence-based lower cut-off value of 0.85 mmol/L (2.07 mg/dL or 1.7 mEq/L) proposed by the US and German research groups [12, 13] specifically prevents the inclusion of individuals with CLMD [12], who usually fall into the lower half of the reference interval of < 0.85 mmol/L (see Fig. 1).
Despite the central role of magnesium in maintaining proper immune, vascular, and pulmonary function, emerging evidence indicates that magnesemia is seldom assessed in patients [17]. Additionally, an evidence-based standard for the upper range of serum magnesium to differentiate between a “safe” and “hypermagnesemic” value is yet to be determined.
Conclusion
The 43 serum magnesium reference range values we collected from 16 countries varied widely, and all but 2 (DE2 and IN1) had a hypomagnesemic cut-off point well below that of the recommended 0.85 mmol/L (2.07 mg/dL or 1.7 mEq/L). Thus, the hypomagnesemic reference values in this informal collection indicate that physicians and researchers are vastly underestimating hypomagnesemia in their patients and institutions, interpreting as “normal” serum magnesium values that fall well below that recently proposed to be safe for good health. It is critically important to appropriately identify, diagnose, and manage low magnesium status, which is often overlooked and may play a role in susceptibility to increasingly common chronic diseases (e.g., CVD, diabetes, and chronic obstructive pulmonary disease), among other conditions.
A consensus serum magnesium to define hypomagnesemia is suggested to be 0.85 mmol/L (2.07 mg/dL or 1.7 mEq/L). An evidence-based, standardized serum magnesium reference range for hypermagnesemia still needs to be determined. MaGNet researchers should continue gathering and monitoring new evidence to further update recommendations for guidelines that define the correct serum magnesium reference range to maintain health.
Disclosures
Christina West received financial support from CMER for her role in this work. She provides editorial consulting services to authors, nonprofit organizations, and publishers, but has no conflicts of interest that influenced or are relevant to this work. Anton Kraus is an employee of Verla-Pharm Arzneimittel.
Abbreviations
- CLMD
Chronic latent magnesium deficit
- CVD
Cardiovascular disease
- MaGNet
Magnesium global network
Author contributions
A.R.: conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; supervision; validation; visualization; roles/writing—original draft; writing—review and editing. C.W.: data curation; visualization; software; writing—review and editing. R.J.E., O.M., S.B., M.B., E.C., F.-C.C., R.B.C., C.G., F.G.-R., N.G.-M., B.v.E., S.I., K.K.H., D.J.K., K.K., M.K., A.K., J.A.M., M.M.-Z., L.M., M.N., G.P., M.S., Y.S., Y.P.T., R.M.T., T.C.W., K.Y., and F.W.: conceptualization; data curation; formal analysis; investigation; methodology; validation; visualization; writing—review and editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Andrea Rosanoff, Email: arosanoff@gmail.com.
Christina West, Email: christina.west@comcast.net.
Ronald J. Elin, Email: ronald.elin@louisville.edu
Oliver Micke, Email: strahlenklinik@web.de.
Shadi Baniasadi, Email: sbaniasadi@yahoo.com.
Mario Barbagallo, Email: mario.barbagallo@unipa.it.
Emily Campbell, Email: Emily.Y.Campbell@hitchcock.org.
Fu-Chou Cheng, Email: vc1035@gmail.com.
Rebecca B. Costello, Email: rbcostello@earthlink.net
Claudia Gamboa-Gomez, Email: clau140382@hotmail.com.
Fernando Guerrero-Romero, Email: guerrero.romero@gmail.com.
Nana Gletsu-Miller, Email: ngletsum@iu.edu.
Bodo von Ehrlich, Email: aioloskalo@t-online.de.
Stefano Iotti, Email: stefano.iotti@unibo.it.
Ka Kahe, Email: kk3399@cumc.columbia.edu.
Dae Jung Kim, Email: djkim@ajou.ac.kr.
Klaus Kisters, Email: klaus.kisters@elisabethgruppe.de.
Martin Kolisek, Email: martin.kolisek@uniba.sk.
Anton Kraus, Email: info@magnesium-ges.de.
Jeanette A. Maier, Email: jeanette.maier@unimi.it
Magdalena Maj-Zurawska, Email: mmajzur@chem.uw.edu.pl.
Lucia Merolle, Email: Lucia.Merolle@ausl.re.it.
Mihai Nechifor, Email: mihainechif@yahoo.com.
Guitti Pourdowlat, Email: pourdowlat_g@yahoo.com.
Michael Shechter, Email: Michael.Shechter@sheba.health.gov.il, Email: shechtes@netvision.net.il.
Yiqing Song, Email: yiqsong@iu.edu.
Yee Ping Teoh, Email: YeePing.Teoh@wales.nhs.uk.
Rhian M. Touyz, Email: Rhian.Touyz@mcgill.ca
Taylor C. Wallace, Email: taylor.wallace@me.com
Kuninobu Yokota, Email: yokota@jikei.ac.jp.
Federica Wolf, Email: federica.wolf@unicatt.it.
References
- 1.Fiorentini D, Cappadone C, Farruggia G, Prata C. Magnesium: biochemistry, nutrition, detection, and social impact of diseases linked to its deficiency. Nutrients. 2021;13(4):1136. doi: 10.3390/nu13041136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehrenpreis ED, Jarrouj G, Meader R, Wagner C, Ellis M. A comprehensive review of hypomagnesemia. Dis Mon. 2022;68(2):101285. doi: 10.1016/j.disamonth.2021.101285. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed F, Mohammed A (2019) Magnesium: the forgotten electrolyte—a review on hypomagnesemia. Med Sci (Basel). 10.3390/medsci7040056 [DOI] [PMC free article] [PubMed]
- 4.Rico M, Martinez-Rodriguez L, Larrosa-Campo D, Calleja S. Dilemma in the emergency setting: hypomagnesemia mimicking acute stroke. Int Med Case Rep J. 2016;9:145–148. doi: 10.2147/imcrj.S101011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fulop T, Agarwal M, Keri KC (2020) Hypomagnesemia clinical presentation. https://emedicine.medscape.com/article/2038394-clinical. Accessed 13 April 2022
- 6.Peled Y, Ram E, Lavee J, Tenenbaum A, Fisman EZ, Freimark D, Klempfner R, Sternik L, Shechter M. Hypomagnesemia is associated with new-onset diabetes mellitus following heart transplantation. Cardiovasc Diabetol. 2019;18(1):132. doi: 10.1186/s12933-019-0939-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Upala S, Jaruvongvanich V, Wijarnpreecha K, Sanguankeo A. Hypomagnesemia and mortality in patients admitted to intensive care unit: a systematic review and meta-analysis. QJM. 2016;109(7):453–459. doi: 10.1093/qjmed/hcw048. [DOI] [PubMed] [Google Scholar]
- 8.Reddy ST, Soman SS, Yee J. Magnesium balance and measurement. Adv Chronic Kidney Dis. 2018;25(3):224–229. doi: 10.1053/j.ackd.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Sawicki CM, Jacques PF, Lichtenstein AH, Rogers GT, Ma J, Saltzman E, McKeown NM. Whole- and Refined-grain consumption and longitudinal changes in cardiometabolic risk factors in the Framingham Offspring Cohort. J Nutr. 2021;151(9):2790–2799. doi: 10.1093/jn/nxab177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosanoff A. Changing crop magnesium concentrations: impact on human health. Plant Soil. 2013;368(1):139–153. doi: 10.1007/s11104-012-1471-5. [DOI] [Google Scholar]
- 11.Elin RJ. Assessment of magnesium status for diagnosis and therapy. Magnes Res. 2010;23(4):S194–198. doi: 10.1684/mrh.2010.0213. [DOI] [PubMed] [Google Scholar]
- 12.Costello RB, Elin RJ, Rosanoff A, Wallace TC, Guerrero-Romero F, Hruby A, Lutsey PL, Nielsen FH, Rodriguez-Moran M, Song Y, et al. Perspective: the case for an evidence-based reference interval for serum magnesium: the time has come. Adv Nutr. 2016;7(6):977–993. doi: 10.3945/an.116.012765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Micke O, Vormann J, Kraus A, Kisters K. Serum magnesium: time for a standardized and evidence-based reference range. Magnes Res. 2021;34:84–89. doi: 10.1684/mrh.2021.0486. [DOI] [PubMed] [Google Scholar]
- 14.Costello RB, Rosanoff A. Magnesium. In: Marriott BP, Birt DF, Stalling VA, Yates AA, editors. Present knowledge in nutrition. 11. San Diego: Academic Press; 2020. pp. 349–373. [Google Scholar]
- 15.Malinowska J, Małecka M, Ciepiela O. Variations in magnesium concentration are associated with increased mortality: study in an unselected population of hospitalized patients. Nutrients. 2020;12(6):1836. doi: 10.3390/nu12061836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosanoff A. US adult magnesium requirements need updating: impacts of rising body weights and data-derived variance. Adv Nutr. 2021;12(2):298–304. doi: 10.1093/advances/nmaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trapani V, Rosanoff A, Baniasadi S, Barbagallo M, Castiglioni S, Guerrero-Romero F, Iotti S, Mazur A, Micke O, Pourdowlat G, et al. The relevance of magnesium homeostasis in COVID-19. Eur J Nutr. 2021;61:625–636. doi: 10.1007/s00394-021-02704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang W, Wang E, Chen W, Chen C, Chen S. Continuous observation of serum total magnesium level in patients undergoing hemodialysis. Blood Purif. 2021;50(2):196–204. doi: 10.1159/000509788. [DOI] [PubMed] [Google Scholar]