Abstract
This network meta-analysis (NMA) assessed the efficacy of remdesivir in hospitalized patients with COVID-19 requiring supplemental oxygen. Randomized controlled trials of hospitalized patients with COVID-19, where patients were receiving supplemental oxygen at baseline and at least one arm received treatment with remdesivir, were identified. Outcomes included mortality, recovery, and no longer requiring supplemental oxygen. NMAs were performed for low-flow oxygen (LFO2); high-flow oxygen (HFO2), including NIV (non-invasive ventilation); or oxygen at any flow (AnyO2) at early (day 14/15) and late (day 28/29) time points. Six studies were included (N = 5245 patients) in the NMA. Remdesivir lowered early and late mortality among AnyO2 patients (risk ratio (RR) 0.52, 95% credible interval (CrI) 0.34–0.79; RR 0.81, 95%CrI 0.69–0.95) and LFO2 patients (RR 0.21, 95%CrI 0.09–0.46; RR 0.24, 95%CrI 0.11–0.48); no improvement was observed among HFO2 patients. Improved early and late recovery was observed among LFO2 patients (RR 1.22, 95%CrI 1.09–1.38; RR 1.17, 95%CrI 1.09–1.28). Remdesivir also lowered the requirement for oxygen support among all patient subgroups. Among hospitalized patients with COVID-19 requiring supplemental oxygen at baseline, use of remdesivir compared to best supportive care is likely to improve the risk of mortality, recovery and need for oxygen support in AnyO2 and LFO2 patients.
Subject terms: Outcomes research, Viral infection
Introduction
Infection with SARS-CoV-2 can cause coronavirus disease 2019 (COVID-19) and, in severe cases, patients may present with acute respiratory distress syndrome or septic shock with multiple organ failure1. Compared to seasonal influenza, patients with COVID-19 are more likely to be hospitalized, need intensive care, have a longer duration of hospitalization, and die in hospital2. Further, severe COVID-19 patients are at a higher risk for hospital-acquired infections, namely ventilator-associated pneumonia, and have increased rates of multiorgan dysfunction3–5.
Remdesivir (GS-5734) is a ribonucleic acid (RNA)-dependent RNA polymerase inhibitor that was identified early as a promising therapeutic candidate for COVID-19 due to its broad inhibitory activity against RNA viruses such as the Middle East Respiratory Syndrome6, and acts as a nucleoside analog, inhibiting the RNA-dependent RNA polymerase of SARS-CoV-27. Clinical trials were initiated in 2020 to evaluate the safety and efficacy of remdesivir, among other drugs, as treatments for COVID-19. These included the National Institute of Allergy and Infectious Diseases Adaptive COVID-19 Treatment Trials (ACTT-1 and ACTT-2) which assessed the impact of remdesivir, alone or in combination, on time to recovery8,9; and the World Health Organization (WHO)-led SOLIDARITY trial which compared remdesivir, lopinavir/ritonavir, lopinavir/ritonavir with interferon-B1a and chloroquine or hydroxychloroquine on mortality10. ACTT-1, the pivotal double-blind, randomized, placebo-controlled trial, found that treatment with remdesivir resulted in shorter median recovery time compared to those who received placebo; post-hoc-analyses among low-flow oxygen patients suggested remdesivir resulted in a 70% reduction in mortality8. While results in SOLIDARITY were not stratified by supplemental oxygen needs, there was a trend towards a clinical benefit of remdesivir for patients on oxygen versus patients who were ventilated10. Despite this, following the interim results of SOLIDARITY10, the WHO concluded that remdesivir had little or no effect on hospitalized patients with COVID-19, as determined by overall mortality.
Given the ongoing global emergency of the disease and rapid viral evolution of SARS-CoV-2, effective and safe treatments for patients with COVID-19 are still urgently needed. Multiple meta-analyses have been conducted in order to determine the clinical significance of remdesivir for patients with COVID-1911–21. However, the role of remdesivir by supplemental oxygen needs is not yet fully understood. This review and meta-analysis includes previously unavailable data to evaluate the efficacy of remdesivir in hospitalized COVID-19 patients requiring low- and/or high-flow oxygen on key endpoints of interest.
Materials and methods
Study design
This study followed the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement for study design (Table S1 Supplementary Materials)22.
Outcomes
Key outcomes of interest were mortality; recovery (defined as either recovery from COVID-19 or discharge from hospital, and was assumed to be interchangeable despite varying definitions of recovery across trials); no longer requiring supplemental oxygen; or progressing to non-invasive ventilation (NIV) or invasive mechanical ventilation (IMV). Outcomes were stratified by the population for which remdesivir has been conditionally approved to treat COVID-19 by the European Medicines Agency (EMA): patients with pneumonia requiring supplemental oxygen (low- or high-flow oxygen or other NIV) at the start of treatment. These were defined as oxygen at any flow, high-flow oxygen (which included, in some trials, patients receiving NIV), or low-flow oxygen. Patients in trials who were on NIV at baseline (included in this analysis when grouped in an ordinal group that included patients with high-flow oxygen or NIV) and remained on NIV, were considered to have progressed as they did not recover.
Search strategy and inclusion criteria
A targeted search was conducted over three months (February to April, 2021) to identify relevant materials in MEDLINE (PubMed), medRxiv, EMBASE and Cochrane Trials (Table S2, Supplementary Materials). Inclusion criteria for studies were either published or in pre-print randomized controlled trials (RCTs) that enrolled patients hospitalized with COVID-19 requiring supplemental oxygen at baseline. Patients in at least one arm of the trial must have been treated with remdesivir and the trial had to report on at least one outcome of interest on day 14/15 or day 28/29. In trials that reported on both patients who did and did not receive supplemental oxygen, only those patients who required supplemental oxygen at baseline were included.
Data extraction & risk of bias evaluation
Data extraction was done by one researcher. Outcomes reported at different time points were considered equivalent: day 14 to day 15 and 28 to day 29. One study reported outcomes at day 2423 and it was assumed to be equivalent to the day 28/29 time point. Risk of bias was evaluated using the revised risk of bias assessment for randomized controlled trials tool by one member of the research team24.
Statistical analysis
Given the lack of statistical difference for 5- versus 10-day treatment of remdesivir in previous meta-analyses13,25,26, this analysis aggregated 5- and 10-day treatment. All outcomes were analyzed using standard Bayesian techniques, adapting previously validated methods27,28. A Bayesian network meta-analysis, using a generalized linear model (with binomial likelihood and log link) for each outcome, was implemented using BUGSnet. Non-informative prior distributions were used for all parameters (Table S3, Supplementary Materials)29. The Markov chain Monte Carlo simulations were specified as a burn-in of 50,000 iterations followed by 100,000 iterations with 10,000 adaptations. Trace plots and density plots were used to evaluate convergence graphically. Both fixed and random effect models have been utilized in prior remdesivir meta-analyses11–19. While model fits were similar for fixed and random effects (Table S4, Supplementary Materials), given the small number of studies included in the analysis, a fixed effects model was selected as the base case. Results of the random effects model are included in the Supplementary Materials. Consistency within the network was assessed using the individual data points’ posterior mean deviance contributions for the consistency model versus the inconsistency model, following recommendations30. Results are presented as risk ratios (RR) between treatment and best supportive care (defined as the non-remdeisivr treatment arm) with forest plots. Surface under the curve cumulative ranking probabilities (SUCRA) plots are also presented to show the ranking of treatments. Credible intervals (CrI) of 95% were used for inference. All data analyses were performed using Microsoft Excel (2019) and the R statistical package.
Scenario analyses were performed to evaluate the robustness of the models’ results. The first included data from the SIMPLE-Severe trial25, via a matched historical control study31, where remdesivir was compared to a control arm of a retrospective cohort of patients with severe COVID-19 (via inverse probability weighted multiple logistic regression). The second scenario analysis excluded ACTT-2 from the analysis, thereby only including comparisons of remdesivir versus standard of care. The third scenario analysis explored 5- and 10-day treatment with remdesivir, separately, versus best supportive care.
Results
Search and study selection process
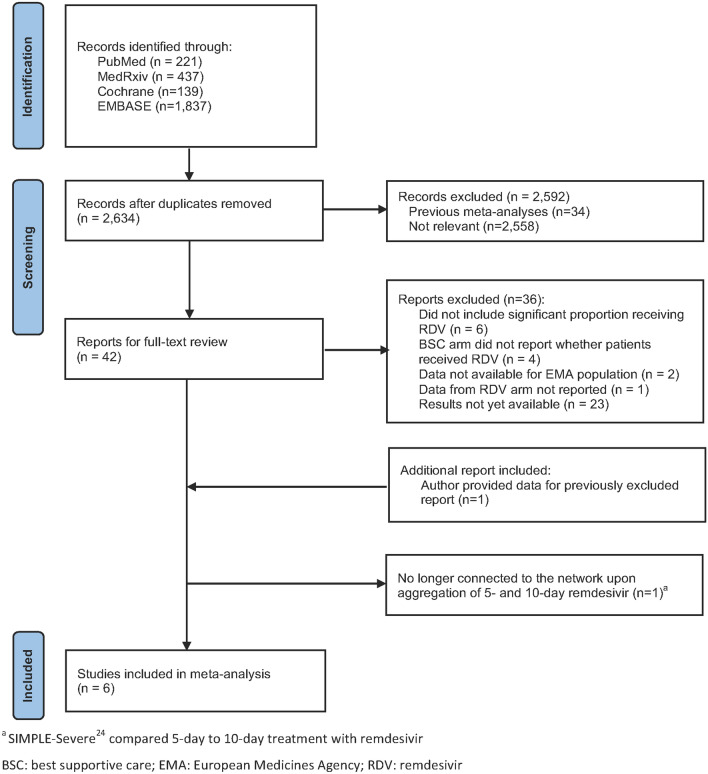
A total of 2634 unique studies were retrieved from the databases and 42 studies were retained for full-text review; a further 36 were excluded (Fig. 1). While SIMPLE-Moderate26 did not report results stratified by the EMA population, the authors were contacted and were able to provide the appropriate data; thus, this study was included. Further, following construction of the networks (Fig. S1, Supplementary Materials), it was determined that when aggregating the 5- and 10-day treatment arms, SIMPLE-Severe25 could no longer be connected to the network and was thus excluded from the base case analysis. Therefore, in the base case, a total of six studies were entered into the meta-analysis8–10,23,26,32 (Fig. 1).
Figure 1.
PRISMA study selection flow diagram.
Risk of bias assessment results
Risk of bias, as assessed by the revised risk of bias assessment tool is presented in Table S5 (Supplementary Materials). While the majority of the included studies had an overall low risk of bias, due to deviations in the intended interventions in three studies10,23,26, and bias in the randomization study in Mahajan et al.23, not all included studies were at low risk of bias.
Characteristics of studies included in the analysis
Characteristics of the included studies are presented in Table 1. Patient characteristics from the included studies are presented in Table 2. Treatment with remdesivir was consistently administered intravenously as 200 mg on day 1 followed by 100 mg for either 4 or 9 days. Across all trials, all patients could receive best supportive care in all treatment arms.
Table 1.
Characteristics of randomized controlled trial studies included.
| Study Country Author |
Design | Phase | Number randomized | Follow-up period | Primary endpoint | Arm 1 | Arm 2 | Arm 3 |
|---|---|---|---|---|---|---|---|---|
|
ACTT-1 Multi-country Beigel8 |
Double-blind, placebo-controlled | 3 | 1062 | 29 days | Time to recovery | RDV by IV as 200 mg on day 1, followed by 100 mg on days 2–10 or until discharge or death plus supportive care | Matching placebo plus supportive care | N/A |
|
ACTT-2 Multi-country Kalil9 |
Double-blind, placebo-controlled | 3 | 1033 | 29 days | Time to recovery | Baricitinib as 4 mg daily for 14 days or until discharge plus RDV by IV as 200 mg on day 1, followed by 100 mg on days 2–10 or until discharge or death plus supportive care | RDV by IV as 200 mg on day 1, followed by 100 mg on days 2–10 or until discharge or death plus placebo plus supportive care | N/A |
|
Hubei China Wang32 |
Double-blind, placebo-controlled | 3 | 236 | 28 days | Time to clinical improvement up to day 28 | RDV by IV as 200 mg on day 1, followed by 100 mg on days 2–10 | Matching placebo | N/A |
|
SOLIDARITY Multi-country WHO Solidarity Consortium10 |
Open-label | 3 | 5475* | 28 days | In-hospital mortality | RDV by IV as 200 mg on day 1, followed by 100 mg on days 2–10 plus supportive care | Standard of care according to local hospital | N/A |
|
SIMPLE-Moderate Multi-country Spinner26 |
Open-label | 3 | 596 | 28 days | Clinical status on day 11 | RDV by IV as 200 mg on day 1, followed by 100 mg on days 2–4 plus supportive care | RDV by IV as 200 mg on day 1, followed by 100 mg on days 2–10 plus supportive care | Best supportive care |
|
Mahajan India Mahajan23 |
Open-label | NR | 82 | 24 days | Improvement in clinical outcome | RDV by IV as 200 mg on day 1, followed by 100 mg on days 2–5 plus supportive care | Standard of care | N/A |
*Remdesivir and control arm only.
IV Intravenous, N/A Not applicable, NIV Non-invasive ventilation, NR Not reported, RDV Remdesivir.
Table 2.
Patient characteristics of included studies include.
| Study | TX arm | Age (years) | Male, n (%) | BMI | Median time from symptom onset to treatment, days | Hospitalized, not requiring O2, % | Hospitalized, requiring O2, % | Hospitalized, requiring NIV or high flow O2, % | Hospitalized, receiving IMV or ECMO, % |
|---|---|---|---|---|---|---|---|---|---|
| ACTT-18 | RDV10 + BSC | Mean: 58.6 | 352 (65.1) | NR | 9.0a | 13.9 | 42.9 | 17.6 | 24.2 |
| BSC | Mean: 59.2 | 332 (63.7) | 9.0a | 12.1 | 39.0 | 18.8 | 29.6 | ||
| ACTT-29 | RDV10 | Mean: 55.8 | 333 (64.3) | Mean: 32.3 | 8.0a | 13.9 | 53.3 | 21.8 | 11.0 |
| BAR + RDV10 | Mean: 55.0 | 319 (61.9) | Mean: 32.2 | 8.0a | 13.6 | 55.9 | 20.0 | 10.5 | |
| Hubei32 | RDV10 + BSC | Median: 66.0 | 89 (56.0) | NR | 11.0 | 0.0 | 82.0 | 18.0 | 0.0 |
| BSC | Median: 64.0 | 51 (65.0) | 11.0 | 4.0 | 83.0 | 12.0 | 1.0 | ||
| SOLIDARITY10 | RDV10 + BSC | NR | 1,706 (62.2) | NR | NR | 24.1 | 66.6 | 9.3 | |
| BSC | 1,725 (63.7) | 24.5 | 66.9 | 8.6 | |||||
| SIMPLE-Moderate26 |
RDV10 + BSC Ordinal score 3b |
Mean: 67.0 Median: 67.0 |
NR |
Mean: 41.0 Mean: 41.0 |
11.0 | 0.0 | 0.0 | 100.0 | 0.0 |
|
RDV5 + BSC Ordinal score 3b |
Mean: 68.0 Median: 68.0 |
Mean: 23.8 Median: 23.8 |
8.0 | 0.0 | 0.0 | 100.0 | 0.0 | ||
|
BSC Ordinal score 3b |
Mean: 43.5 Median: 44.0 |
Mean: 40.2 Median: 40.2 |
13.0 | 0.0 | 0.0 | 100.0 | 0.0 | ||
|
RDV10 + BSC Ordinal score 4c |
Mean: 51.6 Median: 51.0 |
Mean: 30.6 Median: 27.9 |
10.0 | 0.0 | 100.0 | 0.0 | 100.0 | ||
|
RDV5 + BSC Ordinal score 4c |
Mean: 54.9 Median: 57.0 |
Mean: 27.3 Median: 26.2 |
9.0 | 0.0 | 100.0 | 0.0 | 100.0 | ||
|
BSC Ordinal score 4c |
Mean: 60.4 Median: 61.0 |
Mean: 28.2 Median: 27.7 |
10.0 | 0.0 | 100.0 | 0.0 | 100.0 | ||
| Mahajan23 | RD5 | Mean: 58.1 | 21 (61.7) | NR | Mean: 6.3 a | 0.0 | 79.4 | 20.6 | 0.0 |
| BSC | Mean: 57.4 | 27 (75.0) | Mean: 7.4 a | 0.0 | 72.2 | 27.8 | 0.0 | ||
aReported as time from symptom onset to randomization.
bHospitalized, receiving non-invasive ventilation or high-flow oxygen devices.
cHospitalized, requiring low-flow supplemental oxygen.
O2 Oxygen, BAR Baricitinib, BSC Best supportive care, ECMO Extracorporeal membrane oxygenation, IMV Invasive mechanical ventilation, IV Intravenous, NIV Non-invasive ventilation, NR Not reported, RDV5 Remdesivir over 5 days, RDV10 Remdesivir over 10 days.
Outcomes
A summary of the outcomes included in the meta-analysis, stratified by subpopulation, are presented in Table 3. Earlier mortality included assessment at day 149,26,32 or day 158; later mortality included assessment at day 289,10,26,32 or day 298. Mahajan23 assessed outcomes at day 24 and was included with the later assessment. Five studies reported recovery or discharges at both the early (day 14/15) and later (day 28/29) time point8–10,26,32; Mahajan23 assessed discharges at day 24 and was considered with the later assessment. There was insufficient data to analyze either no longer requiring oxygen support or progressing to NIV or IMV at the later time point of assessment; thus, only the early timing of assessment for these outcomes is reported.
Table 3.
Summary of outcomes by oxygen flow requirements.
| Study | Treatment arm | Mortality | Recovery or discharges | No longer requiring O2 | Requiring NIVa | Requiring IMVa | ||
|---|---|---|---|---|---|---|---|---|
| Early | Later | Early | Later | Early | Early | Early | ||
| Any non-invasive oxygen flow | ||||||||
| ACTT-18 | RDV10 + BSC | 20/327 | 28/327 | 206/327 | 263/327 | 235/327 | 17/327 | 29/327 |
| BSC | 38/301 | 45/301 | 157/301 | 217/301 | 174/301 | 18/301 | 41/301 | |
| ACTT-29 | RDV10 | 4/391 | 9/389 | 293/391 | 344/391 | 314/391 | 16/391 | 23/391 |
| BAR + RDV10 | 12/391 | 25/389 | 258/389 | 316/389 | 266/389 | 17/389 | 47/389 | |
| Hubei32 | RDV10 + BSC | 15/153 | 22/158 | 39/153 | 92/150 | 60/153 | 13/153 | 4/153 |
| BSC | 7/78 | 10/78 | 18/78 | 45/77 | 28/78 | 8/78 | 7/78 | |
| SOLIDARITY10 | RDV10 + BSC | NR | 192/1828 | 1234/1828 | 1507/1828 | NR | NR | NR |
| BSC | NR | 219/1811 | 1241/1811 | 1468/1811 | NR | NR | NR | |
| Mahajan23 | RD5 | NR | 5/34 | NR | 2/34 | NR | NR | NR |
| BSC | NR | 3/36 | NR | 3/34 | NR | NR | NR | |
| SIMPLE-Moderate26 | RDV10 + BSC | 0/24 | 0/24 | 22/24 | 23/24 | 23/24 | 0/24 | 0/24 |
| RDV5 + BSC | 0/31 | 0/31 | 24/31 | 28/31 | 27/31 | 2/31 | 0/31 | |
| BSC | 4/38 | 4/38 | 23/38 | 29/38 | 26/38 | 2/38 | 3/38 | |
| Low flow oxygenb | ||||||||
| ACTT-18 | RDV10 + BSC | 7/232 | 9/232 | 166/232 | 206/232 | 183/232 | 5/232 | 13/232 |
| BSC | 21/203 | 25/203 | 124/203 | 156/203 | 137/203 | 7/203 | 21/203 | |
| ACTT-29 | RDV10 | 3/288 | 5/288 | 236/288 | 262/288 | 250/288 | 9/288 | 8/288 |
| BAR + RDV10 | 4/276 | 12/276 | 217/276 | 243/276 | 224/276 | 1/276 | 19/276 | |
| SIMPLE-Moderate26 | RDV10 + BSC | 0/23 | 0/23 | 22/23 | 22/23 | 23/23 | 0/23 | 0/23 |
| RDV5 + BSC | 0/29 | 0/29 | 24/29 | 28/29 | 27/29 | 0/29 | 0/29 | |
| BSC | 4/36 | 4/36 | 22/36 | 27/36 | 25/36 | 1/36 | 3/36 | |
| High flow oxygenc | ||||||||
| ACTT-18 | RDV10 + BSC | 13/95 | 19/95 | 40/95 | 57/95 | 52/95 | 12/95 | 16/95 |
| BSC | 17/98 | 20/98 | 33/98 | 61/98 | 37/98 | 11/98 | 20/98 | |
| ACTT-29 | RDV10 | 1/103 | 5/113 | 57/103 | 82/103 | 64/103 | 7/103 | 15/103 |
| BAR + RDV10 | 7/103 | 13/113 | 41/113 | 73/113 | 44/113 | 16/113 | 28/113 | |
| SIMPLE-Moderate26 | RDV10 + BSC | 0/1 | 0/1 | 0/1 | 1/1 | 0/1 | 0/1 | 0/1 |
| RDV5 + BSC | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 2/2 | 0/2 | |
| BSC | 0/2 | 0/2 | 1/2 | 2/2 | 1/2 | 1/2 | 0/2 | |
aOr worse.
bLow-flow oxygen defined as either hospitalized and requiring any supplemental oxygen or hospitalized requiring low-flow supplemental oxygen, depending on the study.
cHigh-flow oxygen defined as hospitalized and requiring non-invasive ventilation or use of high-flow oxygen devices, depending on the study.
BAR Baricitinib, BSC Best supportive care, IMV Invasive mecahanical ventilation, NIV Non-invasive ventilation, NR Not reported, O2 Oxygen; RDV5 Remdesivir over 5 days, RDV10 Remdesivir over 10 days.
Overall, there was a lack of evidence to suggest inconsistency within the networks (Figs. S2–S6, Supplementary Materials).
Mortality
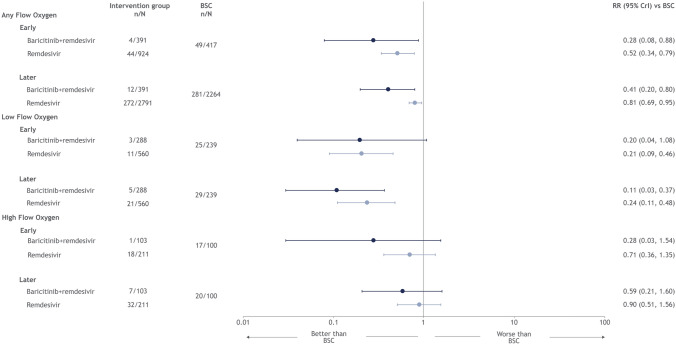
Treatment with remdesivir was superior in lowering the risk of mortality among patients receiving any supplemental oxygen (early assessment RR [95% CrI]: 0.52 [0.34, 0.79]; late assessment RR: 0.81 [0.69, 0.95]) and those receiving only low-flow oxygen at both the early (RR: 0.21 [0.09, 0.46]) and late assessment (RR: 0.24 [0.11, 0.48]) (Fig. 2). Treatment with remdesivir, however, did not lower the risk of mortality among patients receiving high-flow oxygen at either the early or later endpoint assessment (Fig. 2). Results were similar for treatment with remdesivir in combination with baricitinib, with the exception of mortality at the early assessment among low-flow oxygen patients. Treatment with remdesivir (with or without baricitinib) was ranked superior to the standard of care across all patient subgroups at both the early and later assessment for the mortality endpoint (Table S6, Supplementary Materials).
Figure 2.
Forest plot for mortality endpoint, by type of non-invasive oxygen support.
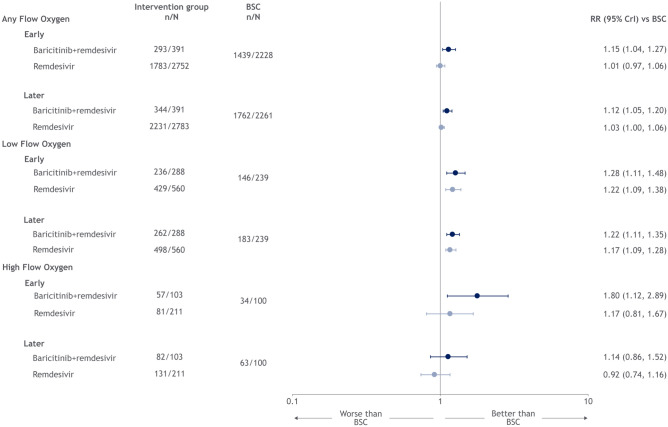
Recovery
Treatment with remdesivir was superior in improving recovery among those on low-flow oxygen at both the early (RR: 1.22 [1.09, 1.38]) and later (RR: 1.17 [1.09, 1.28]) assessment; treatment with remdesivir did not improve recovery in patients receiving any supplemental oxygen or on high-flow oxygen (Fig. 3). Treatment with remdesivir in combination with baricitinib was superior in improving recovery in all patients, with the exception of those on high-flow oxygen at the later assessment. Treatment with remdesivir was ranked superior to standard of care across all patient subgroups at both the early and later assessment for the recovery endpoint (Table S6, Supplementary Materials).
Figure 3.
Forest plot for recovery endpoint, by type of non-invasive oxygen support.
No longer requiring oxygen support
Treatment with remdesivir increased the likelihood of no longer requiring oxygen support among all patient subgroups at day 14 (Fig. 4). Among patient subgroups, the RR (95% CrI) varied from 1.22 (1.11, 1.35) among low-flow oxygen patients to 1.37 (1.01, 1.88) among high-flow oxygen patients. Treatment with remdesivir was ranked superior to the standard of care for no longer requiring oxygen support endpoint across all patient subgroups for the oxygen support endpoint (Table S6, Supplementary Materials). Similar results were observed for remdesivir in combination with baricitinib (Fig. 4).
Figure 4.
Forest plot for free from oxygen support endpoint, by type of non-invasive oxygen support.
Progressing to NIV or IMV
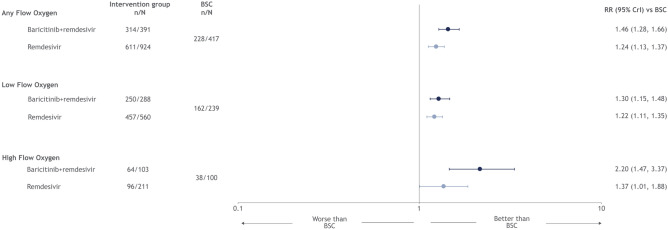
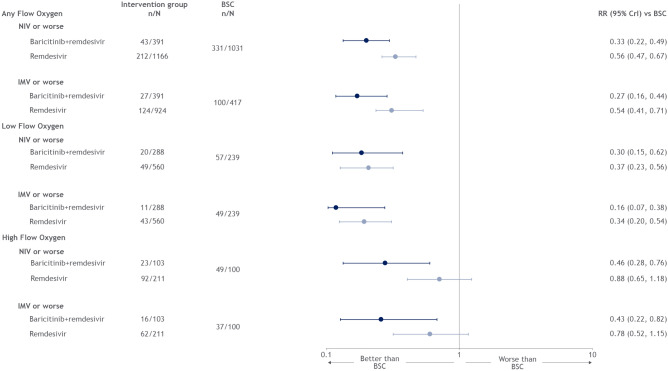
Treatment with remdesivir lowered the risk of progression to NIV or worse among patients on any supplemental oxygen (RR: 0.56 [0.47, 0.67]) and low-flow oxygen (RR: 0.37 [0.23, 0.56]) and lowered the risk of progression to IMV or worse among patients on any supplemental oxygen (RR: 0.54 [0.41, 0.71]) and low-flow oxygen (RR: 0.34 [0.20, 0.54]) (Fig. 5). For both NIV and IMV, treatment with remdesivir was ranked superior to the standard of care across all patient subgroups (Table S6, Supplementary Materials). Treatment with remdesivir in combination with baricitinib lowered the risk of progression to NIV or worse, or IMV or worse, across all patient subgroups at both the early and late time assessment.
Figure 5.
Forest plot for need for non-invasive ventilation or invasive medical ventilation support endpoint, by type of non-invasive oxygen support.
Scenario analyses
When treatment with remdesivir was disaggregated for 5- and 10-days, results were similar to the base case analysis (Fig. S7). However, given the few patients available to the network for 5-day remdesivir, effect estimates are uncertain as reflected by the wide credible intervals.
ACTT-2 compared treatment with remdesivir to remdesivir in combination with baricitinib. When ACTT-2 was excluded from the network, results for remdesivir were similar to the base case analysis. Remdesivir significantly decreased mortality among patients on any flow and on low-flow oxygen (Fig. S8). Results for the endpoints recovery, no longer requiring oxygen support and progressing to more intensive oxygen support (either NIV or IMV or worse, depending on baseline oxygen status) were similar to the base case analysis (Figs. S9–S11). For all endpoints for the low- and high-flow oxygen subgroups, only ACTT-1 and SIMPLE-Moderate informed the analyses.
SIMPLE-Severe25 only reported outcomes for the early time assessment, therefore, this scenario analysis only explored outcomes at day 14/15. When data from SIMPLE-Severe was included in the network via its historical control31, results were similar to the base case analysis (Fig. S12).
Discussion
Clinical studies8, along with recent real-world evidence8,33–36, have demonstrated a mortality benefit for remdesivir in patients hospitalized with COVID-19 requiring supplemental oxygen. Our network meta-analysis demonstrates that among patients receiving low-flow oxygen, treatment with remdesivir consistently improved clinical outcomes including lowering the risk of mortality, improving recovery, increasing the likelihood of no longer requiring oxygen support and lowering the risk of progression to NIV or worse; results were similar when excluding the ACTT-2 trial. In patients treated with remdesivir in combination with baricitinib, the magnitude of effect was higher, indicating potentially synergistic effects, particularly in the high-flow oxygen group. These results support the conditional approval by the EMA and multiple jurisdictions globally that have recommended remdesivir for the treatment of patients with COVID-1919,37,38.
As observed in clinical practice39, and supported by the results of this meta-analysis, the effect of remdesivir on clinical outcomes varies depending on the degree of respiratory support at baseline. This meta-analysis suggests that the degree of respiratory support may be a useful indicator for treatment decisions. However, optimized surrogate markers for disease progression, including the identification of the pathophysiologic stages of COVID-1940,41 and the biological plausibility of the association between viral replication and pathophysiologic processes, are needed to further understand the clinical benefit of phase-specific treatments in COVID-19.
We also found that remdesivir in combination with baricitinib was superior to remdesivir monotherapy across all endpoints: the combination of an antiviral (remdesivir) with an anti-inflammatory (such as baricitinib, corticosteroids, or tocilizumab), as recommended in the National Institute of Health guidelines for the treatment of COVID-19, may be an effective treatment strategy for COVID-19 and should be further assessed42. While treatment with remdesivir monotherapy resulted in significant improvements in mortality, recovery and progression among patients on low flow oxygen, the presence of baricitinib increased the magnitude of benefit observed across all endpoints.
The results of this meta-analysis differ from previous studies due to various reasons. Prior meta-analyses that have assessed the efficacy of remdesivir have included studies evaluating patients with heterogenous severity of COVID-19 disease or when less RCT evidence was available10–13,15–20. In situations where meta-analyses were used to inform guideline recommendations43, imprecision in severity assessment may have compromised the validity of the recommendation44. Other key differences include that prior meta-analyses included a more variable, smaller, study sample, in some cases without regard to receipt of supplemental oxygen at baseline. For example, remdesivir’s impact on mortality reported by the WHO in the SOLIDARITY publication did not reach statistical significance in the overall population10. However, in the subgroup of patients with low- and high-flow supplemental oxygen/non-mechanical ventilation, there was a numerical trend towards benefit of treatment with remdesivir, with 28-day mortality lower among those treated with remdesivir (9.4%) versus standard of care (10.6%)10. Our results, when exploring low- and high-flow supplemental oxygen separately, have shown a more pronounced benefit for remdesivir in the low-flow oxygen population as observed elsewhere21,44. The lack of observed clinical benefit in the high-flow oxygen population may indicate that clinical benefit of remdesivir is most pronounced in patients receiving low-flow oxygen; however, observed differences may also be due to smaller sample size in the high-flow oxygen population and the inclusion of patients on NIV in the high-flow oxygen population in some studies, which may have confounded the results. Prior analyses generally considered treatment with remdesivir separately as 5-day or 10-day courses, versus aggregate treatment as in our analysis. As noted in the methods, prior analyses identified no difference in 5- versus 10-day treatment13,25,26; further, treatment up to 10 days has been recommended in clinical practice45. Differences in heterogeneity of the standard of care arm and in reporting may prevent meaningful comparisons in certain cases; the impact of these differences on results is difficult to ascertain. Further methodological differences may also explain the differences observed in results. Previous analyses have differentially reported outcomes as odds ratios15,17,18,43,46, versus risk ratios in our analysis, which only approximate each other when event rates are low, which is not the case for all endpoints. The Cochrane review did not consider the proportion of patients who recovered, but looked at time to recovery and determined these data were not able to be synthesized; therefore, recovery was not assessed in their meta-analysis20. Other analyses, such as the meta-analysis for mortality published alongside the SOLIDARITY trial, have drawn conclusions regarding statistical significance based on 99% confidence intervals44, as opposed to the more standard 95% intervals employed in our analysis. Further, given their large sample size and contribution to the network, this confounding factor may bias the results of any meta-analysis that includes this data.
To the best of our knowledge, this is the first meta-analysis performed in remdesivir’s EMA-indicated population that incorporates patient-level data from SIMPLE-Moderate. The similar model fits and results across the fixed and random effect models underlines the consistency and robustness of our results. However, the evaluation and synthesis of evidence in a rapidly evolving field is inherently associated with limitations. First, SIMPLE-Severe could not connect to a network in the base case analysis as it compared 5- versus 10-day treatments of remdesivir (with no further control arm)25; however, a scenario analysis where it was included through its historical control did not meaningfully impact the results. Second, the heterogeneity of the included trials may limit the generalizability of the results. For example, SOLIDARITY did not require all patients to have a confirmed infection of COVID-19, and the inclusion of patients was left at the discretion of the enrolling physician; further, the protocol exclusion criteria were ambiguous. For reasons unknown, mortality rates observed in SOLIDARITY’s best supportive care arm were higher than those observed across other studies conducted in a similar time period. Given SOLIDARITY’s large sample size, these limitations may contribute disproportionately to the results of this analysis. Third, this meta-analysis excludes the recent results of the ACTT-347 and the DisCoVeRy trial48, both of which were published after our search. While ACTT-3 showed similar effects to studies included in this meta-analysis of remdesivir alone on mortality rates, DisCoVeRy was a sub-study of SOLIDARITY and the DisCoVeRy trial would have been excluded to avoid potential bias due to double-counting patients. Fourth, across our included trials, the definition of recovery varied and for the purpose of synthesizing our evidence, we assumed discharge to be equivalent to recovery where recovery was not reported as a distinct outcome. Fifth, we assumed that outcomes reported at day 24 were equivalent to those reported at day 28 in the analysis. Sixth, the trials included enrolled patients from across multiple geographic regions with varying definitions of best supportive care that have evolved since the beginning of the pandemic; these differences have likely impacted mortality not only between regions but also over time, as evidence emerges on best supportive care for patients with COVID-19. Seventh, while the data informing this meta-analysis came from RCTs, not all identified studies were at low risk of bias; results should be interpreted within the context of the limitations of the included studies. Finally, the data informing our meta-analysis was identified through a targeted, rather than a systematic, literature review. However, given the constrained nature of the disease area and the ability to extensively validate the included studies using other recently conducted meta-analyses, this is likely not a limitation.
In patients with COVID-19 requiring any or low-flow supplemental oxygen at baseline, based on available RCT evidence, this analysis found that treatment with remdesivir lowered mortality, accelerated recovery and reduced progression to NIV, compared to best supportive care. Future studies exploring both the impact and timing of intervention of antivirals, notably baricitinib, in patients may provide additional data to explain these findings. The results of this study suggest that remdesivir should be considered as part of a multi-faceted care strategy for these patients.
Supplementary Information
Author contributions
All authors contributed to the design of the research. R.B., N.S. and S.J. performed the analysis of the results with all authors contributing to the discussion and interpretation of the results. R.B. took the lead in writing the manuscript, with all authors providing critical feedback on all drafts of the manuscript.
Funding
This work was supported by Gilead Sciences Inc.
Data availability
All data analyzed during this study are included in this published article and its supplementary information files.
Competing interests
AG has received research grants, advisory board fees, and travel grants from Angelini, Menarini, Gilead Sciences, Janssen, MSD, Novarti, Pfizer, ViiV, and GSK. JJM received consulting fees from Maple Health Group and Atriva Therapeutics GmbH, reimbursements for travel expenses from Gilead Sciences, ViiV Healthcare and Correvio Pharma, institutional research funding from the National Institutes of Health. PP has research grants, advisory boards fees from Technophage, MSD, Abionic and Gilead Sciences. RP has received research support (awarded to his institution) and participated in advisory boards from Gilead, ViiV Healthcare, MSD, Lilly and Theratechnologies. RB, SJ, RP, MJS and ATP have no competing interests to declare.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-13680-6.
References
- 1.Helmy YA, Fawzy M, Elaswad A, Sobieh A, Kenney SP, Shehata AA. The COVID-19 pandemic: A comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J. Clin. Med. 2020;9(4):1225. doi: 10.3390/jcm9041225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piroth L, Cottenet J, Mariet A-S, et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: A nationwide, population-based retrospective cohort study. Lancet Respir. Med. 2021;9(3):251–259. doi: 10.1016/S2213-2600(20)30527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021 doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 4.Nseir S, Martin-Loeches I, Povoa P, et al. Relationship between ventilator-associated pneumonia and mortality in COVID-19 patients: A planned ancillary analysis of the coVAPid cohort. Crit. Care. 2021;25(1):177. doi: 10.1186/s13054-021-03588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grasselli G, Scaravilli V, Mangioni D, et al. Hospital-acquired infections in critically Ill patients with COVID-19. Chest. 2021 doi: 10.1186/s13054-021-03672-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malin JJ, Suárez I, Priesner V, Fätkenheuer G, Rybniker J. Remdesivir against COVID-19 and other viral diseases. Clin. Microbiol. Rev. 2020;34(1):e00162–e220. doi: 10.1128/CMR.00162-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kokic G, Hillen HS, Tegunov D, et al. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 2021;12(1):279. doi: 10.1038/s41467-020-20542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—FINAL report. N. Engl. J. Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N. Engl. J. Med. 2020;384(9):795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO Solidarity Trial Consortium Repurposed antiviral drugs for Covid-19—Interim WHO solidarity trial results. N. Engl. J. Med. 2020;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Abdouh A, Bizanti A, Barbarawi M, et al. Remdesivir for the treatment of COVID-19: A systematic review and meta-analysis of randomized controlled trials. Contemp. Clin. Trials. 2021;101:106272–106272. doi: 10.1016/j.cct.2021.106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander, P. E., Piticaru, J., Lewis, K. et al. Remdesivir use in patients with coronavirus COVID-19 disease: A systematic review and meta-analysis of the Chinese Lancet trial with the NIH trial. medRxiv 2020:2020.2005.2023.20110932. (2020)
- 13.Rezagholizadeh A, Khiali S, Sarbakhsh P, Entezari-Maleki T. Remdesivir for treatment of COVID-19; an updated systematic review and meta-analysis. Eur. J. Pharmacol. 2021;897:173926–173926. doi: 10.1016/j.ejphar.2021.173926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elsawah HK, Elsokary MA, Abdallah MS, ElShafie AH. Efficacy and safety of remdesivir in hospitalized Covid-19 patients: Systematic review and meta-analysis including network meta-analysis. Rev. Med. Virol. 2020 doi: 10.1002/rmv.2187. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y, Chen D, Cai D, Yi Y, Jiang S. Effectiveness of remdesivir for the treatment of hospitalized COVID-19 persons: A network meta-analysis. J. Med. Virol. 2021;93(2):1171–1174. doi: 10.1002/jmv.26443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piscoya A, Ng-Sueng LF, Parra Del Riego A, et al. Efficacy and harms of remdesivir for the treatment of COVID-19: A systematic review and meta-analysis. PLoS ONE. 2020;15(12):e0243705. doi: 10.1371/journal.pone.0243705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shrestha DB, Budhathoki P, Syed N-IH, Rawal E, Raut S, Khadka S. Remdesivir: A potential game-changer or just a myth? A systematic review and meta-analysis. Life Sci. 2021;264:118663–118663. doi: 10.1016/j.lfs.2020.118663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yokoyama Y, Briasoulis A, Takagi H, Kuno T. Effect of remdesivir on patients with COVID-19: A network meta-analysis of randomized control trials. Virus Res. 2020;288:198137–198137. doi: 10.1016/j.virusres.2020.198137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris, A., Juni, P., Odutayo, A. Remdesivir for hospitalized patients with COVID-19. Sci. Briefs Ontario COVID 19 Sci. Advis. Table2(27) (2021).
- 20.Ansems K, Grundeis F, Dahms K, et al. Remdesivir for the treatment of COVID-19. Cochrane Database Syst. Rev. 2021;8:Cd014962. doi: 10.1002/14651858.CD014962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, T. C., Murthy, S., Del Corpo, O. et al. Remdesivir for the treatment of COVID-19: An updated systematic review and meta-analysis. medRxiv. 2022:2022.2001.2022.22269545.
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahajan, L., Singh, A. P, Gifty. Clinical outcomes of using remdesivir in patients with moderate to severe COVID-19: A prospective randomised study. Indian J. anaesth.65(Suppl 1), S41–S46 (2021). [DOI] [PMC free article] [PubMed]
- 24.Sterne JAC, Savović J, Page MJ, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 25.Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N. Engl. J. Med. 2020;383(19):1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of Remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: A randomized clinical trial. JAMA. 2020;324(11):1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dias S, Welton NJ, Sutton AJ, Ades AE. Evidence synthesis for decision making 1: Introduction. Med. Decis. Making. 2013;33(5):597–606. doi: 10.1177/0272989X13487604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med. Decis. Making. 2013;33(5):607–617. doi: 10.1177/0272989X12458724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Béliveau A, Boyne DJ, Slater J, Brenner D, Arora P. BUGSnet: An R package to facilitate the conduct and reporting of Bayesian network Meta-analyses. BMC Med. Res. Methodol. 2019;19(1):196. doi: 10.1186/s12874-019-0829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dias, S., Welton, N. J., Sutton, A. J., Caldwell, D. M., Lu, G. & Ades, A. E. NICE decision support unit technical support documents. In NICE DSU Technical Support Document 4: Inconsistency in Networks of Evidence Based on Randomised Controlled Trials (2014). [PubMed]
- 31.Olender SA, Perez KK, Go AS, et al. Remdesivir for severe COVID-19 versus a cohort receiving standard of care. Clin. Infect. Dis. 2020;73(11):e4166–e4174. doi: 10.1093/cid/ciaa1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mozaffari, E., Chandak, A., Zhang, Z. et al. Remdesivir treatment is associated with improved survival in hospitalized patients with COVID-19. Paper presented at: World Microbe Forum2021; Virtual.
- 34.Go, A., Malenica, I., Fusco, D. et al. Remdesivir versus standard of care for severe COVID-19. Paper presented at: World Microbe Forum2021; Virtual.
- 35.Falcone, M., Suardi, L. R., Tiseo, G. et al. Early use of remdesivir and risk of disease progression in hospitalized patients with mild to moderate COVID-19. Clin. Ther. (2022). [DOI] [PMC free article] [PubMed]
- 36.Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J. Antimicrob. Chemother. 2021;76(12):3296–3302. doi: 10.1093/jac/dkab321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Institute for Health and Care Excellence. COVID-19 rapid guideline: Managing COVID-19. NICE guideline [NG191] (2021). [PubMed]
- 38.Remdesivir for COVID-19. Aust Prescr.43(5), 176–177. 10.18773/austprescr.2020.060 (2020). [DOI] [PMC free article] [PubMed]
- 39.Garibaldi BT, Wang K, Robinson ML, et al. Comparison of time to clinical improvement with vs without Remdesivir treatment in hospitalized patients with COVID-19. JAMA Netw. Open. 2021;4(3):e213071–e213071. doi: 10.1001/jamanetworkopen.2021.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: A systematic review and meta-analysis. Lancet Microbe. 2021;2(1):e13–e22. doi: 10.1016/S2666-5247(20)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim MC, Cui C, Shin KR, et al. Duration of culturable SARS-CoV-2 in hospitalized patients with Covid-19. N. Engl. J. Med. 2021;384(7):671–673. doi: 10.1056/NEJMc2027040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Institutes of Health. COVID-19 Treatment Guideliens Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines, accessed 25 June 2021. https://www.covid19treatmentguidelines.nih.gov/ (2021). [PubMed]
- 43.Rochwerg B, Siemieniuk RAC, Agoritsas T, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- 44.Guérin, P. J., McLean, A. R. D., Rashan, S. et al. Definitions matter: Heterogeneity of COVID-19 disease severity criteria and incomplete reporting compromise meta-analysis. medRxiv. 2021:2021.2006.2004.21257852 (2021). [DOI] [PMC free article] [PubMed]
- 45.European Medicines Agency Human Medicines Committee. Annex I: Conditions for use of remdesivir (2020).
- 46.Siemieniuk RAC, Bartoszko JJ, Ge L, et al. Drug treatments for covid-19: Living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalil AC, Mehta AK, Patterson TF, et al. Efficacy of interferon beta-1a plus remdesivir compared with remdesivir alone in hospitalised adults with COVID-19: A double-bind, randomised, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2021;9:1365–1376. doi: 10.1016/S2213-2600(21)00384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ader F, Bouscambert-Duchamp M, Hites M, et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): A phase 3, randomised, controlled, open-label trial. Lancet Infect. Dis. 2022;22(2):209–221. doi: 10.1016/S1473-3099(21)00485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analyzed during this study are included in this published article and its supplementary information files.