Abstract
Streptococcus thermophilus autolytic strains are characterized by a typical bell-shaped growth curve when grown under appropriate conditions. The cellular mechanisms involved in the triggering of lysis and the bacteriolytic activities of these strains were investigated in this study. Lactose depletion and organic solvents (ethanol, methanol, and chloroform) were shown to trigger a premature and immediate lysis of M17 exponentially growing cells. These factors and compounds are suspected to act by altering the cell envelope properties, causing either the permeabilization (organic solvents) or the depolarization (lactose depletion) of the cytoplasmic membrane. The autolytic character was shown to be associated with lysogeny. Phage particles, most of which were defective, were observed in the culture supernatants after both mitomycin C-induced and spontaneous lysis. By renaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis, a bacteriolytic activity was detected at 31 kDa exclusively in the autolytic strains. This enzyme was detected during both growth and spontaneous lysis with the same intensity. We have shown that it was prophage encoded and homologous to the endolysin Lyt51 of the streptococcal temperate bacteriophage φ01205 (M. Sheehan, E. Stanley, G. F. Fitzgerald, and D. van Sinderen, Appl. Environ. Microbiol. 65:569–577, 1999). It appears from our results that the autolytic properties are conferred to the S. thermophilus strains by a leaky prophage but do not result from massive prophage induction. More specifically, we propose that phagic genes are constitutively expressed in almost all the cells at a low and nonlethal level and that lysis is controlled and achieved by the prophage-encoded lysis proteins.
Autolysis of lactic acid bacteria used as starters appears to be a crucial step in the flavor development of fermented dairy products (12, 15). It indeed causes the opening of cells and the subsequent release into the curd of intracellular enzymes that are involved in the flavor compound formation. The activity of these enzymes can therefore be enhanced by better accessibility to their substrates, proteins, lipids, or carbohydrates from the milk. It has been shown that the main consequence of cell autolysis in cheese is peptidolysis acceleration, leading to an increased rate of free amino acid production and to a decrease in bitter taste (2, 3, 13, 16, 25, 32, 34, 35, 55).
Bacterial autolysis results from the degradation of the peptidoglycan, which is the major structural component of the bacterial cell wall, by enzymes called peptidoglycan hydrolases. Bacteria synthesize their own peptidoglycan hydrolases, named autolysins (45). These cell wall-associated enzymes are potentially lethal for the cell and thus require stringent regulation. It has been proposed that autolysins are involved in different cellular processes including cell wall expansion and turnover, cell division, and transformation (47). Autolysis would result from an uncontrolled action of the bacterial autolysins after inhibition of peptidoglycan synthesis (47). For lysogenic strains, lysis can be caused by induction of the resident prophage. The prophage lysis system is then responsible for the host lysis. It contains in most cases two effectors, a peptidoglycan hydrolase, named endolysin, and a second protein, a so-called holin. Holins are small proteins causing nonspecific lesions in the membrane. They thus allow the endolysin, usually devoid of a signal peptide, access to the peptidoglycan to cause subsequent host envelope disruption (56).
Hitherto, most of the studies dealing with lactic acid bacteria autolysis concern the genus Lactococcus and to a lesser extent Lactobacillus. Autolysis was investigated by physiological studies and also by analysis of the peptidoglycan hydrolase content. The autolytic character of Lactococcus and Lactobacillus strains was studied in liquid medium (2, 3, 28, 33, 41, 54) and for some of them during cheese ripening (5, 13, 16, 32, 35, 53, 55). It appears to vary from strain to strain. Various factors could account for the different autolytic behaviors: the cellular content in peptidoglycan hydrolases, the regulation of either the expression or the activity of these enzymes, or the cell wall composition. Another alternative is the involvement of a prophage in the cell lysis, as demonstrated for the lactococcal strain AM2 (32). The bacteriolytic enzymes of Lactococcus lactis (31, 38, 41) and Lactobacillus spp. (10, 52) have been studied by renaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), which allows detection of peptidoglycan hydrolase activities after renaturation in a substrate-containing gel. In L. lactis, an endogenous autolysin (9) and a prophage-encoded enzyme (31) were identified by this technique.
Streptococcus thermophilus received little attention regarding autolysis despite its industrial significance as starter used in the manufacture of yogurts and Italian and Swiss type cheeses. For S. thermophilus, spontaneously autolytic strains have been previously identified (43, 50, 58). Lysis occurs at the end of the exponential growth phase, resulting in a typical bell-shaped growth curve. Independent studies of S. thermophilus temperate bacteriophages led furthermore to the observation that lysogens exhibit an identical autolytic phenotype (19). The aim of our study was to investigate the cellular mechanisms involved in the triggering of lysis of S. thermophilus and to specify the link between the autolytic phenotype and lysogeny in this species. For this purpose, 6 different S. thermophilus strains identified as autolytic out of 146 S. thermophilus strains screened were further characterized in this work. All of them were found to be lysogenic. Different environmental factors, such as lactose depletion and organic solvents, were identified as triggers of premature lysis. A bacteriolytic enzyme of 31 kDa was detected by renaturing SDS-PAGE exclusively in the autolytic strains. It was shown to be prophage encoded and homologous to the endolysin Lyt51 of the streptococcal temperate bacteriophage φ01205 (44). From all these results, we propose a mechanism of lysis triggering according to which S. thermophilus lysis is triggered and achieved under unfavorable environmental conditions via the lysis proteins of a leaky prophage.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The autolytic S. thermophilus strains were obtained from two different collections: strains DN-001065 and DN-001561 were from the Centre International de Recherche Daniel Carasso collection (Danone, Le Plessis-Robinson, France), and strains CNRZ 701, CNRZ 1358 (type strain), CNRZ 1205, and CNRZ 1209 were from the CNRZ collection (Institut National de la Recherche Agronomique [INRA], Jouy-en-Josas, France). Nonautolytic S. thermophilus strains CNRZ 302 and CNRZ 1446 were obtained from the INRA collection. Strain CNRZ 1446 is an isogenic representative of the type strain, which was nonautolytic, as opposed to strain CNRZ 1358. The nonautolytic strains were grown at 42°C in M17 broth (49) (Difco Laboratories, Detroit, Mich.) supplemented with 0.75% (wt/vol) lactose. The autolytic strains were grown in M17 medium containing a high lactose concentration (1.5% [wt/vol]) to prevent lysis or a limited lactose concentration (1% [wt/vol] for strain DN-001065 and 0.5% [wt/vol] for the other autolytic strains) to ensure lysis. Growth and lysis were monitored by measuring the optical density at 580 nm (OD580) in 1-cm cuvettes with a spectrophotometer (model Uvikon 931; Kontron Instruments Inc., Everett, Mass.). Escherichia coli XL1-Blue recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZ ΔM15 Tn10 (Tetr)] (Stratagene) was cultured in Luria-Bertani broth (42) containing tetracycline (12.5 μg ml−1) at 37°C with shaking. The pQE30 plasmid vector (Qiagen) conferring ampicillin resistance was used for high-level expression of six-His-tagged proteins. When needed, ampicillin was used at a concentration of 100 μg ml−1.
Lysis experiments.
The effect of lactose depletion on the triggering of lysis was studied as follows. Bacteria grown in M17 medium containing a limited lactose concentration were harvested after different culture times by centrifugation at 7,500 × g for 15 min at 15°C and washed once in distilled water at room temperature. The cells were then resuspended in the same volume of either fresh M17 broth or 50 mM Tris-HCl (pH 7.0) buffer, either supplemented with 0.5% (wt/vol) lactose or devoid of lactose. When specified, the purified recombinant endolysin (six-His-tagged) Lyt51 (see below) was added at various concentrations. The cell suspensions were then incubated at 42°C. Cell lysis was monitored by measuring the OD580 of the bacterial suspensions. The extent of lysis was expressed as the percent decrease of OD580 after a given time, and the rate of lysis was expressed as the decrease in OD580 per minute during the first 60 min.
Lactose concentration was determined in cell-free culture supernatants by enzymatic analysis with the lactose/d-galactose determination kit (Boehringer Mannheim) according to the supplier's instructions.
Compounds tested for their ability to trigger a premature lysis were added to exponential-phase cultures. The resulting volume changes were always less than 2%, and temperature was held constant.
Mitomycin C induction.
S. thermophilus strains were grown in M17 broth at 42°C to an OD580 of 0.3. Mitomycin C (Sigma Chemical Co., St. Louis, Mo.) was added at a final concentration of 0.2 μg ml−1 as described previously (20). The culture was further incubated at 42°C, and the OD580 was monitored regularly. A culture grown in M17 broth at 42°C was used as the control.
Bacteriophage preparation and electron microscopy observation.
The autolytic strains were grown in 250 ml of M17 medium. Fifty milliliters of the culture was induced with mitomycin C as described above, and the remainder of the culture was grown until lysis occurred spontaneously. In both cases, after lysis was completed, the lysate was incubated for 1 h at 37°C with DNase I (5 μg ml−1), RNase (12.5 μg ml−1), and MgCl2 (1 mM). Lysozyme (1 mg ml−1) and mutanolysin (Sigma) (500 U ml−1) were also added at this step to ensure an extensive release of phage particles. After addition of NaCl at the final concentration of 0.5 M, the lysate was further incubated on ice for 20 min. Cell debris was subsequently eliminated by a low-speed centrifugation (5,000 × g, 15 min). Phage particles were collected from the supernatant by centrifugation at 100,000 × g for 2 h at 15°C in an ultracentrifuge (model Centrikon T-1080; Kontron Instruments Inc.). The phage pellet was resuspended in 240 μl of TM buffer (10 mM Tris-HCl, 10 mM MgSO4, pH 8.0), and the suspension was filtered through a 0.45-μm-pore-size filter (Millex-HA; Millipore S.A., Bedford, Mass.).
Phage particles were then negatively stained with 2% (wt/vol) uranyl acetate, as previously described (1), and observed with a Zeiss model EM-10 electron microscope at an accelerating voltage of 80 kV.
DNA techniques.
Restriction enzymes and T4 DNA ligase were respectively obtained from Eurogentec and Boehringer Mannheim and were used as recommended by the suppliers. Molecular cloning, purification, and analysis of DNA were performed by standard procedures (42).
PCR was carried out in a model 2400 Gene Amp PCR system (Perkin-Elmer, Norwalk, Conn.) with Taq DNA polymerase according to the instructions of the manufacturer (Appligene-Oncor, Inc., Gaithersburg, Md.). Total DNA was isolated from S. thermophilus strains as reported by Chapot-Chartier et al. (14).
Total DNA was digested with the appropriate restriction enzymes, electrophoresed in a 0.7% agarose gel, and blotted onto a Hybond-N+ nylon membrane (Amersham International, Amersham, United Kingdom) by the Southern method as described by Sambrook et al. (42). A DNA probe corresponding to an 825-bp fragment of the S. thermophilus temperate phage φ01205 endolysin gene, lyt51 (48) was amplified by PCR from the total DNA of S. thermophilus CNRZ 1205. Primers LYS-1 (5′-ATGAGCGTAAAACAAAAACTA; position 1 to 21) and LYS-2 (5′-GTCGTCCTTATTCCAGCAAGA; position 825 to 805) used for this purpose were deduced from the previously published lyt51 sequence (48). The probe was labeled with [α-32P]dCTP by using the Nick Translation DNA labeling kit (Amersham). Hybridization experiments were done under high-stringency conditions (50% formamide, 42°C) according to a standard protocol (42).
SDS-PAGE and renaturing SDS-PAGE.
SDS-PAGE was carried out as described by Laemmli (27) with a Mini Protean II cell unit (Bio-Rad Laboratories, Inc., Hercules, Calif.) and a gel size of 75 by 55 mm. Samples to be analyzed were mixed with sample loading buffer containing (final concentrations) 62.5 mM Tris-HCl (pH 6.8), 2.3% (wt/vol) SDS, 50 mM dithiothreitol, 10% (vol/vol) glycerol, and 0.01% (wt/vol) bromophenol blue. They were then heated for 3 min at 100°C.
Renaturing SDS-PAGE was performed as previously described (29, 40). Micrococcus lysodeikticus ATCC 4698 (Sigma) autoclaved cells (0.2% [wt/vol]) or S. thermophilus CNRZ 302 autoclaved cells (0.4% [wt/vol]) were included in 12.5% polyacrylamide gels as a substrate for the bacteriolytic enzymes. After electrophoresis, the gel was washed in distilled water for 30 min at room temperature with gentle shaking. It was thereafter transferred into renaturation buffer containing 50 mM MES (2-morpholinoethanesulfonic acid) (Sigma), NaOH (pH 6.0), and 0.1% (wt/vol) Triton X-100. It was incubated for 16 h at 37°C under gentle shaking. It was rinsed with distilled water, stained with 0.1% (wt/vol) methylene blue in 0.01% (wt/vol) KOH for 2 h at room temperature with gentle shaking, and destained with distilled water. The bacteriolytic activities appear as clear bands on a blue background.
Molecular mass was determined by comparison with prestained molecular mass standards separated by electrophoresis on the same gel. The prestained standards were purchased from Bio-Rad Laboratories and contained phosphorylase B (107,000 Da), bovine serum albumin (74,000 Da), ovalbumin (49,300 Da), carbonic anhydrase (36,400 Da), soybean trypsin inhibitor (28,500 Da), and lysozyme (20,900 Da).
Preparation of cell extracts for renaturing SDS-PAGE.
SDS cell extracts were prepared from exponential-phase cells recovered by centrifugation at 7,500 × g for 15 min at 4°C. The cell pellet was resuspended in sample loading buffer, heated for 3 min at 100°C, and centrifuged at 10,000 × g for 15 min. The supernatant (SDS cell extract) was analyzed by renaturing SDS-PAGE.
In order to monitor the expression levels of bacteriolytic enzymes during growth and lysis, cells were harvested by centrifugation at 7,500 × g for 15 min at 4°C from cultures at different times. The culture supernatant was filtered through 0.45-μm-pore-size filters (Milex-HA) and was tested for the presence of bacteriolytic activities. The cells were resuspended in 50 mM Tris-HCl buffer (pH 7.0) containing 1 mM EDTA and 1 mM AEBSF [4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride] (Interchim) and were disrupted with glass beads 0.1 mm in diameter in a Mini Beadbeater T8M cell disrupter (Biospec Products, Bartlesville, Ill.) by three 1-min cycles with 1 min cooling on ice after each cycle. Glass beads, unbroken cells, and debris were removed by centrifugation at 8,000 × g for 20 min. The protein concentration in the supernatants (glass bead cell extracts) was determined by the Bradford method with the Coomassie protein assay reagent as specified by Pierce Chemical Company (Rockford, Ill.) with bovine serum albumin as the standard. The same protein amount in each cell extract was analyzed by renaturing SDS-PAGE for the presence of bacteriolytic activities.
Expression of the Lyt51 endolysin in E. coli, purification of the recombinant protein, and preparation of antiserum.
The Lyt51 endolysin (44) was expressed in E. coli XL1-Blue with an N-terminal six-His tag by using expression vector pQE30 (Qiagen). The lyt51 endolysin gene was amplified by PCR from S. thermophilus CNRZ 1205 total DNA with the primers LYS-3 (5′-GGCATGCAGCGTAAAACAAAA [position 4 to 17]) and LYS-4 (5′-GGAAGCTTCGTGGTCTATTTG [position 852 to 840]) containing, respectively, SphI and HindIII sites at their 5′ ends and cloned in frame downstream of the six-His box coding sequence in the pQE30 expression vector. E. coli XL1-Blue competent cells (Stratagene) were transformed with the resulting pTIL72 plasmid. The nucleotide sequence of the pTIL72 construction was verified.
The expression of the six-His-tagged Lyt51 was induced by IPTG (isopropyl-β-d-thiogalactopyranoside; 1 mM), according to the Qiagen procedure, in E. coli XL1-Blue harboring the pTIL72 plasmid. After 4 h of induction, the cells were collected by centrifugation at 4,000 × g for 15 min and resuspended in 1/25 volume of column binding buffer (50 mM NaH2PO4 [pH 8.0], 300 mM NaCl, 10 mM imidazole). The cell suspension was then frozen at −20°C. After 2 h it was thawed at room temperature and replaced in the freezer. This procedure was repeated four times, and the cells were finally broken by three ultrasonication pulses of 20 s with 1-min cooling intervals in an ice bath. Insoluble cell debris was removed by centrifugation at 10,000 × g for 15 min, and the His-tagged Lyt51 was purified from the supernatant (cleared lysate) by metal affinity chromatography on a nickel-nitrilotriacetic acid (Ni-NTA) spin column (Qiagen) according to the manufacturer's instructions. Following elution with imidazole, the purified Lyt51 protein was dialyzed for 16 h against phosphate-buffered Saline (PBS; 10 mM sodium phosphate [pH 7.4], 100 mM NaCl) at 4°C. The protein concentration was determined with the Coomassie protein assay reagent (Pierce).
Purified Lyt51 was injected into a rabbit to raise antibodies (Biological Services Unit, University College of Cork, Cork, Ireland). Four injections of 50 μg of Lyt51 were performed at 1-week intervals.
The antibodies directed against the His-tagged Lyt51 were purified by affinity chromatography from the rabbit serum obtained 7 days after the third booster injection, according to the procedure of Gu et al. (22). Briefly, the His-tagged Lyt51 from an E. coli-cleared lysate was bound to a Ni-NTA spin column and then 600 μl of the crude rabbit antiserum was applied to the column and left to stand for 20 min. The serum was then allowed to filter through, and the column was washed five times with 600 μl of 150 mM NaCl–50 mM Tris-HCl, pH 7.4, followed by five washes with 600 μl of 2 M NaCl–50 mM Tris-HCl, pH 7.4. The Lyt51-specific antibodies were thereafter eluted with 600 μl of 4 M MgCl2 solution (pH 4.5) and dialyzed against water for 1 h and then against PBS for 16 h at 4°C.
Immunoblotting analysis.
Immunoblotting was carried out as described by Towbin and Gordon (51) using the purified anti-Lyt51 antibodies. The same protein amount from each SDS cell extract and the purified His-tagged Lyt51 (2 ng) were electrophoresed on a 12.5% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane (Schleicher & Schuell). After incubation with the primary anti-Lyt51 antibodies (1/500), the membrane was incubated with protein G-horseradish peroxidase conjugate (Bio-Rad) (1/3,000). Antigen-antibody complexes were subsequently detected using chemiluminescence with the ECL Plus Western blotting detection kit (Amersham) according to the manufacturer's instructions.
RESULTS
Lactose depletion triggers the lysis of the autolytic S. thermophilus strains.
S. thermophilus DN-001065 (Fig. 1A) as well as the other five S. thermophilus autolytic strains (data not shown) exhibited a typical bell-shaped growth curve when grown at 42°C in M17 medium supplemented with lactose in limited concentrations (1% for strain DN-001065 and 0.5% for the other strains). After reaching maximal growth, cells lysed rapidly, resulting in a sharp decrease of OD580. The onset of lysis was coincident with lactose exhaustion from the medium (data not shown).
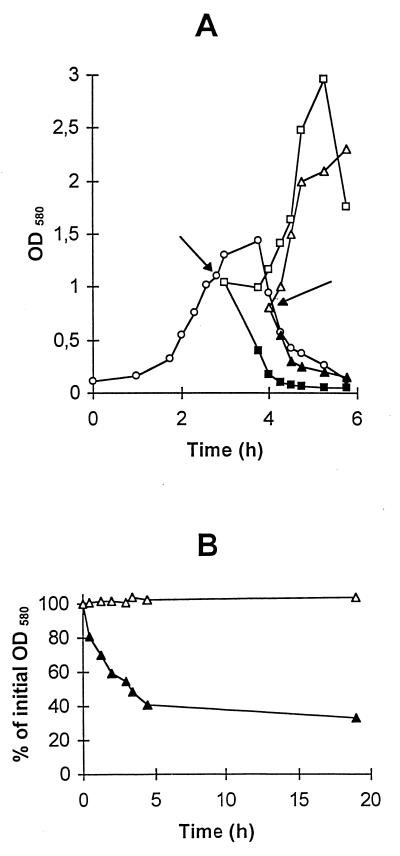
FIG. 1.
Effect of lactose depletion on S. thermophilus DN-001065 lysis. (A) S. thermophilus DN-001065 was grown in M17 medium with 1% lactose at 42°C (○). Cells were harvested during the exponential-growth phase (□, ■) or during the lysing phase (▵, ▴). They were resuspended in the same volume of fresh M17 medium with 0.5% lactose (□, ▵) or devoid of lactose (■, ▴) and further incubated at 42°C. Arrows indicate the times at which cells were harvested. (B) Cells were harvested at the beginning of the lysing phase, washed in distilled water at room temperature, and resuspended in the same volume of 50 mM Tris-HCl buffer, pH 7.0, with 0.5% lactose (▵) or devoid of lactose (▴). The cell suspensions were further incubated at 42°C.
These results suggested that lactose depletion was the triggering factor for cell lysis. To confirm this hypothesis, the lytic behavior of exponential-phase cells transferred in fresh M17 medium devoid of lactose was examined. Upon their transfer, the cells lysed immediately with a lysis rate (1.4 × 10−2 OD580 unit min−1 for strain DN-001065) similar to that of spontaneous lysis occurring at the end of the exponential-growth phase (Fig. 1A). When transferred in M17 medium containing lactose, the exponential-phase cells did not lyse and resumed growth after a short lag phase (Fig. 1A). The lysis phenomenon observed in the absence of lactose was specific to the autolytic strains. Indeed, when nonautolytic strain CNRZ 1446 cells were used in the same experiment, they stopped growing but did not lyse (data not shown).
In addition, we observed that lactose can arrest the lysis of the autolytic strains. When a lysing culture was transferred into fresh M17 medium containing lactose, lysis stopped immediately and a renewed growth phase was observed. In absence of lactose, lysis continued (Fig. 1A).
The protective role of lactose against lysis was also investigated with buffer solutions. For this purpose, S. thermophilus autolytic strains at the beginning of the lysing phase in M17 medium were transferred in 50 mM Tris-HCl buffer, pH 7.0, supplemented with 0.5% lactose or not supplemented, and incubated at 42°C. In the absence of lactose, the cell suspension turbidity decreased linearly during 2 h (Fig. 1B). For strain DN-001065 the extent of lysis reached 70% after 19 h of incubation. By contrast, when lactose was present in the buffer, the OD580 remained stable over the same period of time. These results thus show that lactose also protects resting cells against lysis.
Thus, the depletion of lactose, which is the unique carbon source in the growth medium, appears to be the trigger of cell lysis. The fact that even exponential-phase cells lyse under lactose depletion suggests that the triggering of lysis does not require the accumulation in the cells of lysis effectors at the end of the exponential-growth phase but rather that these lysis effectors are present in the cells at any stage of growth.
Identification of compounds that trigger the premature lysis of autolytic S. thermophilus strains.
In order to specify the cellular mechanisms involved in the triggering of lysis, we have searched for compounds causing the premature lysis of exponentially growing cells of the autolytic strains. In a previous study, we have shown that NaCl triggers the lysis of S. thermophilus autolytic strains (23). Here, we have tested the effect of organic solvents (2% [vol/vol] ethanol, 0.6% [vol/vol] methanol, and 2% [vol/vol] chloroform). As shown on Fig. 2 for chloroform, their addition to an exponential-phase culture was followed in less than 15 min by a dramatic lysis of the culture, which resulted in a sudden drop of OD580. Lysis occurred earlier than in the control culture upon lactose exhaustion. The characteristics of the lysis were similar to those of one triggered by NaCl (23). When challenged with the same compounds, the nonautolytic strain CNRZ 1446 used as a control stopped growing but did not lyse (data not shown). Organic solvents as well as NaCl were thus able to overcome the protective role of lactose and triggered a specific, rapid, and premature lysis of the autolytic strains grown in M17.
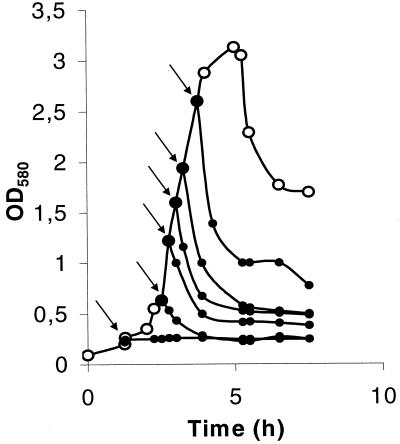
FIG. 2.
Effect of chloroform on the lysis of S. thermophilus DN-001065 exponentially growing cells. The strain was grown in M17 medium (○). At the times indicated by arrows, chloroform (2%) (●) was added to the medium.
The triggering of lysis occurred very quickly after the addition of organic solvents or NaCl (23). Furthermore, it was not inhibited by chloramphenicol (data not shown), and lysis could be triggered from the very beginning of exponential growth (Fig. 2). These results suggest that de novo protein synthesis is not required for lysis and support the hypothesis that the lysis effectors are present in the autolytic cells irrespective of the culture age.
Correlation between the autolytic phenotype and lysogeny in S. thermophilus.
In the literature, S. thermophilus strains previously identified as lysogenic by mitomycin C treatment were shown to be autolytic (19, 36). This prompted us to evaluate whether the autolytic strains of this study were lysogenic.
The six S. thermophilus autolytic strains were treated with mitomycin C as described in Materials and Methods. As shown for strain DN-001065 on Fig. 3, following mitomycin C addition, cells went on growing as well as the control during 40 min and then a dramatic lysis occurred, resulting in a sudden drop of OD580 to a value close to the initial value. The onset of the mitomycin C-induced lysis happened earlier than the spontaneous lysis occurring in the nontreated control. We thereby succeeded in inducing the premature lysis of the six autolytic strains.
FIG. 3.
Effect of mitomycin C on the growth of S. thermophilus DN-001065 in M17 medium. Symbols: ○, untreated culture; ■, mitomycin C induction. The arrow indicates the time of mitomycin C addition (0.2 μg/ml).
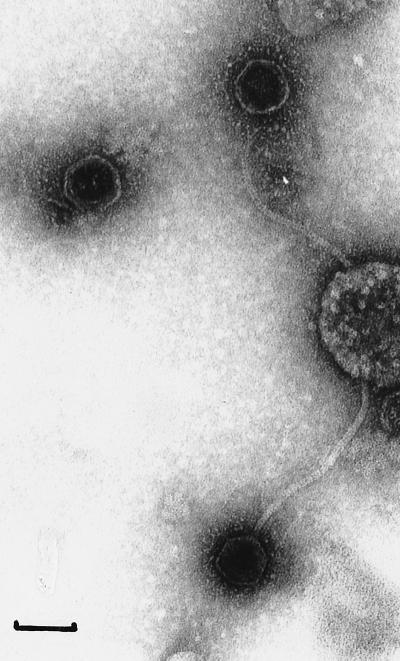
Electron microscopy observations revealed the presence of bacteriophage particles in the DN-001065 lysates after both mitomycin C-induced and spontaneous lysis. The number of phage particles observed was, however, lower after spontaneous lysis than after mitomycin C induced lysis. Under both conditions, intact particles were rare and were accompanied by ample numbers of tailless heads and headless tails. The observed intact phages presented the same morphology. They possessed isometric heads with hexagonal outlines (45 nm in diameter) and flexible noncontractile tails (200 nm in length) (Fig. 4). Tail fibers were not observed in our preparations. It therefore appears that these phages belong, like the other S. thermophilus bacteriophages analyzed so far, to the Siphoviridae family defined by Francki et al. (21) or to group B as defined by Bradley (6).
FIG. 4.
Electron micrograph of bacteriophage particles observed in an S. thermophilus DN-001065 lysate after mitomycin C induction. Bar, 50 nm.
These results could be extended to the five other selected autolytic strains. The six autolytic S. thermophilus strains are thus lysogenic.
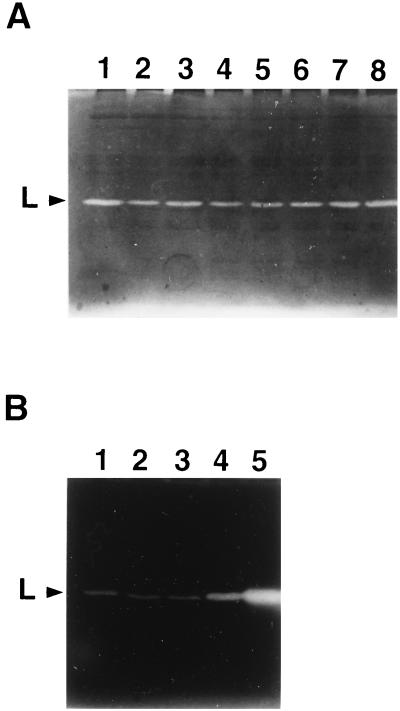
The autolytic S. thermophilus strains contain a 31-kDa bacteriolytic activity absent from the nonautolytic strains.
We tested whether autolytic and nonautolytic S. thermophilus strains could be distinguished on the basis of their contents of bacteriolytic activities. These were analyzed by renaturing SDS-PAGE with two different substrates included in the gels: autoclaved M. lysodeikticus or S. thermophilus cells. The presence of bacteriolytic enzymes in SDS cell extracts from each strain grown in M17 medium was tested. Experiments presented in this section were performed with the DN-001065 autolytic strain and the CNRZ 302 nonautolytic strain.
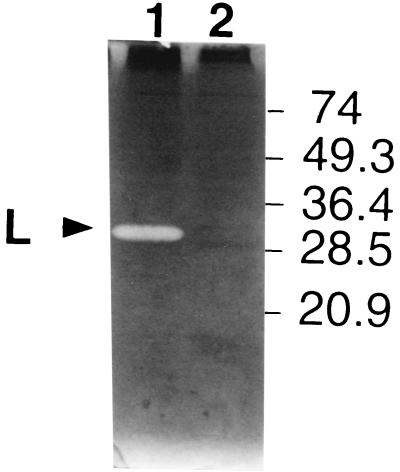
With the M. lysodeikticus autoclaved cells, which are classically used as the substrate for bacteriolytic enzymes, no activity band was detected, either in the SDS cell extract of strain DN-001065 or in that of the nonautolytic strain CNRZ 302 (data not shown). With the S. thermophilus substrate, one activity band, named L, was detected at 31 kDa in the DN-001065 strain (Fig. 5, lane 1). Band L was absent from nonautolytic strain CNRZ 302 (Fig. 5, lane 2). Its detection required the presence of a reducing agent, such as dithiothreitol or β-mercaptoethanol, in the sample loading buffer (data not shown). An identical activity band was also present in the five other autolytic strains and was absent from the nonautolytic strains tested as well as from a prophage-cured derivative strain (data not shown).
FIG. 5.
Detection of bacteriolytic activities in S. thermophilus DN-001065 and CNRZ 302 cells by renaturing SDS-PAGE. The gel contained autoclaved S. thermophilus cells. Lane 1, DN-001065 SDS cell extract; lane 2, CNRZ 302 SDS cell extract. The position of lytic band L is indicated by an arrowhead. The numbers on the right are molecular masses (in kilodaltons).
It is noteworthy that band L was detected with the same intensity during growth and lysis (Fig. 6A) and that no induction could be evidenced before the lysis. The culture lysis was accompanied by a concomitant increase of the bacteriolytic activity detected in the culture supernatant (data not shown).
FIG. 6.
Effect of mitomycin C on the bacteriolytic activity profile of S. thermophilus DN-001065. During the experiment shown on Fig. 3, samples were removed from the untreated culture (A) and from the mitomycin C induced culture (B) after different times, and cell extracts were prepared with glass beads as described in Materials and Methods. The same protein amount of each cell extract was analyzed by renaturing SDS-PAGE. (A) Samples taken at zero time and at 10, 20, 30, 40, 50, 120, and 165 min (lanes 1 to 8, respectively) after the time of mitomycin C addition. (B) Samples taken at zero time and at 10, 20, 30, and 35 min (lanes 1 to 5, respectively) after mitomycin C addition. The position of bacteriolytic band L is indicated by an arrowhead.
The 31-kDa bacteriolytic enzyme is a prophage-encoded enzyme homologous to the endolysin Lyt51 of the streptococcal temperate bacteriophage φ01205.
The intensity of the bacteriolytic activity detected at 31 kDa in the autolytic strains was monitored by renaturing SDS-PAGE during mitomycin C induction. As above, results are shown for S. thermophilus DN-001065 and were verified for the five other S. thermophilus autolytic strains. Cell extracts were prepared at different culture times following mitomycin C addition with glass beads as described in Materials and Methods, and the same protein quantity was loaded on a gel containing S. thermophilus autoclaved cells.
Thirty minutes after mitomycin C induction, the intensity of activity band L increased significantly (Fig. 6B). It went on increasing until lysis took place, 45 min after mitomycin C addition. At the time of lysis, band L was detected in the culture supernatant (data not shown). The intensity of activity band L did not vary during the same time period in the absence of mitomycin C (Fig. 6A).
Thus, the 31-kDa bacteriolytic activity appeared to be mitomycin C inducible. It was absent from a prophage-cured derivative strain and was detected exclusively in the S. thermophilus autolytic strains, which were furthermore lysogenic. In addition, it presented the same properties in renaturing SDS-PAGE (molecular mass, requirement for a reducing agent, and substrate specificity) as the bacteriolytic activities detected in the lysates of S. thermophilus cultures infected with virulent phages (data not shown). All together, these findings suggested that the 31-kDa enzyme was encoded by a prophage.
As it was shown that genes involved in the lysis module of S. thermophilus bacteriophages were highly conserved at the DNA sequence level (17, 44), we examined whether activity band L could be a bacteriolytic enzyme homologous to the recently identified endolysin Lyt51 of S. thermophilus temperate bacteriophage φ01205 (44).
We first investigated the presence of a gene homologous to lyt51 in the six autolytic strains by probing strain DNA digests with the PCR-generated fragment consisting of nucleotides 1 to 825 of lyt51 (data not shown). The results showed that the six autolytic strains contain a DNA fragment which is not present in the nonautolytic strain CNRZ 302 and which hybridizes with the lyt51 probe.
We then examined whether a protein similar to Lyt51 could be detected in the autolytic strains. For this purpose, antibodies were raised against Lyt51, which was overexpressed as a six-His-tagged fusion protein in E. coli and purified by Ni-NTA affinity chromatography. The antibodies were used in immunoblotting experiments on SDS cell extracts of the autolytic strains.
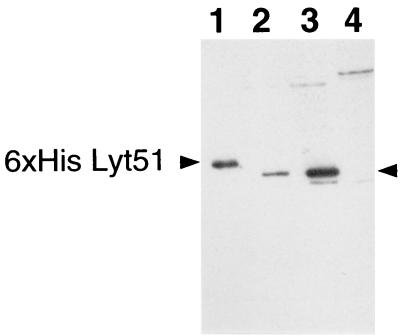
In the S. thermophilus DN-001065 SDS cell extract, a protein of 31 kDa was recognized by the purified antibodies (Fig. 7, lane 2) and the band intensity was higher in the mitomycin C-induced cells (Fig. 7, lane 3). One band was observed at the same molecular mass in the cell extracts of the five other autolytic strains as well (data not shown). The purified His-tagged Lyt51 used for antibody production was used as the control (Fig. 7, lane 1). The observed difference in electrophoretical mobility between the His-tagged endolysin and the protein detected in the DN-001065 cell extracts can be attributed to the fact that the molecular mass of the recombinant protein (32.2 kDa) is higher than that of the native protein (31.1 kDa) due to the six-His tag fusion. As expected, no band was detected at 31 kDa in the SDS cell extract of S. thermophilus CNRZ 302 (Fig. 7, lane 4). Protein bands detected at about 74 kDa, as well as the one at 29 kDa, were equally present in strain CNRZ 302 and thus appear nonspecific.
FIG. 7.
Immunoblotting with anti-Lyt51 antibodies. Lane 1, purified six-His-tagged Lyt51; lane 2, DN-001065; lane 3, mitomycin C-induced DN-001065; lane 4, CNRZ 302. The SDS cell extracts were prepared 40 min after mitomycin C induction of strain DN-001065. For each cell extract, the same protein amount was transferred on a nitrocellulose membrane. The blot was incubated with the purified antibodies directed against the six-His-tagged Lyt51 and then with protein G coupled to horseradish peroxidase. Positions of the six-His-tagged Lyt51 and the 31-kDa protein reacting with the anti-Lyt51 antibodies are indicated by arrowheads.
Thus, the 31-kDa bacteriolytic activity detected in the S. thermophilus autolytic strains is encoded by a prophage and is homologous to the Lyt51 endolysin identified in the φ01025 temperate bacteriophage of S. thermophilus.
Effect of exogenous endolysin on the growth and lysis of S. thermophilus autolytic strains.
The effect of exogenous endolysin on the growth and lysis of the autolytic DN-001065 strain was tested. For this purpose, exponential-phase cells were collected and resuspended in fresh M17 medium supplemented with lactose and several amounts (0, 10, 25, and 100 ng ml−1) of purified six-His-tagged Lyt51. The concentration of 100 ng ml−1 corresponds to the maximum amount that is released in the culture supernatant by spontaneous lysis, as estimated by renaturing SDS-PAGE.
At concentrations of 25 ng ml−1 and below, the endolysin Lyt51 has no effect on growth and lysis triggering; the cultures behave like the control without endolysin (data not shown). At a concentration of 100 ng ml−1, the growth rate was reduced but lysis was triggered at the same time as for the control (data not shown).
These results thus rule out the hypothesis that the cells lysed from the outside, that is, from the action of endolysin released by a fraction of the cell population. They rather indicate that most of the lysis observed in response to the identified triggering factors is from within.
DISCUSSION
S. thermophilus autolytic strains are characterized by a typical bell-shaped growth curve, reflecting their propensity to dramatically lyse after a normal growth phase. The cellular mechanisms involved in triggering the lysis of these strains were investigated in the present study. Lysis was rapidly triggered under unfavorable environmental conditions such as lactose depletion and organic solvent addition. This autolytic phenotype appears to be linked to the lysogenic character of the strains. It is also associated with the presence of a 31-kDa prophage-encoded endolysin, homologous to Lyt51 of the streptococcal temperate bacteriophage φ01205 (44) and detected in the cells during both growth and lysis.
Lysis of the autolytic strains appears to be triggered by the depletion of lactose, the unique carbon source in the growth medium. In addition, our results indicate that lactose prevents the lysis of starved or resting cells. As the triggering of lysis of S. thermophilus autolytic strains grown on lactose and also on saccharose was previously reported to be concomitant with sugar depletion from the medium (50, 58), we assumed that lysis is caused by the carbon source depletion irrespective of its nature. The cellular integrity of S. thermophilus autolytic strains thus appears to depend on the presence of metabolic energy in the medium as observed for some strains in other bacterial genus such as Enterococcus hirae (46), L. lactis (41), Propionibacterium spp. (30), and Bacillus subtilis (24).
Since S. thermophilus does not contain storage polymers, lactose starvation may immediately result in energy starvation and in the subsequent rapid dissipation of the proton motive force (PMF), as described previously for L. lactis (39). Organic solvents as well as NaCl (23) were shown to remove the protective effect of lactose against lysis. Following their addition, a sharp and immediate lysis, which does not seem to require de novo protein synthesis, occurred. It is worth noting that organic solvents are known to permeabilize the cytoplasmic membrane and that NaCl was also shown to lower the PMF at concentrations above 50 mM (26). The environmental factors triggering the cell lysis thus present the common feature of affecting the cell envelope properties by causing either the depolarization or the permeabilization of the cytoplasmic membrane.
In the present survey all the strains selected for their autolytic character are lysogenic. Reciprocally, according to the literature (19, 36), all the S. thermophilus lysogenic strains identified by mitomycin C induction are autolytic. The unique exception is the lysogen St18 described by Carminati and Giraffa (11), which did not exhibit the autolytic phenotype; this was most probably due to inappropriate growth conditions that led to lysis inhibition. The relation between the autolytic phenotype and the presence of a mitomycin C-inducible prophage in the strain genomes thus appears to be symmetrical in S. thermophilus. The involvement of a prophage in the autolytic phenotype is moreover supported by the nonautolytic character of strains cured for their prophage and the restoration of the autolytic phenotype by relysogenization (19, 36). The uniformity of the S. thermophilus lysogen phenotype might be linked to the recently evidenced genetic similarity among the streptococcal temperate bacteriophages (8, 17, 18, 20, 48) and their lysogeny modules (37).
A unique bacteriolytic enzyme of 31 kDa was detected by renaturing SDS-PAGE exclusively in the autolytic strains. We showed that this enzyme is prophage encoded and homologous to the endolysin Lyt51 of the streptococcal temperate bacteriophage φ01205 (44). The identification of the 31-kDa enzyme as an endolysin homologous to Lyt51 was based first on the similar biochemical properties of both enzymes observed by renaturing SDS-PAGE, i.e., their molecular masses and their requirement for a reducing agent to be detected as well as their apparently narrow substrate specificities. The 31-kDa enzyme identification was further substantiated by the detection of DNA homologous to lyt51 in the autolytic strains, and finally confirmed by the immunodetection of a 31-kDa protein reacting with anti-Lyt51 antibodies.
Although the autolytic phenotype of the S. thermophilus strains is linked to their lysogenic character and is observed under unfavorable physiological conditions, cell lysis does not appear to result from massive prophage induction in response to a stress. Indeed, lysis resulting from lysogenic induction, such as that caused by mitomycin C treatment, occurs after about 45 min, and, over this period of time, expression of phage genes is massively induced, as shown for the 31-kDa endolysin. By contrast, following lactose depletion and organic solvent or NaCl addition, lysis occurs more quickly and does not appear to require de novo protein synthesis. In addition, the 31-kDa endolysin is detected with the same intensity up to the onset of lysis. It thus appears that the two lysis events do not result from the same triggering mechanism and therefore that the autolytic phenotype is not caused by massive prophage induction.
It rather seems that the autolytic phenotype results from an incomplete prophage repression. Several observations suggest that the S. thermophilus lysogens failed to efficiently control the prophage state; linearized phage DNA (7, 19) and the 31-kDa endolysin were detected in cells that were not treated with mitomycin C, and phage particles were liberated by the spontaneous lysis of the lysogens. As an extensive culture lysis was triggered under appropriate conditions and as most of the cell lysis appears to come from within, we assumed that the prophage induction takes place in almost all the cells and not only in a fraction of the population. According to this hypothesis, the prophage lysis proteins are also expressed in the cells, as shown for the endolysin. In S. thermophilus phages, as in most bacteriophages, the lysis system contains, in addition to the endolysin, holin proteins which are required by the endolysin to pass through the cytoplasmic membrane and reach the peptidoglycan (17, 44, 48). The high cell toxicity of these membrane-embedded proteins implies that the prophage induction occurs at a low and therefore nonlethal level. We propose that the prophage lysis proteins control and achieve cell lysis and are thus responsible for the autolytic phenotype. It has been suggested that the culture lysis resulting from the endolysin action can be viewed as a reporter event for holin function (4). The involvement of holin proteins in lysis triggering was suggested by the fact that the environmental triggers of S. thermophilus lysis, which are factors leading to the membrane depolarization, are also known to trigger premature lysis of induced E. coli lambda lysogens by causing premature hole formation by holins (4, 56, 57). These data suggest that noninduced S. thermophilus lysogens express holin proteins at a nonlethal level and that, according to the well-documented model of lysis triggering in lambda lysogens, collapse of an already unstable membrane potential, which is weakened because of the expression of holins, would cause the conversion of holins from a dormant to an active state. Our hypothesis is thus that the prophage lysis proteins, which are the 31-kDa endolysin and the holins, would accomplish lysis and that the scheduling of lysis would be controlled by mechanisms governing the activity of the holins present in small amounts in the membranes during growth.
Because they can lyse in response to several specific environmental signals such as NaCl (23), lactose depletion, and organic solvents, S. thermophilus lysogens appear to be a very useful tool to evaluate the impact of cell lysis on the flavor development of fermented dairy products. In respect to the significant problem of phage infection in the dairy industry, the restriction to their wide industrial use is their lysogenic character, although we did not detect virulent phages in the lysis supernatants. Further studies will thus be required in order to specify the role of S. thermophilus lysogeny in phage infection.
ACKNOWLEDGMENTS
This work was supported by the Centre International de Recherche Daniel Carasso of the Danone Group. C.H.-K. was subsidized by a CIFRE convention (no. 95762) from the French Association de la Recherche Technique and the T3Net program from the Commission of the European Communities.
We thank Patrick Taillez for the access to the randomly amplified polymorphic DNA classification of the S. thermophilus strains from the CNRZ collection. We acknowledge Michelle Sheehan for helpful advice on Lyt51 purification and Kate Pollock for antiserum production. We are very grateful to Jean-Claude Gripon and Gérard Denariaz for their interest in this work. We warmly thank Bertrand Nicolas for photographic work.
REFERENCES
- 1.Accolas J-P, Spillman H. The morphology of six bacteriophages of Streptococcus thermophilus. J Appl Bacteriol. 1979;47:135–144. [Google Scholar]
- 2.Bie R, Sjöström G. Autolytic properties of some lactic acid bacteria used in cheese production. Part I: Material and methods. Milchwissenschaft. 1975;30:653–657. [Google Scholar]
- 3.Bie R, Sjöström G. Autolytic properties of some lactic acid bacteria used in cheese production. Part II. Experiments with fluid substrates and cheese. Milchwiss. 1975;30:739–747. [Google Scholar]
- 4.Bläsi U, Young R. Two beginnings for a single purpose: the dual-start holins in the regulation of phage lysis. Mol Microbiol. 1996;21:675–682. doi: 10.1046/j.1365-2958.1996.331395.x. [DOI] [PubMed] [Google Scholar]
- 5.Botazzi V, Battistotti B, Vescovo M, Rebecchi A, Bianchi F. Development and lysis of homofermentative thermophilic lactobacilli microcolonies in grana cheese. Ann Microbiol Enzimol. 1992;42:227–247. [Google Scholar]
- 6.Bradley D E. Ultrastructure of bacteriophages and bacteriocins. Bacteriol Rev. 1967;31:230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brüssow H, Bruttin A. Characterization of a temperate Streptococcus thermophilus bacteriophage and its genetic relationship with lytic phages. Virology. 1995;212:632–640. doi: 10.1006/viro.1995.1521. [DOI] [PubMed] [Google Scholar]
- 8.Brüssow H, Probst A, Frémont M, Sidoti J. Distinct Streptococcus thermophilus bacteriophages share an extremely conserved DNA fragment. Virology. 1994;200:854–857. doi: 10.1006/viro.1994.1256. [DOI] [PubMed] [Google Scholar]
- 9.Buist G, Kok J, Leenhouts K J, Dabrowska M, Venema G, Haandrikman A J. Molecular cloning and nucleotide sequence of the gene encoding the major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell separation. J Bacteriol. 1995;177:1554–1563. doi: 10.1128/jb.177.6.1554-1563.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cappa F, Botazzi V. Characterization of autolytic enzymes in Lactobacillus casei subsp. casei 1St261. Ann Microbiol Enzimol. 1996;46:299–310. [Google Scholar]
- 11.Carminati D, Giraffa G. Evidence and characterization of temperate bacteriophage in Streptococcus thermophilus subsp. thermophilus St18. J Dairy Res. 1992;59:71–79. doi: 10.1017/s0022029900030260. [DOI] [PubMed] [Google Scholar]
- 12.Chapot-Chartier M-P. Les autolysines des bactéries lactiques. Lait. 1996;76:91–109. [Google Scholar]
- 13.Chapot-Chartier M-P, Deniel C, Rousseau M, Vassal L, Gripon J C. Autolysis of two different strains of Lactococcus lactis during cheese ripening. Int Dairy J. 1994;4:251–269. [Google Scholar]
- 14.Chapot-Chartier M-P, Rul F, Nardi M, Gripon J-C. Gene cloning and characterization of PepC, a cysteine aminopeptidase from Streptococcus thermophilus, with sequence similarity to the eukaryotic bleomycin hydrolase. Eur J Biochem. 1994;224:497–506. doi: 10.1111/j.1432-1033.1994.00497.x. [DOI] [PubMed] [Google Scholar]
- 15.Crow V L, Coolbear T, Gopal P K, Martley F G, McKay L L, Riepe H. The role of autolysis of lactic acid bacteria in the ripening of cheese. Int Dairy J. 1995;5:855–875. [Google Scholar]
- 16.Crow V L, Martley F G, Coolbear T, Roundhill S J. The influence of phage-assisted lysis of Lactococcus lactis subsp. lactis ML8 on Cheddar cheese ripening. Int Dairy J. 1995;5:454–472. [Google Scholar]
- 17.Desiere F, Lucchini S, Brüssow H. Evolution of Streptococcus thermophilus bacteriophage genomes by modular exchanges followed by point mutations and small deletions and insertions. Virology. 1998;241:345–356. doi: 10.1006/viro.1997.8959. [DOI] [PubMed] [Google Scholar]
- 18.Desiere F, Lucchini S, Bruttin A, Zwahlen M C, Brüssow H. A highly conserved DNA replication module from Streptococcus thermophilus phages is similar in sequence and topology to a module from Lactococcus lactis phages. Virology. 1997;234:372–382. doi: 10.1006/viro.1997.8643. [DOI] [PubMed] [Google Scholar]
- 19.Fayard B. PhD thesis. Nancy, France: Nancy I; 1993. [Google Scholar]
- 20.Fayard B, Haefliger M, Accolas J-P. Interactions of temperate bacteriophages of Streptococcus salivarius subsp. thermophilus with lysogenic indicators affect phage DNA restriction patterns and host ranges. J Dairy Res. 1993;60:385–399. [Google Scholar]
- 21.Francki R I B, Fauquet C M, Knudson D L, B D L. Classification and nomenclature of viruses. Fifth report of the International Committee on Taxonomy of Viruses. Arch Virol Suppl. 1991;2:1–450. [Google Scholar]
- 22.Gu J, Stephenson C G, Iadarola M J. Recombinant proteins attached to a nickel-NTA column: use in affinity purification of antibodies. BioTechniques. 1994;17:257–262. [PubMed] [Google Scholar]
- 23.Husson-Kao C, Mengaud J, Gripon J-C, Benbadis L, Chapot-Chartier M-P. The autolysis of S. thermophilus DN-001065 is triggered by several food-grade environmental signals. Int Dairy J. 2000;9:715–723. [Google Scholar]
- 24.Joliffe L K, Doyle R J, Streips U N. The energized membrane and cellular autolysis in Bacillus subtilis. Cell. 1981;25:753–763. doi: 10.1016/0092-8674(81)90183-5. [DOI] [PubMed] [Google Scholar]
- 25.Kawabata S, Vassal L, LeBars D, Cesselin B, Nardi M, Gripon J-C, Chapot-Chartier M-P. Phage-induced lysis of Lactococcus lactis during Saint Paulin cheese ripening and its impact on proteolysis. Lait. 1997;77:229–239. [Google Scholar]
- 26.Konings W N, Poolman B, Driessen A J M. Bioenergetics and solute transport in lactococci. Crit Rev Microbiol. 1989;16:419–476. doi: 10.3109/10408418909104474. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Langsrud T, Landaas A, Castberg H B. Autolytic properties of different group N streptococci. Milchwissenschaft. 1987;42:556–560. [Google Scholar]
- 29.Leclerc D, Asselin A. Detection of bacterial cell wall hydrolases after denaturing polyacrylamide gel electrophoresis. Can J Microbiol. 1989;35:749–753. doi: 10.1139/m89-125. [DOI] [PubMed] [Google Scholar]
- 30.Lemée R, Lortal S, Cesselin B, van Heijenoort J. Involvement of an N-acetylglucosaminidase in autolysis of Propionobacterium freudenreichii CNRZ 725. Appl Environ Microbiol. 1994;60:4351–4358. doi: 10.1128/aem.60.12.4351-4358.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lepeuple A S, van Gemert E, Chapot-Chartier M-P. Analysis of the bacteriolytic enzymes of the autolytic Lactococcus lactis subsp. cremoris strain AM2 by renaturing polyacrylamide gel electrophoresis: identification of a prophage-encoded enzyme. Appl Environ Microbiol. 1998;64:4142–4148. doi: 10.1128/aem.64.11.4142-4148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lepeuple A-S, Vassal L, Cesselin B, Delacroix-Buchet A, Gripon J-C, Chapot-Chartier M-P. Involvement of a prophage in the lysis of Lactococcus lactis subsp. cremoris AM2 during cheese ripening. Int Dairy J. 1998;8:667–674. [Google Scholar]
- 33.Lortal S, Boyaval P, van Heijenoort J. Influence de plusieurs facteurs sur l'autolyse de Lactobacillus helveticus CNRZ 414. Lait. 1989;69:223–231. [Google Scholar]
- 34.Lowrie R J, Lawrence R C, Peberdy M F. Cheddar cheese flavour. V. Influence of bacteriophage and cooking temperatures on cheese made under controlled bacteriological conditions. New Zealand J Dairy Sci Technol. 1974;9:116–121. [Google Scholar]
- 35.Meijer W, Dobbelaar C, Hugenholtz J. Thermoinducible lysis in Lactococcus lactis subsp. cremoris SK 110: implications for cheese ripening. Int Dairy J. 1998;8:275–280. [Google Scholar]
- 36.Mercenier A. Molecular genetics of Streptococcus thermophilus. FEMS Microbiol Rev. 1990;87:61–78. doi: 10.1016/0378-1097(90)90697-o. [DOI] [PubMed] [Google Scholar]
- 37.Neve H, Zenz K I, Desiere F, Koch A, Heller K J, Brüssow H. Comparison of the lysogeny module from the temperate Streptococcus thermophilus bacteriophages TP-J34 and Sfi 21: implications for the modular theory of phage evolution. Virology. 1998;241:61–72. doi: 10.1006/viro.1997.8960. [DOI] [PubMed] [Google Scholar]
- 38.Ostlie H, Vegarud G, Langsrud T. Autolysis of lactococci: detection of lytic enzymes by polyacrylamide gel electrophoresis and characterization in buffer systems. Appl Environ Microbiol. 1995;61:3598–3603. doi: 10.1128/aem.61.10.3598-3603.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otto R, Vije J, ten Brink B, Klont B, Konings W N. Energy metabolism in Streptococcus cremoris during lactose starvation. Arch Microbiol. 1985;141:348–352. [Google Scholar]
- 40.Potvin C, Leclerc D, Tremblay G, Asselin A, Bellemare G. Cloning, sequencing and expression of a Bacillus bacteriolytic enzyme in Escherichia coli. Mol Gen Genet. 1988;214:241–248. doi: 10.1007/BF00337717. [DOI] [PubMed] [Google Scholar]
- 41.Riepe H R, Pillidge C J, Gopal P K, McKay L L. Characterization of the highly autolytic Lactococcus lactis subsp. cremoris strains CO and 2250. Appl Environ Microbiol. 1997;63:3757–3763. doi: 10.1128/aem.63.10.3757-3763.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 43.Sandholm E, Sarimo S S. Autolysis of Streptococcus thermophilus. FEMS Microbiol Lett. 1981;11:125–129. [Google Scholar]
- 44.Sheehan M M, Stanley E, Fitzgerald G F, van Sinderen D. Identification and characterization of a lysis module present in a large proportion of bacteriophages infecting Streptococcus thermophilus. Appl Environ Microbiol. 1999;65:569–577. doi: 10.1128/aem.65.2.569-577.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shockman G D, Barrett J F. Structure, function, and assembly of cell walls of gram-positive bacteria. Annu Rev Microbiol. 1983;37:501–527. doi: 10.1146/annurev.mi.37.100183.002441. [DOI] [PubMed] [Google Scholar]
- 46.Shockman G D, Conover M J, Kolb J J, Philipps P M, Riley L S, Toennies G. Lysis of Streptococcus faecalis. J Bacteriol. 1961;81:36–43. doi: 10.1128/jb.81.1.36-43.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shockman G D, Höltje J-V. Microbial peptidoglycan (murein) hydrolases. New comprehensive biochemistry. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. London, United Kingdom: Elsevier Science; 1994. pp. 131–166. [Google Scholar]
- 48.Stanley E, Fitzgerald G, Le Marrec C, Fayard B, van Sinderen D. Sequence analysis and characterization of φ01205, a temperate bacteriophage infecting Streptococcus thermophilus CNRZ1205. Microbiology. 1997;143:3417–3429. doi: 10.1099/00221287-143-11-3417. [DOI] [PubMed] [Google Scholar]
- 49.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas T D, Crow V L. Lactose and sucrose utilization by Streptococcus thermophilus. FEMS Microbiol Lett. 1983;17:13–17. [Google Scholar]
- 51.Towbin H, Gordon J. Immunoblotting and dot immunobinding—current status and outlook. J Immunol Methods. 1984;72:313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- 52.Valence F, Lortal S. Zymogram and preliminary characterization of Lactobacillus helveticus autolysins. Appl Environ Microbiol. 1995;61:3391–3399. doi: 10.1128/aem.61.9.3391-3399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valence F, Richoux R, Thierry A, Palva A, Lortal S. Autolysis of Lactobacillus helveticus and Propionobacterium freudenreichii in Swiss cheeses: first evidence by using species-specific lysis markers. J Dairy Res. 1998;65:609–620. [Google Scholar]
- 54.Vegarud G, Castberg H B, Langsrud T. Autolysis of group N streptococci. Effect of media composition modifications and temperature. J Dairy Sci. 1983;66:2294–2303. [Google Scholar]
- 55.Wilkinson M G, Guinee T P, O'Callaghan D M, Fox P F. Autolysis and proteolysis in different strains of starter bacteria during Cheddar cheese ripening. J Dairy Res. 1994;61:249–262. [Google Scholar]
- 56.Young R. Bacteriophage lysis: mechanism and regulation. Microbiol Rev. 1992;56:430–481. doi: 10.1128/mr.56.3.430-481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Young R, Bläsi U. Holins: form and function in bacteriophage lysis. FEMS Microbiol Rev. 1995;17:191–205. doi: 10.1111/j.1574-6976.1995.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 58.Zourari A. PhD thesis. Paris, France: INA-PG; 1991. [Google Scholar]