Abstract
Objectives:
To examine maximal strength and fatigability of the knee extensors, and mechanomyographic amplitude (MMGRMS)–force relationships of the vastus lateralis (VL) during repetitive muscle actions for 5 aerobically-(AT), 5 resistance-trained-(RT), and 5 sedentary (SED) individuals.
Methods:
Participants performed maximal voluntary contractions before (MVCPRE) and after (MVCPOST) attempting 20 isometric trapezoidal muscle actions at 50% MVCPRE. MMG was recorded from the VL. b terms (slopes) were calculated from the natural log-transformed MMGRMS-force relationships for each participant (increasing and decreasing segments). MMGRMS was averaged during steady force.
Results:
RT had greater MVCPRE (P<0.001) and MVCPOST (P=0.001–0.004) than AT and SED. Only AT completed 20 muscle actions and exhibited no decrease in MVCPOST (P=0.149). The b terms were greater for RT than AT during the increasing segment of the first contraction (P=0.001) and decreasing segment of the last contraction (P=0.033). The b terms were also greater for RT (P=0.006) during the increasing than decreasing segment for the first contraction. MMGRMS during steady force was greater during the last contraction when collapsed across training status (P=0.021).
Conclusion:
Knee extensor MVC and fatigability, and motor unit control strategies for the VL during a series of repetitive contractions were influenced by chronic training status.
Keywords: Isometric Trapezoidal Muscle Action, Log-Transform Model, Mechanomyography, Motor Unit Control Strategies, Vastus Lateralis
Introduction
Numerous studies have investigated the influence of chronic aerobic- and resistance-training on physiological adaptations[1,2]. Traditionally, aerobic training focuses on repetitive movements of moderate to long durations with low resistance, whereas resistance training involves lifting moderate to heavy external loads with low to moderate repetition ranges. Consequently, muscular strength and endurance, skeletal muscle structure, and myosin heavy chain (MHC) expression differs among chronic training statuses[3,4].
Interestingly, few studies have examined the influence of chronic training status on motor unit (MU) firing rate behavior among chronic aerobically- (AT), resistance-trained (RT), and sedentary (SED) individuals. Previously, De Luca et al.[5] utilized needle electromyography (EMG) and reported greater initial firing rates of the deltoid for Olympic caliber swimmers and healthy controls than Olympic caliber power lifters, whereas the power lifters had greater firing rates than the swimmers at derecruitment. In addition, Herda et al.[6] reported greater firing rates of the vastus lateralis (VL) for chronic AT than RT at a targeted force as examined by surface EMG signal decomposition. Subsequently, it is suggested that firing rate differences are partially influenced by training related changes in the physical properties (%MHC expression) of the MU pool[5,6]. The research techniques in the aforementioned studies require advanced equipment that is not commonly found in research laboratories, thus, it should be investigated if measurements that are relatively inexpensive and require minimal signal processing are also sensitive to differences in MU behavior among chronic training statuses.
While intramuscular EMG and surface EMG signal decomposition quantify individual MU properties (i.e. firing rates, action potential size), surface mechanomyography (MMG) is the mechanical counterpart to neural activation and is defined as the recording and quantification of low-frequency lateral oscillations from active, unfused muscle fibers during a contraction[7]. The amplitude of the MMG signal (MMGRMS) is influenced by several factors, such as the active stiffness of the fibers modulated by the number of MUs recruited, as well as the firing rates of the active MUs[7,8]. MMGRMS-force relationships have previously differentiated MU activation and deactivation strategies among chronically trained individuals during moderate- and high-intensity contractions[9,10]. In addition, Beck et al.[11] previously reported greater MMGRMS of the VL for RT in comparison to AT during a 30-s step contraction at 50% MVC. Thus, it is plausible that MMGRMS may be sensitive to adjustments in MU behavior as a function of chronic training status during repetitive muscle actions; however, this has yet to be investigated.
Due to the amount of variability reported among individuals, it has been suggested MMGRMS-force relationships should be examined on a subject-by-subject basis[12,13]. A natural log-transformation of the MMGRMS-force relationships can provide a quantitative method for describing the linearity of the force-related patterns of responses for MMG amplitude[12]. For example, if the slopes (b terms) equal 1 or the 95% confidence intervals (CIs) of the slope include 1, then the relationship is linear. If the b term is less than 1 and the 95% CIs do not include 1, the relationship decelerates downward across the force spectrum. Furthermore, a b term that equals zero or has 95% CIs that include zero indicates no slope for MMGRMS across the force spectrum. The b terms have displayed similar reliability to relationships derived from MU behavior examined with surface EMG signal decomposition[14,15], and in agreement with advanced single MU techniques, have been sensitive to alterations in MU behavior due to MHC expression[3,11,16], short-term aerobic training[17], and chronic training status[9]. Thus, examining the log-transformed MMGRMS–force relationships during a series of isometric trapezoidal contractions that include a linearly increasing and decreasing segment may provide insight on the influence of chronic training on adjustments in MU activation and deactivation strategies during fatigue.
In addition, the steady force segment of the isometric trapezoidal muscle action is similar to a step contraction[8,18,19] and will allow comparisons of MMGRMS among AT, RT, and SED at 50% MVC. Previously, MMGRMS has differentiated MU activation and deactivation strategies among chronic training statuses in a similar cohort of individuals during a single contraction[9]. Due to the strict eligibility requirements for this study, we would expect group differences for MHC expression of the VL[3,11,20], which influences the mechanical behavior of muscle[16]. Therefore, the purpose of this study was to examine the influence of chronic training status on MU control strategies during repetitive isometric trapezoidal muscle actions that contained a linearly increasing, steady force, and linearly decreasing muscle actions. We hypothesized the RT will exhibit greater slopes (b terms) than the AT during the linearly increasing segment of the first contraction due to a greater amplitude of force twitches. In addition, we hypothesize greater b terms for the RT than the AT during the linearly decreasing segment of the last contraction as a result of greater contribution of MU recruitment due to the accumulation of fatigue[21]. Lastly, we hypothesized MMGRMS will be greater for the RT during the steady force segment based on the previous findings of Beck et al.[11].
Methods
Subjects
Fifteen healthy participants (mean±SD; age=22±4 yrs; body mass=74±23 kg; height=173±12 cm) volunteered for this investigation. Depending on training status, they were categorized as AT (five participants; age=19±1 yrs; body mass=59±12 kg; height=72±16 cm), RT (five participants; age=25±5 yrs; body mass=99±18 kg; height=179±8 cm), or SED (five participants; age=22±4 yrs; body mass=63±11 kg; height=168±10 cm) for further statistical analysis. Differences in neuromuscular behavior among chronic training statuses have been reported with as few as 3 participants per group[5]. Thus, 5 participants were assigned to each group to ensure potential differences were not a function of type I error and is in accordance with previous investigations examining chronic training statuses with MMG[3,9,11]. The AT participants engaged in a structured running program for at least 3 years prior and ran an average of 61±15 miles ⋅ wk-1 for 7 – 10 h ⋅ wk-1 with no resistance training. The RT engaged in a structured weight training program for 4 years prior for 4 – 8 h ⋅ wk-1 without any aerobic activity and self-reported a one-repetition back squat at least twice their body mass. The SED participants did not participate in any form of structured exercise within the past 3 years prior to participating in the study. None of the participants reported any history of current or ongoing neuromuscular disease or musculoskeletal injuries to the ankle, knee, and hip joints. This study was approved by the University’s institutional review board for human subjects research. Each subject read and signed an informed consent form and completed a pre-exercise health status questionnaire.
Isometric testing
Each participant visited the laboratory for one experimental trial. During the experimental trial, isometric strength testing for the right knee extensors was assessed for each participant using the force signal (N) from a load cell (LC, Omegadyne, Inc., Sunbury, OH, USA) that was fitted to a Biodex System 3 isokinetic dynamometer (Biodex Medical Systems, Shirley, NY). Participants were seated with restraining straps over their pelvis, trunk, and contralateral thigh, and the lateral condyle of the femur aligned with the input axis of the dynamometer in accordance with the Biodex User’s Guide (Biodex Pro Manual, Applications/Operations, 1998). All isometric leg extension testing was assessed at a knee flexion of 90°.
During the experimental trial, participants performed 3, 5-s MVCs with strong verbal encouragement for motivation and were given 3 minutes of rest between each attempt. The highest force output among 3 MVCs was designated as the maximal force output (MVCPRE). Following three to five minutes of rest, participants attempted 20 submaximal isometric trapezoidal muscle actions at 50% MVC. A 50% MVC was examined as MMGRMS previously differentiated between AT and RT during a 30-s step contraction[11], thus, repetitive trapezoidal muscle actions performed at 50% MVC may reveal differences in MU activation and deactivation strategies among training statuses during fatigue. Task failure was defined as when the force of the participants dropped 5% MVC below the required output[21]. The trapezoid trajectory contained five segments: a 5-s baseline, a linear increase from baseline at rate of 10% MVC/s, a constant force of the targeted %MVC for 12-s, a linear decrease to baseline at a rate of 10% MVC/s, and a final 3-s baseline period. Therefore, an 8 to 9-s rest interval was given between trapezoidal muscle actions. Prior to the 50% MVC, participants practiced the isometric trapezoid muscle action at 20% MVC to familiarize themselves with tracing the template. Participants were given a second attempt if they had an issue tracing the template. Participants were instructed to maintain their force output as close as possible to the target force presented digitally in real time on a computer monitor. Immediately following the series of contractions, participants performed one MVC for the knee extensors (MVCPOST).
Mechanomyography
MMG was collected during the isometric trapezoidal muscle actions with an active miniature accelerometer (EGAS-FS-10-/VO5; Measurement Specialties, Inc., Hampton, VA, USA) that was pre-amplified with a gain of 200, frequency response of 0 – 200 Hz, sensitivity of 68.5 mV m ⋅ s-1 and range of ± 98.1 m ⋅ s-1. The accelerometer was placed on the VL on the lateral/anterior portion of the muscle at 50% of the distance between the greater trochanter and the lateral condyle of the femur[3,9,11]. Double-sided tape was used to attach the accelerometer to the skin.
Signal processing
The MMG (m⋅s-2) and force (N) signals were simultaneously sampled at 2 kHz with a Biopac data acquisition system (MP150; Biopac Systems, Inc., Santa Barbara, CA) during each isometric trapezoidal muscle action. All subsequent signals were stored and processed off-line with custom-written software (LabVIEW, version 11; National Instruments, Austin, TX). The MMG signals were bandpass filtered (fourth-order Butterworth) at 5 – 100 Hz. During the isometric trapezoidal contractions, consecutive, non-overlapping 0.25-s epochs were analyzed for the force and MMG signals. An epoch of 0.25-s has previously been utilized[9] to minimize the non-stationarity reported in MMG signals[22]. The amplitude of the MMG signal was calculated with root mean square (RMS).
Skinfold thickness
Previously, Bahamonde et al.[23] reported acute bouts of exercise did not affect skinfold thickness and, thus, measurements were taken after experimental testing to ensure values were recorded at the MMG sensor site. Measurements were taken in accordance to Jackson and Pollock[24] with a calibrated Harpenden caliper (John Bull, UK). The average of three measurements was used to represent skinfold thickness. It has been previously suggested that skinfold thickness may act as a low-pass filter for the MMG signal[25].
Statistical Analysis
For the linearly increasing and decreasing segments of the isometric trapezoid (Figure 1), simple linear regression models were fit to the log-transformed MMGRMS-force relationships[3,9,17]. The equations were represented as:
Figure 1.
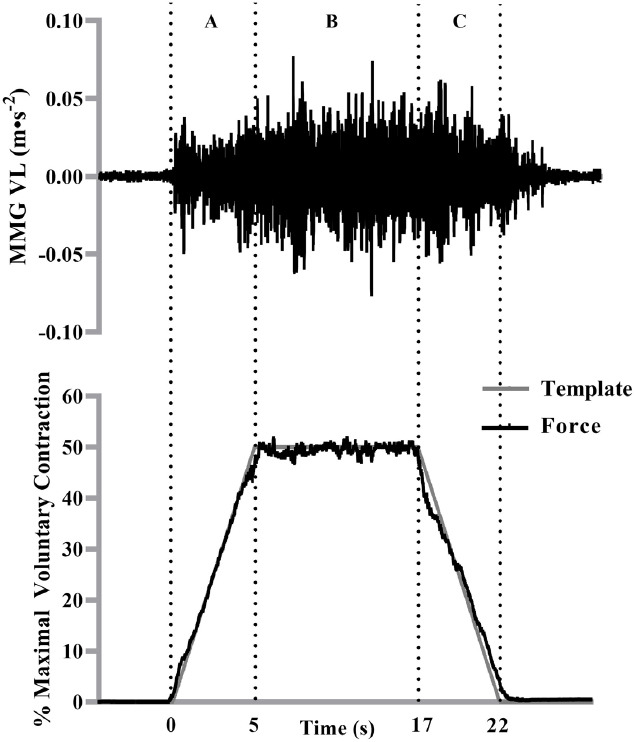
The mechanomyographic (MMG) signal from the vastus lateralis during a 50% isometric trapezoidal contraction from one participant. The force signal (bottom) is overlaid onto the trapezoidal template as it appeared for the participant during the trial. The vertical lines represent the (A) linear force increase, (B) the steady force and (C) the linear force decrease segments of the 50% isometric trapezoidal contraction. The MMG signal that corresponded with the contraction segments (A-C) was selected for analysis.
(1) ln[Y] = b(ln[X]) + ln[a]
where ln[Y] = the natural log of the MMGRMS values, ln[X] = the natural log of the force values, b = the slope and ln[a] = the natural log of the y-intercept. This can also be expressed as an exponential equation after the antilog transformation:
(2) Y = aXb
where Y = predicts MMGRMS values, X = force, b = slope of Eq. (1), and a = the antilog of the y-intercept from Eq. (1). Slopes and the y-intercepts were calculated using Microsoft Excel® version 2010 (Microsoft, Inc., Redmond, WA, USA). For the steady force segment of the trapezoid, MMGRMS was calculated by averaging the values from each 0.25-s epoch during the entire 12-s steady targeted contraction force.
Three separate one-way ANOVAs (group [AT vs. RT vs. SED]) were used to examine differences in skinfold thickness, contractions completed, and the percent change from MVCPRE to MVCPOST. A two-way mixed factorial ANOVA (group [AT vs. RT vs. SED] x time [PREMVC vs. POSTMVC]) was used to assess differences in MVC. A two-way mixed factorial ANOVA (group [AT vs. RT vs. SED] x contraction [first vs. last]) was used to assess differences in MMGRMS during the steady force segment. Furthermore, a three-way mixed factorial ANOVA (group [AT vs. RT vs. SED] x contraction [first vs. last] x segment [linear increase vs. linear decrease] was used to examine differences in the b terms (slopes) from the MMGRMS-force relationships during the linear increasing and decreasing segments of the submaximal isometric trapezoid muscle actions. Lastly, six separate Pearson’s product moment correlation coefficients were calculated comparing skinfold thickness with the b terms from the linearly increasing and decreasing segments and MMGRMS of the steady force segment for the first and last contraction, respectively. When appropriate, follow-up analyses included additional ANOVAs, independent samples Τ-tests, and paired samples t-tests with Bonferroni corrections. The level of significance was set at P≤0.05 and all statistical analyses were performed using SPSS 20 (IBM Corporation, Armonk, New York, USA). Effect sizes were estimated using Hedges’s g and effect size estimates were classified as minimal (0 – 0.2), small (0.2 – 0.5), medium (0.5 – 0.8) or large (> 0.8).
Results
Isometric Strength Testing
For percent change from MVCPRE to MVCPOST, the one-way ANOVA indicated there was a significant difference among groups (F[2,12]=8.488, P=0.005, ηp2=0.586). The percentage change for RT (-35.21±4.80%) was significantly greater than AT (-9.71±12.23%; P=0.005; g=2.744). However, SED (-25.39%±10.94%) was not different in comparison to AT (P=0.082; g=1.351) or RT (P=0.425; g=1.162).
For maximal strength, there was a significant two-way interaction (group x time; F[2,12]=16.746, P<0.001, ηp2=0.736). For MVCPRE, RT (1035.40±197.09 N) was significantly greater than AT (473.20±132.63 N; P<0.001; g=3.347) and SED (505.00±160.21 N; P<0.001; g=2.953); however, there was no significant difference between AT and SED (P>0.999; g=0.216) at MVCPRE. For MVCPOST, RT (667.00±102.21 N) was significantly greater than AT (419.20±83.32 N; P=0.004; g=2.658) and SED (369.80±96.14 N; P<0.001; g=2.995); however, there were no significant differences between AT and SED (P>0.999; g=0.549) at MVCPOST. MVCPOST was significantly less than MVCPRE for RT (P=0.002; g=2.347) and SED (P=0.020; g=1.023), but not for AT (P=0.149; g=0.488) (Figure 2).
Figure 2.
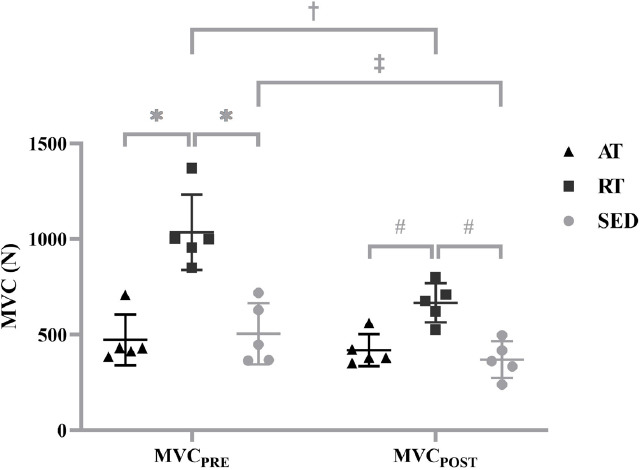
Individual values for maximal voluntary isometric contraction (MVC) before (MVCPRE) and after (MVCPOST) the repetitive isometric trapezoidal contractions for the aerobically-trained (AT), resistance-trained (RT) and sedentary (SED). Horizontal bars represent the means and standard deviations for the respective groups. * indicates MVCPRE was greater for RT than AT (P<0.001) and SED (P<0.001). # indicates MVCPOST was greater for RT than AT (P=0.004) and SED (P<0.001). † indicates MVCPRE was greater than MVCPOST for RT (P=0.002). ‡ indicates MVCPRE was greater than MVCPOST for SED (P=0.020).
Contractions Completed
There were significant differences (F[2,12]=7.387, P=0.008, ηp2=0.552) among the groups. AT (20.00±0.00) completed significantly more contractions than RT (10.40±6.12; P=0.029; g=2.218) and SED (9.00±5.96; P=0.012; g=2.610). However, there was no significant difference between RT and SED (P>0.999; g=0.232).
Linear increasing and decreasing MMGRMS-force relationships
There was a significant three-way interaction (group x contraction x segment; F[2,12]=6.310, P=0.013, ηp2=0.513). During the linearly increasing segment of the first contraction, the b terms for the RT (0.953±0.390) were significantly greater than AT (-0.039±0.251; P=0.001; g=3.024). Additionally, the b terms for the RT during the linearly increasing segment were significantly greater than the linearly decreasing segment (0.449±0.440; P=0.006; g=1.092) of the first contraction. Furthermore, the RT (1.006±0.727) had significantly greater b terms than the AT (0.119±0.222; P=0.033; g=1.650) during the linearly decreasing segment of the last contraction. There were no other differences reported among the contractions, segments, or training statuses (P>0.05) (Figure 3). The means ± standard deviation values and the 95% confidence intervals for the b terms for each group, segment, and contraction are presented in Table 1. Figure 4 illustrates the MMGRMS patterns of the response during the linearly increasing and decreasing segments for the AT, RT, and SED.
Figure 3.
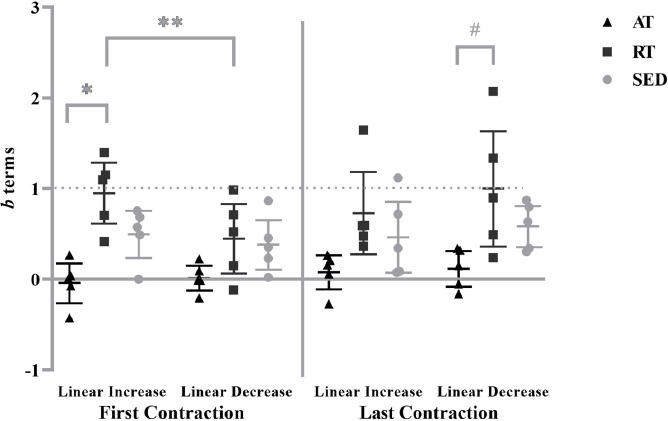
Individual values for the b terms from the mechanomyographic amplitude (MMGRMS) vs. force relationship for the aerobically-trained (AT), resistance-trained (RT), and sedentary (SED) from the linearly increasing and decreasing segments of the isometric trapezoidal contractions. Horizontal bars represent the means and 95% confidence intervals for the respective groups. * indicates greater b terms for RT than AT (P=0.001) during the linear increasing segment of the first contraction. # indicates greater b terms for RT than AT (P=0.033) during the linearly decreasing segment of the last contraction. ** Indicates a greater b term during the linearly increasing than decreasing segment for the RT (P=0.006).
Table 1.
Mean (SD) values and 95% confidence intervals for the b terms from the log-transformed mechanomyographic amplitude (MMGRMS)-force relationships for the aerobically-trained (AT), resistance-trained (RT), and sedentary (SED) participants.
| Linearly increasing segment | Linearly decreasing segment | |||||||
|---|---|---|---|---|---|---|---|---|
| AT | RT | SED | AT | RT | SED | |||
| First contraction | b terms | mean 95% CI | -0.038 (0.251) | 0.953 (0.390) | 0.500 (0.296) | 0.016(0.159) | 0.449 (0.440) | 0.384 (0.314) |
| -0.259-0.181 | 0.610-1.295 | 0.240 - 0.760 | -0.123-0.155 | 0.063 - 0.835 | 0.110-0.659 | |||
| Last contraction | b terms | mean 95% CI | 0.081 (0.213) | 0.732 (0.518) | 0.467 (0.447) | 0.119(0.222) | 1.006 (0.727) | 0.588 (0.260) |
| -0.105 - 0.268 | 0.278- 1.186 | 0.075 - 0.859 | -0.077-0.314 | 0.369- 1.644 | 0.361-0.815 | |||
Figure 4.
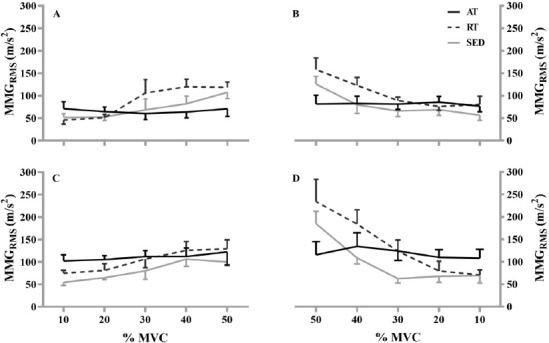
Plotted means and standard error of the mean for the aerobically-trained (AT; black line), resistance-trained (RT; black dashed line), and sedentary (SED; gray line) during the linearly increasing (A, C) and decreasing (B, D) segments of mechanomyographic amplitude (MMGRMS)–force relationship from 10% to 50% maximal voluntary contraction (%MVC) for the first (top) and last (bottom) contraction.
Steady force segment
The analyses indicated no significant two-way interaction (group x contraction; F[2,12]=1.979, P=0.181, ηp2=0.248) or main effect for group (F[2,12]=0.742, P=0.497, ηp2=0.110). However, there was a main effect for contraction (F[2,12]=4.934, P=0.046 ηp2=0.291). MMGRMS for the last contraction (135.71±44.38 m⋅s-2) was significantly greater than the first contraction (115.01±36.49 m⋅s-2; P=0.046; g=0.510) when collapsed across groups (Figure 5).
Figure 5.
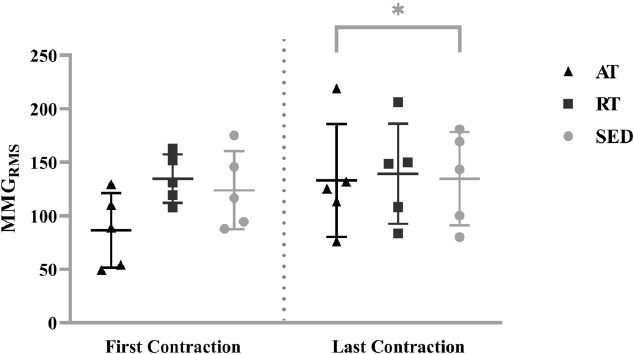
Individual values for mechanomyographic amplitude (MMGRMS) from the steady force segment of the first and last isometric trapezoidal contraction for the aerobically-trained (AT), resistance-trained (RT) and sedentary (SED). Horizontal bars represent the means and standard deviations for the respective groups. * indicates greater MMGRMS for the last contraction than the first contraction when collapsed across groups (P=0.046).
Skinfold thickness
There were no significant differences (F[2,12]=2.306, P=0.142, ηp2=0.278) among the AT (10.66±6.12 mm), RT (14.80±2.53 mm) and SED (21.37±12.08 mm).
Correlations
Pearson’s product moment correlations were not significant (P=0.296–0.944; r=-0.020-0.289) when comparing skinfold thickness to the b terms from the linearly increasing and decreasing segments or MMGRMS from the steady force of the first and last contraction, respectively (Figure 6).
Figure 6.
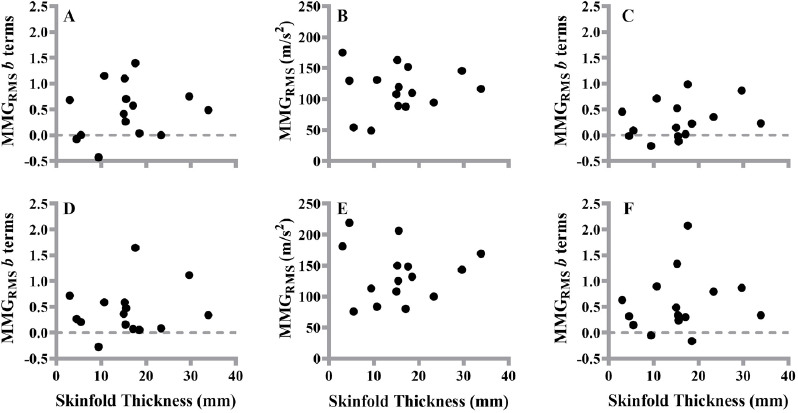
The plotted relationships between skinfold thickness (mm) and the b terms during the linearly increasing (A, D) and decreasing (C, F) segments and mechanomyographic amplitude (MMGRMS) (B, E) during the steady force segment of the first (top graphs) and last (bottom graphs) 50% maximal voluntary contraction.
Discussion
Similar to previous studies[20,26], the RT demonstrated greater maximal strength than the AT and SED. Strength differences may be attributed to training adaptations that favor maximal force production, such as increases in muscle cross-sectional area[4], type II muscle fiber hypertrophy[27,28], and a greater increase in the size of higher- in relation to lower-threshold MUs[29,30]. In addition, only the AT completed all 20 submaximal muscle actions and maintained maximal strength at MVCPOST, which is likely due to the influence of greater type I%MHC[3,20]. Nevertheless, differences in maximal strength and fatigue resistance were influenced by chronic training status.
There were chronic training status related differences in MU activation strategies during the first trapezoidal muscle action, such as greater b terms for the RT compared to the AT[3,9]. It is well documented that type I fibers have slower twitch relaxation rates[31], which allows tetanus at lower frequencies than type II fibers[32]. Herda et al.[3], Fry et al.[20] and Beck et al.[11] previously reported that type I%MHC expression for the VL was significantly greater for chronic AT- compared to RT- and SED-individuals when using a similar criteria as the current study to categorize individuals. In addition, it has been reported that MU firing rates for the VL are significantly increased with chronic aerobic training[6] and greater type I%MHC expression[33]. Thus, due to the aforementioned chronic training related neurological and MHC adaptations, the AT would likely possess a greater percentage of MUs that fuse[16,34], which would negatively affect MMGRMS[35] and may explain the lower b terms for the AT compared to the RT in the current study. The b terms for the SED were not different than the AT. It is plausible our 50% contraction did not recruit enough of the highest threshold MUs to result in group differences previously reported between the SED and AT[3,9].
During the first trapezoidal muscle action, only the RT exhibited muscle action-related differences, such as, greater b terms during the linearly increasing in comparison to the linearly decreasing segment. The 12-s steady force segment may have increased active muscle stiffness or altered MU deactivation strategies for the RT. A likely explanation is MU potentiation, which would allow MUs to derecruit at higher force levels than recruitment due to increased MU twitch forces throughout the contraction, and has previously been reported for RT individuals[6]. Thus, the same level of force (%MVC) could be produced with less MUs for the linearly decreasing segment and, subsequently, reduce MMGRMS for the RT. Conversely, it has been reported that chronic AT individuals derecruit MUs at lower force values compared to recruitment, and it is suggested the divergent behavior between chronic AT and RT individuals was due to a greater type II MHC for the RT[6]. In addition, the b terms between the linearly increasing and decreasing segments were not different for the SED. These findings provide further support that MU activation and deactivation strategies of the VL are not different for SED individuals during moderate intensity isometric trapezoidal contractions[10]. Nonetheless, training status resulted in muscle action-related differences for only the RT during a 50% isometric trapezoidal contraction.
No study has previously examined the effects of chronic training status on MMGRMS-force relationships during multiple isometric trapezoidal contractions. During the last contraction, there were training status related differences in MU deactivation strategies, such as greater b terms for the RT in comparison to the AT. It is well documented that MU twitch forces decrease during fatigue; consequently, the central nervous system will increase input excitation to the MU pool to meet the targeted contraction intensity[5,36]. As fatigue increases, MU recruitment thresholds decrease and the subsequent increase of input excitation recruits additional higher-threshold MUs[36-38]. The lack of differences between groups for the linearly increasing segment suggests RT recruited additional higher threshold MUs during steady force, which would decrease overall active muscle stiffness since they do not tetanize[34,39] and result in greater MMGRMS at the onset of the linearly decrease segment (Figure 3.). Even though the SED did not finish all 20 contractions, their b terms were not different among the AT and RT. It has been reported SED individuals possess difficulties activating the entire MU pool due to greater corticospinal inhibition[40,41]. Thus, it is possible the SED used a similar contribution of MU recruitment and firing rate increases to modulate force, whereas the RT relied primarily on recruitment while the AT relied on firing rate increases (95% CIs included 0). Nonetheless, the b terms differentiated MU deactivation strategies for the RT in comparison to the AT during the linearly deceasing segment, while the SED was not different among groups.
The steady force segment of the isometric trapezoidal muscle action is similar to a step contraction[8,19] and may provide additional insight on adjustments in MU behavior between the linearly increasing and decreasing segments. Contrary to Beck et al.[11] but in agreement with Trevino and Herda[9], there were no training status related differences for the current study. It is plausible a longer steady force segment is necessary to differentiate among training status as Beck et al.[11] utilized a 30-s muscle action at 50% MVC, whereas we used a 12-s steady force segment during an isometric trapezoidal muscle action. Interestingly, MMGRMS was greater for all groups during the steady force segment of the last contraction. The amplitude of the MMG signal reflects MU recruitment[7] and, thus, the greater value for the last contraction suggest all groups increased the activation of higher threshold MUs to maintain the targeted force. The findings support the use of all three force segments when examining the mechanical behavior of muscle as the b terms for the linearly decreasing segment without a preceding steady force segment would suggest only the RT increased recruitment to match the targeted force for the last contraction.
It should be noted that skinfold thickness may low pass filter the MMG signal[25,42]. Skinfold thicknesses were not significantly different among the AT, RT, and SED, tentatively suggesting that skinfold thickness was not an explanation for the chronic training related differences in the linearity of the MMGRMS patterns of response during the increasing and decreasing muscle actions, and the lack of group differences at steady force. However, it is possible there would have been significant differences in skinfold thickness among groups with a larger sample size. We believe researchers should always examine the relationship between skinfold thickness/subcutaneous fat at the sensor site with the MMG variables of interest to provide confidence filtering is not influencing the results. For the current study, the lack of relationships between skinfold thickness and the b terms for all muscle actions is in agreement with previous investigations[3,9,16,17,25] and provides further evidence skinfold thickness was not a contributing factor for the findings.
Conclusion
This is the first study to investigate MU control strategies among chronic training statuses during repetitive isometric trapezoidal muscle actions. The MMGRMS-force relationships during the linearly increasing segment of the first contraction and the linearly decreasing segment of the last contraction suggest a greater reliance on motor unit firing rates to modulate force or greater active muscle stiffness for the AT compared to the RT. In addition, only the RT exhibited muscle action-related differences. The greater acceleration in the MMGRMS-force patterns during the linearly increasing than decreasing segment during the first contraction suggests the relative contributions of recruited MUs and their firing rates were less during the ramp down for the RT. MMGRMS-force relationships for the SED were not different among groups and between muscle actions despite not being able to complete all 20 contractions, suggesting alterations in the mechanical behavior of the muscle occur following chronic aerobic- and resistance-training. In addition, the RT demonstrated the greatest maximal strength for pre- and post-testing; however, only the AT were able to complete all 20 isometric trapezoidal contractions and maintain maximal strength. Therefore, knee extensor maximal strength and fatigability, and MU control strategies for the VL during a series of repetitive contractions were influenced by chronic training status. Future research should utilize higher contraction intensities and/or longer steady force segments to examine MU control activation and deactivation strategies during task failure for chronic aerobically trained individuals.
Authors’ contributions
TJH and MAT designed the experiments, MAT, TJH, SAS, and AAO conducted the experiments, MAT, TJH, SAS, and AAO analyzed the data. AAO wrote the manuscript. MAT, TJH, SAS, and AAO edited and revised the manuscript. All authors approved the final version of the manuscript submitted for publication and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Acknowledgments
We are grateful for the contributions of the undergraduate research assistants in the Neuromechanics Laboratory for their assistance in performing the experiments.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Kraemer WJ, Häkkinen K, Newton RU, Nindl BC, Volek JS, McCormick M, Gotshalk LA, Gordon SE, Fleck SJ, Campbell WW. Effects of heavy-resistance training on hormonal response patterns in younger vs. older men. Journal of Αpplied Πhysiology. 1999;87(3):982–992. doi: 10.1152/jappl.1999.87.3.982. [DOI] [PubMed] [Google Scholar]
- 2.Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. American Journal of Physiology-Endocrinology and Metabolism. 2004;286(1):E92–E101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- 3.Herda TJ, Housh TJ, Fry AC, Weir JP, Schilling BK, Ryan ED, Cramer JT. A noninvasive, log-transform method for fiber type discrimination using mechanomyography. Journal of Electromyography and Kinesiology. 2010;20(5):787–794. doi: 10.1016/j.jelekin.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Häkkinen K, Keskinen K. Muscle cross-sectional area and voluntary force production characteristics in elite strength-and endurance-trained athletes and sprinters. European journal of applied physiology and occupational physiology. 1989;59(3):215–220. doi: 10.1007/BF02386190. [DOI] [PubMed] [Google Scholar]
- 5.De Luca C, LeFever R, McCue M, Xenakis A. Behaviour of human motor units in different muscles during linearly varying contractions. The Journal of physiology. 1982;329(1):113–128. doi: 10.1113/jphysiol.1982.sp014293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herda TJ, Siedlik JA, Trevino MA, Cooper MA, Weir JP. Motor unit control strategies of endurance-versus resistance-trained individuals. Muscle &nerve. 2015;52(5):832–843. doi: 10.1002/mus.24597. [DOI] [PubMed] [Google Scholar]
- 7.Orizio C. Muscle sound:Bases for the. Journal of Critical Reviews in Biomedical Engineering. 1993;21(3):201–43. [PubMed] [Google Scholar]
- 8.Beck TW, Housh TJ, Johnson GO, Weir JP, Cramer JT, Coburn JW, Malek MH. Mechanomyographic amplitude and mean power frequency versus torque relationships during isokinetic and isometric muscle actions of the biceps brachii. Journal of Electromyography and Kinesiology. 2004;14(5):555–564. doi: 10.1016/j.jelekin.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Trevino MA, Herda TJ. The effects of chronic exercise training status on motor unit activation and deactivation control strategies. Journal of sports sciences. 2016;34(3):199–208. doi: 10.1080/02640414.2015.1046396. [DOI] [PubMed] [Google Scholar]
- 10.Trevino MA, Herda TJ. Mechanomyographic mean power frequency during an isometric trapezoid muscle action at multiple contraction intensities. Physiological measurement. 2015;36(7):1383. doi: 10.1088/0967-3334/36/7/1383. [DOI] [PubMed] [Google Scholar]
- 11.Beck TW, Housh T, Fry A, Cramer J, Weir J, Schilling B, Falvo M, Moore C. The influence of muscle fiber type composition on the patterns of responses for electromyographic and mechanomyographic amplitude and mean power frequency during a fatiguing submaximal isometric muscle action. Electromyography and clinical neurophysiology. 2007;47(4-5):221–232. [PubMed] [Google Scholar]
- 12.Herda TJ, Weir JP, Ryan ED, Walter AA, Costa PB, Hoge KM, Beck TW, Stout JR, Cramer JT. Reliability of absolute versus log-transformed regression models for examining the torque-related patterns of response for mechanomyographic amplitude. Journal of neuroscience methods. 2009;179(2):240–246. doi: 10.1016/j.jneumeth.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 13.Orizio C, Perini R, Veicsteinas A. Muscular sound and force relationship during isometric contraction in man. European journal of applied physiology and occupational physiology. 1989;58(5):528–533. doi: 10.1007/BF02330708. [DOI] [PubMed] [Google Scholar]
- 14.Parra ME, Miller JD, Sterczala AJ, Kelly MR, Herda TJ. The reliability of the slopes and y-intercepts of the motor unit firing times and action potential waveforms versus recruitment threshold relationships derived from surface electromyography signal decomposition. European Journal of Applied Physiology. 2021;121(12):3389–3398. doi: 10.1007/s00421-021-04790-6. [DOI] [PubMed] [Google Scholar]
- 15.Colquhoun RJ, Tomko PM, Magrini MA, Muddle TW, Jenkins ND. The influence of input excitation on the inter-and intra-day reliability of the motor unit firing rate versus recruitment threshold relationship. Journal of neurophysiology. 2018;120(6):3131–3139. doi: 10.1152/jn.00490.2018. [DOI] [PubMed] [Google Scholar]
- 16.Trevino MA, Herda TJ, Fry AC, Gallagher PM, Vardiman JP, Mosier EM, Miller JD. The influence of myosin heavy chain isoform content on mechanical behavior of the vastus lateralis in vivo. Journal of Electromyography and Kinesiology. 2016;28:143–151. doi: 10.1016/j.jelekin.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Sontag SA, Trevino MA, Herda TJ, Sterczala AJ, Miller JD, Parra ME, Dimmick HL, Deckert J. Endurance training alters motor unit activation strategies for the vastus lateralis, yet sex-related differences and relationships with muscle size remain. European Journal of Applied Physiology. 2021;121(5):1367–1377. doi: 10.1007/s00421-021-04622-7. [DOI] [PubMed] [Google Scholar]
- 18.Herda TJ, Ryan ED, Beck TW, Costa PB, DeFreitas JM, Stout JR, Cramer JT. Reliability of mechanomyographic amplitude and mean power frequency during isometric step and ramp muscle actions. Journal of Νeuroscience Μethods. 2008;171(1):104–109. doi: 10.1016/j.jneumeth.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Ryan ED, Beck TW, Herda TJ, Hartman MJ, Stout JR, Housh TJ, Cramer JT. Mechanomyographic amplitude and mean power frequency responses during isometric ramp vs. step muscle actions. Journal of Νeuroscience Μethods. 2008;168(2):293–305. doi: 10.1016/j.jneumeth.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Fry AC, Housh TJ, Cramer JB, Weir JP, Beck TW, Schilling BK, Miller JD, Nicoll JX. Noninvasive assessment of skeletal muscle myosin heavy chain expression in trained and untrained men. The Journal of Strength &Conditioning Research. 2017;31(9):2355–2362. doi: 10.1519/JSC.0000000000001645. [DOI] [PubMed] [Google Scholar]
- 21.Contessa P, De Luca CJ, Kline JC. The compensatory interaction between motor unit firing behavior and muscle force during fatigue. Journal of Νeurophysiology. 2016;116(4):1579–1585. doi: 10.1152/jn.00347.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beck TW, DeFreitas JM, Stock MS, Dillon MA. An examination of mechanomyographic signal stationarity during concentric isokinetic, eccentric isokinetic and isometric muscle actions. Physiological Μeasurement. 2010;31(3):339. doi: 10.1088/0967-3334/31/3/005. [DOI] [PubMed] [Google Scholar]
- 23.Bahamonde R, De Witt BS M, Mikesky A. Effects of acute exercise on skinfold measurements. International Journal of Fitness. 2009;5(1) [Google Scholar]
- 24.Jackson AS, Pollock ML. Practical assessment of body composition. The Physician and Sportsmedicine. 1985;13(5):76–90. doi: 10.1080/00913847.1985.11708790. [DOI] [PubMed] [Google Scholar]
- 25.Cooper MA, Herda TJ, Vardiman JP, Gallagher PM, Fry AC. Relationships between skinfold thickness and electromyographic and mechanomyographic amplitude recorded during voluntary and non-voluntary muscle actions. Journal of Electromyography and Kinesiology. 2014;24(2):207–213. doi: 10.1016/j.jelekin.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Kanehisa H, Ikegawa S, Fukunaga T. Force-velocity relationships and fatiguability of strength and endurance-trained subjects. International journal of Sports Medicine. 1997;18(02):106–112. doi: 10.1055/s-2007-972604. [DOI] [PubMed] [Google Scholar]
- 27.Staron R, Karapondo D, Kraemer W, Fry A, Gordon S, Falkel JE, Hagerman F, Hikida R. Skeletal muscle adaptations during early phase of heavy-resistance training in men and women. Journal of applied physiology. 1994;76(3):1247–1255. doi: 10.1152/jappl.1994.76.3.1247. [DOI] [PubMed] [Google Scholar]
- 28.Staron R, Malicky E, Leonardi M, Falkel J, Hagerman F, Dudley G. Muscle hypertrophy and fast fiber type conversions in heavy resistance-trained women. European Journal of Applied Physiology and Occupational Physiology. 1990;60(1):71–79. doi: 10.1007/BF00572189. [DOI] [PubMed] [Google Scholar]
- 29.Sterczala AJ, Miller JD, Dimmick HL, Wray ME, Trevino MA, Herda TJ. Eight weeks of resistance training increases strength, muscle cross-sectional area and motor unit size, but does not alter firing rates in the vastus lateralis. European Journal of Applied Physiology. 2020;120(1):281–294. doi: 10.1007/s00421-019-04273-9. [DOI] [PubMed] [Google Scholar]
- 30.Pope ZK, Hester GM, Benik FM, DeFreitas JM. Action potential amplitude as a noninvasive indicator of motor unit-specific hypertrophy. Journal of Neurophysiology. 2016;115(5):2608–2614. doi: 10.1152/jn.00039.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiles C, Young A, Jones D, Edwards R. Relaxation rate of constituent muscle-fibre types in human quadriceps. Clinical Science. 1979;56(1):47–52. doi: 10.1042/cs0560047. [DOI] [PubMed] [Google Scholar]
- 32.Mealing D, Long G, McCarthy P. Vibromyographic recording from human muscles with known fibre composition differences. British Journal of Sports Medicine. 1996;30(1):27–31. doi: 10.1136/bjsm.30.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trevino MA, Herda TJ, Fry AC, Gallagher PM, Vardiman JP, Mosier EM, Miller JD. Influence of the contractile properties of muscle on motor unit firing rates during a moderate-intensity contraction in vivo. Journal of Neurophysiology. 2016;116(2):552–562. doi: 10.1152/jn.01021.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Luca CJ, Contessa P. Biomechanical benefits of the onion-skin motor unit control scheme. Journal of Biomechanics. 2015;48(2):195–203. doi: 10.1016/j.jbiomech.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bichler E. Mechanomyograms recorded during evoked contractions of single motor units in the rat medial gastrocnemius muscle. European Journal of Applied Physiology. 2000;83(4):310–319. doi: 10.1007/s004210000261. [DOI] [PubMed] [Google Scholar]
- 36.Adam A, De Luca CJ. Firing rates of motor units in human vastus lateralis muscle during fatiguing isometric contractions. Journal of Applied Physiology. 2005;99(1):268–280. doi: 10.1152/japplphysiol.01344.2004. [DOI] [PubMed] [Google Scholar]
- 37.Contessa P, Luca CJD. Neural control of muscle force:indications from a simulation model. Journal of Neurophysiology. 2013;109(6):1548–1570. doi: 10.1152/jn.00237.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Contessa P, Adam A, De Luca CJ. Motor unit control and force fluctuation during fatigue. Journal of Applied Physiology. 2009;107(1):235–243. doi: 10.1152/japplphysiol.00035.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akataki K, Mita K, Watakabe M. Electromyographic and mechanomyographic estimation of motor unit activation strategy in voluntary force production. Electromyography and Clinical Neurophysiology. 2004;44(8):489–496. [PubMed] [Google Scholar]
- 40.Kidgell DJ, Pearce AJ. Corticospinal properties following short-term strength training of an intrinsic hand muscle. Human Movement Science. 2010;29(5):631–641. doi: 10.1016/j.humov.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Christie A, Kamen G. Cortical inhibition is reduced following short-term training in young and older adults. Age. 2014;36(2):749–758. doi: 10.1007/s11357-013-9577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaskólska A, Brzenczek W, Kisiel-Sajewicz K, Kawczyński A, Marusiak J, Jaskólski A. The effect of skinfold on frequency of human muscle mechanomyogram. Journal of Electromyography and Kinesiology. 2004;14(2):217–225. doi: 10.1016/j.jelekin.2003.08.001. [DOI] [PubMed] [Google Scholar]


