Abstract
Objective:
To evaluate the effects of a core stability exercise program on balance, coordination, and severity of ataxia in children with cerebellar ataxic cerebral palsy (CP).
Methods:
Forty children with cerebellar ataxic CP (mean age: 6.75±1.35 years) were randomly assigned to a control group and an intervention group for 2 months of follow-up. The control group received a standard physical therapy program three times weekly (1 h per session), while the intervention group received a core stability program for 30 min, in addition to the selected physical therapy program. Both groups were evaluated pre-treatment and post-treatment using the Scale for the Assessment and Rating of Ataxia, the Balance Error Scoring Systems scale, Bruininks-Oseretsky tests of motor proficiency, and HUMAC balance system scores.
Results:
We found statistically significant reductions in the severity of ataxia, as well as improved balance and coordination in both groups, with stronger effects observed in the intervention group (P<0.05).
Conclusion:
The core stability program can improve balance and coordination in children with cerebellar ataxic CP when incorporated with a standard physical therapy program.
Keywords: Ataxic Children, Balance, Coordination, Core Stability, HUMAC balance system
Introduction
Ataxic cerebral palsy (CP) accounts for approximately 5–10% of children with CP and is classified into subtypes (cerebellar ataxia and ataxic diplegia due to lower limb spasticity)[1]. One in four children with ataxic CP is classified as having ataxic diplegic CP[2]. Ataxic diplegia has pathogenetic and clinical presentations, such as spastic diplegia, rather than simple ataxia. In ataxic diplegic children, all extremities are neurologically affected. There is more voluntary control in the upper extremities, spasticity and stiffness in the lower extremities that affect children’s ability to roll, sit, stand, and ambulate; as well as signs and symptoms of loss of balance and coordination[1].
Children with cerebellar ataxic CP present with hypoplasia or malformations of the cerebellum, which impairs integration of the neural information required to coordinate smooth movement and balance[3].
Children with cerebellar ataxic CP have poor coordination, poor balance, fine motor problems, axial and appendicular ataxia, dysmetria, generalized hypotonia, intention tremors, and movement performance with abnormal force, rhythm, and accuracy[4]; they do not develop contractures and experience cognitive delays and slurred speech[5]. Cerebellar ataxia results in a staggering gait, synergic movement, and nystagmus due to the incoordination of eye movements[6]. Children with cerebellar ataxic CP have poor trunk control, stand with a wide base of support, walk with an unsteady gait (veering toward the more affected side), sway and stop during walking, and may take backward steps to avoid falling[7]. Children with cerebellar ataxic CP have poor coordination; dysmetria during finger-to-finger, finger-to-nose, and heel-to-knee maneuvers; and dysregulation during rapid alternating hand movements[8]. They have positive Romberg signs with their eyes closed[8]. Cerebellar ataxia leads to problems in regulating the force, range, direction, velocity, and rhythm of muscle contractions, leading to asthenia, asynergy, delayed reaction time, and dyschronometria[9].
Postural control is impaired in children with cerebellar ataxic CP due to an imbalance between agonist and antagonist muscles, weak equilibrium, and protective reactions[10]. Children with cerebellar ataxic CP have poor core stability due to weakness of the abdominal and back muscles, and there is a positive correlation between core stability and functional activities[11]. Core stability programs have been shown to improve muscle strength, fix trunk stability and endurance, and enhance limb movement[12,13]. Core stability improves abdominal, back, pelvic, and shoulder muscle strength, all of which are responsible for maintaining trunk stability and endurance during static and dynamic activities[14,15].
Core stability training improves standing and walking abilities, as well as endurance and balance in children with ataxic CP[16-18]. Although there are several studies on the effects of core stability programs on motor function in children with CP, no currently available studies have determined the efficacy of such a training model in children with cerebellar ataxic CP. Therefore, the purpose of the current study was to explore the efficacy of a core stability exercise program on balance and coordination in children with cerebellar ataxic CP.
Methods
Study Design
This study was a pretest-posttest randomized controlled trial conducted between October 2018 and May 2021. The study procedures were conducted in accordance with the 1975 Declaration of Helsinki’s ethical standards and its latest 2013 update. Informed consent was obtained from all participants’ caregivers, and the clinical trial was registered with ClinicalTrials.gov (NCT04823936).
Patients
Forty-five children with cerebellar ataxic CP were selected from private pediatric physical therapy centers. The participants were aged 5–9 years. They were randomly assigned to a control or an intervention group using the Statistical Package for the Social Sciences (SPSS) software (SPSS, Inc., Chicago, IL, USA). Included children had a motor function level IV as per the Gross Motor Function Classification System[19], had an ataxia severity score ranging from 10 to 25 based on the Scale for Assessment and Rating of Ataxia (SARA)[19]. Children were excluded if they had visual impairments, spasticity, uncontrolled convulsions, any other neuromuscular diseases, ataxia-telangiectasia, spinocerebellar ataxia, and/or Joubert syndrome.
Sample size calculation was performed using G*POWER statistical software (version 3.1.9.2: Franz Faul, Universität Kiel, Germany) and revealed that the required sample size for this study was 17 patients per group. Calculations were made using α=0.05, β=0.2. The sample size was increased to 45 children to overcome the dropout rate.
Outcome Measures
Comparative measurements were performed for both groups prior to the intervention and after 2 months of intervention. Measurements were conducted by an independent assessor blinded to patient allocation to eliminate experimental biases that arise from a participant’s expectations and assessor effect on the participants. The severity of ataxia was measured via SARA scoring and was characterized by assessments of gait, stance, sitting, speech, fast alternating movements, and the finger-chase, nose-finger, and heel-shin tests[20]. Stability and posture steadiness were measured using the balance error scoring systems scale (BESS), with testing performed on the ground and on a foam block[21]. Coordination was measured using the Bruininks-Oseretsky Test of Motor Proficiency (BOT-2); subtests 4 and 7 were applied to measure bilateral and upper limb coordination, respectively[22]. Balance was measured using the HUMAC Balance System scoring, a high-quality computerized balance system developed by Computer Sports Medicine, Inc. (CSMi, Stoughton, MA, USA)[23].
Intervention
The control group received the standard selected physical therapy program for 1 h three times weekly for two successive months. This program included facilitation of balance and protective reactions while kneeling, half kneeling, and standing; standing alone on a balance board; standing on one leg; open gait training on a balance beam and stepper; walking on wedges; stair climbing; and strengthening back and abdominal muscles. The intervention group received the selected physical therapy program for 1 h three times weekly, in addition to the core stability program for 30 min. The frequency of repetitions of each exercise depended on the child’s tolerance and was increased according to the child’s ability[24], as illustrated in Table 1.
Table 1.
Illustration of the core stability program.
| Description | Repetition |
|---|---|
| Abdominal draw-in with a double knee to chest | 10- 15 times |
| Trunk twist while sitting on a medicine ball | 10- 15 times |
| Lying in a supine position on a medicine ball and rotating the trunk from side-to-side | 10- 15 times |
| Lying in a supine position on a mat and pulling upper and lower limbs upward | 10- 15 times |
| Bridging from a supine lying position on the left leg, while raising the right leg | 10- 15 times |
| Bridging from a supine lying position on the right leg, while raising the left leg | 10- 15 times |
| Quadrupled position - raising the right upper limb and the left lower limb | 5 minutes |
| Quadrupled position - raising the left upper limb and the right lower limb | 5 minutes |
Statistical Analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 26 (SPSS, Inc., Chicago, IL, USA). The mean and standard deviation (SD) were calculated for all demographic characteristics. A paired t-test was used for within-group comparisons before and after the intervention. An independent t-test was applied to compare the demographic data and baseline characteristics of both groups, as well as the pre-treatment and post-treatment mean differences in all measured variables. The threshold for statistical significance was set at P<0.05.
Results
Forty-five children with ataxic CP were assessed for eligibility at the beginning of the study; 40 children met the study inclusion criteria and were assigned to one of two groups. Four children did not complete the study (two due to infrequent attendance and two withdrew from the study because of changing their residence). Finally, 36 children completed the study procedure. The data were statistically analyzed, as shown in Figure 1.
Figure 1.
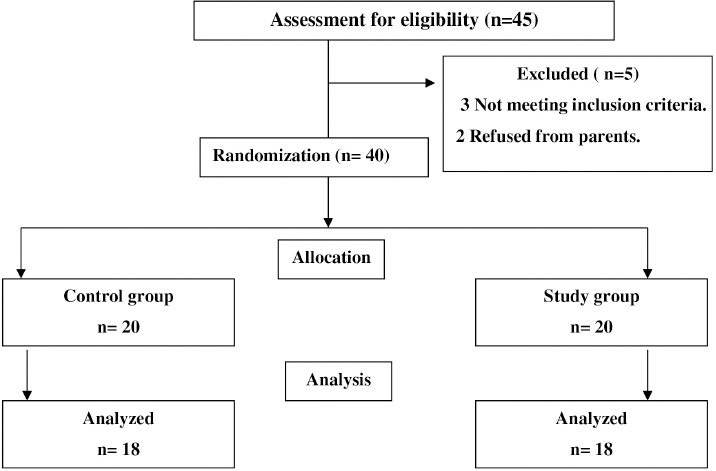
Flow chart.
General Patient Characteristics
There were no statistically significant differences between the groups in terms of baseline characteristics. The mean ages of the control and intervention groups were 6.5±1.5 and 7.0±1.2 years, respectively (P=0.19). The mean body mass indices (BMIs) for the control and intervention groups were 23±1.5 and 24±0.5 kg/m2, respectively (P=0.11). There were no statistically significant differences between the two groups in terms of sex, with a Chi-squared value of 0.21 (P=0.71).
Pre-treatment Comparison between Both Groups
As shown in Table 2, there were no statistically significant differences between the groups prior to the study intervention in any of the measured variables (P>0.05).
Table 2.
Pre-treatment comparison between both groups.
| Control group x¯ ±SD | Study group x¯ ±SD | P-Value | ||
|---|---|---|---|---|
| Severity of ataxia (scale score) | 24±5 | 23±1 | 0.13* | |
| Balance Error Scoring Systems (scale score) | 30 | 31 | 0.29* | |
| Bilateral coordination (point score) | 10 | 11 | 0.67* | |
| Upper limb coordination (point score) | 20 | 21 | 0.12* | |
| Mobility Standing Balance Bilateral (%) | 45.34±11.22 | 46.83±12.8 | 0.22* | |
| Stability Standing Balance Bilateral (%) | 49.57±7.91 | 50.45±8.34 | 0.17* | |
| The limit of stability test | 20.77±5.42 | 21.61±6 | 0.26* | |
| Modified clinical test of sensory integration of balance. | Eye open soft surface (%) | 65.25±10.29 | 64.87±9.45 | 0.21* |
| Eye open firm surface (%) | 58.94±8.92 | 59.27±7.34 | 0.76* | |
| Eye closed soft surface (%) | 70.24±15.27 | 71.41±11.39 | 0.2* | |
| Eye closed firm surface (%) | 62.4±14.65 | 63.18±13.03 | 0.87* | |
x¯ ±SD: mean±standard deviation, P: level of significant,
: non-significant.
Pre-treatment and Post-treatment Comparison for Both Groups
As shown in Table 3, there was a statistically significant improvement in all measured variables following the intervention (P<0.05).
Table 3.
Pre and post-treatment comparison between both groups.
| Variable | Control group Mean ± SD | P-value | Study group Mean ± SD | P-value | |||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||
| Severity of ataxia (scale score) | 24±5 | 18±2 | 0.002** | 23±1 | 14±4 | 0.0001** | |
| Balance Error Scoring Systems (scale score) | 30 | 23 | 0.001** | 31 | 16 | 0.001** | |
| Bilateral coordination (point score) | 10 | 14 | 0.001** | 11 | 19 | 0.002** | |
| Upper limb coordination (point score) | 20 | 27 | 0.003** | 21 | 34 | 0.003** | |
| Mobility Standing Balance Bilateral (%) | 45.34±11.22 | 56.74±12.62 | 0.0001** | 46.83±12.8 | 70.42±14.61 | 0.0001** | |
| Stability Standing Balance Bilateral (%) | 49.57±7.91 | 60.81±8.12 | 0.004** | 50.45±8.34 | 73.05±9.11 | 0.001** | |
| The limit of stability test (%) | 20.77±5.42 | 28.41±6.13 | 0.001** | 21.61±6 | 40.7±2.21 | 0.003** | |
| Modified clinical test of sensory integration of balance. | Eye open soft surface (%) | 65.25±10.29 | 76.17±8.25 | 0.002** | 64.87±9.45 | 88.19±7.15 | 0.001** |
| Eye closed soft surface (%) | 58.94±8.92 | 71.66±10.17 | 0.0001** | 59.27±7.34 | 82.72±8.25 | 0.004** | |
| Eye open firm surface (%) | 70.24±15.27 | 81.24±7.07 | 0.001** | 71.41±11.39 | 90.96±12.38 | 0.001** | |
| Eye closed firm surface (%) | 62.4±14.65 | 71.59±13.19 | 0.003** | 63.18±13.03 | 83.09±10.82 | 0.0001** | |
x¯ ±SD: mean ± standard deviation, P: level of significant,
: significant.
Post-treatment Comparison
As presented in Table 4, there was a statistically significant difference in both groups after the intervention, with stronger effects observed for the intervention group in all measured variables (P<0.05).
Table 4.
Post-treatment comparison between both groups.
| Control group x¯ ±SD | Study group x¯ ±SD | P-Value | ||
|---|---|---|---|---|
| Severity of ataxia (scale score) | 18±2 | 14±4 | 0.0003** | |
| Balance Error Scoring Systems (scale score) | 23 | 16 | 0.002** | |
| Bilateral coordination (point score) | 14 | 19 | 0.001** | |
| Upper limb coordination (point score) | 27 | 34 | 0.0001** | |
| Mobility Standing Balance Bilateral (%) | 56.74±12.62 | 70.42±14.61 | 0.002** | |
| Stability Standing Balance Bilateral (%) | 60.81±8.12 | 73.05±9.11 | 0.001** | |
| The limit of stability test (%) | 28.41±6.13 | 40.7±2.21 | 0.0004** | |
| Modified clinical test of sensory integration of balance | Eye open soft surface (%) | 76.17±8.25 | 88.19±7.15 | 0.001** |
| Eye closed soft surface (%) | 71.66±10.17 | 82.72±8.25 | 0.002** | |
| Eye open firm surface (%) | 81.24±7.07 | 90.96±12.38 | 0.001** | |
| Eye closed firm surface (%) | 71.59±13.19 | 83.09±10.82 | 0.0003** | |
x¯ ±SD: mean ± standard deviation, P: level of significant,
: significant.
Discussion
Limited research studies have investigated the efficacy and validity of physical therapy rehabilitation programs and techniques for treating children with ataxic CP. To our knowledge, no prior study has evaluated the effects of core stability rehabilitation programs on children with cerebellar ataxic CP on the severity of ataxia, balance, and upper and lower limb coordination. Children with cerebellar ataxic CP level IV according to GMFCS are independent in their functional activities at home, but they show loss of balance and weak coordination, disturbing standing and walking abilities. This study aimed to determine the effects of core stability training on the severity of ataxia, balance, and coordination in children with cerebellar ataxic CP.
In addition to BESS, the HUMAC Balance System scoring was used to evaluate balance, posture steadiness, and the ability to stand over a soft and hard surface. Functional activities were measured by SARA as it evaluated functional activities such as standing, walking, and sitting; both SARA and BESS are strong indicators of functional activity improvement in children with cerebellar ataxic CP. Coordination was evaluated in the upper extremities and bilateral coordination was measured by BOT-2 together with SARA, which evaluates fast alternating movements, and the finger-chase, nose-finger, and heel-shin tests[20].
The results of the current study showed a statistically significant reduction in the severity of ataxia measured by SARA. This reduction was associated with statistically significant increases in upper limb and bilateral coordination measured by BOT-2 in both groups. There was a statistically significant improvement in balance, as measured by the BESS and HUMAC balance system scores in both groups.
There was a significant improvement in the intervention group compared to the control group, as core stability training programs improved the strength and endurance of the muscles stabilizing the trunk and improving the relationship between neural control and the musculoskeletal system. Core stability training programs focus on increasing trunk control and stability by increasing strength and muscle control in the trunk, shoulder girdle, and pelvis. Core stability training improves the ability of the nervous system to predict loss of balance, thus activating trunk musculature as it stimulates proprioception. Somatosensory control of the vestibular system is also stimulated during the application of the core stability exercise program as it detects body position in a static and dynamic position. This agrees with the results of a previous case series study that evaluated the effect of a multidimensional physical therapy program incorporating strength and stability exercise training in patients with ataxia. The study reported significant progress in coordination, balance, and balance confidence, which translated into an improvement in several areas of impairment and activity limitations[25]. Our results are also consistent with those of a previous single-patient research report, which examined the effects of trunk stabilization training on balance, gait, function, and trunk performance in an individual with ataxia and showed improvement in each of the measured variables after 10 weeks of training[25].
Children with cerebellar ataxic CP have weak posture reactions, trunk instability, loss of balance, and poor coordination. Core stability improved the connections between the abdominal, spine, shoulder girdle, and pelvic girdle muscles to stabilize the trunk and provide support for extremity activities. This, in turn, improves static and dynamic balance and balance on soft and hard objects with the eyes open and closed, improving upper and limb coordination and bilateral coordination. These observations agree with that in Stevens et al. (2007), who concluded that core stability education improves postural alignment in healthy children[26].
In the current study, core stability training improved trunk control, leading to an improvement in upper and lower limb function activities that appear in the statistics of the SARA scale results. This is consistent with the fact that proximal stability leads to proper distal localization and execution of motor activities, thus improving hand function activities. The core stability program evaluated in the current study improved corrective postural control mechanisms, thus improving upper and lower limb coordination and performance patterns of motor function leading to more harmonious, appropriate, and purposeful movements. Rose et al. stated that the abdominal and back muscles are bilaterally activated to improve balance and stabilization of the spine[27]. Core stability improves upper and lower limb activity, and enhances postural control[28]. Sterba et al. (2002) reported that trunk and pelvic strengthening exercises improved equilibrium, protective reactions, and reciprocal coordination of the lower extremities in children with CP[29].
In the current study, core stability training improved children’s ability to use the vestibular, somatosensory, and visual systems to maintain balance and stability. This is clearly demonstrated through the improvement of bilateral mobility standing and bilateral stability standing balance tests, as well as balance measured by a modified clinical test of sensory integration via the HUMAC balance system. This finding agrees with Ali et al. (2016), who stated that strengthening core muscle activation improved total stability limits, as well as anteroposterior and mediolateral balance as measured by the Biodex Balance System[30]. Core stabilization improved static and dynamic balance within standing tests and time up to go tests in children with spastic diplegic CP[31].
Systematic reviews have revealed that physical therapy programs based on muscle strengthening and balance training in patients with cerebellar ataxia improve balance and decrease the severity of ataxia (as measured by SARA)[32]. Balance training and muscle-strengthening exercises improve SARA scores and gait in ataxic adults[33]. Similarly, Ahmed et al. (2014) concluded that core stability is a cornerstone for postural control; developing and strengthening the core muscles allow for maintaining stability in upright positions and gives freedom to activities of the upper limb[34].
The generalizability of these results is subject to certain limitations. First, there was a limited number of the selected sample due to the COVID-19 pandemic and the low incidence of children with cerebellar ataxic CP. Second, the study was limited to ambulant children with ataxic CP with greater motor function activities, and the age range in the selected sample was 5–9 years. Third, the study measured balance and coordination only and did not consider the effect of core stability on muscle strength. Finally, this study lacked follow-up assessment beyond the post-treatment occasion.
For further investigations, researchers should focus on the following:
• the long-term effects of core stability programs on balance and coordination in children with ataxic CP.
• the effect of a core stability program on other functional activities such as sitting, rising reaction, and standing in non-ambulant children with ataxic CP.
• the effect of a core stability program on the fine motor activities of the upper limb in children with ataxic CP.
• the effect of core stability on the dyskinetic and spastic types of children with CP.
Conclusion
The core stability program can improve balance and coordination in children with cerebellar ataxic CP when incorporated with a standard physical therapy program.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.McHale DP, Jackson AP, Campbell, Levene MI, Corry P, Woods CG, Lench NJ, Mueller RF, Markham AF. A gene for ataxic cerebral palsy maps to chromosome 9p12-q12. Eur J Hum Genet. 2000;8(4):267–72. doi: 10.1038/sj.ejhg.5200445. [DOI] [PubMed] [Google Scholar]
- 2.Teive HA, Ashizawa T. Primary and secondary ataxias. Curr Opin Neurol. 2015;28(4):413–22. doi: 10.1097/WCO.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen A, Holzinger SD, Oskoui M, Shevell M, Registry CCP. Losing a diagnosis of cerebral palsy:a comparison of variables at 2 and 5 years. Dev Med Child Neurol. 2020;62(1):83–8. doi: 10.1111/dmcn.14309. [DOI] [PubMed] [Google Scholar]
- 4.Sankar C, Mundkur N. Cerebral palsy-definition, classification, etiology and early diagnosis. Indian J Pediatr. 2005;72(10):865–68. doi: 10.1007/BF02731117. [DOI] [PubMed] [Google Scholar]
- 5.Fahey MC, Maclennan AH, Kretzschmar D, Gecz J, Kruer MC. The genetic basis of cerebral palsy. Dev Med Child Neurol. 2017;59(5):462–69. doi: 10.1111/dmcn.13363. [DOI] [PubMed] [Google Scholar]
- 6.Pavone P, Praticò AD, Pavone V, Lubrano R, Falsaperla R, Rizzo R, Ruggieri M. Ataxia in children:early recognition and clinical evaluation. Ital J Pediatr. 2017;43(1):1–9. doi: 10.1186/s13052-016-0325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kruer MC. Pediatric movement disorders. Pediatr Rev. 2015;36(3):104–15;quiz 116, 129. doi: 10.1542/pir.36.3.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nass RD, Koch D. Child and adolescent neurology. Mosby's neurology psychiatric access series. St. Louis: Mosby; 1998. The neurologic examination of the young child. In:David RB, editor; pp. 53–70. [Google Scholar]
- 9.Tada M, Nishizawa M, Onodera O. Redefining cerebellar ataxia in degenerative ataxias:lessons from recent research on cerebellar systems. Journal of Neurology, Neurosurgery, and Psychiatry. 2015;86(8):922–8. doi: 10.1136/jnnp-2013-307225. [DOI] [PubMed] [Google Scholar]
- 10.de Graaf-Peters VB, Blauw-Hospers CH, Dirks T, Bakker H, Bos AF, Hadders-Algra M. Development of postural control in typically developing children and children with cerebral palsy:possibilities for intervention? Neurosc Biobehav Rev. 2007;31(8):1191–200. doi: 10.1016/j.neubiorev.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Sediek RH, El-Tohamy AM, Nassar I. Relation Between Core-Stability and Functional Abilities in Children with Spastic Cerebral Palsy. Trends Applied Sci Res. 2016;11(1):19–5. [Google Scholar]
- 12.Haruyama K, Kawakami M, Otsuka T. Effect of core stability training on trunk function, standing balance, and mobility in stroke patients:a randomized controlled trial. Neurorehabil Neural Repair. 2017;31(3):240–49. doi: 10.1177/1545968316675431. [DOI] [PubMed] [Google Scholar]
- 13.Reed CA, Ford KR, Myer GD, Hewett TE. The effects of isolated and integrated 'core stability'training on athletic performance measures. Sports Med. 2012;42(8):697–6. doi: 10.2165/11633450-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briggs AM, Greig AM, Wark JD, Fazzalari NL, Bennell KL. A review of anatomical and mechanical factors affecting vertebral body integrity. Int J Med Sci. 2004;1(3):170–80. doi: 10.7150/ijms.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zazulak BT, Hewett TE, Reeves NP, Goldberg B, Cholewicki J. The effects of core proprioception on knee injury:a prospective biomechanical-epidemiological study. Am J Sports Med. 2007;35(3):368–73. doi: 10.1177/0363546506297909. [DOI] [PubMed] [Google Scholar]
- 16.El Shemy SA. Trunk endurance and gait changes after core stability training in children with hemiplegic cerebral palsy:A randomized controlled trial. J Back Musculoskelet Rehabil. 2018;31(6):1159–67. doi: 10.3233/BMR-181123. [DOI] [PubMed] [Google Scholar]
- 17.Huang C, Chen Y, Chen G, Xie Y, Mo J, Li K, Huang R, Pan G, Cai Y, Zhou L. Efficacy and safety of core stability training on gait of children with cerebral palsy:A protocol for a systematic review and meta-analysis. Medicine. 2020;99(2):1–5. doi: 10.1097/MD.0000000000018609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang WR, WU XP, Zhang QF, Xin L, YU LM. Effects of Strengthened Core Stability Training with Band on Motor Function and Balance in Children with Spastic Cerebral Palsy. Chinese Journal of Rehabilitation Theory and Practice. 2018;24(1):97–100. [Google Scholar]
- 19.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 20.Weyer A, Abele M, Schmitz-Hübsch T, Schoch B, Frings M, Timmann D, Klockgether T. Reliability and validity of the scale for the assessment and rating of ataxia:a study in 64 ataxia patients. Mov Disord. 2007;22(11):1633–37. doi: 10.1002/mds.21544. [DOI] [PubMed] [Google Scholar]
- 21.Melo RDS, Lemos A, Macky CFDST, Raposo MCF., Ferraz K. M. Postural control assessment in students with normal hearing and sensorineural hearing loss. Braz J Otorhinolaryngol. 2015;81(4):431–38. doi: 10.1016/j.bjorl.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deitz JC, Kartin D, Kopp K. Review of the Bruininks-Oseretsky test of motor proficiency, (BOT-2) Phys Occup Ther Pediatr. 2007;27(4):87–102. [PubMed] [Google Scholar]
- 23.Blosch C, Schäfer R, de Marées M, Platen P. Comparative analysis of postural control and vertical jump performance between three different measurement devices. PloS One. 2019;14(9):1–16. doi: 10.1371/journal.pone.0222502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farid R, Norasteh AA, Hatamian H. The effect of core stability exercise program on the balance of patients with multiple sclerosis. Caspian J Neurol Sci. 2016;2(4):9–17. [Google Scholar]
- 25.Sartor-Glittenberg C, Brickner LA. multidimensional physical therapy program for individuals with cerebellar ataxia secondary to traumatic brain injury:a case series. Physiother Theory Pract. 2014;30(2):138–48. doi: 10.3109/09593985.2013.819952. [DOI] [PubMed] [Google Scholar]
- 26.Stevens VK, Vleeming A, Bouche KG, Mahieu NN, Vanderstraeten GG, Danneels LA. Electromyographic activity of trunk and hip muscles during stabilization exercises in four-point kneeling in healthy volunteers. Eur Spine J. 2007;16(5):711–18. doi: 10.1007/s00586-006-0181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose J, Wolff DR, Jones VK, Bloch DA, Oehlert JW, Gamble JG. Postural balance in children with cerebral palsy. Dev Med Child Neurol. 2002;44(1):58–63. doi: 10.1017/s0012162201001669. [DOI] [PubMed] [Google Scholar]
- 28.Dodd K, Taylor N, Damiano D. Systemic review of strengthening for individuals with cerebral palsy. Arch Phys Med Rehabil. 2002;83(8):1157–64. doi: 10.1053/apmr.2002.34286. [DOI] [PubMed] [Google Scholar]
- 29.Sterba JA, Rogers BT, France AP, Vokes DA. Horseback riding in children with cerebral palsy:effect on gross motor function. Dev Med Child Neurol. 2002;44(5):301–8. doi: 10.1017/s0012162201002122. [DOI] [PubMed] [Google Scholar]
- 30.Ali MSM, Elazem F, Anwar GM. Effect of core stabilizing program on balance in spastic diplegic cerebral palsy children. Int J PharmTech Res. 2016;9(5):129–36. [Google Scholar]
- 31.Chung EJ, Kim JH, Lee BH. The effects of core stabilization exercise on dynamic balance and gait function in stroke patients. J Phys Ther Sci. 2013;25(7):803–6. doi: 10.1589/jpts.25.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akiyoshi M. Physical Therapy for Cerebellar Ataxia Neurological Physical Therapy. Toshiaki Suzuki, IntechOpen. 2017;10:157–70. [Google Scholar]
- 33.Miyai I, Ito M, Hattori N, Mihara M, Hatakenaka M, Yagura H, Sobue G, Nishizawa M. Cerebellar ataxia rehabilitation trial in degenerative cerebellar diseases. Neurorehabil Neural Repair. 2012;26(5):515–22. doi: 10.1177/1545968311425918. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed MA, Abd El Azeim FH, Abd El Raouf ER. The problem solving strategy of poor core stability in children with cerebral palsy:a clinical trial. J Pediatr Neonatal Care. 2014;1(6):1–6. [Google Scholar]


