Abstract
Structural musculoskeletal adaptations following amputation, such as bone mineral density (BMD) or muscle architecture, are often overlooked despite their established contributions to gait rehabilitation and the development of adverse secondary physical conditions. The purpose of this review is to provide a summary of the existing literature investigating musculoskeletal adaptations in individuals with major lower-limb amputations to inform clinical practice and provide directions for future research. Google Scholar, PubMed, and Scopus were searched for original peer-reviewed studies that included individuals with transtibial or transfemoral amputations. Summary data of twenty-seven articles indicated reduced BMD and increased muscle atrophy in amputees compared to controls, and in the amputated limb compared to intact and control limbs. Specifically, BMD was reduced in T-scores and Z-scores, femoral neck, and proximal tibia. Muscle atrophy was evidenced by decreased thigh cross-sectional area, decreased quadriceps thickness, and increased amounts of thigh fat. Overall, amputees have impaired musculoskeletal health. Future studies should include dysvascular etiologies to address their effects on musculoskeletal health and functional mobility. Moreover, clinicians can use these findings to screen increased risks of adverse sequelae such as fractures, osteopenia/porosis, and muscular atrophy, as well as target specific rehabilitation exercises to reduce these risks.
Keywords: Anatomy, Limb Loss, Physiology, Remodeling, Symmetry
Introduction
Major lower-limb amputations occurring proximal to the tarsometatarsal joint are experienced by a variety of adults, ranging from young veterans with traumatic etiologies to elderly individuals with dysvascular etiologies[1]. Gait adaptations post-amputation lead to changes in loading and muscle recruitment strategies[2], which influences asymmetries between amputated and intact limbs. Specifically, less force is transmitted through the amputated limb compared to the intact limb, resulting in musculoskeletal remodeling[3,4]. These adaptations often result in adverse clinical sequelae in this population, such as osteopenia and muscle atrophy, which may influence functional mobility and quality of life[5,6].
Structural musculoskeletal properties are defined in this manuscript as anatomical or physiological components, such as bone mineral density (BMD) and muscle architecture. Structural musculoskeletal properties have been understudied despite their known contributions to gait and rehabilitation post-amputation[7]. Several original research articles exist on the epidemiology or prevalence of outcomes associated with structural musculoskeletal properties[8,9], and existing literature reviews address the clinical presentations such as leg strengthening exercise[6], low back pain[10], and walking ability[11,12]. A review by Gailey et al. (2015)[5] discussed musculoskeletal factors associated with negative secondary health effects, such as osteopenia, but only two of forty-four articles discussed structural musculoskeletal properties. A current review dedicated to structural musculoskeletal adaptations following amputation is lacking from the literature.
Since studies have found increased risks of osteopenia and muscle atrophy[13,14] along with increased adverse events such as falls and fractures[15,16] in the amputee population, a better understanding of the underlying structural changes can broaden the range of clinical solutions to increase symmetry between prosthetic and intact limbs and assess these risks. A summary of findings could also provide evidence to proactively monitor musculoskeletal health to predict fracture or fall risks, as well as inform recommendations of specific rehabilitation exercises.
Therefore, this review aims to summarize literature investigating structural musculoskeletal adaptations in individuals with unilateral major lower-limb amputations to inform clinical practice and provide directions for future research.
Methods
A search was performed on Dec 1st, 2021 in Google Scholar, PubMed, and Scopus to encompass peer-reviewed original research articles of structural musculoskeletal adaptations in individuals post-amputation, such as BMD and muscle architecture. References from included studies were also examined for inclusion.
The following search terms were used:
• Google Scholar: musculoskeletal OR muscle OR skeletal OR bone OR physiol* OR anatom* AND amput* AND below-knee OR “below knee” transtibial OR above-knee OR “above knee” transfemoral OR leg OR lower-limb OR lower limb
• PubMed: ((((soft tissue injuries[MeSH Terms]) OR ((muscle, skeletal[MeSH Terms]) AND (pathology[MeSH Subheading])) OR ((muscular atrophy[MeSH Terms]) AND (pathology[MeSH Subheading])) OR ((adiposity[MeSH Terms]) AND (physiology[MeSH Subheading])) OR (adipose tissue[MeSH Terms])) OR (bone mineral density[MeSH Terms] OR bone mineral densit*[Title/Abstract] OR bone densit[Title/Abstract] OR BMD[Title/Abstract])) AND ((((amputees[MeSH Terms]) OR (amputation[MeSH Terms])) OR (amput*[Title/Abstract])) AND ((below-knee OR transtibial OR trans-tibial OR above-knee OR transfemoral OR trans-femoral OR leg OR lower-limb OR lower limb OR “above knee” OR “below knee”) OR ((extremities, lower[MeSH Terms]) ))))
• Scopus: musculoskeletal OR muscle OR skeletal OR bone OR physiol* OR anatom* AND amput* AND below-knee OR “below knee” transtibial OR above-knee OR “above knee” transfemoral OR leg OR lower-limb OR lower limb
Original research articles were included if they investigated underlying structural musculoskeletal properties, defined as anatomical or physiological components (e.g. BMD, muscle architecture), in the lower-limbs of individuals of any age after transtibial or transfemoral amputation surgery. Title screening excluded articles that did not include individuals with amputation or were not original research studies (e.g. literature reviews). Abstracts were excluded if they were not peer-reviewed or if they were case reports, defined as less than five participants. Full-text articles were excluded if they were not available in English, used computer modeling in place of participant testing, or did not contain structural musculoskeletal methodology. For example, while skin morphology is an important consideration[17], it was not the purpose of this review.
MGF adapted and applied search terms from a librarian at the University of North Texas Health Science Center and performed title screening. MGF, SK, and WN performed abstract and full-text screening. MGF and SK discussed all articles that passed full-text screening to ensure inclusion of appropriate articles and to ensure data was summarized accurately.
Results
A total of twenty-seven studies were included after applying inclusion and exclusion criteria (Figure 1). All studies are summarized in Table 1 and are grouped by whether they utilized skeletal (n=15, where n represents the number of studies) or muscular (n=12) methodology. Bemben et al. (2017) and Cavedon et al. (2021) were the only studies that utilized both skeletal and muscular methodology and both primarily utilized skeletal methodology[18,19].
Figure 1.
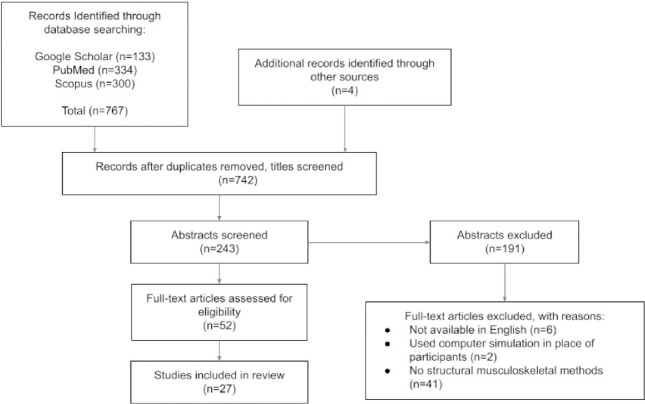
Flow diagram of the inclusion process.
Table 1.
Summary of Included Studies
| Author, Year | Title | Participants With Amputation | Methods | Time Points | Comparison | |
|---|---|---|---|---|---|---|
| Skeletal | Bemben et al. (2017) | Acute bone changes after lower limb amputation resulting from traumatic injury | 8 TT; Mean age 35.4 (SD 11.1); All traumatic etiologies | DXA measured BMD for total body, lumbar spine, femoral neck, proximal femur; pQCT measured residual limb volumetric BMD, stress-strain index, and muscle cross-sectional area | Prior to prosthesis fitting; 6 months post-prosthesis; 12 months post-prosthesis; additional blood draw occurred at time of surgery | Amputated vs intact limbs; over time points |
| Cavedon et al. (2021) | Body composition and bone mineral density in athletes with a physical impairment | 18 total 7 TT and 11 TF Mean age 34.4; All athletes of at least two years in adaptive sports | DXA measured whole-body and regional: total mass, lean mass, fat mass, % fat mass, fat mass/lean mass, BMC, and BMD | Cross-sectional; inclusion stated all athletes of at least two years in adaptive sports | Amputated vs intact limbs; amputee group vs spinal cord injury group vs control group | |
| Haket et al. (2017) | Periprosthetic cortical bone remodeling in patients with an osseointegrated leg prosthesis | 27 TF with osseointegration; 21 males, 6 females Mean age 48 (range 23-68); Mean TSA 18 years (range 2-45) | DXA measured BMD at the femoral neck (DXA only included 24 patients); X-ray measured periprosthetic cortical thickness; | Immediately post-op; 1 year post-op; 2 years post-op | Amputated vs intact; time points | |
| Hansen et al. (2019) | Changes in periprosthetic bone mineral density and bone turnover markers after osseointegrated implant surgery: A cohort study of 20 transfemoral amputees with 30-month follow-up | 19 TF with osseointegration; 12 males, 7 females; Mean age 49 (SD 11.17) | DXA measured BMD in lumbar spine, proximal femur and seven periprosthetic regions (zones 1-7 may or may not be similar to other studies) | Pre-op (2-21 days before surgery), and 1, 3, 6, 7, 9, 12, 18, 24 and 30 months after the S1 surgery or until implant was removed | Amputees vs controls; removed OI implant over nonremoved OI implant; over time points | |
| Hoyt et al. (2021) | Femoral Neck Hounsfield Units as an Adjunct for Bone Mineral Density After Combat-Related Lower Extremity Amputation | 26 individuals with 30 amputations total; 17 TT amputations and 13 TF amputations; All males; Mean age 26.4 (range 22-29); All traumatic etiologies | DXA measured BMD at femoral neck; CT measured Hounsfield units at femoral neck | Cross-sectional; inclusion criteria stated DXA and CT scans within 6 months of each other; DXA scans taken 5-11 months post-injury (mean 6 months) | Correlation b/t hounsfield units from CT scans and BMD from DXA scans | |
| Ramírez et al. (2011) | Analysis of bone demineralization due to the use of exoprosthesis by comparing Young’s modulus of the femur in unilateral transfemoral amputees | 20 TF; 3 females and 17 males; Mean age 44.6 (range 23–71); Mean TSA 10.9 years; All used SACH foot and mechanical monocentric knee | CT measured Young’s Modulus (no BMD data presented- just correlations) | Cross-sectional; no inclusion criteria stated | Amputated vs intact proximal femur at three locations= femoral neck, metaphysis just below lesser trochanter, and proximal quarter of the diaphysis | |
| Royer and Koenig (2005) | Joint loading and bone mineral density in persons with unilateral, trans-tibial amputation | 9 TT; 8 male 1 female; Mean age 41.7 (SD 10.6); Mean TSA 16.7 years (STD 10.9); All used ESAR feet; 4 traumatic etiologies, 1 diabetic, 2 congenital, 1 blood clot, 1 infection; | DXA measured BMD in proximal femur and tibia | Cross-sectional | Amputated vs intact vs averaged matched control limb value | |
| Rush et al. (1994) | Osteopenia in patients with above knee amputation | 16 TF; All male; Mean age 48 range (23-66) All ischial weight bearing sockets; 9 suction sockets and 7 silesian belt suspension; 8 traumatic etiologies, 6 cancer, 2 vascular | DXA measured BMD for L2 and femoral neck | Cross-sectional; inclusion says prosthesis users for over 5 years | Amputated vs intact; amputee group vs controls | |
| Sherk et al. (2008) | BMD and bone geometry in transtibial and transfemoral amputees | 14 total; 7 TT (5 males and 2 females); Mean age 43.4 (SD 6.0); 7 TF (6 males and 1 female); Mean age 45.7 (SD 5.7); TSA (14.7 TT and 15.5 TF), and hours/day of prosthesis wear (15 TT and 11 TF); 11 traumatic etiologies, 1 secondary to diabetes, 1 secondary to circulation issues, and 1 secondary to osteomyelitis; both groups had similar numbers of years wearing a prosthesis (14.4 TT and 15.4 TF), | DXA measured areal BMD of the dual proximal femur, lumbar spine, and total body; pQCT measured volumetric BMD and bone geometry at the distal ends of both limbs | Cross-sectional; inclusion stated ambulatory with a prosthesis for at least 6 months | Amputated vs intact limbs; group comparisons for both levels and two groups of nonamputee controls (one transtibial control group and one transfemoral control group) | |
| Smith et al. (2009) | A study of bone mineral density in adults with disability | 52 lower-limb amputees (no further details) | DXA measured BMD for total lumbar spine, femoral neck, total proximal femur | Cross-sectional; inclusion stated they had to have their disability for at least 3 months | Amputees vs other groups with musculoskeletal deficits (e.g. spinal cord injury) | |
| Smith et al. (2011) | A study of BMD in lower limb amputees at a national prosthetics center | 52 total; 24 TT; 19 TF; 8 bilateral; 1 hip disarticulation; 39 males and 13 females Mean age 61.9 (SD 12.8) | DXA measured BMD in lumbar spine, femoral neck, and proximal femur | Cross-sectional | Amputated vs intact; male vs female | |
| Thomson et al. (2019A) | Proximal Bone Remodeling in Lower Limb Amputees Reconstructed With an Osseointegrated Prosthesis | 48 total with osseointegration; 15 TT (12 males and 3 females) and 33 TF (22 males and 11 females); Mean age 51 (SD 13.5); TF group split into 2 groups depending on presence of femoral neck lag screw | DXA measured BMD at lumbar spine and femoral neck | Pre-op; 1 year post-op; and 3 years post-op | Amputated vs intact limbs; between amputation level/femoral neck screw groups; over time points | |
| Thomson et al. (2019B) | Radiographic Evaluation of Bone Remodeling Around Osseointegration Implants Among Transfemoral Amputees | 28 TF with osseointegration; 15 received integral leg prosthesis (10 male and 5 female) and 13 received osseointegration prosthetic limb type A (8 male and 5 female); Mean age 48 years (SD 12.4) | X-rays measured bone density, longitudinal bone coverage, and bone width | About 6 months post-op (0.4 with STD of 0.5 years); about 3 years post-op(3.0 with STD of 0.8 years) | 7 femoral (inverse Gruen) zones; between osseointegration implant groups; over time points | |
| Tugcu et al. (2009) | Muscle strength and bone mineral density in mine victims with transtibial amputation | 15 TT; All male; Mean age 26.2 (SD 3.9); Mean TSA 57.9 months (SD 47.5) All traumatic etiologies; All PTB sockets | DXA measured BMD at femoral neck, Ward’s triangle, total femur, and total tibia | Cross-sectional | Amputated vs intact | |
| Yazicioglu et al. (2008) | Osteoporosis: A factor on residual limb pain in traumatic trans-tibial amputations | 36 TT; All male; Mean age 26.8 (SD 3.5); Mean TSA 62.8 months (SD 37); All traumatic etiologies | DXA measured BMD for femoral neck, Ward’s triangle, total hip, and proximal tibia | Cross-sectional | Amputated vs intact | |
| Muscular | Bramley et al. (2021) | Changes in Tissue Composition and Load Response After Transtibial Amputation Indicate Biomechanical Adaptation | 10 TT; (6 males and 4 females); Mean age 41 (range 25-62); Mean TSA 7.5 years; 2 chronic regional pain disease etiologies, 2 congenital, 5 traumatic, 1 vascular; Mean daily socket use 12.5 hours (range 6-16) | MRI measured fatty infiltration of limbs | Cross-sectional | Amputated vs intact vs control |
| de Palma et al. (2011) | Involvement of the muscle-tendon junction in skeletal muscle atrophy: an ultrastructural study | 15 TT Group A= 12 elderly (mean age 79 years; range 65-85) 10 males and 2 females; 10 vascular etiologies, 1 osteomyelitis, 1 cancer Group B= 3 healthy young adults (mean age 32 range 25-35); All male; All traumatic etiologies | Histology measured fiber structures; EM measured base/perimeter ratio in musculotendinous junction | Cross-sectional | Group A vs B | |
| George et al. (2021) | Circumference Method Estimates Percent Body Fat in Males U.S. Service Members with Lower Limb Loss | 47 total; 23 unilateral TT; 4 bilateral TT; 14 unilateral TF; 3 bilateral TF; 3 TT/TF; Mean age 27.6 years (SD 5.7) | DXA measured percent body fat | Cross-sectional | Amputees vs controls | |
| Henson et al. (2021) | Understanding lower limb muscle volume adaptations to amputation | 12 total; 6 unilateral TT; mean age 33.7 years (SD 1.9); mean TSA 7.5 years 6 bilateral TF; mean age 31.8 years (SD 2.9); mean TSA 7.2 years; All male; All traumatic etiologies; All used dynamic response feet; All TF used MPKs | MRI measured gross skeletal measurements and muscle volume | Cross-sectional | Amputated vs intact (in TT) vs control | |
| Jaegers et al. (1995) | Changes in hip muscles after above-knee amputation | 12 TF; Mean age 38.2 (SD of 18); TSA 3- 35 years (mean 9.4); 7 traumatic etiology and 5 osteosarcomic etiology | MRI measured femur and muscle volume | Cross-sectional; inclusion said at least 2 years post-amputation | Amputated vs intact vs control | |
| Onat et al. (2016) | Ultrasonographic assessment of the quadriceps muscle and femoral cartilage in transtibial amputees using different prostheses | 38 TT; 13 using vacuum suspension; 11 male and 2 female; Mean age 41.9 years with SD 11.8; TSA 10.8 years; Prosthesis use 5.6 years); 25 using pin-lock suspension; 20 males and 5 females; Mean age 40.6 years with SD 11.6; Mean TSA 16.3 years; prosthesis use 6.6 years) | Ultrasound of femoral cartilage thickness (intercondylar area, lateral femoral condyle, medial femoral condyle) and quadriceps muscle thickness (rectus femoris, vastus intermedius, vastus intermedius, and vastus medialis) | cross-sectional; inclusion states at least 6 months of prosthesis use | Amputated vs intact limbs; two suspension groups | |
| Putz et al. (2017) | Structural changes in the thigh muscles following trans-femoral amputation | 12 TF; 6 males and 6 females; Mean age 44.1 at amputation (range 21-69); All cancer | MRI measured fatty infiltration and degeneration at the middle and distal end of specific muscles within the residual limb | About 1 year post-op (avg 10.6 months SD 12.6); about 2 years post-op (avg 25.6 months SD 21.4); 12 patients included at time 1 but only 7 patients included at time 2 | Middle vs end of residual limb; time points | |
| Renström et al. (1983) | Thigh muscle atrophy in below-knee amputees | 10 TT; 8 males and 2 females; Mean age 56; 4 vascular etiologies, 2 infection, 4 trauma; Mean TSA 24 months (SD 37) | Histology measured fast and slow-twitch fibers, fiber sizes, and fiber area; CT measured mean fiber area of muscles in the thigh; measuring tape determined cross-sectional area of the thigh | Cross-sectional | Amputated vs intact; type 1 vs 2 fibers | |
| Schmalz et al. (2001) | Selective thigh muscle atrophy in trans-tibial amputees: an ultrasonographic study. | 17 TT; 15 male and 2 female; Mean age 47 (SD 18); 14 traumatic etiologies, 1 due to infection, 1 due to tumor, and 1 due to venous thrombosis; All had patellar tendon bearing prostheses | Ultrasound measured cross-sectional area and thickness of the quadriceps femoris, sartorius, gracillis, semitendinosus, and biceps femoris | Cross-sectional; demographics state at least 6 months of prosthesis use (range 0.5 - 19 years with median of 5 years) | Amputated vs intact vs control limb | |
| Sharma et al. (2019) | Fast and slow myosin as markers of muscle regeneration in mangled extremities: a pilot study | 15 lower-limb amputees (no level details); All trauma | Histology measured fast and slow myosin in residual limb | During amputation surgery, at 7 day follow-up | Fast vs slow myosin; time points | |
| Sherk et al. (2010) | Interlimb muscle and fat comparisons in persons with lower-limb amputation | 12 total 7 TT; Mean age 43.4 (SD 15.8) 5 TF; Mean age 38.5 (SD 10.6) | DXA measured thigh and lower-leg fat mass and bone-free lean body mass; qQCT measured muscle cross-sectional areas and fat cross-sectional areas of the end of residual and intact limbs with thresholding technique to determine the composition of fat vs muscle | Cross-sectional; inclusion states ambulatory for at least 6 months | Amputated vs intact limbs; amputee vs control groups | |
| Sibley et al. (2020) | The effects of long-term muscle disuse on neuromuscular function in unilateral transtibial amputees | 9 TT; All male; Mean age 40.3 (SD 8.5); All traumatic etiologies | Ultrasound of the vastus lateralis measured muscle thickness, pennation angle, and fascicle length | Cross-sectional; inclusion states amputation performed at least 6 months prior | Amputated vs intact vs control |
Studies are categorized by skeletal or muscular methodologies. All individuals with amputation were unilaterally affected unless otherwise specified. Mean age is in years unless otherwise specified. Abbreviations: TT= transtibial, TF= transfemoral, SD= standard deviation, TSA = time since amputation, DXA= Dual Energy X-ray Absorptiometry, pQCT= peripheral quantitative computed tomography, BMD= bone mineral density, CT= computed tomography, MRI= magnetic resonance imaging, MPKs= microprocessor knees.
Participant Demographics: Included studies were composed of transtibial amputees (n=10), transfemoral amputees (n=7), both (n=9), or unclear (n=2). The majority of studies included twenty participants with amputation or less (n=18). Eleven studies included females. Fifteen studies included individuals with traumatic etiology, with eight of those exclusively studying individuals with traumatic etiologies. In contrast, only a few studies included individuals with etiologies due to dysvascular issues (n=6), cancer (n=7), or congenital limb deficiency (n=2). Most studies had a mean participant age of 40- 49 years (n=13). Few studies recorded time since amputation (n=9), activity level (n=7), prosthetic wear time (n=7), or prosthetic componentry (n=8).
Comparisons: There were a wide variety of comparisons conducted across all studies. While most studies compared each individual’s amputated limb to their intact limb (n=18), some also compared individuals with amputations to control groups of individuals without amputations (n=11) or individuals with spinal cord injury (n=2). Subgroups of individuals with amputation (n=9) were also compared by transtibial and transfemoral (n=4), osseointegration type (n=3), prosthetic suspension (n=1), and age (n=1). Studies were either cross-sectional (n=20) or compared data collected at multiple time points (n=7). Of these seven studies, two-time points (n=3), three-time points (n=3), and ten-time points (n=1) were compared.
Methodology & Parameters: Studies employed a variety of methodologies to measure skeletal and muscular properties. The studied parameters were inconsistent across studies, but those reported by a minimum of two or more studies are summarized in Table 2 and 3 for skeletal and muscular properties, respectively.
Table 2.
Summary of Mean Skeletal Data.
| Study | TT Group | TF Group | Amputee Group (level unspecified) | Control Group | TT Limb | Intact Limb | TF limb | Intact Limb | Amputated Limb (level unspecified) | Intact Limb | Control Limb | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T-Scores | Femoral neck | |||||||||||
| Smith et al. (2011) | - | - | - | - | - | - | - | - | -1.91 male, - 2.63 female | -1.3 male, -1.96 female | - | |
| Tugcu et al. (2009) | - | - | - | - | -0.4 | 0.8 | - | - | - | - | - | |
| Yazicioglu et al. (2008) | - | - | - | - | -0.69 | 0.35 | - | - | - | - | - | |
| Hip | ||||||||||||
| Tugcu et al. (2009) | - | - | - | - | -0.5 | 1.1 | - | - | - | - | - | |
| Yazicioglu et al. (2008) | - | - | - | - | -0.88 | 0.59 | - | - | - | - | - | |
| Ward’s triangle | ||||||||||||
| Tugcu et al. (2009) | - | - | - | - | 0.3 | 1.5 | - | - | - | - | - | |
| Yazicioglu et al. (2008) | - | - | - | - | -0.12 | 0.84 | - | - | - | - | - | |
| Z-Scores | Lumbar spine | |||||||||||
| Smith et al. (2011) | - | - | - | - | - | - | - | - | - | 0.11 male, 0.63 female | - | |
| Thomson et al. (2019A) | 0.466 | 0.19 without femoral lag screw, -0.3 with | 0.163 | - | - | - | - | - | - | - | - | |
| Femoral neck | ||||||||||||
| Smith et al. (2011) | - | - | - | - | - | - | - | - | -0.38 male, 0.19 female | -1.01 male, -0.48 female | - | |
| Thomson et al. (2019A) | - | - | - | - | -0.32 | 0.4428 | -2.309 without femoral lag screw, -2.291 with | 0.0476 without femoral lag screw, 0.3273 with | - | - | - | |
| BMD (g/cm^2) | Whole-body | |||||||||||
| Bemben et al. (2017) | 1.271 pre, 1.279 6MO, 1.271 12MO | - | - | - | - | - | - | - | - | - | - | |
| Cavedon et al. (2021) | 1.2 | 1.15 | 1.17* | - | - | - | - | - | - | - | ||
| Sherk et al. (2008) | 1.272 | 1.227 | - | 1.275 for TT controls, 1.264 for TF controls | - | - | - | - | - | - | - | |
| Lumbar spine | ||||||||||||
| Bemben et al. (2017) | 1.266 pre, 1.244 6MO, 1.257 12MO | - | - | - | - | - | - | - | - | - | - | |
| Hansen et al. (2019) | - | 1.13 | - | 1.18 | - | - | - | - | - | - | - | |
| Sherk et al. (2008) | 1.296 | 1.241 | - | 1.336 for TT controls 1.441 for TF controls | - | - | - | - | - | - | - | |
| Smith et al. (2009) | - | - | 0.994 | - | - | - | - | - | - | - | - | |
| Smith et al. (2011) | - | - | 1.039 male, 0.865 female | - | - | - | - | - | - | - | - | |
| Thomson et al. (2019A) | 1.3164 | 1.268 without femoral lag screw 1.173 with femoral lag screw | 1.261 | - | - | - | - | - | - | - | - | |
| Femoral neck | ||||||||||||
| Bemben et al. (2017) | - | - | - | - | 1.087 pre, 0.996 6MO, 0.984 12MO | 1.119 pre, 1.087 6MO, 1.095 12MO | - | - | - | - | - | |
| Haket et al. (2017) | - | - | - | - | - | - | 0.68 preop, 0.67 12MO, 0.69 24MO | - | - | - | ||
| Rush et al. (1994) | - | - | - | - | - | - | 0.68 | 1.01 | - | - | - | |
| Sherk et al. (2008) | - | - | - | - | 1.015 | 1.077 | 0.704 | 1.064 | - | - | 1.072 for TT, 1.146 for TF | |
| Smith et al. (2009) | - | - | 0.724 | - | - | - | - | - | - | - | ||
| Smith et al. (2011) | - | - | - | - | - | - | - | - | 0.672 male, 0.556 female | 0.753 male, 0.632 female | - | |
| Thomson et al. (2019A) | - | - | - | - | 0.9747 | 1.072 | 0.709 without femoral lag screw, 0.6725 with | 1.016 without femoral lag screw, 1.01 with | 0.783 | 1.031 | - | |
| Tugcu et al. (2009) | - | - | - | - | 1.01 | 1.55 | - | - | - | - | - | |
| Yazicioglu et al. (2008) | - | - | - | - | 0.97 | 1.11 | - | - | - | - | - | |
| Ward’s triangle | ||||||||||||
| Tugcu et al. (2009) | - | - | - | - | 0.99 | 1.15 | - | - | - | - | - | |
| Yazicioglu et al. (2008) | - | - | - | - | 0.94 | 1.06 | - | - | - | - | - | |
| Proximal femur | ||||||||||||
| Bemben et al. (2017) | - | - | - | - | 0.862 pre, 0.734 6MO, 0.739 12MO | 0.904 pre, 0.911 6MO, 0.912 12MO | - | - | - | - | - | |
| Hansen et al. (2019) | - | - | - | - | - | - | 0.66 | 1.03 | - | - | 1.04 | |
| Royer and Koenig, (2005) | - | - | - | - | 0.82* | 0.94* | - | - | - | - | 0.92* | |
| Sherk et al. (2008) | - | - | - | - | 0.817 | 0.93 | 0.527 | 0.937 | - | - | 0.915 for TT, 0.904 for TF | |
| Smith et al. (2009) | - | - | 0.897 | - | - | - | - | - | - | - | - | |
| Smith et al. (2011) | - | - | - | - | - | - | - | - | 0.807 male, 0.617 female | 0.947 male, 0.738 female | - | |
| Proximal tibia | ||||||||||||
| Royer and Koenig, (2005) | - | - | - | - | 0.75* | 1.09* | - | - | - | - | 0.99* | |
| Tugcu et al. (2009) | - | - | - | - | 0.56 | 0.86 | - | - | - | - | - | |
| Yazicioglu et al. (2008) | - | - | - | - | 0.6 | 0.95 | - | - | - | - | - | |
| Volumetric BMD (mg/cm^3) | Total | |||||||||||
| Bemben et al. (2017) | - | - | - | - | - | - | 701.1 pre, 564.9 6MO, 551.3 12MO | 788.3 pre, 722.1 6MO, 798.1 12MO | - | |||
| Sherk et al. (2008) | - | - | - | 512.3 | 757.3 | 462.7 | 812.3 | - | - | 749.7 for TT controls, 927.7 for TF controls | ||
Summary of skeletal data reported in at least two studies included in this review. Asterisk (*) indicates value was estimated from a graph. Dash (-) indicates data was not reported. Abbreviations: TT= transtibial, TF= transfemoral, BMD= bone mineral density, MO= months.
Table 3.
Summary of Mean Muscle and Fat Data.
| Study | TT Group | TF Group | Control Group | TT Limb | Intact Limb | TF Limb | Intact Limb | Amputated Limb (level unspecified) | Intact Limb | Control Limb | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cross-sectional area (mm2 or % compared to intact limb or % atrophy) | Thigh | ||||||||||
| Bemben et al., (2017) | - | - | - | - | - | - | - | 6324.8 pre, 4117.8 6MO, 3554.7 12MO | 6479.1 pre, 69(20.5 6MO, 6515.5 12MO | - | |
| Renstrom et al., (1983) | - | - | - | 86% | - | - | - | - | - | - | |
| Sherk et al., (2010) | - | - | - | 1621.3mm2 | 5320.4mm2 | 4818.9mm2 | 17122.8mm2 | - | - | 5675.9 mm2 TT, 17,028.3 mm2 TF | |
| Sartorius | |||||||||||
| Jaegers et al., (1995) | - | - | - | 40.1% atrophy compared to intact | - | - | - | - | - | - | |
| Schmalz et al., (2001) | - | - | - | 88.30% | - | - | - | - | - | - | |
| Gracilis | |||||||||||
| Jaegers et al., (1995) | - | - | - | 24.6% atrophy compared to intact | - | - | - | - | - | - | |
| Schmalz et al., (2001) | - | - | - | 95.10% | - | - | - | - | - | - | |
| Semitendinosus | |||||||||||
| Jaegers et al., (1995) | - | - | - | 44.3% atrophy compared to intact | - | - | - | - | - | - | |
| Schmalz et al., (2001) | - | - | - | 91.90% | - | - | - | - | - | - | |
| Biceps femoris (long head) | |||||||||||
| Jaegers et al., (1995) | - | - | - | 32.9% atrophy compared to intact | - | - | - | - | - | - | |
| Schmalz et al., (2001) | - | - | - | 92.00% | - | - | - | - | - | - | |
| Thickness (mm or % compared to intact limb) | Vastus lateralis | ||||||||||
| Onat et al., (2016) | - | - | - | 9.63mm | 11.06mm | - | - | - | - | - | |
| Schmalz et al., (2001) | - | - | - | 80.20% | - | - | - | - | - | - | |
| Sibley et al., (2020) | - | - | - | 15.4mm | 26.3mm | - | - | - | - | 25.0mm | |
| Rectus femoris | |||||||||||
| Onat et al., (2016) | - | - | - | 15.6mm | 17.63mm | - | - | - | - | - | |
| Schmalz et al., (2001) | - | - | - | 84.30% | - | - | - | - | - | - | |
| Vastus medialis | |||||||||||
| Onat et al., (2016) | - | - | - | 11.6mm | 17.04mm | - | - | - | - | - | |
| Schmalz et al., (2001) | - | - | - | 76.20% | - | - | - | - | - | - | |
| Vastus intermedius | |||||||||||
| Onat et al., (2016) | - | - | - | 12.23mm | 16.66mm | - | - | - | - | - | |
| Schmalz et al., (2001) | - | - | - | 69.60% | - | - | - | - | - | - | |
| Fat Mass (%) | Whole-Body % Fat Mass | ||||||||||
| Cavedon et al., (2021) | 21.47 | 21.45 | 16* | - | - | - | - | - | - | - | |
| Sherk et al., (2010) | 33.5 | 32.4 | 24.2 TT controls 25.5 TF controls | - | - | - | - | - | - | - | |
| George et al., (2021) | uni 23.1; bi 23.1 | uni 23.4; bi 17.2 | 19.5 | ||||||||
| Affected Thigh % Fat Mass | |||||||||||
| Cavedon et al., (2021) | 26.5 | 34.58 | - | - | - | - | - | - | - | - | |
| Sherk et al., (2010) | 38* | 42* | 30.0 TT controls, 29.0 TF controls* | - | - | - | - | - | - | - | |
| Intact Thigh % Fat Mass | |||||||||||
| Cavedon et al., (2021) | 20.66 | 21.72 | - | - | - | - | - | - | - | - | |
| Sherk et al., (2010) | 36* | 33* | 30.5 TT controls, 28.5 TF controls* | - | - | - | - | - | - | - | |
Summary of muscle and fat data reported in at least two studies included in this review. Asterisk (*) indicates value was estimated from a graph. Dash (-) indicates data was not reported. Abbreviations: TT= transtibial, TF= transfemoral, uni= unilateral limb loss, bi= bilateral limb loss.
The majority used dual x-ray absorptiometry (DXA) (n=13) to measure T-scores of the femoral neck (n=3), hip (n=2), and Ward’s triangle (n=2), and Z-scores of the lumbar spine (n=2), and femoral neck (n=2). The same methodologies were utilized to report bone mineral density (BMD) in parameters of whole-body (n=3), lumbar spine (n=6), femoral neck (n=10), Ward’s triangle (n=2), proximal femur (n=6), proximal tibia (n=3), and volumetric BMD (n=2). Other methodologies utilized to obtain the above properties were peripheral quantitative computed tomography (pQCT) scans (n=2), computed tomography (CT) scans (n=2), and x-rays (n=2).
Muscular methodologies utilized for each parameter were even less consistent. Histology (n=3) was employed to measure the fiber structures, fiber composition/size, ultrasound was employed to measure various muscle thicknesses the vastus lateralis (n=3), rectus femoris (n=2), vastus medialis (n=2), and vastus intermedius (n=2). MRI (n=4) was used in measuring fatty degeneration as well as femur and muscle volume, DXA (n=3) was utilized to measure whole-body, thigh, and lower-leg fat mass, qQCT (n=1) for muscle and fat CSA at the end of each limb. Muscle fiber typing could not be summarized in Table 3, because all four studies examined different muscles or properties[6,20-22].
Discussion
Despite varying methodologies and reported parameters, studies agreed amputees compared to control groups and the amputated limb compared to the intact had reduced BMD and increased muscle atrophy. Specifically, reduced BMD was found in multiple parameters, T-scores and Z-scores, whole-body, lumbar spine, femoral neck, Ward’s triangle, proximal femur, and proximal tibia. Additionally, muscle atrophy was found in parameters of decreased thigh cross-sectional area (CSA) and quadriceps thickness with higher ratios of fat to muscle within the thigh. Overall, the correlation between altered structural parameters (e.g. reduced BMD and increased muscle atrophy) with adverse clinical outcomes, such as increased risks of fractures, osteopenia, osteoporosis, and reduced mobilities in this population was repeatedly reported by multiple studies.
Skeletal Adaptations
The majority of articles included in this review investigated BMD using DXA, which is the gold standard for screening osteopenia and osteoporosis but is rarely used in post-amputation management[23]. Consistently, these studies revealed that individuals with amputations compared to control groups, and amputated limbs compared to intact/control limbs had reduced BMD. Moreover, there was increased prevalence of osteopenia or osteoporosis in amputees, shown by a lower T- and Z-scores[15,24-27]. In accordance with the World Health Organization classification, an individual’s T-score is a comparison of their bone density to a healthy individual of the same sex who is 30 years of age, while a Z-score compares an individual’s bone density to an individual of the same age and sex[28]. Therefore, T-scores are only sex-matched, while Z-scores are age and sex-matched.
Studies consistently found reduced BMD in the femoral neck and Ward’s triangle in amputated limbs compared to intact limbs[24-27,29]. Ward’s triangle is a small space within the femoral neck, located between the principal compressive, secondary compressive, and primary tensile trabeculae. BMD of Ward’s triangle is a region of initial bone loss[30], with low BMD and high flexing strain[31] and is accepted as a sensitive indicator of osteoporosis[32]. Femoral neck BMD was reduced in amputated limbs regardless of amputation level. Ward’s triangle BMD was only reported by two studies in individuals with transtibial amputation, and both indicated reduced BMD in the amputated limb. Overall, it is important to note that femoral neck and proximal femur BMD represents the greatest predictive power of fracture at that site[24,33] indicating that individuals with amputations are at an increased risk of femoral neck fractures compared to controls.
In congruence with the femoral neck and Ward’s triangle results, the proximal femur (femoral neck, trochanter, total hip) and proximal tibia had reduced BMD compared to the intact limb and controls across studies. Three studies that reported proximal tibia BMD all compared transtibial limbs to intact limbs and found reduced BMD on the amputated limb[25,26,34]. Royer and Koenig[34] also included limbs from a control population, with values in between amputated and intact limbs. This finding further supports that the amputated limb is underused, and the intact limb is overused compared to control limbs. Reduced amputated limb BMD in the proximal tibia could be a factor in the development of intact limb knee osteoarthritis, which is prevalent in this population[34,35].
All studies that reported whole-body or lumbar BMD found that BMD was most preserved in controls, followed by individuals with transtibial amputations, then individuals with transfemoral amputations. In a longitudinal study, whole-body and lumbar BMD over time in individuals with transtibial amputations had recovered baseline values at the 12-month follow-up despite having reduced values at the 6-month follow-up[18], suggesting a rehabilitation and adaptation period over the first six months post-amputation, consistent with the findings in the lower limb BMD.
The two studies that reported volumetric BMD both found reduced values on the amputated limb compared to the intact limb[18,36]. Volumetric BMD, defined as BMC per volume of bone, can only be measured through computed tomography and is considered a more accurate measure of BMD compared to areal BMD[37-39]. Individuals with transfemoral amputation had greater reductions in volumetric BMD on the amputated limb than those with transtibial amputation[36]. These studies, again, highlight the importance of knee joint sparing, when possible, and show structural adaptations occur within the first six months after the amputation.
Overall, these studies suggest that those with an amputated limb suffer from loss of BMD in their amputated limb as well as central whole-body and lumbar regions, but have potential to reach baseline with proper rehabilitation. Proactive skeletal screening post-amputation may help identify and reduce the prevalent risks of osteopenia and osteoporosis in this population through targeted rehabilitation exercises to increase loading at the affected limbs. In addition, decreasing the time between amputation and ambulation with a prosthesis within the first six months could be critical to maintaining bone health.
Muscular Adaptations
Studies that investigated muscular adaptations found a decrease in thigh CSA and quadriceps thickness, an increase in amounts of thigh fat, and more muscle fiber atrophy in the amputated limb compared to the intact limb.
Thigh muscle CSA was reduced in the amputated limb compared to the intact limb in three studies, despite reporting data in different units[2,18,40]. Only one study to include a control group and found control limbs had similar CSA values to intact limbs in both individuals with transtibial and transfemoral amputations[2]. Reduced thigh CSA and volume indicates reduced ability to generate force, which can impede push-off during gait[14,41] and contribute to the asymmetry between limbs[42].
All three studies that reported quadriceps muscle thickness found reduced quadriceps muscle thickness in the amputated limb compared to the intact limb[20,43,44], indicating reductions in quadriceps strength. Additionally, the intact limb had greater thickness compared to the control limb, which aligns with many previous studies that have found that individuals will compensate by overusing their intact limb[20]. The quadriceps are important in prosthetic control, particularly in terms of knee extension for stability and hip flexion for prosthetic clearance throughout gait, regardless of amputation level. Therefore, lack of quadriceps strength could potentially increase the prevalence of gait deviations and fall risk.
Fat mass was consistently greater in individuals with amputation compared to controls, especially in the thighs. Studies conflicted on whether individuals with transfemoral amputation had similar or greater fat mass compared to individuals with transtibial amputation, which may have been due to differences in included participants. Regardless of amputation level, thigh fat mass can be an important factor in achieving optimal prosthetic socket fit and effective prosthetic control[2]. Quantifying fat mass can inform prosthetic modeling and fitting, as well as gait rehabilitation exercises to improve functional prosthesis use.
Although the muscle architecture data could not be included in Table 3 due to the wide range of studied muscle groups and reported parameters, structural changes in fiber type, pennation angle, and fascicle length, are important indicators of muscle atrophy[20-22,40]. Despite lack of consistency in methodology across studies, findings include a complete reduction in fast-twitch compared to slow-twitch fibers in the amputated limb within seven days[21], and a reduction in slow-twitch fibers[40] and shorter fascicle length[20] in the vastus lateralis of the amputated limb compared to the intact limb. Additionally, elderly individuals with vascular etiologies of transtibial amputation had split muscle fibers, fiber atrophy, reduced cross-sectional fiber area and length, and adipose tissue in the gastrocnemius compared to younger individuals with traumatic etiologies[22]. Fiber atrophy and shorter fascicle lengths in the amputated limb indicate impaired ability to generate force, which can impede gait.
Overall, these studies suggest that amputated limbs had less thigh CSA and quadriceps thickness, more thigh fat, and more muscle fiber atrophy with shorter fascicle lengths compared to the intact limb, which could impact gait through reduced knee stabilization and decreased propulsion. Proactive screening for lower limb muscular atrophy along with targeted exercises to specific thigh muscles could help prevent muscle atrophy reduction, and lead to increased symmetry between amputated and intact limbs.
Clinical Considerations
Osteopenia and osteoporosis: Studies in this review found increased risks of fractures, osteopenia, and osteoporosis, particularly at the femoral neck and distal end of the amputated limb. This aligns with retrospective studies in this population[15,45], and agrees with previous literature that decreased loading results in reduced BMD[34,46]. Coupled with muscle atrophy, which has also been associated with decreased loading, this population is particularly vulnerable to local and generalized osteoporosis[13,25,26,29]. Clinicians could focus on recommending targeted rehabilitation exercises that increase weight-bearing load through the amputated limb, particularly within the first six months post-amputation.
Osseointegration: Individuals with osseointegration may not experience the same reductions in bone health as individuals who use traditional prostheses. After 30 months, individuals with removed implants had reduced BMD, but individuals with non-removed implants had BMD values normalized to baseline values[47]. Regarding preservation of femoral neck Z-scores in individuals with osseointegration, studies were inconsistent with results ranging from decreased, no significant change, and increased Z-scores. Differences in findings may be explained by amputation levels of included participants or different time points for follow-up measurements[27,48,49]. Maintenance or increase of BMD may be evidence of more activity on the prosthetic limb or more frequent ambulation[27]. Two studies agreed periprosthetic cortical thickness around the implant increased over two years[24,49]. Muscles contribute to femur stabilization with osseointegrated implants more than with a traditional prosthetic socket, which may lead to increased periprosthetic cortical thickness[48,49]. Due to the loading differences between osseointegration and traditional prosthetic sockets, individuals with osseointegration may not experience the same declines in BMD. However, localized bone declines in the femoral neck and distal end as stated above could put individuals at increased risk of periprosthetic fracture and surgery, potentially resulting in more proximal amputation levels.
Muscle strength: Studies that investigated structural muscle properties in this review found reduced thigh CSA, indicative of reduced force production. This finding aligns with many studies that have measured muscle contraction strength or force generation and absorption during gait[50]. The reduced ability of the amputated limb to produce force can impede gait and factor into the development of secondary health conditions, such as osteoarthritis[5,51-53]. Studies in this review also found reduced quadriceps strength and increased thigh fat mass in the amputated limb, so exercises that increase quadriceps strength and reduce thigh fat mass could be goals prioritized in rehabilitation. Additionally, daily socket use was found to be negatively correlated with adipose infiltrating muscle in one study[54], indicating less daily socket use was associated with more muscle atrophy. This supports the importance of daily prosthesis use to limit residual limb atrophy.
Level of amputation: Studies that directly compared individuals with transtibial and transfemoral amputations typically found a greater degree of BMD reduction and muscle atrophy in individuals with transfemoral amputations[2,19,27,36]. This aligns with numerous studies in this population that have found individuals with transfemoral amputations are more affected than individuals with transtibial amputations due to more proximal functional loss[5]. Therefore, individuals with transfemoral amputations have more structural and functional limitations than individuals with transtibial amputations, generally resulting in decreased musculoskeletal health. Less force is transferred to the body, particularly the femur, because force is transferred through the prosthetic socket to soft tissue and the ischial tuberosity. Force passes through more soft tissue in a transfemoral prosthesis compared to a transtibial prosthesis, due to amputation surgery and prosthesis design[36].
Time since amputation: Nearly all studies that measured multiple time points found significantly reduced BMD and increased muscle atrophy within six months or one year following amputation surgery. Findings suggest six months for skeletal rehabilitation and one year for muscular rehabilitation post-amputation may be a critical threshold of time to strengthen musculoskeletal health in rehabilitation[18,55], but more research across multiple time points is needed.
Regional differences within the amputated limb: Studies that directly compared regional differences in musculoskeletal architecture along the amputated limb found reduced BMD and increased muscle atrophy at the distal end of the amputated limb compared to proximal or middle sections[2,36,55]. Throughout the proximal femur, found the lowest Young’s modulus values and BMD at the femoral neck and the highest values at the proximal quarter diaphysis of the femur[56]. These results suggest the femoral neck and distal end of the amputated limb may be important regions to monitor musculoskeletal health and target in rehabilitation.
Participant demographics: Findings in this review are not representative of the majority of individuals with amputations. The majority of participants included in the studies were adults between 18-65 years of age with traumatic etiologies. However, 42% of individuals with limb loss are 65 years of age or older regardless of etiology, and 54% have dysvascular etiologies, such as diabetes mellitus and peripheral artery disease that have been associated with increased bone loss, fracture risks, osteoporosis/penia[1,57-62]. Additionally, studies rarely compared results by amputated limb length, time since amputation, activity level, or prosthetic wear time, and findings typically conflicted. Additionally, two studies found significant differences between prosthetic wear time and BMD, but in different parameters[24,36]. More research is needed to generalize findings to the majority of individuals in this population.
Clinical outcome measures: While all twenty-seven manuscripts included in this review stated clinical considerations, only two collected clinical outcome measures[27,44]. Clinical outcome measures included the Houghton survey to assess prosthetic use[44], as well as the 6-Minute Walk Test and Timed Up and Go[27]. However, the Houghton scores were not tied to findings in the study, and pre-osseointegration surgery 6-Minute Walk Test scores were positively correlated with BMD values at one-year follow-up. Thus, there is not yet a body of evidence that demonstrates how structural adaptations may potentially influence clinical outcome measure performance aside from one study in individuals with osseointegration. More research is needed to directly correlate structural findings summarized throughout this review to clinical outcome measure scores, as an indicator of functional mobility.
Limitations and Potential for Future Work
This review only summarized structural musculoskeletal properties, defined as anatomical or physiological components such as BMD and muscle architecture, published in the English language. Several articles measured other parameters, such as muscle activation using electromyography or muscle strength using dynamometers. These relationships should also be considered to understand how musculoskeletal architecture relates to functional mobility.
Future research can include larger sample sizes of participants with a variety of demographics, such as time since amputation and activity level. Additionally, studies can include participants that reflect the majority of individuals with limb loss, such as individuals who are older or have dysvascular etiologies. Including these individuals can also provide evidence to differentiate the effects of musculoskeletal adaptations due to amputation, aging, and dysvascular conditions such as diabetes. One study stated inclusion of individuals with congenital deficiencies, which were only two of nine participants, and did not compare etiologies[34]. Individuals with a congenital deficiency may have differences in musculoskeletal architecture than individuals who undergo amputation, but no current literature has compared individuals with congenital etiologies to other etiologies.
Future work can also compare parameters such as time since amputation, prosthetic componentry, and activity level to provide a variety of musculoskeletal health expectations based on these factors. Multiple time points were compared by several studies, but more work is needed to confirm critical thresholds that could be important landmarks in rehabilitation. Additionally, few studies measured the same musculoskeletal parameters or included raw values, especially in terms of muscular adaptations, which made results difficult to compare across studies. This population also has a combination of a high risk of falls and fractures and lower BMD on the amputated hip. Proactive assessment of fracture risk and prevention could be an initial crucial piece of long-term care in this population[24]. More work is needed to confirm findings by studies included in this review, investigate muscle architecture, and translate findings to clinical outcome measure performance.
Conclusion
This review summarized literature investigating structural musculoskeletal adaptations in individuals with major unilateral lower-limb amputations to inform clinical considerations and guide directions for future research. Findings in this review aligned with findings from non-anatomical studies that have suggested increased risks of fractures, osteopenia, osteoporosis, and muscle atrophy. BMD was reduced in individuals with amputation compared to controls and amputated limbs compared to intact limbs in T-scores and Z-scores, whole-body, lumbar spine, femoral neck, Ward’s triangle, proximal femur, proximal tibia, and BMC. These findings indicate increased risks of experiencing fractures, osteopenia, and osteoporosis, particularly in the femoral neck and amputated limb. Amputated limbs also had more muscle atrophy compared to the intact limb, specifically in parameters of decreased thigh CSA and quadriceps thickness with more thigh fat and muscle fiber atrophy. These findings were more pronounced in individuals with transfemoral amputations compared to transtibial amputations, and in individuals with amputations compared to control groups. Studies that measured multiple time points indicated the first six months to one-year post-amputation may be a critical threshold for musculoskeletal rehabilitation. Musculoskeletal adaptations could eventually be screened by clinicians to inform rehabilitation techniques and improve functional mobility. However, more research is needed to directly inform clinical outcome measure performance and functional mobility.
Authors’ contributions
Conceptualization: MGF. Investigation: MGF, SK, and WN. Visualization: MGF, SK, WN. Writing- original draft: MGF and SK. Writing- review and editing: WN, RAM. Supervision: RAM. All authors accept responsibility for the integrity of the data analysis.
Acknowledgements
The authors would like to thank research librarian Laura Haygood at the University of North Texas Health Science Center for formulating the search criteria.
Footnotes
MG Finco was supported by the National Institutes of Health/National Institute on Aging (T32 AG020494) and the Institute for Healthy Aging. S. Kim was supported by Grant (# RP170301) from Cancer Prevention and Research Institutes of Texas. The remaining authors have nothing to declare.
Edited by: G. Lyritis
References
- 1.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, et al. Estimating the prevalence of limb loss in the United States:2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422–9. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Sherk VD, Bemben MG, Bemben DA. Interlimb muscle and fat comparisons in persons with lower-limb amputation. Arch Phys Med Rehabil. 2010;91(7):1077–81. doi: 10.1016/j.apmr.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am. 1984;66(3):397–402. [PubMed] [Google Scholar]
- 4.Frost HM. Wolff's Law and bone's structural adaptations to mechanical usage:an overview for clinicians. Angle Orthod. 1994;64(3):175–88. doi: 10.1043/0003-3219(1994)064<0175:WLABSA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Gailey R, Allen K, Castles J, et al. Review of secondary physical conditions associated with lower-limb amputation and long-term prosthesis use. J Rehabil Res Dev. 2008;45(1):15–29. doi: 10.1682/jrrd.2006.11.0147. [DOI] [PubMed] [Google Scholar]
- 6.Renstrom PAFH, Alaranta H, Pohjolainen T. Review:leg strengthening of the lower limb amputee. 1995;7(1):11–32. [Google Scholar]
- 7.Finco MG, Menegaz RA. Skeletal asymmetries in anatomical donors with lower-limb amputations. PM R. 2021 Mar 29; doi: 10.1002/pmrj.12599. doi:10.1002/pmrj.12599. Epub ahead of print. PMID:33780165. [DOI] [PubMed] [Google Scholar]
- 8.Liu K, Tang T, Wang A, et al. Surgical revision for stump problems after traumatic above-ankle amputations of the lower extremity. BMC Musculoskelet Disord. 2015;16(1) doi: 10.1186/s12891-015-0508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yepson H, Mazzone B, Eskridge S, et al. The influence of tobacco use, alcohol consumption, and weight gain on development of secondary musculoskeletal injury after lower limb amputation. Arch Phys Med Rehabil. 2020;101(10):1704–1710. doi: 10.1016/j.apmr.2020.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Highsmith MJ, Kahle JT, Miro RM, et al. Prosthetic interventions for people with transtibial amputation:systematic review and meta-analysis of high-quality prospective literature and systematic reviews. J Rehabil Res Dev. 2016;53(2):157–84. doi: 10.1682/JRRD.2015.03.0046. [DOI] [PubMed] [Google Scholar]
- 11.van Velzen JM, van Bennekom CA, Polomski W, et al. Physical capacity and walking ability after lower limb amputation:a systematic review. Clin Rehabil. 2006;20(11):999–1016. doi: 10.1177/0269215506070700. [DOI] [PubMed] [Google Scholar]
- 12.Kahle JT, Highsmith MJ, Schaepper H, et al. Predicting walking ability following lower limb amputation:an updated systematic literature review. Technol Innov. 2016;18(2-3):125–137. doi: 10.21300/18.2-3.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulkarni J, Adams J, Thomas E, et al. Association between amputation, arthritis and osteopenia in british male war veterans with major lower limb amputations. Clin Rehabil. 1998;12(4):348–53. doi: 10.1191/026921598672393611. [DOI] [PubMed] [Google Scholar]
- 14.Isakov E, Burger H, Gregorič M, et al. Stump length as related to atrophy and strength of the thigh muscles in trans-tibial amputees. Prosthet Orthot Int. 1996;20(2):96–100. doi: 10.3109/03093649609164425. [DOI] [PubMed] [Google Scholar]
- 15.Haleem S, Yousaf S, Hamid T, et al. Characteristics and outcomes of hip fractures in lower limb amputees. Injury. 2021;52(4):914–917. doi: 10.1016/j.injury.2020.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg N, Gottlieb A, Siev-Ner I, et al. Fall incidence and associated risk factors among people with a lower limb amputation during various stages of recovery - a systematic review. Disabil Rehabil. 2019;41(15):1778–1787. doi: 10.1080/09638288.2018.1449258. [DOI] [PubMed] [Google Scholar]
- 17.Swanson EC, Friedly JL, Wang RK, et al. Optical coherence tomography for the investigation of skin adaptation to mechanical stress. Skin Res Technol. 2020;26(5):627–638. doi: 10.1111/srt.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bemben DA, Sherk VD, Ertl WJJ, et al. Acute bone changes after lower limb amputation resulting from traumatic injury. Osteoporos Int. 2017;28(7):2177–2186. doi: 10.1007/s00198-017-4018-z. [DOI] [PubMed] [Google Scholar]
- 19.Cavedon V, Sandri M, Peluso I, et al. Body composition and bone mineral density in athletes with a physical impairment. PeerJ. 2021;9:e11296. doi: 10.7717/peerj.11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sibley AR, Strike S, Moudy SC, et al. The effects of long-term muscle disuse on neuromuscular function in unilateral transtibial amputees. Exp Physiol. 2020;105(3):408–418. doi: 10.1113/EP088087. [DOI] [PubMed] [Google Scholar]
- 21.Sharma GR, Kumar V, Kanojia RK, et al. Fast and slow myosin as markers of muscle regeneration in mangled extremities:pilot Study. Eur J Orthop Surg Traumatol. 2019;29(7):1539–1547. doi: 10.1007/s00590-019-02448-w. [DOI] [PubMed] [Google Scholar]
- 22.de Palma L, Marinelli M, Pavan M, et al. Involvement of the muscle-tendon junction in skeletal muscle atrophy:an ultrastructural study. Rom J Morphol Embryol. 2011;52(1):105–9. [PubMed] [Google Scholar]
- 23.Hoyt BW, Lundy AE, Clark DM, et al. Femoral neck hounsfield units as an adjunct for bone mineral density after combat-related lower extremity amputation. J Orthop Trauma. 2021;35(5):e158–e164. doi: 10.1097/BOT.0000000000001980. [DOI] [PubMed] [Google Scholar]
- 24.Smith É, Comiskey C, Carroll Á, et al. A study of bone mineral density in lower limb amputees at a national prosthetics center. J Prosthet Orthot. 2011;23(1):14–20. [Google Scholar]
- 25.Tugcu I, Safaz I, Yilmaz B, et al. Muscle strength and bone mineral density in mine victims with transtibial amputation. Prosthet Orthot Int. 2009;33(4):299–306. doi: 10.3109/03093640903214075. [DOI] [PubMed] [Google Scholar]
- 26.Yazicioglu K, Tugcu I, Yilmaz B, et al. Osteoporosis:A factor on residual limb pain in traumatic trans-tibial amputations. Prosthet Orthot Int. 2008;32(2):172–8. doi: 10.1080/03093640802016316. [DOI] [PubMed] [Google Scholar]
- 27.Thomson S, Lu W, Zreiqat H, et al. Proximal bone remodeling in lower limb amputees reconstructed with an osseointegrated prosthesis. J Orthop Res. 2019;37(12):2524–2530. doi: 10.1002/jor.24445. [DOI] [PubMed] [Google Scholar]
- 28.Lewiecki EM. Osteoporosis:clinical evaluation. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000. [Google Scholar]
- 29.Rush PJ, Wong JS, Kirsh J, et al. Osteopenia in patients with above knee amputation. Arch Phys Med Rehabil. 1994;75(1):112–5. [PubMed] [Google Scholar]
- 30.Cardadeiro G, Baptista F, Zymbal V, et al. Ward's area location, physical activity, and body composition in 8- and 9-year-old boys and girls. J Bone Miner Res. 2010;25(11):2304–12. doi: 10.1002/jbmr.229. [DOI] [PubMed] [Google Scholar]
- 31.Nardi A, Ventura L, Rossini M, et al. The importance of mechanics in the pathogenesis of fragility fractures of the femur and vertebrae. Clinical cases in mineral and bone metabolism:the official journal of the Italian Society of Osteoporosis. Mineral Metabolism, and Skeletal Diseases. 2010;7(2):130–134. [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshihashi AK, Drake AJ, 3rd, Shakir KM. Ward's triangle bone mineral density determined by dual-energy x-ray absorptiometry is a sensitive indicator of osteoporosis. Endocr Pract. 1998;4(2):69–72. doi: 10.4158/EP.4.2.69. [DOI] [PubMed] [Google Scholar]
- 33.Kanis JA, Glüer CC. An update on the diagnosis and assessment of osteoporosis with densitometry. Committee of Scientific Advisors, International Osteoporosis Foundation. Osteoporos Int. 2000;11(3):192–202. doi: 10.1007/s001980050281. [DOI] [PubMed] [Google Scholar]
- 34.Royer T, Koenig M. Joint loading and bone mineral density in persons with unilateral, trans-tibial amputation. Clin Biomech (Bristol, Avon) 2005;20(10):1119–25. doi: 10.1016/j.clinbiomech.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Melzer I, Yekutiel M, Sukenik S. Comparative study of osteoarthritis of the contralateral knee joint of male amputees who do and do not play volleyball. J Rheumatol. 2001;28(1):169–72. [PubMed] [Google Scholar]
- 36.Sherk VD, Bemben MG, Bemben DA. BMD and bone geometry in transtibial and transfemoral amputees. J Bone Miner Res. 2008;23(9):1449–57. doi: 10.1359/jbmr.080402. [DOI] [PubMed] [Google Scholar]
- 37.Prentice A, Parsons TJ, Cole TJ. Uncritical use of bone mineral density in absorptiometry may lead to size-related artifacts in the identification of bone mineral determinants. Am J Clin Nutr. 1994;60(6):837–42. doi: 10.1093/ajcn/60.6.837. [DOI] [PubMed] [Google Scholar]
- 38.Compston JE. Bone Density:BMC, BMD, or Corrected BMD? Bone. 1995;16(1):5–7. doi: 10.1016/8756-3282(95)80004-A. [DOI] [PubMed] [Google Scholar]
- 39.Baroncelli GI, Bertelloni S, Ceccarelli C, et al. Measurement of volumetric bone mineral density accurately determines degree of lumbar undermineralization in children with growth hormone deficiency. J Clin Endocrinol Metab. 1998;83(9):3150–4. doi: 10.1210/jcem.83.9.5072. [DOI] [PubMed] [Google Scholar]
- 40.Renström P, Grimby G, Morelli B, et al. Thigh muscle atrophy in below-knee amputees. Scand J Rehabil Med Suppl. 1983;9:150–62. [PubMed] [Google Scholar]
- 41.Adamczyk PG, Kuo AD. Mechanisms of gait asymmetry due to push-off deficiency in unilateral amputees. IEEE Trans Neural Syst Rehabil Eng. 2015;23(5):776–785. doi: 10.1109/TNSRE.2014.2356722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutkowska-Kucharska A, Kowal M, Winiarski S. Relationship between asymmetry of gait and muscle torque in patients after unilateral transfemoral amputation. Appl Bionics Biomech. 2018;2018:5190816. doi: 10.1155/2018/5190816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmalz T, Blumentritt S, Reimers CD. Selective thigh muscle atrophy in trans-tibial amputees:an ultrasonographic study. Arch Orthop Trauma Surg. 2001;121(6):307–12. doi: 10.1007/s004020000227. [DOI] [PubMed] [Google Scholar]
- 44.Şahin Onat Ş, Malas F, Öztürk GT, et al. Ultrasonographic assessment of the quadriceps muscle and femoral cartilage in transtibial amputees using different prostheses. Prosthet Orthot Int. 2016;40(4):484–9. doi: 10.1177/0309364615592701. [DOI] [PubMed] [Google Scholar]
- 45.Denton JR, McClelland SJ. Stump fractures in lower extremity amputees. J Trauma. 1985;25(11):1074–8. [PubMed] [Google Scholar]
- 46.Pearson OM, Lieberman DE. The Aging of Wolff's “Law”:Ontogeny and responses to mechanical loading in cortical bone. Am J Phys Anthropol. 2004;(Suppl 39):63–99. doi: 10.1002/ajpa.20155. [DOI] [PubMed] [Google Scholar]
- 47.Hansen RL, Langdahl BL, Jørgensen PH, et al. Changes in periprosthetic bone mineral density and bone turnover markers after osseointegrated implant surgery:A cohort study of 20 transfemoral amputees with 30-month follow-up. Prosthet Orthot Int. 2019;43(5):508–518. doi: 10.1177/0309364619866599. [DOI] [PubMed] [Google Scholar]
- 48.Haket LM, Frölke JPM, Verdonschot N, et al. Periprosthetic cortical bone remodeling in patients with an osseointegrated leg prosthesis. J Orthop Res. 2017;35(6):1237–1241. doi: 10.1002/jor.23376. [DOI] [PubMed] [Google Scholar]
- 49.Thomson S, Thomson A, Tetsworth K, et al. Radiographic evaluation of bone remodeling around osseointegration implants among transfemoral amputees. J Orthop Trauma. 2019;33(8):e303–e308. doi: 10.1097/BOT.0000000000001488. [DOI] [PubMed] [Google Scholar]
- 50.Prinsen EC, Nederhand MJ, Rietman JS. Adaptation strategies of the lower extremities of patients with a transtibial or transfemoral amputation during level walking:a systematic review. Arch Phys Med Rehabil. 2011;92(8):1311–25. doi: 10.1016/j.apmr.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 51.Silverman AK, Fey NP, Portillo A, et al. Compensatory mechanisms in below-knee amputee gait in response to increasing steady-state walking speeds. Gait Posture. 2008;28(4):602–9. doi: 10.1016/j.gaitpost.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Beyaert C, Grumillier C, Martinet N, et al. Compensatory mechanism involving the knee joint of the intact limb during gait in unilateral below-knee amputees. Gait Posture. 2008;28(2):278–84. doi: 10.1016/j.gaitpost.2007.12.073. [DOI] [PubMed] [Google Scholar]
- 53.Norvell DC, Czerniecki JM, Reiber GE, et al. The Prevalence of knee pain and symptomatic knee osteoarthritis among veteran traumatic amputees and nonamputees. Arch Phys Med Rehabil. 2005;86(3):487–93. doi: 10.1016/j.apmr.2004.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bramley JL, Worsley PR, Bader DL, et al. Changes in tissue composition and load response after transtibial amputation indicate biomechanical adaptation. Ann Biomed Eng. 2021;49(12):3176–3188. doi: 10.1007/s10439-021-02858-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Putz C, Block J, Gantz S, et al. Structural changes in the thigh muscles following trans-femoral amputation. Eur J Orthop Surg Traumatol. 2017;27(6):829–835. doi: 10.1007/s00590-017-1929-5. [DOI] [PubMed] [Google Scholar]
- 56.Ramírez JF, Isaza JA, Mariaka I, et al. Analysis of bone demineralization due to the use of exoprosthesis by comparing young's modulus of the femur in unilateral transfemoral amputees. Prosthet Orthot Int. 2011;35(4):459–66. doi: 10.1177/0309364611420478. [DOI] [PubMed] [Google Scholar]
- 57.Pasqualini L, Ministrini S, Macura A, et al. Increased bone resorption:a possible pathophysiological link between hypovitaminosis d and peripheral arterial disease. Eur J Vasc Endovasc Surg. 2016;52(3):352–9. doi: 10.1016/j.ejvs.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 58.Hyde Z, Mylankal KJ, Hankey GJ, et al. Peripheral arterial disease increases the risk of subsequent hip fracture in older men:the health in men study. Osteoporos Int. 2013;24(5):1683–8. doi: 10.1007/s00198-012-2218-0. [DOI] [PubMed] [Google Scholar]
- 59.Ungprasert P, Wijarnpreecha K, Thongprayoon C, et al. Peripheral arterial disease and risk of hip fracture:a systematic review and meta-analysis of cohort studies. J Postgrad Med. 2018;64(4):220–225. doi: 10.4103/jpgm.JPGM_685_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiao H, Xiao E, Graves DT. Diabetes and its effect on bone and fracture healing. Curr Osteoporos Rep. 2015;13(5):327–35. doi: 10.1007/s11914-015-0286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baldwin MJ, Policha A, Maldonado T, et al. Novel association between bone mineral density scores and the prevalence of peripheral artery disease in both sexes. Vasc Med. 2017;22(1):13–20. doi: 10.1177/1358863X16672740. [DOI] [PubMed] [Google Scholar]
- 62.Krakauer JC, McKenna MJ, Buderer NF, et al. Bone loss and bone turnover in diabetes. Diabetes. 1995;44(7):775–82. doi: 10.2337/diab.44.7.775. [DOI] [PubMed] [Google Scholar]


