Abstract
Coccidioidomycosis is a significant health problem of dogs and humans in endemic regions, especially California and Arizona in the U.S. Both species would greatly benefit from a vaccine to prevent this disease. A live avirulent vaccine candidate, Δcps1, was tested for tolerability and efficacy to prevent pulmonary coccidioidomycosis in a canine challenge model. Vaccine injection-site reactions were transient and there were no systemic effects observed. Six of seven vaccine sites tested and all draining lymph nodes were sterile post-vaccination. Following infection with Coccidioides posadasii, strain Silveira, arthroconidia into the lungs, dogs given primary and booster vaccinations had significantly reduced lung fungal burdens (P = 0.0003) and composite disease scores (P = 0.0002) compared to unvaccinated dogs. Dogs vaccinated once had fungal burdens intermediate between those given two doses or none, but disease scores were not significantly different from unvaccinated (P = 0.675). Δcps1 was well-tolerated in the dogs and it afforded a high level of protection when given as prime and boost. These results drive the Δcps1 vaccine toward a licensed veterinary vaccine and support continued development of this vaccine to prevent coccidioidomycosis in humans.
Keywords: Coccidioidomycosis, Vaccine, Dogs, Avirulent, Fungal
1. Introduction
Coccidioidomycosis, also known as Valley Fever, is a systemic fungal disease endemic to regions of the southwestern United States, Mexico, Central America and South America [1]. Its incidence and range in the US are both expanding because of changing demography, travel, and climate [2–5]. Coccidioidomycosis is contracted by inhalation of Coccidioides posadasii or C. immitis arthroconidia, and disease occurs in both animals and humans [6,7].
Over half of human coccidioidal infections are asymptomatic and confer resistance to illness from future exposure [8]. The others result in a clinical illness, estimated to annually affect approximately 1% of persons in the heavily endemic areas of Arizona and the Central Valley of California [9]. Most frequently this produces a weeks- to months-long respiratory illness which is eventually self-limited in the majority of patients [10]. Per 100,000 patients, approximately 10% develop pulmonary or disseminated disease complications that result in long-term to lifetime antifungal treatment and medical care, permanent disability, or death [10,11]. The yearly economic impact of coccidioidomycosis to California and Arizona is nearly $1.5 billion dollars [12,13].
Dogs are also very susceptible to coccidioidomycosis, and the infection rate is higher than in humans [14]. The range of illness is similar but complications approach 25% of cases, which results in all symptomatic dogs being treated with protracted courses of antifungal medication [15–18]. The cost of treatment is a burden to owners that sometimes leads to euthanasia and relinquishment of dogs to shelters. Thus, the morbidity and mortality from this disease is significant in dogs and humans and an effective vaccine to prevent coccidioidomycosis would be beneficial for both.
The mutant strain, Δcps1, from which the 6 kb CPS1 gene has been deleted, proved to be avirulent in mice [19]. Vaccinating mice with viable arthroconidia of Δcps1 produced excellent protection against subsequent lethal infection with either C. posadasii or C. immitis [19,20]. In mice, the immunity has been shown to be durable for at least six months with no diminution of protection [20]. We have also demonstrated that it is the result of adaptive immunity based on detecting increased CD4+ T-cells secreting IFN-γ in lung and spleen cells of vaccinated and vaccinated/challenged mice [20]. Finally, live arthroconidia do not require an adjuvant to protect mice and therefore a vaccine formulation could be relatively inexpensive to manufacture, making commercialization feasible for both a veterinary and a human vaccine.
Because there is significant need to prevent coccidioidomycosis in dogs and because a successful veterinary vaccine provides documentation of safety and efficacy in a relevant animal species with respect to FDA evaluation [21], a licensed vaccine for dogs was pursued first. USDA regulations for licensure of animal vaccines require demonstration of efficacy in the target species. Groups of dogs were vaccinated subcutaneously with Δcps1 arthroconidia and subsequently infected by intratracheal nebulization of virulent C. posadasii arthroconidia [22]. We assessed safety, tolerability and efficacy of the Δcps1 vaccine candidate to protect dogs against pulmonary coccidioidomycosis. The results show there is a feasible path towards Δcps1 as a vaccine in humans.
2. Materials and methods
2.1. Fungal organisms
2.1.1. Vaccine strain
The generation of avirulent Δcps1 has been previously described [19]. Δcps1 was grown on 2X glucose yeast extract (GYE) agar (2% glucose, 1% yeast extract, 1.5% agar) at 30 °C for 6–8 weeks until colonies appeared mature [19]. Arthroconidia were harvested in sterile, pyrogen-free water using the spin-bar method as previously described [23], and filtered through Pellon Thermolam® Plus material to remove hyphal fragments [24]. Arthroconidia were enumerated by hemocytometer and 10-fold serial dilutions grown on GYE agar for 4 days at 37 °C to determine viable colony forming units (CFU). Growth and use of Δcps1 was permitted at BSL2/ABSL2 or higher under approval of the Institutional Biosafety Committee of the University of Arizona.
2.1.2. Infection strain
C. posadasii, strain Silveira, (Silveira) (ATCC #28868) was grown to maturity (4 weeks) on GYE agar, harvested, and enumerated as described above. Silveira grows more rapidly than Δcps1, so colonies from serial dilutions were enumerated after growth at 37 °C for 3 days instead of 4 days. All growth and handling of Silveira occurred at BSL3 and ABSL3.
For vaccinations and infections, arthroconidia of Δcps1 and Silveira, respectively, were shipped overnight as concentrated suspensions of ≥ 90% viability for use in dogs within two weeks of arrival. Suspensions were diluted in 0.9% USP saline immediately before use.
2.2. Animals
One to two year old beagle and beagle mix dogs of both genders were housed in same sex pairs according to Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals at Veterinary and Biomedical Research Center, Inc., (VBRC) (Manhattan, KS), a BSL2 facility. Dogs were fed once daily and water was available ad libitum. Procedures were approved by the institutional animal care and use committee of Kansas State University (Manhattan, KS). Following the vaccination protocol and monitoring, dogs were transported using a USDA-approved transportation company to the Biosafety level 3 Animal Disease Laboratory (ADL), Colorado State University (CSU)(Ft. Collins, CO). In the ADL, dogs were socially housed according to PHS standards in single gender groups of 7–8 dogs in rooms with raised areas, supplemental sleeping boxes, and sterilizable enrichment toys. Dogs were fed once daily and water was available ad libitum. All procedures were approved by the CSU institutional animal care and use committee and were performed at ABSL3.
2.3. Vaccination
Dogs underwent veterinary assessment for normal health prior to vaccination. Dogs were individually identified by tattoo and randomized to receive one of four vaccine conditions or saline only. Δcps1 arthroconidia were diluted to deliver the specified dose in 1 mL of saline and the dose was injected subcutaneously (SC) in the back of the neck, alternating sides to differentiate prime on study day 0 and booster vaccination on day 28. Injection sites were inspected daily and each scored separately. If sites appeared normal, this was recorded without measurement. If swelling was present, the site was measured in two or three dimensions with calipers and recorded until resolved. Dogs were bled (~10 mls) before vaccination (day 0), and on days 14, 28, and 42. Serum was frozen (−20 °C) for later antibody testing. On day 42, one randomly selected dog from each vaccination group was humanely euthanized, observations of the injection sites were recorded, and the injection sites and draining lymph nodes were collected into sterile Whirl-pak specimen bags (Nasco, Washington, IN) for fungal culture.
2.4. Infectious challenge
All procedures were performed at ABSL3. Upon arrival at ADL, dogs were examined to assure health and fitted with a Lifechip Bio-thermo sensor (Destron Fearing, South St. Paul, MN) subcutaneously for identification and monitoring of body temperature during the studies. Dogs were acclimated for ≥ 7 days prior to infection. The day before infection, body weight, serum chemistries, CBC, and three-view thoracic radiographs were collected for each dog. For infection, dogs were sedated with xylazine (1–2 mg/kg IM), anesthetized intravenously with propofol (4–6 mg/kg titrated to effect) and intubated with a cuffed endotracheal tube. Silveira arthroconidia, diluted in 5 mL of 0.9% saline, were delivered as an intratracheal fine particle suspension over the course of 10–15 min with an LC Sprint nebulizer and Vios air compressor (PARI Respiratory Equipment, Inc, Midlothian, VA) by the method of Soffler. et.al. [22] Dogs’ muzzles were cleaned with 70% ethanol after extubation to diminish the chance of exposure of personnel.
2.5. Infection monitoring
Following infection, activity level, eating, and drinking were monitored daily and body temperature recorded every 2–3 days using the Lifechip bio-thermo sensor. Dogs were weighed weekly. Three-view thoracic radiographs were obtained every two weeks until the end of the study using a portable digital radiography machine (Next Equine DR System 1417E, Sound, Carlsbad, CA). Serum chemistries, CBC, and serum archival for antibody testing were performed every two weeks. Serum was centrifuged and sterilized using a 0.8 μm filter. Serum was split and 1 mL sent to the Diagnostic Medicine Center at CSU for biochemistry analysis while the remainder was archived at −20 °C for later serology. CBCs were completed on a blood analyzer (Heska Element HT-5, Heska, Love-land, CO) within the ABSL3 facility. Dogs were euthanized by injection of pentobarbital overdose at the study endpoint 56 days post-infection, or if they had deteriorating clinical condition that met criteria for early removal. Though the model was not designed to produce mortality in 56 days, criteria for early removal included ≥ 15% loss of body weight, respiratory distress, unable to eat/drink/ambulate or a combination of any three: persistent cough several times a day > 7 days, persistent fever > 104.5 for > 5 days, anorexia > 7 days, progressive depression/lethargy over 7 days.
2.6. Necropsy
Gross observations were recorded with particular focus on the lungs and thoracic cavity. The six lung lobes were dissected and weighed individually prior to homogenization for culture. One mediastinal lymph node was also collected for culture. For histopathology, specimens of approximately 1-cm3 from each lung lobe, liver, spleen, and kidney, and a whole mediastinal lymph node were preserved in 10% neutral-buffered formalin. The lymph nodes selected for fungal culture and histopathology were the two largest observed in the mediastinum. Samples of other abnormal-appearing tissues were also preserved in 10% neutral-buffered formalin.
2.7. Fungal burden
Lung and lymph node tissue was weighed prior to homogenizing with either a Glas-Col 099C K54 variable speed homogenizer (Glas-Col, LLC, Terra Haute, IN) or a blender in a premeasured quantity of saline under BSL3 containment. Serial 10-fold dilutions were made and 100 μL of suspension distributed on GYE plates and incubated for 3 days. For specimens that had minimal or no evidence of gross disease, 400–500 μL of the residual undiluted homogenate was also dispensed on a GYE plate and enumerated as described. Colonies were counted and fungal burden expressed as CFU/g of tissue.
2.8. Serology
Serum samples were shipped on dry ice to the University of Arizona, where they were stored at −20 °C until testing. One mL serum samples from the blood collected post-vaccination day 28, post-vaccination day 63 or 70 (prior to infectious challenge), and at termination were submitted to a commercial veterinary reference laboratory for Coccidioides screen and titer (Antech Diagnostics, Fountain Valley, CA); the test detects IgM and IgG using IDCF and IDTP antigens [25]. All serum specimens (9 per dog) from the vaccination and challenge studies were tested by a commercial enzyme immunoassay (EIA) (MiraVista Canine Coccidioides IgG Antibody EIA, Mira Vista Diagnostics, Indianapolis, IN) according to their protocol [26]. Results are reported in EIA Units (EU): <8 EU = negative; 8-<10 EU = intermediate; ≥10 EU = positive. The antigen for detection of antibody is proprietary [26].
2.9. Histopathological scoring
Five-micrometer sections of paraffin-embedded, formalin-fixed tissues were stained routinely with hematoxylin and eosin (H&E) and scored blindly by a single veterinary pathologist (HBO). The parameters scored were overall lesion extent, inflammation, necrosis, spherule score (estimated number of organisms in lesion), mineralization, and fibrosis (Table S3). Scores from each slide were summed to provide a total.
2.10. Radiography scoring
Radiographic images were submitted electronically for scoring by a single veterinary radiologist (JH), who was blinded to treatment conditions of the dogs. The thorax was scored for extent and distribution of pulmonary pattern, pulmonary lesions, thoracic lymphadenopathy, pleural disease, and pericardial or bone involvement (Table S4). The latter was limited to bones visualized on the thoracic radiographs. A cumulative score was determined for each dog by summing the scores for all time points.
2.11. Composite disease scoring
A composite score was derived for each dog in the vaccine/challenge study. Parameters that reflected the severity of disease were selected. These included, Log10 of the total lung fungal burden, Log10 of one mediastinal lymph node (LN) fungal burden, reciprocal of the AGID serology titer, number of lung nodules visualized at necropsy, lymph node enlargement score (0–4), total histopathology score and spherule score (Table S3), radiology score (Table S4), neutrophilia, monocytosis, hyperglobulinemia, hypoalbuminemia, (1 point for each abnormal value at 4 time points) and reciprocal of the albumin:globulin ratio (A/G ratio) in the terminal serum. Increased neutrophils, monocytes and globulin and decreased serum albumin are common abnormalities in clinically ill dogs with coccidioidomycosis [18].
2.12. Statistical analysis
Non-parametric Kruskal-Wallis tests were performed on the data from the vaccine-challenge study using Prism 8.1 (GraphPad Software, San Diego, CA).
3. Results
3.1. Vaccination of dogs with Δcps1 and assessment of injection sites
The primary objective of this portion of the study was to assess safety and tolerance of Δcps1 in dogs. A secondary goal was to determine if antibody response could be a surrogate marker of vaccine immunogenicity. Dogs were randomized into five groups (n = 6–7/grp) and vaccinated with 1 × 104, 5 × 104, or 1 × 105 viable arthroconidia of Δcps1 twice 28 days apart (10 K PB [prime/boost], 50 K PB, and 100 K PB, respectively), or once only with 1 × 105 arthroconidia (100 K P [prime only]) on day 0. Control dogs were given saline twice on day 0 and 28. Injection site reaction frequency, approximate size, and time to resolution or reduction in swelling is summarized in Table 1. The majority of dogs developed localized swelling at the injection sites characterized by a soft to moderately firm, moveable, subcutaneous swelling that resolved in 2 weeks or less in most animals. Generally, increased doses of Δcps1 generated more frequent injection site reactions, especially booster reactions, with increased likelihood of greater size and time to resolution of the swelling. The dogs did not exhibit pain to palpation of the swelling, and none of them developed abscesses or ulceration. They had no systemic adverse reactions (fever, pain, lameness, lack of appetite, lethargy, vomiting, or diarrhea). In the final evaluation of the injection sites at necropsy following infection, none of the injection sites were identified (119 or 125 days after primary vaccination). Overall, the vaccine was well-tolerated in dogs at the doses tested. Two weeks after booster vaccination (day 42), one dog randomly selected from each vaccinated group (n = 4 dogs total) was euthanized and the injection sites and prescapular lymph nodes were collected for fungal culture. Injection site granulomas were diminutive (<3 mm) or not visualized. The only sample that had fungal growth, which was not quantitated, was the undiluted residual homogenate from the primary vaccination site of the 100 K PB dog. Lymph nodes from all four dogs appeared normal and were negative for fungal growth. We documented infrequent persistence of the vaccine strain at injection sites (1 of 7 sites) and no detectable spread to draining lymph nodes.
Table 1.
Injection site reactions following subcutaneous primary (P) and booster (B) vaccination with Δcps1 arthroconidia. Vaccine was administered in the back of the neck on alternating sides.
| No. Viable Arthro-conidia | Vx Dose (# Dogs) | No. Inj Site Rxns | Size range and Days to Reduction/Resolution of Swelling | |||
|---|---|---|---|---|---|---|
| Small (cm3) | Days to Resolution | Large (cm3) | Days to Resolution | |||
| 1 × 10 4 | P (7) | 5/7 | 0.2 – 1.0 (5) | 10–14 (3), 33 (1), 42 (1) | None | N/A |
| B (7) | 2/7 | 0.2 – 1.0 (2) | 10–14 (2) | None | N/A | |
| 5 × 10 4 | P (7) | 5/7 | 0.125 – 1 (4) | 11–14 (4) | 3.5 (1) | Reduced by 14 days |
| B (7) | 5/7 | 0.3 – 1.0 | 11–14 (5) | None | N/A | |
| 1 × 10 5 | P (14) | 13/14 | 0.2 – 1.0 (11) | 11–14 (11) | 2.0 (1) 2.0 (1) 3.5 (1) |
12, 12, 42 |
| B (7) | 5/7 | 0.125–1.0 (3) | 14 | 1.5 (1) 3.5 (1) |
Reduced by 14 days | |
Serum collected on study days 0, 14, 28, and 42 was tested for anticoccidioidal IgG antibody production by EIA [26]. One dog in the 100 K PB group had a positive test (21.8 EU) on day 42, and all other sera were negative. Serum collected on day 28 and immediately prior to infectious challenge (day 63 or day 70 from initial vaccination) was tested by AGID as well. None of the dogs had IgG or IgM detected by AGID on day 28 after the primary vaccination, and a single dog was positive for IgG only on day 63 post-vaccination. Overall, antibody detection proved a poor surrogate marker of dogs’ response to the Δcps1 vaccine. However, it appears it will be unlikely to interfere with antibody testing, the standard assay to detect infection in dogs.
3.2. Infectious challenge and clinical course of vaccinated dogs
To test vaccine efficacy, 30 dogs (n = 6/group) from the safety and tolerability study were transported to the biosafety level 3 ADL at CSU, and randomized into same-sex housing groups of 7–8 dogs. CSU investigators were blind to the dogs’ treatment. The dogs were divided into two cohorts of 15, separated by one week, for infection and monitoring to manage the workload of the procedures. Using the dose found in preliminary experiments to result in a reliable and robust infection, dogs were infected with 1 × 104 arthroconidia of Silveira by intratracheal nebulization on day 63 or day 70 post-vaccination for the two cohorts. Animals were observed daily and the rest of the data collection procedures were performed every two weeks through euthanasia. All 30 dogs survived until study termination, with clinical abnormalities recorded for only four dogs, all of them in the control and prime only groups. Clinical signs included coughing (2 dogs), lethargy (1 dog), fever (transient −1 dog, persistent −1 dog), and weight loss > 5% (1 dog).
3.3. Disease scores and lung fungal burdens
The quantitative measures used to assess vaccine efficacy were lung and lymph node fungal burden (log10), reciprocal of the terminal AGID IgG antibody titer, gross necropsy lung and lymph node scores, total histopathology score, spherule score, radiology score, presence of neutrophilia, monocytosis, hyperglobulinemia, and hypoalbuminemia, and reciprocal of the A/G ratio. The score in each category was summed to provide a composite disease score for each dog. The minimum score was zero for a dog with no evidence of infection; higher scores, which could be > 100, correlated with more severe disease. (See Table S1 for composite disease scoring details.)
The 10 K PB, 50 K PB, and 100 K PB vaccinations all greatly reduced disease. Group mean composite disease scores of 9.5, 10.7, and 11.7, respectively, were significantly lower than unvaccinated controls (123.7, p = 0.0002) and 100 K P dogs (55.9, p = 0.037) (Kruskal-Wallis). (Fig. 1) There were no statistical differences in the group composite disease scores between the three groups vaccinated twice, and all three doses were highly protective. Prime only vaccination did not significantly reduce the composite disease scores compared to the unvaccinated dogs (p = 0.675, Kruskal-Wallis). Lung and lymph node fungal burden, presence of immunodiffusion titers, the finding of a disseminated rib lesion, and increasing perturbations in white blood cell counts, albumin, and globulin demonstrated that dogs given a single vaccine fared less well than the prime/boost groups.
Fig. 1.
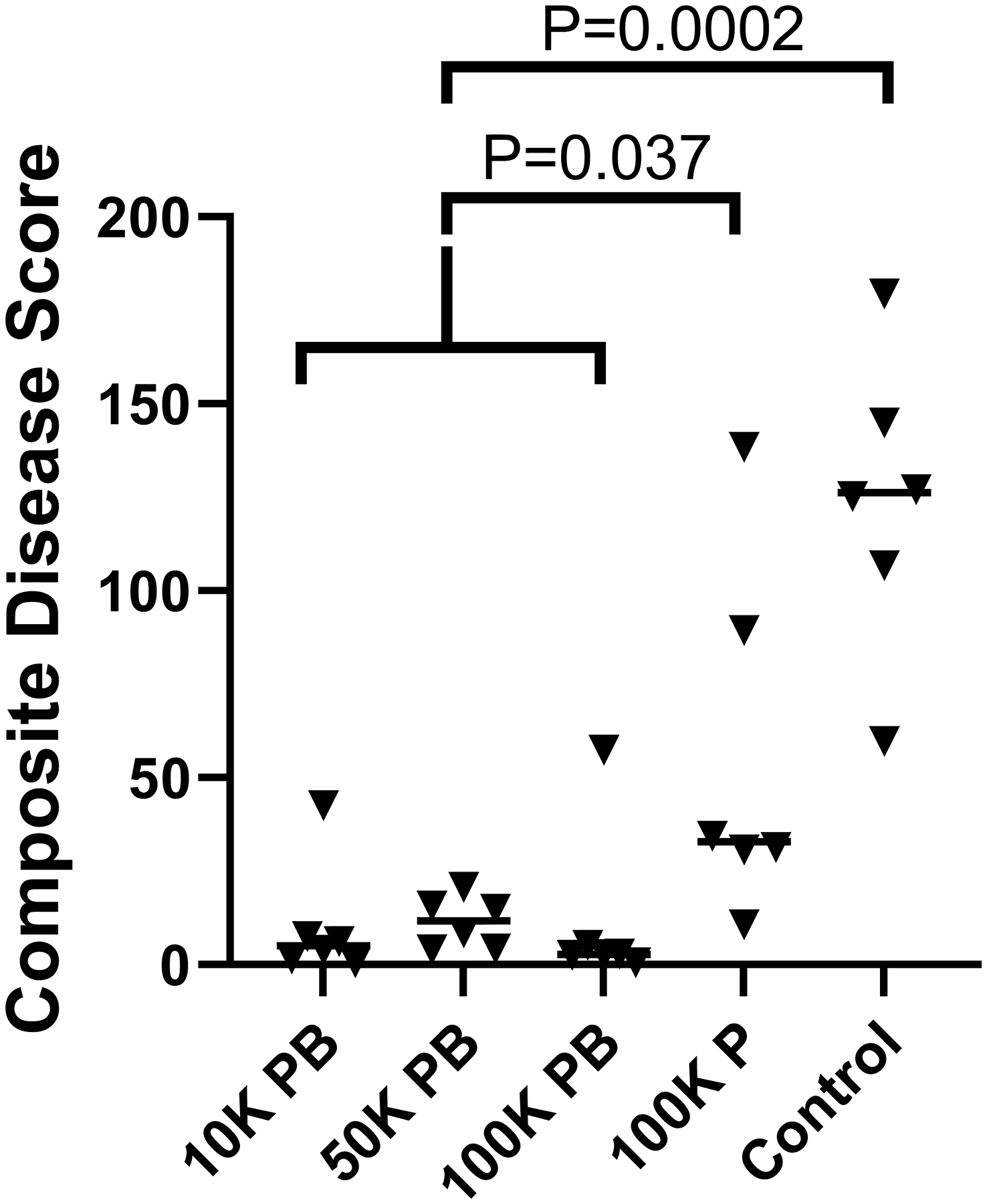
Composite disease scores by vaccination group. The mean disease scores were similarly low (P = 0.23) in the three groups that received both prime and booster vaccinations so they were combined for further analysis. Two vaccinations resulted in significantly lower disease scores than one vaccination or no vaccinations. Bar = median score. (Statistical analysis – Kruskal-Wallis).
To determine total lung fungal burden, individual lung lobes were completely homogenized in a quantified volume of saline, a 1-mL aliquot of the homogeneous suspension was cultured by serial dilution, and the total lung fungal burden was calculated. The median lung fungal burdens were ≤ 100 CFU for the three prime/boost groups, with no significant differences between them (p = 0.37, Kruskal-Wallis). The prime/boost groups were combined for further comparison. Lung fungal burdens of prime/boost dogs were significantly lower than prime only (p = 0.0036) or controls (p = 0.0003, Kruskal-Wallis). (Fig. 2) Lung fungal burden measurement and statistical significance correlated well with the total composite disease scores in these dogs, indicating a direct relationship between disease burden and in-life measures of infection, such as radiographic abnormalities, antibody titers, neutrophilia, monocytosis, hyperglobulinemia, and reduced A/G ratio.
Fig. 2.
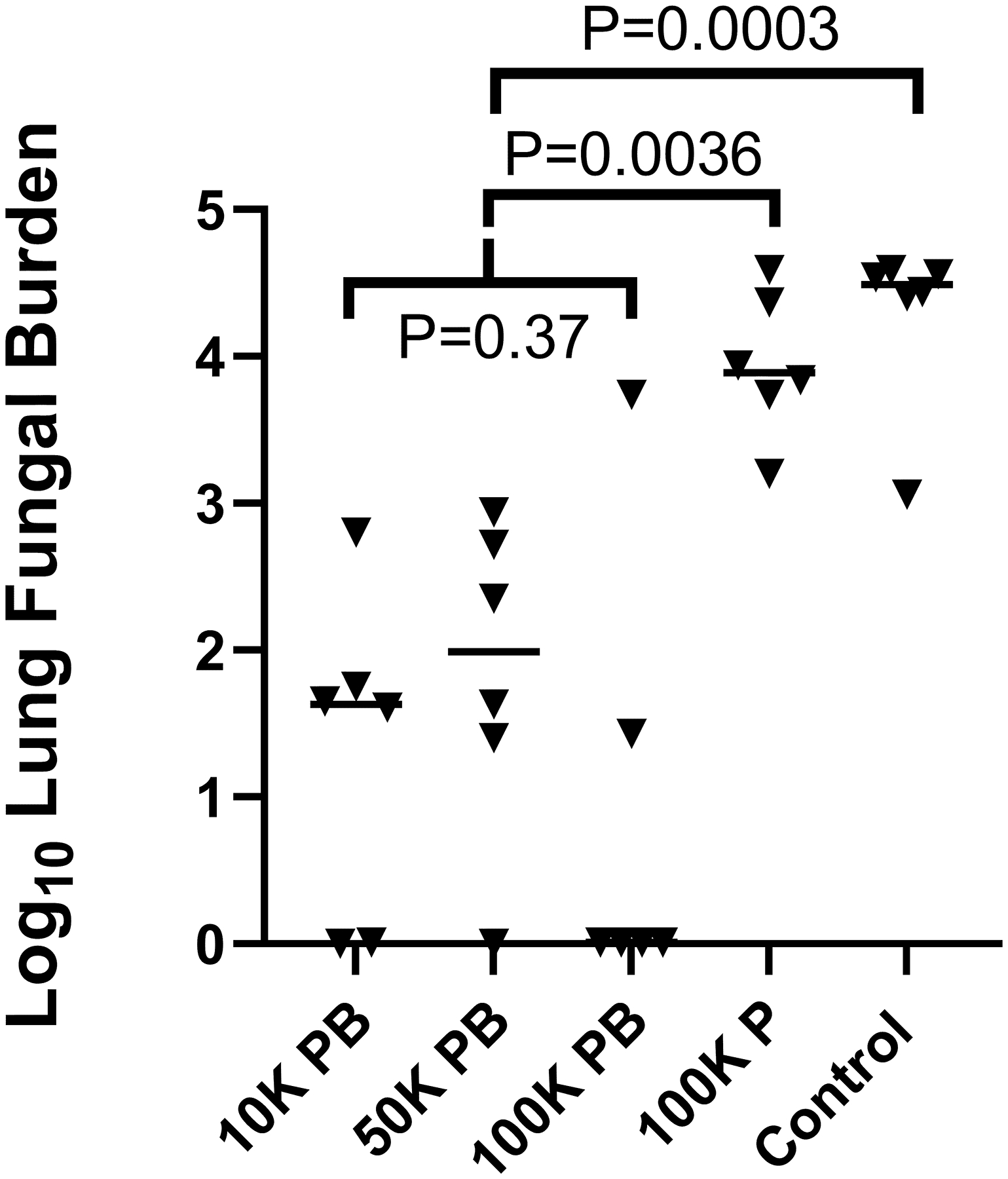
Lung fungal burdens of dogs vaccinated twice, once, or not at all with Δcps1. Differences between the 10 K PB, 50 K PB, and 100 K PB groups were not significant (P = 0.37), so they were combined for further comparison. Prime/boost significantly reduced total lung fungal burdens compared to prime only (100 K P) or no vaccination (Control) eight weeks after intratracheal inoculation of dogs with 1 × 104 Silveira arthroconidia. Lung fungal burdens were not significantly reduced by prime only vs. no vaccine (P = 0.3). Bar = median. (Statistical analysis - Kruskal-Wallis).
3.4. Serological responses of dogs after challenge
Serum was collected and banked every two weeks from one day prior to infection to euthanasia. All samples were tested by EIA for IgG antibody, and the pre-challenge and terminal sera were tested by AGID for IgG and IgM at the commercial laboratory. Prior to infection, all dogs were seronegative by both tests except for one dog in the 10 K PB group which had both a transient AGID IgG titer of 1:4 and and 9.8 EU (intermediate, below positive cutoff) by EIA. After infection, all the control dogs and 4/6 100 K P dogs were seropositive for IgG antibody by one or both tests. (Fig. 3) (Table S2) Among the dogs vaccinated twice, three were seropositive, one MVEIA only, one AGID only, and one both. AGID-positive dogs had higher composite disease scores than the other prime/boost dogs by visual examination of the data. (Table S2)
Fig. 3.
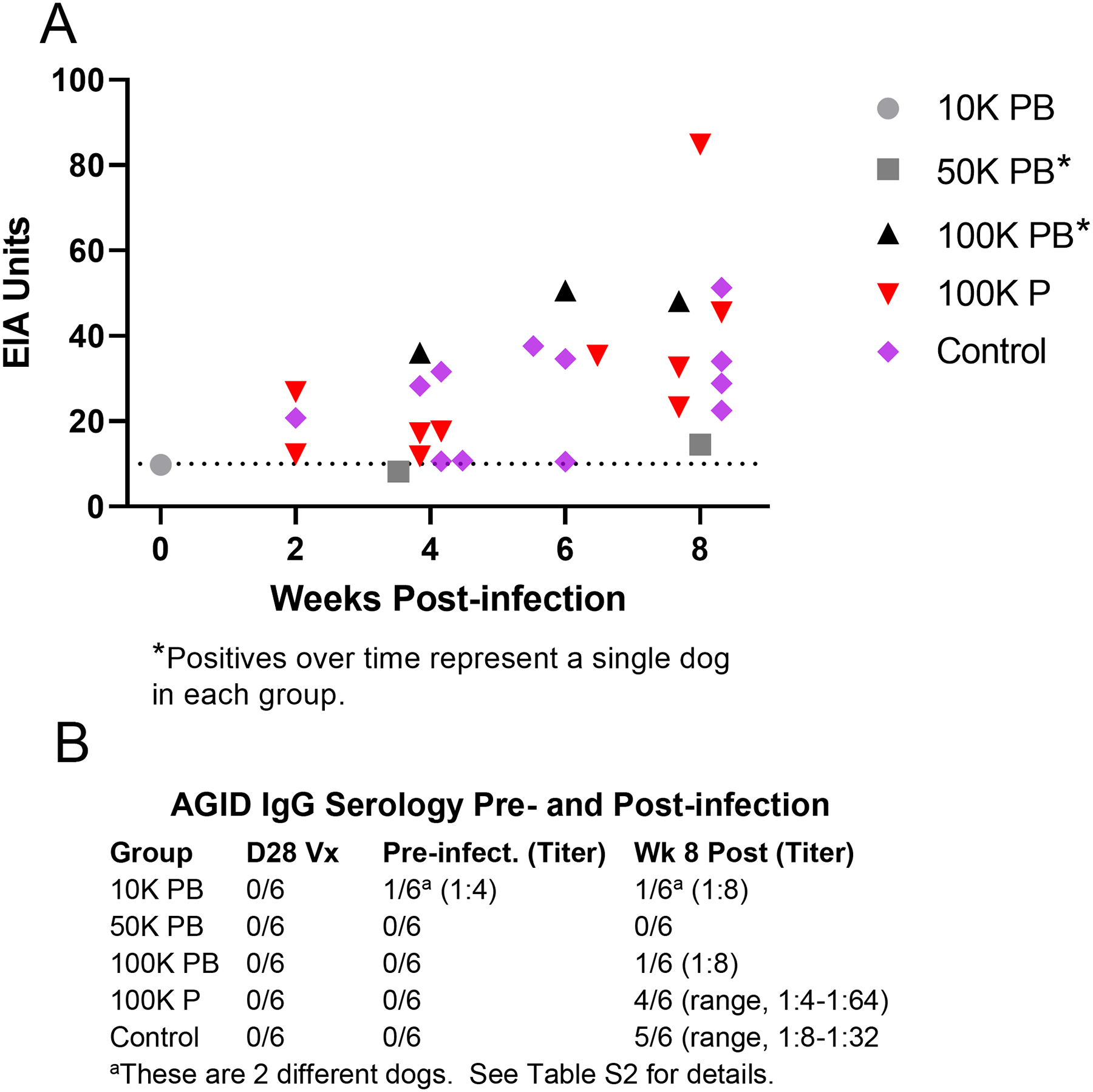
Serology of vaccinated dogs challenged with Silveira arthroconidia. Serum from 30 dogs (n = 6/grp) was tested every two weeks from prior to vaccination until 42 days post-vaccination, and immediately prior to infectious challenge until euthanasia 8 weeks post-infection. (A) MVEIA values ≥ 8 EIA Units (EU) in dogs pre-infection until 8 weeks post-infection (termination). Dogs in the 100 K P and Control groups had IgG detected as early as 2 weeks post-infection and had more IgG positives over time, indicating greater disease activity. All dogs not represented on the graph had negative EIA results. (B) IgG by agar gel immunodiffusion was not detected in any dogs 28 days after primary vaccination (D28Vx), and one dog had a transient, low titer (1:4) 63 days after primary vaccination when tested prior to infection. There were no false positive results in unvaccinated Control dogs. Terminal IgG titers (range 1:4–1:64) were frequent in the 100 K P and Control groups but not in Prime/Boost groups. Only two Prime/Boost dogs had terminal IgG titers, and the dog with a titer pre-challenge had resolved by end of study.
3.5. Radiographic assessment
Radiographs were scored by a single board-certified veterinary radiologist blind to the treatment groups. In 13/18 dogs vaccinated twice, lung radiographs were normal (Score = 0) at all time points. Of dogs with radiography scores above 0, four were ≤ 3 and one had a score of 11. The abnormalities in the latter dog correlated with a higher histopathology score and higher lung fungal burden than other dogs vaccinated twice.
Radiographic abnormalities were seen in 3/6 100 K P dogs, and all six control dogs. Fig. 4 shows representative terminal radiographs comparing dogs with prime and booster vaccinations to prime only or no vaccination. One dog in the 100 K P group had a lytic-proliferative rib bone lesion, presumptively disseminated coccidioidomycosis, that was observed on day 14 p.i. and continued to enlarge throughout the study. (Figure S1)
Fig. 4.
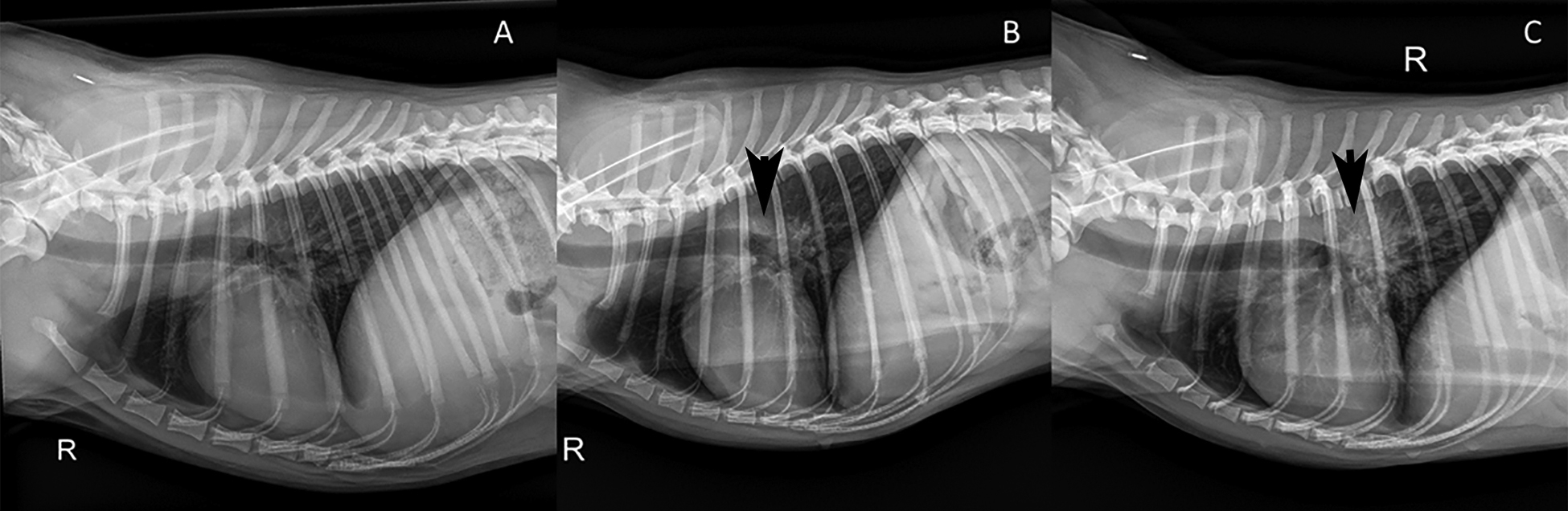
Example right lateral radiographs from vaccine-challenge study dogs. (A) Normal lungs (score = 0) [100 K PB]; (B) interstitial infiltrates in a hilar pattern with moderate hilar lymphadenopathy (arrow) (score = 11) [100 K P]; (C) moderate, diffuse reticulonodular infiltrates with severe hilar lymphadenopathy (arrow) and enlarged sternal lymph node (score = 21) [Control].
3.6. Gross and microscopic pathology of infected dogs
At necropsy, 56 days p.i., grossly visible lesions were limited to the thoracic cavity and included lung nodules, mediastinal lymph node enlargement, and occasional sternal lymph node enlargement (n = 4). Twelve dogs in the prime/boost groups had normal-appearing lungs and thoracic lymph nodes. A normal gross appearance did not preclude the presence of spherules as evidenced by the fact that seven of the 12 dogs had growth from the lungs and/or mediastinal lymph node. Tiny granulomas not easily visualized may have organisms with a high likelihood of being cultured by the whole-lobe homogenization used for this study. In the 100 K P and control groups, lesions were grossly visible in all dogs. No injection site nodules were seen at necropsy.
Histologically, 12/18 dogs among the groups vaccinated twice had pyogranulomatous lesions in the lungs and/or lymph nodes that were characteristic of coccidioidomycosis, but spherules were identified in sections from only two dogs, indicating that organisms were sparse or absent. In the 100 K P and control groups combined, 11/12 dogs had lesions and spherules were visualized in nine. Lung lesions in the latter two groups were described as multifocal to coalescing, pyogranulomatous to suppurative, with fibrosis and necrosis (Fig. 5, A and B). In these dogs also, severe pyogranulomatous inflammation typically effaced the structure of the mediastinal lymph nodes as shown in Fig. 5, C and D. Outside of the thoracic cavity, the only lesion identified was a small granuloma without spherules in the renal cortex of one dog. It could not be determined if the lesion was caused by coccidioidomycosis.
Fig. 5.
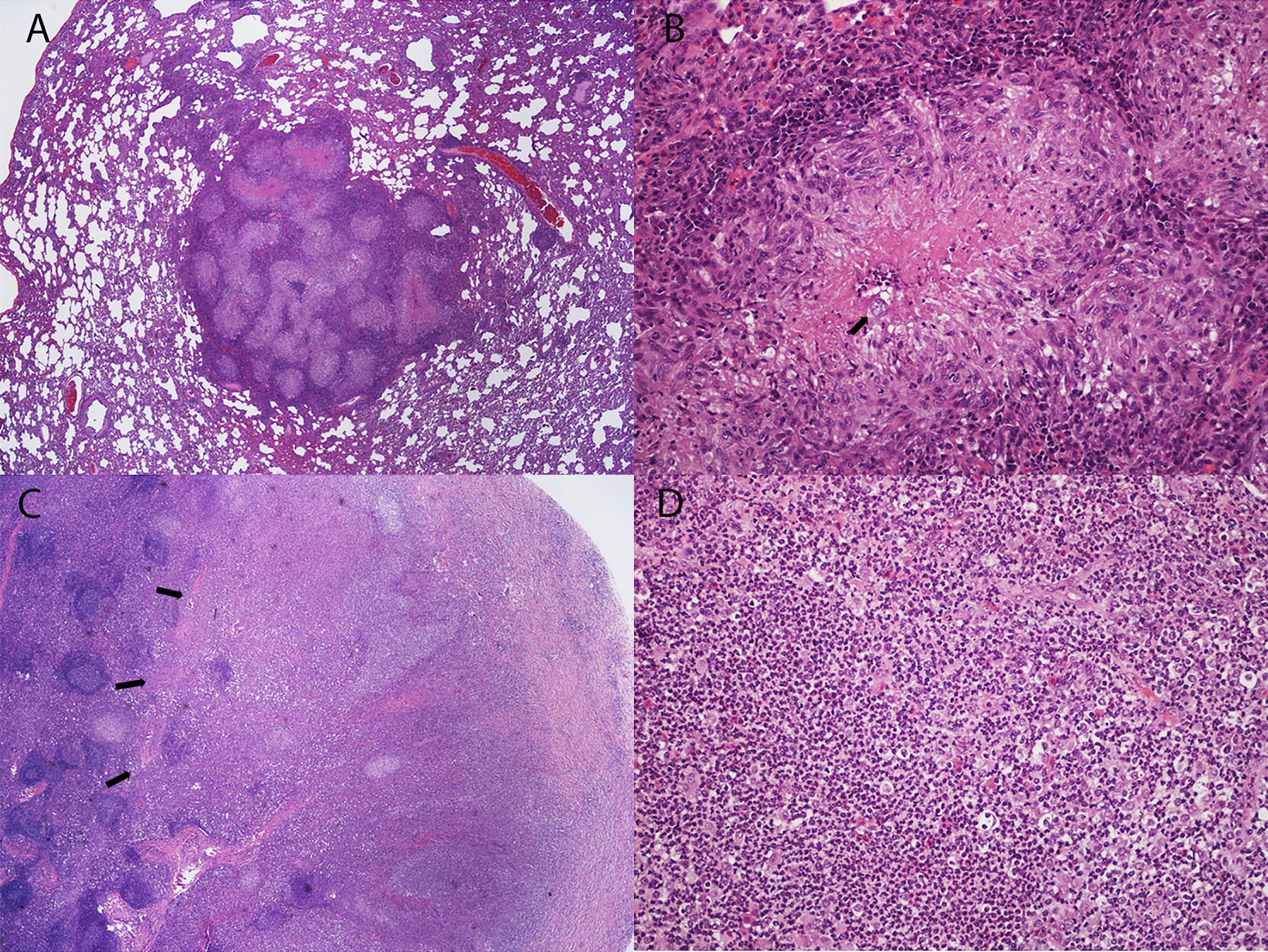
Examples of lung and mediastinal lymph node histopathology in Coccidioides-infected dogs from the Control group. (A) Multifocal to coalescing granulomatous and pyogranulomatous lesions with multifocal necrosis in lung lobe. (B) Spherule (arrow) in a necrotic center surrounded by pyogranulomatous inflammation. (C) There is effacement of normal architecture of the mediastinal lymph node due to severe pyogranulomatous inflammation (arrows). (D) Higher power image of lymph node effacement in (C) shows diffuse neutrophilic inflammation. Stain: hematoxylin and eosin. Magnification: A, C – 20X; B, D – 200X.
4. Discussion
Subcutaneous prime plus booster vaccination of young adult dogs with doses of 1 × 104 to 1 × 105 live Δcps1 arthroconidia prevented or greatly mitigated coccidioidomycosis in a pulmonary challenge model. The challenge was designed to produce significant measures of disease in the lungs by radiography, gross and histological observations, and lung fungal cultures. Only minimal measures of disease were seen in nearly 90% of the dogs vaccinated twice, and none of them exhibited illness that would have prompted veterinary evaluation in a clinical setting. In essence, all the dogs in these groups were protected from clinically relevant coccidioidomycosis. By contrast, a single high dose of vaccine, did not significantly reduce either lung fungal burdens or global disease scores compared to unvaccinated controls, thus demonstrating that a booster immunization is necessary to induce protection, even though these dogs did not exhibit very much illness either in the time frame of the study.
In addition to preventing disease, a vaccine needs to be safe and well-tolerated, and the lack of systemic adverse effects and resolution of swelling at the injection sites supports both of these features in the dogs. Though a few dogs had relatively large initial vaccine reactions, the majority resolved in 14 days or less, and no injection site reactions were identified on necropsy at the end of the challenge study. A killed spherule vaccine previously tested in humans not only failed to show a significant reduction in diagnosed coccidioidomycosis in the vaccinated cohort, but was unacceptably irritating at the injection site [27]. The dogs in this study, though they frequently developed subcutaneous swelling, did not exhibit pain, fever, lameness, or other indications of discomfort. We have previously shown in mice that the majority of viable Δcps1 arthroconidia undergo degradation before completing spherule development [19]. This appears to be similar in dogs based on failure to grow the vaccine strain from 6/7 injection sites in dogs by 42 days post-vaccination. However, we believe that initiation of spherule growth is required for the antigen presentation which affords the extraordinary level of protection observed in this study, since we have shown that administering even high doses of dead Δcps1 arthroconidia provides no protection from virulent challenge in mice [19].
A surrogate marker of a vaccine response is highly desirable and antibody detection is commonly used for this [28]. However serology failed to detect consistent antibody responses to the Δcps1 vaccine in dogs. Since durable immunity to Coccidioides is cell-mediated [20,29], lack of antibody does not indicate the dogs failed to respond, especially considering the high level of protection in the prime/boost groups following challenge. In fact, dogs with higher lung fungal burdens and composite disease scores were the ones which developed antibodies, while the majority of dogs with minimal disease remained seronegative after infection as they were after immunization.
The quantitative measures of disease used in these dogs are a combination of in-life assessments (radiography, CBC, serum chemistry analysis) and post-mortem findings (lung and lymph node fungal burdens, gross thoracic disease assessment, and histopathology). As this study did not include a large number of dogs, we did not compare the ability of the in-life measures alone to assess disease severity in the model versus the post-mortem measures. In murine coccidioidomycosis models, lung fungal burdens are a critical measure of vaccine efficacy [30,31], and, for our dog study, a much more humane endpoint than mortality/survival. For monitoring of vaccine efficacy in community dogs in the future, the in-life measures of disease, especially prevention of primary pulmonary and disseminated disease, will be very important, as would be the case in human clinical trials.
A limitation of these studies is that they were not designed to investigate cell-mediated responses of the dogs to the vaccine, other than protection from disease after challenge. Though unpublished, we have shown that protection by Δcps1 can be passively transferred with CD4+ splenocytes but not T-depleted splenic cells or immune serum from vaccinated mice, and that Rag-1−/− mice lacking T- and B-cells do not generate protective responses following vaccination (Powell and Shubitz). Elucidating the vaccine’s protective mechanisms in dogs was beyond the scope of this study, for which the primary objective was to satisfy regulatory requirements for demonstration of efficacy. Currently, resources are lacking to study additional dogs for cell-mediated immune responses. The lack of antibody as a surrogate marker of vaccine immunity is also potentially limiting, but primarily in the context of assessing vaccine responses in humans, where a challenge trial cannot be performed. It will need to be determined empirically if humans make antibody responses or detectable cell-mediated responses to Δcps1. In humans, there are some well-defined assays for assessing cell-mediated immunity, such as skin test delayed hypersensitivity, and CD69 upregulation on CD3+ lymphocytes [32,33].
Finally, though we have determined that the immunity from Δcps1 in mice lasts at least six months [20], there are no data on durability of immunity in dogs. Due to the expense of such a study, coupled with the large number of dogs required, it is unlikely a duration of immunity study will be done experimentally. It is anticipated that post-approval monitoring for clinical illness in vaccinated populations of dogs would detect immunity that wanes in less than a year from vaccination.
In summary, Δcps1 appears well-tolerated in dogs and significantly protects them from experimentally induced coccidioidomycosis. This study is a large step toward meeting the regulatory requirements to license a Δcps1 vaccine for dogs. These studies were driven not only by the need for a vaccine to prevent clinical coccidioidomycosis in dogs, but as a stepping stone on the path to a vaccine to prevent disease in humans. The public health benefit to humans for a preventative vaccine for coccidioidomycosis is justified by the financial impact of the disease [12,13]. Morbidity is also substantial for those who experience the illness, including those with self-limited disease, because their ability to engage in work and other normal life activities is diminished for weeks to months [29]. The per capita public health problem in the endemic regions of the United States is similar to that for polio, measles, mumps, or rubella before there were effective vaccines [34,35]. Deaths attributable to coccidioidomycosis average 236 per year [36], and an effective vaccine has the potential to prevent most of them, along with the high costs of care associated with severe disease. Despite the strong public health argument, finding investment for a small market vaccine represents a major obstacle to commercialization. If the Δcps1 vaccine candidate to prevent coccidioidomycosis in dogs proves successful, this would encourage public health-based investment, perhaps in conjunction with private enterprises as with the canine vaccine, to develop this vaccine for humans.
Supplementary Material
Acknowledgements
We thank DJ Rezac and staff at Veterinary and Biomedical Research Center, Inc, for vaccinating and assessing reactions in the dogs; Lourdes Lewis and Margaret McDermott for preparation of fungal cultures; Joe Wheat, Nicole Bridges, and Michelle Durkin at MiraVista Diagnostics for collaboration on serology. We thank Tom Monath and Lynda Tussey for reading the manuscript and suggesting insightful improvements.
Funding
This work was funded by the National Institutes of Allergy and Infectious Disease [grant number RO1-AI-132140] and Anivive Lifesciences, LLC, Long Beach, CA.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [John N Galgiani reports financial support was provided by National Institutes of Health. Edward Robb reports financial support and equipment, drugs, or supplies were provided by Anivive Life Sciences, LLC. Edward Robb reports a relationship with Anivive Lifesciences, LLC that includes: employment.. Lisa Shubitz has patent #Fungal lmmunogens and Related Materials and Methods WO2014164843-A1 issued to Arizona Board of Regents. Marc Orbach has patent #Fungal lmmunogens and Related Materials and Methods WO2014164843-A1 issued to Arizona Board of Regents.].
Abbreviations:
- ADL
Animal Disease Laboratory
- CO
Ft. Collins
- AGID
agar gel immunodiffusion
- A/G
albumin:globulin
- BSL
Biosafety Level
- w/A
Animal
- CFU
colony-forming units
- CSU
Colorado State University
- EIA
Enzyme immunoassay
- EU
EIA Units
- GYE
glucose-yeast extract agar
- SC
subcutaneously
Footnotes
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.10.029.
References
- [1].McCotter OZ, Benedict K, Engelthaler DM, Komatsu K, Lucas KD, Mohle-Boetani JC, et al. Update on the Epidemiology of coccidioidomycosis in the United States. Med Mycol 2019;57:S30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Weaver E, Kolivras KN, Thomas RQ, Thomas VA, Abbas KM. Environmental factors affecting ecological niche of Coccidioides species and spatial dynamics of valley fever in the United States. Spatial Spatio-temporal Epidemiol 2020;32:100317. 10.1016/j.sste.2019.100317. [DOI] [PubMed] [Google Scholar]
- [3].Litvintseva AP, Marsden-Haug N, Hurst S, Hill H, Gade L, Driebe EM, et al. Valley Fever: Finding New Places for an Old Disease: Coccidioides immitis Found in Washington State Soil Associated With Recent Human Infection. Clin Infect Dis 2015;60(1):e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gorris ME, Cat LA, Zender CS, Treseder KK, Randerson JT. Coccidioidomycosis Dynamics in Relation to Climate in the Southwestern United States. GeoHealth 2018;2(1):6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Benedict K, McCotter OZ, Brady S, Komatsu K, Sondermeyer Cooksey GL, et al. Surveillance for Coccidioidomycosis - United States, 2011–2017. MMWR Surveillance Summary 2019;68(7):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nguyen C, Barker BM, Hoover S, Nix DE, Ampel NM, Frelinger JA, et al. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin Microbiol Rev 2013;26(3):505–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shubitz LF. Comparative aspects of coccidioidomycosis in animals and humans. Ann New York Acad Sci 2007;1111(1):395–403. [DOI] [PubMed] [Google Scholar]
- [8].Drutz DJ, Catanzaro A. Coccidioidomycosis. Am Rev Respiratory Dis 1978;117. 559–85-727-71. [Google Scholar]
- [9].Freedman M, Anderson SM, Benedict K, McCotter O, Derado G, Hoekstra R, et al. Preliminary Estimates of Annual Burden of Coccidioidomycosis in the United States, 2010–2014. CA: Seventh International Coccidioidomycosis Symposium. Stanford; 2017. [Google Scholar]
- [10].Galgiani JN, Blair JE, Ampel NM, Thompson GR. Treatment for Early, Uncomplicated Coccidioidomycosis: What Is Success? Clin Infect Dis 2020;70(9):2008–12. [DOI] [PubMed] [Google Scholar]
- [11].Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Geertsma F, Hoover SE, et al. 2016 Infectious Diseases Society of America (IDSA) Clinical Practice Guideline for the Treatment of Coccidioidomycosis. Clin Infectious Dis: An Off Publication Infectious Dis Soc Am 2016;63(6):e112–46. [DOI] [PubMed] [Google Scholar]
- [12].Wilson L, Ting J, Lin H, Shah R, MacLean M, Peterson M, et al. The Rise of Valley Fever: Prevalence and Cost Burden of Coccidioidomycosis Infection in California. Int J Environ Res Public Health [Electronic Resource]. 2019;16 (7):1113. 10.3390/ijerph16071113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kent Hill HK. The Cost of Valley Fever In Arizona. Seidman Research Institute. Seidman Research Institute: Arizona State University; 2020:1–12. [Google Scholar]
- [14].Shubitz LF, Butkiewicz CD, Dial SM, Lindan CP. Incidence of Coccidioides infection among dogs residing in a region in which the organism is endemic. J Am Veterinary Medical Assoc 2005;226(11):1846–50. [DOI] [PubMed] [Google Scholar]
- [15].Graupmann-Kuzma A, Valentine BA, Shubitz LF, Dial SM, Watrous B, Tornquist SJ. Coccidioidomycosis in dogs and cats: A review. J Am Animal Hospital Assoc 2008;44:226–35. [DOI] [PubMed] [Google Scholar]
- [16].Davidson AP, Shubitz LF, Alcott CJ, Sykes JE. Selected Clinical Features of Coccidioidomycosis in Dogs. Med Mycol 2019;57(Supplement_1):S67–75. [DOI] [PubMed] [Google Scholar]
- [17].Hector RF. An overview of antifungal drugs and their use for treatment of deep and superficial mycosis in animals. Clin Techniques Small Animal Practice 2005;20:240–9. [DOI] [PubMed] [Google Scholar]
- [18].Johnson LR, Herrgesell EJ, Davidson AP, Pappagianis D. Clinical, clinicopathologic, and radiographic findings in dogs with coccidioidomycosis: 24 cases (1995–2000). J Am Vet Med Assoc 2003;222 (4):461–6. [DOI] [PubMed] [Google Scholar]
- [19].Narra HP, Shubitz LF, Mandel MA, Trinh HT, Griffin K, Buntzman AS, et al. A Coccidioides posadasii CPS1 Deletion Mutant Is Avirulent and Protects Mice from Lethal Infection. Infect Immun 2016;84(10):3007–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shubitz LF, Powell DA, Trinh HT, Lewis ML, Orbach MJ, Frelinger JA, et al. Viable spores of Coccidioides posadasii Dcps1 are required for vaccination and provide long lasting immunity. Vaccine 2018;36(23):3375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Allio T. Product Development Under FDA’s Animal Rule: Understanding FDA’s Expectations and Potential Implications for Traditional Development Programs. Therapeutic Innovation Regulatory Sci. 2016;50(5):660–70. [DOI] [PubMed] [Google Scholar]
- [22].Soffler C, Bosco-Lauth AM, Aboellail TA, Marolf AJ, Bowen RA, Morici LA. Development and Characterization of a Caprine Aerosol Infection Model of Melioidosis. PLoS ONE 2012;7(8):e43207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Huppert M, Sun SH, Gross AJ. Evaluation of an experimental animal model for testing antifungal substances. Antimicrob Agents Chemother 1972;1 (5):367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shubitz LF, Powell DA, Butkiewicz CD, Lewis ML, Trinh HT, Frelinger JA, et al. A Chronic Murine Disease Model of Coccidioidomycosis Using Coccidioides posadasii, Strain 1038. J Infect Dis 2021;223(1):166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pappagianis D, Zimmer BL. Serology of coccidioidomycosis. Clin Microbiol Rev 1990;3(3):247–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Holbrook ED, Greene RT, Rubin SI, Renschler JS, Book BP, Hanzlicek AS, et al. Novel canine anti-Coccidioides immunoglobulin G enzyme immunoassay aids in diagnosis of coccidioidomycosis in dogs. Med Mycol 2019;57(7):800–6. [DOI] [PubMed] [Google Scholar]
- [27].Pappagianis D. Evaluation of the protection efficacy of the killed Coccidioides immitis spherule vaccine in humans. Am Rev Respiratory Dis 1993;148:656–60. [DOI] [PubMed] [Google Scholar]
- [28].Hogrefe WR. Biomarkers and assessment of vaccine responses. Biomarkers 2005;10(sup1):50–7. [DOI] [PubMed] [Google Scholar]
- [29].Kirkland TN. The Quest for a Vaccine Against Coccidioidomycosis: A Neglected Disease of the Americas. J Fungi 2016;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fierer J, Waters C, Walls L. Both CD4+ and CD8+ T cells can mediate vaccine-induced protection against Coccidioides immitis infection in mice. J Infect Dis 2006;193(9):1323–31. [DOI] [PubMed] [Google Scholar]
- [31].Abuodeh R, Orbach M, Mandel MA, Das A, Galgiani J. Genetic Transformation of Coccidioides immitis Facilitated by Agrobacterium tumefaciens. J Infect Dis 2000;181(6):2106–10. [DOI] [PubMed] [Google Scholar]
- [32].Ampel NM, Kramer LA, Li L, Carroll DS, Kerekes KM, Johnson SM, et al. In Vitro Whole-Blood Analysis of Cellular Immunity in Patients with Active Coccidioidomycosis by Using the Antigen Preparation T27K. Clin Vaccine Immunol 2002;9(5):1039–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mafi N, Murphy CB, Girardo ME, Blair JE. Coccidioides (spherulin) skin testing in patients with pulmonary coccidioidomycosis in an endemic region†. Med Mycol 2020;58(5):626–31. [DOI] [PubMed] [Google Scholar]
- [34].White CC, Koplan JP, Orenstein WA. Benefits, risks and costs of immunization for measles, mumps and rubella. Am J Public Health 1985;75(7):739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nathanson N, Martin JR. The epidemiology of poliomyelitis : enigmas surrounding its appearance, epidemicity, and disappearance. Am J Epidemiol 1979;110:672–92. [DOI] [PubMed] [Google Scholar]
- [36].Jones JM, Koski L, Khan M, Brady S, Sunenshine R, Komatsu KK. Coccidioidomycosis: An underreported cause of death—Arizona, 2008–2013. Med Mycol 2018;56(2):172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


