Abstract
Black, Latinx and Indigenous people in the United States experience a disproportionate burden of asthma and atopic dermatitis. The study of these disease disparities has focused on proximal socioenvironmental exposures and on the biomechanistic, including genetic, differences between racial and ethnic groups. While biomedical research in allergy and immunology stands to benefit from the inclusion of diverse study populations, the narrow focus on biologic mechanisms disregards the complexity of interactions across biological and structural factors, including the effects of structural racism. Structural racism is the totality of ways in which society fosters discrimination by creating and reinforcing inequitable systems through intentional policies and practices sanctioned by government and institutions. It is embedded across multiple levels, including the economic, educational, health care and judicial systems, which manifest in inequity in the physical and social environment. In this review, we present a conceptual framework and pull from the literature to demonstrate how structural racism is a root cause of atopic disease disparities, by way of residential segregation, socioeconomic position, and mass incarceration that may lead to aberrations in the innate and adaptive immune response and the augmentation of physiological stress responses, contributing to a disproportionate disease burden for racial and ethnic populations.
Keywords: Racism, Health Disparities, Asthma, Allergic Dermatitis, Atopy
INTRODUCTION
Racial and ethnic health disparities have persisted, and in the case of atopic diseases have worsened(1) despite scientific research dedicated to untangling the contributing biomechanisms and dedicated efforts to address poorly controlled disease(2–4), including the rampant introduction of precision medicine therapy(5,6). Asthma and atopic dermatitis are two of the most common chronic conditions of childhood in the United States (US), often persisting into adulthood(7,8). Asthma affects over 5.1 million children and over 20 million adults, corresponding to 7.8% of the US population(7). Approximately 7.3% of adults and 13–15% of children have atopic dermatitis(8–10). Like many chronic health conditions, the burden of atopic diseases disproportionally affects Black and Brown communities(11–17). The persistence of disparities require a shift of the biomedical framework to incorporate root causes, particularly the direct and indirect roles of structural racism(18). Structural racism is both historically rooted and culturally reinforced(19). It is the totality of ways in which society fosters discrimination by creating and reinforcing inequitable systems through intentional policies and practices sanctioned by various levels of government and institutions(19–21). Structural racism is embedded across society, including in our economic, educational, health care and justice systems, manifesting as inequitable distribution of resources(19). This is distinct from interpersonal racism, which is the differential treatment with regards to race, skin color, ethnic origin, or immigration status(22).
While we focus this review on role of structural racism contributing to the disparities within the Black, Latinx, and Indigenous communities(11–14,16,23–25), we recognize that the pervasive effects of structural racism has likely influenced disease outcomes in many – if not all – minoritized communities in the US. For this review, we use the term “Latinx”; while not fully adopted by all of the population, given the topic of the manuscript, we felt it was important to select a term that represents a global movement toward more gender inclusive terms(26). We also recognize that “Latinx” encompass diverse subgroups with unique migration to the US; however, these groups also share cultural and environmental risk factors for atopic diseases that are influenced by structural and societal factors(27). Indigenous populations refer to descendants of the peoples who inhabited the Americans prior to European colonization.
A deeper understanding of the history and the ongoing oppression of Black, Latinx, and Indigenous populations in the US is needed to understand the deep rooted and broad effect of structural racism on health outcomes and, specifically, on asthma and atopic dermatitis. This review examines the direct and indirect pathways in which structural racism may increase disease and worsen morbidity for asthma and atopic dermatitis. In the sections that follow, we provide a conceptual framework of structural racism and mechanisms of how it has manifested in the US, review the racial and ethnic disparities for asthma and atopic dermatitis, summarize the literature examining pathways of structural racism, and conclude with recommendations for future research and efforts that move towards reducing disease disparities.
CONCEPTUAL FRAMEWORK
Racial categories in the US were originally established by European settlers in order to assert power over enslaved African and Indigenous People, who were falsely thought to be innately, intellectually, and morally inferior(19). Over centuries, the concept of race and racial differences has been propagated by the field of medicine with the use of racial taxonomies in research and clinical practice(28). To understand how structural racism contributes to disparities in asthma and atopic dermatitis, we propose the following conceptual framework (Figure 1) pulling from the work of Williams and Mohammed(29,30), Bailey(19,21), and the World Health Organization’s Commission on Social Determinants of Health framework(31). The determinants of health include biology, geographic origin & ancestry, health behavior, and the social context as influenced by societal institutions and structural racism (Figure 1)(29,31). These determinants of health interact and, as is the case with health behaviors, may be influenced heavily by socioenvironmental conditions(32). In Figure 1, the concept of race, and the resulting perceived hierarchy, stems from the perpetuation of structural racism in legal, political, cultural, and economic institutions, which has led to residential segregation, disparate socioeconomic opportunities, and mass incarceration. Structural racism and resulting intermediate pathways create racial inequity which contributes to interpersonal racism. The toll of interpersonal racism and discrimination additionally contribute to inequitable access to health promoting resources (disease prevention) and healthcare access as well as chronic psychosocial stress, while also enhancing the effects of structural racism (Figure 1).
Figure 1. Structural Racism as a Root Cause of Allergy and Immunology Disparities.
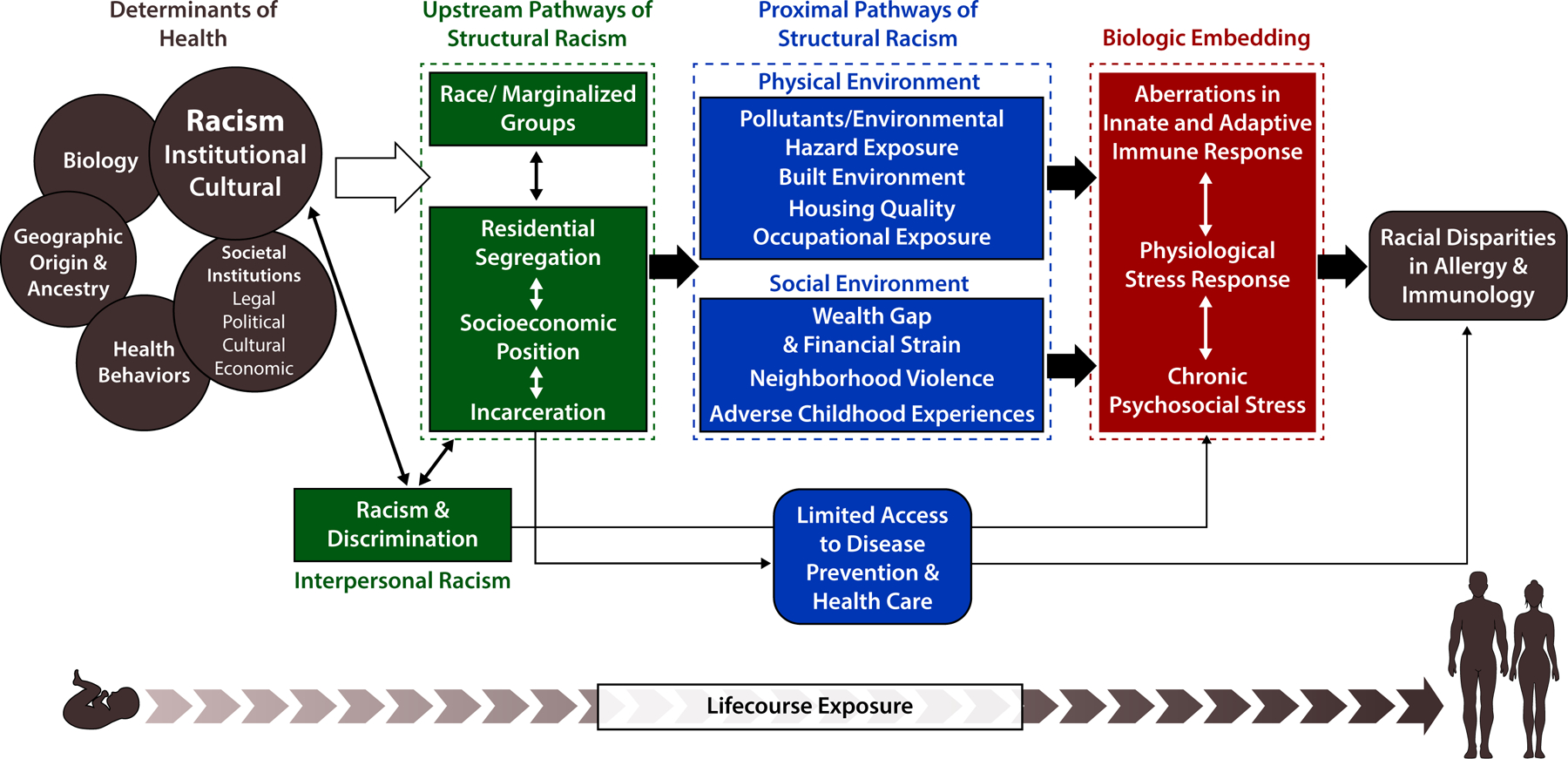
Conceptual framework of the upstream and proximal pathways of structural racism and its effect on health. Framework adapted from the work of Williams and Mohammed(29,30), Bailey(19,21), and the World Health Organization’s Commission on Social Determinants of Health framework(31)
In our framework, structural racism leads to proximal pathways of inequity affecting the physical and social environments. In the physical environment, Black, Latinx, and Indigenous communities are exposed disproportionately to pollutants/environmental hazards, occupational hazards, and poorer housing quality(21,33,34), all factors with a strong evidence-base of association with asthma and atopic dermatitis(25,34–36). In the social environment, Black Latinx, and Indigenous people are more likely to experience financial strain, have less wealth, decreased access to education, live in neighborhoods with higher rates of violence and have a greater number of adverse childhood experiences(33,37–41). Several epidemiologic studies have demonstrated that these proximal pathways are associated with higher physiologic stress burden (Brody 2014, Theal 2012). In addition, discrimination and segregation have been linked to increased inflammation, independent of SEP (Carlson 2017, citation #22 and Simons 2018). Social adversity also produced inflammation in animal models (Cole 2014). Based on the evidence, we hypothesize that these proximal pathways, along with experiences of interpersonal racism, become embedded in the biology through aberrations in the innate and adaptive immune response(42,43). In the sections below, we critically appraise the gaps in our current knowledge and summarize the evidence supporting our framework, which extends from animal to human studies and spans the psychosocial stress to epidemiology research fields.
DISPARITIES IN ASTHMA AND ATOPIC DERMATITIS
In the US, asthma prevalence is substantially higher in Black and Indigenous populations, 10.6% and 10.7%, respectively, compared to 7.7% prevalence within the non-Hispanic White population(7). In addition, Black and Indigenous populations experience higher asthma morbidity with frequent asthma attacks and higher rates of asthma-related health care utilization(11–14). When considered as whole, the prevalence of asthma in Latinx population is lower (6.6%) when compared to most other racial and ethnic groups(7). However, when examined by National subgroup, the prevalence of asthma in Mexican Americans (5.3%) is lower as compared to other subpopulations of Latinx people (8.5%)(7) with Puerto Ricans having the highest lifetime prevalence of asthma(44). Notably, the prevalence of asthma in Mexican Americans is increasing at a greater rate than all other racial and ethnic groups(11). Mexican American children also have a two-fold increased risk of hospitalization as compared to White children, which is similar to the hospitalization rate of Black children with asthma(11). The lower overall prevalence of asthma in Mexican Americans likely underestimates the impact of acculturation, which reflects an adoption of cultures of the dominant society and captures the duration of time spent in host country(44,45). Higher acculturation has been shown to be associated with higher prevalence of asthma in Mexican Americans(44,46) and other Latinx subgroups(44); similar patterns are seen in several disease outcomes(27,47). Proposed mechanisms include adoption of cultural and behavioral practices that lead to increased disease risk and increased exposure to environmental hazards(27,44). It is important to consider the harmful health effects of being a racialized immigrant in the US(48). Structural racism may uniquely impart its influence on first generation Mexican Americans and other racialized immigrants in the form of economic exploitation, including labor exploitation and curbing access to economic resources and opportunities, and neighborhood disinvestment, contributing to concentration of poverty and increased exposure to environmental hazards(48).
The prevalence of atopic dermatitis is estimated to be 1.7 times higher in Black children as compared to White children, particularly in early childhood(15–17). Black children also have higher risk of persistent and severe disease(16). While the incidence of atopic dermatitis in Latinx children is similar to White children in early childhood, Latinx children have higher risk of persistent and severe disease(9,16). Similar to asthma, the cultural and environmental changes associated with acculturation may play a significant role for Latinx children, as children not born in the US but who have resided in the US for greater than 10 years have higher odds of developing atopic dermatitis as compared to those who recently migrated(49,50). Extrapolating from small population-based studies, the prevalence of atopic dermatitis in the US Indigenous population is an estimated 8–11%(51,52). There are even fewer studies which have examined the severity of atopic dermatitis in Indigenous populations in the US. This is a needed area for research, specifically to identify and address risk factors that may be unique to this community.
Even after accounting for traditionally measured socioeconomic factors, racial and ethnic disparities in asthma and atopic dermatitis persist, contributing to controversy over the reason for this residual difference and used to support theories of biologic determinism(15,53). However, there is sparse evidence supporting that biological differences between racial groups drive disparity in disease prevalence and severity. For example, Abuabara et al. demonstrated that neither genetic ancestry, a polygenetic risk score, nor a genetic skin pigment score explained the prevalence or morbidity disparities in atopic dermatitis between Black and White participants(54). Several studies have identified that loss-of-function (LOF) mutations of the filaggrin (FLG-LOF) gene result in epidermal barrier dysfunction. For people of European ancestry (15,55), FLG1-LOF mutations are associated with more severe atopic dermatitis. Past studies have noted that the four most common FLG-LOF mutations are rare or even absent in individuals of African ancestry(15,55,56). However, with advancement of measurement and availability of whole genome sequencing, more recent reviews have identified the presence of variants across the FLG-LOF gene in individuals of African ancestry, with mutations of this gene also causing more severe disease regardless of mutation type(54–56) These studies highlight why biomedical research in allergy and immunology stands to benefit from the inclusion of more diverse study populations; however, narrowly focusing on genetic variations disregards the complexity of interactions across biological and structural factors, which together contribute to disease risk. Instead, we argue that the persistent racial and ethnic disparities in asthma and atopic dermatitis likely reflect residual confounding from both unmeasured social and structural determinants, including the downstream effects of structural racism(57). In this work, there is also need to consider the degree to which structural racism is transmitted transgenerationally through epigenetic modification and the consequences of such modifications on health across populations(58–62).
UPSTREAM PATHWAYS OF STRUCTURAL RACISM
Residential Segregation
Soon after the abolishment of slavery, in the Reconstruction Era, segregation policies were established in the form of black codes to ensure continuation of a cheap labor force and, later, as “Jim Crow laws” under the premise of “separate but equal”(63). While zoning laws segregating neighborhoods were viewed unconstitutional by the Supreme Court in 1917, in recovery from the Great Depression, the federal government sanctioned this practice with preferential investment in current and future homeowners through the Home Owners’ Loan Corporation (HOLC)(64). HOLC created maps of at least 239 US cities ranking communities on mortgage-worthiness(21). Inner-city communities with large Black or immigrant populations were systematically graded and outlined in red (“redlining”), flagging them as hazardous investment areas(21,33,64). This practice barred residents from these communities from receiving HOLC loans and thus systematically preventing the procurement of assets and accumulation of transferable wealth(33). While overt redlining is illegal today, prohibited under the Fair Housing Act of 1968, its effects have endured(65). Communities previously redlined have a persistent pattern of economic inequality and segregation(65).
Nardone and colleagues demonstrated that historical redlined census tracts have 2.4 times higher rates of asthma-related emergency department visits compared to census tracts deemed to be good investments(33). There are several indirect mechanisms in which redlining may lead to worse asthma and atopic dermatitis outcomes for Black and Latinx communities. Redlining led to an inequitable distribution of wealth and over generations has resulted in concentration of poverty, low rates of home ownership, and poor housing quality in Black and immigrant communities(33). In asthma and atopic dermatitis, there is a large body of literature documenting the negative effects of poor housing quality on outcomes(25,34–36). Presently, structural racism continues to operate by denying improved housing opportunities for communities of color via housing and mortgage discrimination(66). Due to these discriminatory practices, despite earning higher incomes, opportunities to move out of disinvested neighborhoods remain limited(66).
The lasting effects of redlining are also seen through the concentration of hazardous air pollutants in Black and immigrant communities(33,64), which have been demonstrated to be associated with asthma morbidity(67). Historically redlined census tracts were subjected to downstream policies, such as eminent domain, industrial zoning, and racial zoning, which affected where highways and toxic hazard sites were built(33,64,68). Historically redlined census tracts have nearly twice as high diesel exhaust particulate (DEP) emissions as compared to non-redlined areas(33). This is particularly important when it comes to disparities in asthma and atopic dermatitis, which have been shown to be triggered by hazardous environmental exposures(25,69–72). Residential segregation, the contemporary manifestation of redlining, Jim Crow laws, and the legal separation of races, has also been linked to higher prevalence of asthma and worse disease outcomes for both asthma and atopic dermatitis(25,36,73,74). In a study examining asthma prevalence in low-birth weight children, Black children had higher rates of asthma as compared to non-Black children(74). However, when non-Black children lived in Black zip codes, defined as having greater than 50% of children living in the zip code who identified as Black, and exposed to the same environments, their rates of asthma were comparable(74). This suggests that structural environment impacts disease disparities, not biological differences between races(74). The systemic disinvestment in public and private sectors and concentrated industrialization within segregated communities of color has led to disproportionate exposure to environmental hazards(73), highlighting one mechanism of how segregation practice and policy lead to greater burden of asthma and atopic dermatitis in Black, Latinx, and Indigenous communities.
As a result of systemic disinvestment of communities of color, residential segregation is also associated with under resourced public services, including healthcare facilities(21,75,76) and healthcare providers(21,75), directly impacting the care that is accessible by Black, Latinx, and Indigenous communities. Residential segregation has led to highly segregated schools with poorer quality of education because of the lack of community resources and funding, caused by concentrated poverty and low property tax revenue(77). This has led to lower average test scores, fewer students in advanced placement courses, limited curriculum, less qualified teachers, less access to academic counseling and higher drop-out rates in segregated schools, all limiting future employment potential(77) and influencing socioeconomic position, a well-described risk factor for asthma prevalence and for morbidity in asthma and atopic dermatitis(9,78–82).
Socioeconomic Position
Similar to socioeconomic status, socioeconomic position (SEP) measures differential access to resources but also encapsulates social class and social stratification(78). Given the overlap in definitions, in this review SEP and SES are used interchangeably. SEP is strongly associated with the development of asthma, worse symptom burden, and higher health care utilization(78–81). There is a two-fold increase in the prevalence of asthma in individuals of low SEP as compared to those with high SEP(7). The link between SEP and the risk of atopic dermatitis is less clear(82). While studies have observed higher prevalence of atopic dermatitis in children and adults with higher SEP, severe atopic dermatitis is associated with lower SEP, lower income, dilapidated housing and living in a community with garbage in the streets(9,82).
While SEP is a strong predictor of atopic disease severity, it cannot be viewed independent of structural racism. In addition to lower educational opportunities, Black, Latinx and Indigenous people have poverty rates (i.e., proportion living below the Federal Poverty Line) as high as 20.8%, 17.6% and 25.4%, respectively, as compared to 8.1% in White populations(83). As highlighted above, residential segregation has contributed to this difference, shaping the distribution of resources and reenforcing racial differences in education and employment opportunities(37,73). Structural racism and discrimination in both institutional and interpersonal levels have also contributed to racial disparities in SEP(37,39,73). Even at the same education level, Black and Latinx people receive less income as compared to their White counterparts and have markedly less wealth at equivalent incomes(37), barring generations of Black and Latinx people from escaping the cycle of poverty and the exposures associated with higher severity of atopic diseases.
Mass Incarceration
The US has the highest rate of incarceration in the world with a prison population of over 2 million(21,63). Structural racism at various levels of the criminal justice system has accounted for a gross over-representation of imprisoned Black, Latinx, and Indigenous people(63). Black Americans have the highest rates of incarceration at a level five times higher than White Americans(84). Latinx and Indigenous people are not far behind at two and four times, respectively, higher rates than their White counterparts(84,85). Despite studies showing that people of all races and ethnicities use and sell drugs at similar rates, Black men are imprisoned on drug charges at rates twenty to fifty times greater than their White counterparts(63).
Over-policing, the imposition of police control in minoritized communities at a level unlikely to occur in the dominant society, and imprisonment of racial and ethnic communities in the US can be traced back to the enslavement of Black and Indigenous people(21,63). Policing began with formation of slave patrols in the 18th century in effort to capture and retrieve runaway slaves(21). After the abolition of slavery, slave patrols evolved into contemporary police, which not only enforced the unequal laws that targeted communities of color, but also participated in the lynching of Black individuals(21). Policies set in this past half century have accelerated incarceration rates for Black, Latinx, and Indigenous populations. In 1971, President Nixon announced the war on drugs, directly leading to a sevenfold increase in the incarcerated population(63). This was followed by the Violent Crime and Law Enforcement Act during the Clinton administration, further enhancing the disparities in the mass incarceration(21,63). Recent evaluation of these policies demonstrates that the “war on drugs” and “tough on crime” policies, including stop-and-frisk laws, are deliberate and systematic examples of racism(86,87).
While literature exploring the relationship between incarceration and atopic diseases is in its infancy, a community-based study of approximately 2,000 adults found that a personal history of incarceration was associated with an increased prevalence of asthma, higher healthcare utilization and more severe asthma symptoms(88). We propose that the impact of mass incarceration on asthma and atopic dermatitis operates through three pathways: by enhancing racial socioeconomic inequities, by amplifying the effects of chronic psychological stress, and by exposing individuals to harmful physical environments (Figure 1). As a result of structural racism in the US legal system and policies disproportionately enforced in minoritized communities, Black individuals in major cities are more likely to have criminal records, despite several reports documenting similar rates of committing crimes when compared to White populations(63,86,87). Upon release from prison, individuals with criminal records are subject to legal barriers, limiting employment and economic opportunities(63,89). This contributes to lower SEP and higher psychosocial stress, both associated with an increased risk of asthma(88–91). Incarceration also exposes individuals to stressful or traumatic environments, which may enhance the psychological stress response and thus predispose individuals to more severe asthma symptoms as discussed below(88,92). Similarly, parental incarceration can also be a source of chronic stress in childhood and has been well studied as one of the ten original factors included in the Adverse Childhood Experiences (ACEs) questionnaire(93,94). Higher exposure to ACEs and has also been associated with higher prevalence asthma in children and adults(95–98). Mass incarceration may also expose individuals to harmful physical environments including secondhand smoke, dilapidated prison conditions, indoor allergens such as pests and hazardous external air pollutants(88), all of which have been associated with asthma(69–71,99,100). Upon release, formerly incarcerated individuals are more likely to live in neighborhoods with substandard housing(88), thus further increasing the potential exposure to conditions known to be associated with asthma and atopic dermatitis(25,34–36).
BIOLOGIC EMBEDDING OF STRUCTURAL RACISM
Structural racism is biologically embedded over the life course(42). Figure 2 highlights empiric pathways by which racism may be embedded and contribute to immune dysregulation and the development or worsening of atopic disease. We recognize one pathway is through increased exposure to environmental hazards, specifically poor indoor and outdoor air quality. For this review, we focus on how structural racism may operate through the physiologic stress response to modulate immune function and promote atopic diseases through the following mechanisms: 1) T-helper (Th)1/Th2 polarization, 2) Th17/regulatory T (T-reg) cell balance, 3) the microbiome, and 4) epigenetic modifications. Much of the evidence presented is drawn from the large empiric-base of biomechanisms of chronic psychosocial stressors at the individual level (i.e., socioeconomic status and perceived stress), as there is limited research directly examining the effects of structural racism on these biological processes. Future research in these areas would advance our understanding of the biomechanisms of structural racism.
Figure 2. Empiric Pathways for Biologic Embedding of Structural Racism.
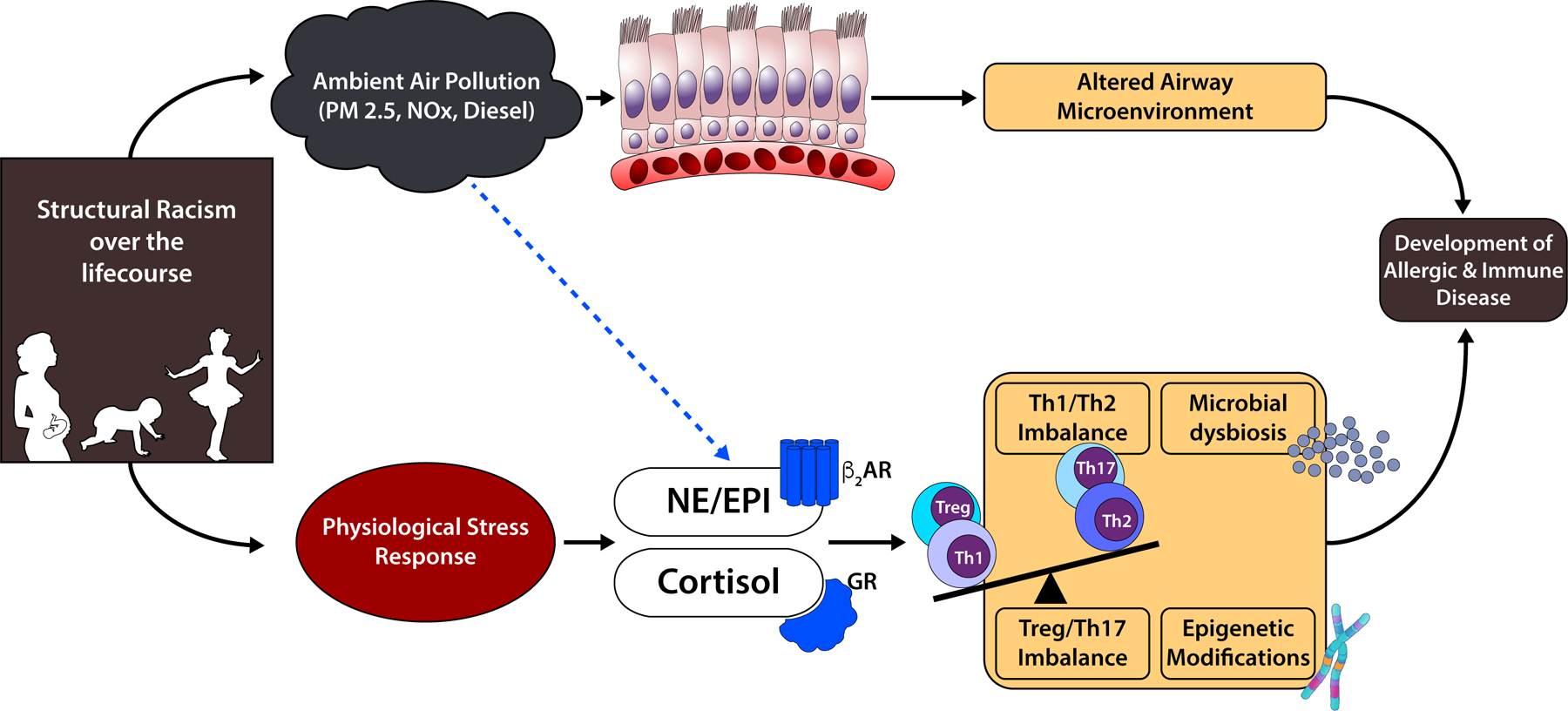
Structural racism may modulate immune function and promote atopic diseases through the following mechanisms: 1) T-helper (Th)1/Th2 polarization, 2) Th17/regulatory T (T-reg) cell balance, 3) the microbiome, and 4) epigenetic modifications
Physiologic Stress Response
Stressful events stimulate activation of the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS), leading to the secretion of glucocorticoids (cortisol) and catecholamines (epinephrine and norepinephrine), respectively, into the bloodstream. These molecules exert effects by binding glucocorticoid receptors and beta(β)-adrenergic receptors present in lymphocytes and other immune cells(101,102). Chronic activation of these receptors leads to aberrant physiologic responses and desensitization, which increases allergic disease morbidity by reducing responsiveness to corticosteroid and β2-agonist treatments(103). Among children with asthma, chronic life stress was associated with decreased expression of the glucocorticoid and β-adrenergic receptors(43).
Th1/Th2 Polarization
T helper (Th) cells present allergens taken up by dendritic cells and have two predominant phenotypes, Th1 and Th2. Th1 cells release Type 1 cytokines (IFN-γ and IL-2), whereas Th2 cells produce Type 2 cytokines (IL-4, IL-5 and IL-13) that induce production of IgE and eosinophilia characteristic of atopic diseases(104). Dendritic cells also induce Th2 cell proliferation when exposed to allergens such as cockroach antigen, a health hazard common in poor housing quality and urban environments(105,106)(107). We show in Figure 2 that differential exposure to environmental hazards may mediate the association between SEP and Th2 polarization, but studies have yet to explicitly investigate these potential pathways with downstream exposures resulting from structural racism. Extrapolating from literature exploring the relationship between SEP, psychosocial stress, and type 2 inflammation(108), one study found that Type 2 cytokines and circulating eosinophils were elevated in children of low SEP when compared to high SEP(92). This relationship between SEP and Th2 polarization was partially mediated by psychological stress in asthmatic children and adolescents(92,109). Subsequently, authors demonstrated that Th2 cytokine production by peripheral blood mononuclear cells (PBMC) stimulated with cockroach or dust mite antigen did not differ by SEP, suggesting that the SEP effect on Th2 polarization may operate through pathways independent of allergen exposures(110). Glucocorticoids and catecholamines may mediate stress-associated Th2 polarization, by inhibiting the production of Type 1 cytokines in Th1 cells and promoting Th2 phenotype(111,112).
Th17/Treg Balance
Recent work has also investigated the role of Th17 cells in allergic pathogenesis(113). While effector Th17 cells promote inflammation, regulatory T cells (Tregs) express forkhead box P3 (FoxP3) and limit excessive inflammatory responses. Psychosocial stressors may promote inflammation by decreasing the number of Tregs and enhancing Th17 cell differentiation. Th cells from low-SEP asthmatic children had elevated expression of RORγt, a transcription factor critical for Th17 cell function(114). The effect of psychosocial stressors on Th17/ Treg balance may be mediated by cortisol(115). PBMCs treated with a synthetic glucocorticoid at concentrations equivalent to cortisol levels observed during high stress periods had decreased FoxP3 expression(116). Th17 differentiation is also regulated by IL-6, a proinflammatory cytokine elevated in individuals from racially segregated communities(117), by activating the signal transducer and activator of transcription 3 (STAT3) protein and releasing Foxp3 from RORγt(118).
Microbiome
Psychosocial stressors may produce shifts in gut microbial diversity that contribute to allergic pathogenesis. For example, infants born to mothers with high prenatal stress and/or elevated cortisol levels had a greater abundance of pathogenic Proteobacteria in their gut microbiome and higher reports of allergic reactions(119). Respiratory microbial communities may also contribute to the development of atopy. Children living in urban environments with uncontrolled asthma had nasopharyngeal microbiomes dominated by Moraxella, a type of Protobacteria, that was also associated with increased eosinophilic inflammation(120). In children with severe asthma, bronchial Proteobacteria positively correlated with TH17-related gene expression in airway epithelia(121).
Epigenetic modifications
Biochemical alterations to the DNA, such as methylation and histone modifications, influence gene expression and accessibility. Allergic asthma was previously associated with differentially methylated genes in the nasal epithelia(122). Psychosocial stressors may also induce epigenetic changes that increase susceptibility to allergic disease(123). Methylation of ADCYAP1R1 was associated with both exposure to violence and increased asthma odds in a study of Puerto Rican children(124). Chronic stress may also impact epigenetic regulation of genes involved in Th cell polarization(125).
CONCLUSION
By fostering discriminatory policies and practices, government and institutions have reinforced the inequitable distribution of resources and opportunities for Black, Latinx and Indigenous people via residential segregation, socioeconomic position, and mass incarceration. These upstream pathways of structural racism have led to inequity in the physical and social environment that are associated with alterations in biological processes, including the physiological stress response, that may contribute to the increased risk for atopic disease and severe symptoms. Exposure to inhaled environmental pollutants may also increase risk of allergic respiratory disease by altering the airway microenvironment and activating neuroendocrine mediators(126,127).
To address racial and ethnic disparities, the field of allergy and clinical immunology must take part in dismantling the proximal effects of structural racism. This is not a simple feat, as these factors are rooted in centuries of discrimination and enslavement. Additional investigation is needed to understand the health impact of racism, including further elucidating the biological processes and physiological responses associated with experiencing the effects of structural racism. Research is also needed to identify ways in which the effects of racism can be mitigated through community based participatory research. This should be done simultaneously with efforts to address the root causes of health disparities. Avenues to achieving a more diverse research workforce reflecting Black, Latinx and Indigenous communities is also needed. To truly address health disparities caused by structural racism, the medical and research community must actively participate in policy reform, advocacy, community re-development and place-based partnerships.
Funding Source:
RD was supported by the University of California President’s Postdoctoral Fellowship. NT was supported by a career development award from the NHLBI (K23- HL125551–01A1). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- US
United States
- FLG-LOF
Filaggrin Loss of Function
- HOLC
Home Owners’ Loan Corporation
- DEP
Diesel Exhaust Particulate
- SEP
Socioeconomic Position
- ACE
Adverse Childhood Experiences
- Th
T-Helper
- T-reg
Regulatory T
- HPA
Hypothalamic-Pituitary-Adrenal
- SNS
Sympathetic Nervous System
- Β
Beta
- PBMC
Peripheral Blood Mononuclear Cells
- FoxP3
Forkhead Box P3
- STAT3
Signal Transducer And Activator Of Transcription 3
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
References
- 1.Odlum M, Moise N, Kronish IM, Broadwell P, Alcántara C, Davis NJ, et al. Trends in Poor Health Indicators among Black and Hispanic Middle-aged and Older Adults in the United States, 1999–2018. JAMA Netw Open 2020;3(11):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eichenfield L, Tom W, Chamlin S, Feldman S, Hanifin J, Simpson E, et al. Guidelines of care for the management of atopic dermatitis. J Am Acad Dermatol [Internet] 2014;70(2):338–51. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0190962213010955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wechsler ME, Szefler SJ, Ortega VE, Pongracic JA, Chinchilli V, Lima JJ, et al. Step-Up Therapy in Black Children and Adults with Poorly Controlled Asthma. N Engl J Med 2019;381(13):1227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Natl Hear Lung Blood Inst 2007;
- 5.Ray A, Camiolo M, Fitzpatrick A, Gauthier M, Wenzel SE. Are we meeting the promise of endotypes and precision medicine in asthma? Physiol Rev 2020;100(3):983–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balakirski G, Novak N. Novel therapies and the potential for a personalized approach to atopic dermatitis. Curr Opin Allergy Clin Immunol 2021;21(4):368–77. [DOI] [PubMed] [Google Scholar]
- 7.CDC. National Asthma Data [Internet] Centers for Disease Control and Prevention. 2019. [cited 2021 Jul 7]. Available from: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm [Google Scholar]
- 8.Chiesa Fuxench ZC, Block JK, Boguniewicz M, Boyle J, Fonacier L, Gelfand JM, et al. Atopic Dermatitis in America Study: A Cross-Sectional Study Examining the Prevalence and Disease Burden of Atopic Dermatitis in the US Adult Population. J Invest Dermatol [Internet] 2019;139(3):583–90. Available from: 10.1016/j.jid.2018.08.028 [DOI] [PubMed] [Google Scholar]
- 9.Silverberg JI, Simpson EL. Associations of childhood eczema severity: A US population-based study. Dermatitis 2014;25(3):107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy, Asthma Immunol [Internet] 2019;123(2):173–178.e1. Available from: 10.1016/j.anai.2019.05.014 [DOI] [PubMed] [Google Scholar]
- 11.Zahran HS, Bailey CM, Damon SA, Garbe PL, Breysse PN. Vital Signs : Asthma in Children — United States, 2001–2016. MMWR Morb Mortal Wkly Rep 2018;67(5):149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keet CA, Matsui EC, McCormack MC, Peng RD. Urban residence, neighborhood poverty, race/ethnicity, and asthma morbidity among children on Medicaid. J Allergy Clin Immunol [Internet] 2017;140(3):822–7. Available from: 10.1016/j.jaci.2017.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowe AA, Bender B, Liu AH, Solomon T, Kobernick A, Morgan W, et al. Environmental Concerns for Children with Asthma on the Navajo. Ann Am Thorac Soc 2018;15(6):745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Washington DM, Curtis LM, Waite K, Wolf MSP-OM. Sociodemographic Factors Mediate Race and Ethnicity-associated Childhood Asthma Health Disparities: a Longitudinal Analysis. J Racial Ethn Heal Disparities 2018;5(5):928–38. [DOI] [PubMed] [Google Scholar]
- 15.Brunner PM, Guttman-Yassky E. Racial differences in atopic dermatitis. Ann Allergy, Asthma Immunol [Internet] 2019;122(5):449–55. Available from: 10.1016/j.anai.2018.11.015 [DOI] [PubMed] [Google Scholar]
- 16.Kim Y, Blomberg M, Rifas-Shiman SL, Camargo CA, Gold DR, Thyssen JP, et al. Racial/Ethnic Differences in Incidence and Persistence of Childhood Atopic Dermatitis. J Invest Dermatol 2019;139(4):827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wegienka G, Havstad S, Joseph CLM, Zoratti E, Ownby D, Woodcroft K, et al. Racial disparities in allergic outcomes in African Americans emerge as early as age 2 years. Clin Exp Allergy 2012;42(6):909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stempel H, Federico MJ, Szefler SJ. Applying a biopsychosocial model to inner city asthma: Recent approaches to address pediatric asthma health disparities. Paediatr Respir Rev [Internet] 2019;32:10–5. Available from: 10.1016/j.prrv.2019.07.001 [DOI] [PubMed] [Google Scholar]
- 19.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet [Internet] 2017;389(10077):1453–63. Available from: 10.1016/S0140-6736(17)30569-X [DOI] [PubMed] [Google Scholar]
- 20.Williams DR, Lawrence JA, Davis BA. Racism and Health: Evidence and Needed Research. Annu Rev Public Health 2019;40:105–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailey ZD, Feldman JM, Bassett MT. How Structural Racism Works — Racist Policies as a Root Cause of U.S. Racial Health Inequities. N Engl J Med [Internet] 2021;384(8):768–73. Available from: https://www.youtube.com/watch?v=KT1vsOJctMk&t=9s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thakur N, Barcelo NE, Borrell LN, Singh S, Eng C, Davis A, et al. Perceived Discrimination Associated With Asthma and Related Outcomes in Minority Youth: The GALA II and SAGE II Studies. Chest 2017;151(4):804–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.OMH. Asthma and American Indians/Alaska Natives. U.S. Department of Heath and Human Services 2021.
- 24.Hanifin JM, Reed ML, Group EP and IW. A population-based survey of eczema prevalence in the United States. Dermatitis 2007;18(2):82–91. [DOI] [PubMed] [Google Scholar]
- 25.Brewer M, Kimbro RT, Denney JT, Osiecki KM, Moffett B, Lopez K. Does neighborhood social and environmental context impact race/ethnic disparities in childhood asthma? Heal Place 2017;44(February 2016):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salinas C, Lozano A. Mapping and recontextualizing the evolution of the term Latinx: An environmental scanning in higher education. J Latinos Educ [Internet] 2019;18(4):302–15. Available from: 10.1080/15348431.2017.1390464 [DOI] [Google Scholar]
- 27.Mcquaid EL, Koinis-Mitchell D, Canino GJ. Acculturation. In: Achieving Respiratory Health Equality. Respiratory Medicine Humana Press, Cham; 2017. [Google Scholar]
- 28.Witzig R The Medicalization of Race: Scientific Legitimization of a Flawed Social Construct. Ann Intern Med 1996;125(8):675–9. [DOI] [PubMed] [Google Scholar]
- 29.Williams DR, Mohammed SA. Racism and Health I: Pathways and Scientific Evidence. Am Behav Sci 2013;57(8):1152–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams DR, Lawrence JA, Davis BA, Vu C. Understanding how discrimination can affect health. Health Serv Res 2019;54(S2):1374–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solar O IA. A Conceptual Framework for Action on the Social Determinants of Health. Social Determinants of Health Discussion Paper 2 (Policy and Practice) 2010; Available from: http://www.who.int/sdhconference/resources/ConceptualframeworkforactiononSDH_eng.pdf
- 32.Cohen DA, Scribner RA, Farley TA. A structural model of health behavior: A pragmatic approach to explain and influence health behaviors at the population level. Prev Med (Baltim) 2000;30(2):146–54. [DOI] [PubMed] [Google Scholar]
- 33.Nardone A, Casey JA, Morello-Frosch R, Mujahid M, Balmes JR, Thakur N. Associations between historical residential redlining and current age-adjusted rates of emergency department visits due to asthma across eight cities in California: an ecological study. Lancet Planet Heal 2020;4(1):e24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenbaum E Racial/ethnic differences in asthma prevalence: The role of housing and neighborhood environments. J Health Soc Behav 2008;49(2):131–45. [DOI] [PubMed] [Google Scholar]
- 35.Hughes HK, Matsui EC, Tschudy MM, Pollack CE, Keet CA. Pediatric Asthma Health Disparities: Race, Hardship, Housing, and Asthma in a National Survey. Acad Pediatr 2017;17(2):127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tackett KJ, Jenkins F, Morrell DS, McShane DB, Burkhart CN. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatr Dermatol 2020;37(1):142–6. [DOI] [PubMed] [Google Scholar]
- 37.Williams DR, Priest N, Anderson N. Understanding Associations between Race, Socioeconomic Status and Health: Patterns and Prospects. Heal Psychol 2016;35(4):407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheon C, Lin Y, Harding DJ, Wang W, Small DS. Neighborhood Racial Composition and Gun Homicides. JAMA Netw open 2020;3(11):e2027591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Logan J The persistence of segregation in the 21st century metropolis. City Community 2013;12(2):160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crouch E, Probst JC, Radcliff E, Bennett KJMS. Prevalence of adverse childhood experiences (ACEs) among US children. Child Abus Negl 2019;92:209–18. [DOI] [PubMed] [Google Scholar]
- 41.Lee RD, Chen J. Adverse childhood experiences, mental health, and excessive alcohol use: Examination of race/ethnicity and sex differences. Child Abus Negl 2017;69:40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krieger N Measures of racism, sexism, heterosexism, and gender binarism for health equity research: From structural injustice to embodied harm-an ecosocial analysis. Annu Rev Public Health 2019;41:37–62. [DOI] [PubMed] [Google Scholar]
- 43.Miller GE, Chen E. Life stress and diminished expression of genes encoding glucocorticoid receptor and β2-adrenergic receptor in children with asthma. Proc Natl Acad Sci U S A 2006;103(14):5496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thakur N, Borrell LN, Ye M, Oh SS, Eng C, Meade K, et al. Acculturation is associated with asthma burden and pulmonary function in Latino youth: The GALA II study. J Allergy Clin Immunol [Internet] 2019;143(5):1914–22. Available from: 10.1016/j.jaci.2018.12.1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iqbal S, Oraka E, Chew GL, Flanders WD. Association between birthplace and current asthma: The role of environment and acculturation. Am J Public Health 2014;104(SUPPL. 1):175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holguin F, Mannino DM, Antó J, Mott J, Ford ES, Teague WG, et al. Country of birth as a risk factor for asthma among Mexican Americans. Am J Respir Crit Care Med 2005;171(2):103–8. [DOI] [PubMed] [Google Scholar]
- 47.Lara M, Gamboa C, Kahramanian MI, Morales LS, Hayes Bautista DE. Acculturation and Latino health in the United States: A review of the literature and its sociopolitical context. Annu Rev Public Health 2005;26:367–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Misra S, Kwon SC, Abraido-Lanza AF, Chebli P, Trinh0Shevrin C, Yi SS. Structural Racism and Immigrant Health in the United States. Heal Educ Behav 2021;48(3):332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia-marcos L, Robertson CF, Anderson HR, Ellwood P, Williams HC, Wong GWK. Does migration affect asthma, rhinoconjunctivitis and eczema prevalence? Global findings from the international study of asthma and allergies in childhood. Int J Epidemiol 2014;43(6):1846–54. [DOI] [PubMed] [Google Scholar]
- 50.Silverberg JI, Simpson EL, Durkin HG. Prevalence of Allergic Disease in Foreign Born American Children. JAMA Pediatr 2013;167(6):554–60. [DOI] [PubMed] [Google Scholar]
- 51.Zullo SW. The epidemiology of xerosis, eczema, and skin care habits of Native Americans. J Am Acad Dermatol 2019;81(4):AB133. [Google Scholar]
- 52.Hua T, Silverberg JI. Atopic dermatitis in US adults: Epidemiology, association with marital status, and atopy. Ann Allergy, Asthma Immunol 2018;121(5):622–4. [DOI] [PubMed] [Google Scholar]
- 53.Daya M, Barnes KC. African American Ancestry Contribution to Asthma and Atopic Dermatitis. Ann Allergy Asthma Immunol 2019;122(5):456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abuabara K, You Y, Margolis DJ, Hoffmann TJ, Risch N, Jorgenson E. Genetic ancestry does not explain increased atopic dermatitis susceptibility or worse disease control among African American subjects in 2 large US cohorts. J Allergy Clin Immunol [Internet] 2020;145(1):192–198.e11. Available from: 10.1016/j.jaci.2019.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu Y, Mitra N, Feng Y, Tishkoff S, Hoffstad O, Margolis D. FLG Variation Differs between European Americans and African Americans. J Invest Dermatol [Internet] 2021;141(7):1855–7. Available from: 10.1016/j.jid.2020.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Margolis DJ, Mitra N, Wubbenhorst B, D-Andrea K, Kraya AA, Hoffstad O, et al. Association of Filaggrin Loss-of-Function Variants With Race in Children With Atopic Dermatitis. JAMA Dermatology 2019;155(11):1269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaufman JS, Cooper RS, McGee DL. Socioeconomic Status and Health in Blacks and Whites: The Problem of Residual Confounding and the Resiliency of Race. Epidemiology 1997;8(6):621. [PubMed] [Google Scholar]
- 58.Conching AKS, Thayer Z. Biological pathways for historical trauma to affect health: A conceptual model focusing on epigenetic modifications. Soc Sci Med 2019;230(March):74–82. [DOI] [PubMed] [Google Scholar]
- 59.Clausing ES, Non AL. Epigenetics as a Mechanism of Developmental Embodiment of Stress, Resilience, and Cardiometabolic Risk Across Generations of Latinx Immigrant Families. Front Psychiatry 2021;12(July):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brockie TN, Heinzelmann M, Gill J. A Framework to Examine the Role of Epigenetics in Health Disparities among Native Americans. Nurs Res Pract 2013;2013:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bombay A, Matheson K, Anisman H. The intergenerational effects of Indian Residential Schools: Implications for the concept of historical trauma. Transcult Psychiatry 2014;51(3):320–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goosby BJ, Heidbrink C. The transgenerational consequences of discrimination on African-American health outcomes. Sociol Compass 2013;7(8):630–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alexander M The New Jim Crow: Mass Incerceration in the Age of Colorblindness New York: New Press; 2010. [Google Scholar]
- 64.Rothstein R The Color of Law New York: Liveright Publishing Corportation; 2017. [Google Scholar]
- 65.Mitchell B, Franco J. HOLC “Redlining” Maps: The persistent structure of segregation and economic inequality. Natl Community Reinvestment Coalit [Internet] 2018; Available from: ncrc.umich.edu/about-ncrc
- 66.Quillian L, Lee JJ, Honoré B. Racial Discrimination in the U.S. Housing and Mortgage Lending Markets: A Quantitative Review of Trends, 1976–2016. Race Soc Probl [Internet] 2020;12(1):13–28. Available from: 10.1007/s12552-019-09276-x [DOI] [Google Scholar]
- 67.Nardone A, Neophytou AM, Balmes J, Thakur N. Ambient Air Pollution and Asthma-Releated Outcomes in Children of Color in the United States: a Scoping Review of Literature Published Between 2013 and 2017. Curr Allergy Asthma Rep 2019;18(5):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whittemore AH. The Experience of Racial and Ethnic Minorities with Zoning in the United States. J Plan Lit 2017;32(1):16–27. [Google Scholar]
- 69.Zheng XY, Ding H, Jiang LN, Chen SW, Zheng JP, Qiu M, et al. Association between Air pollutants and asthma emergency room visits and hospital admissions in time series studies: A systematic review and meta-Analysis. PLoS One 2015;10(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nishimura KK, Galanter JM, Roth LA, Oh SS, Thakur N, Nguyen EA, et al. Early-Life air pollution and asthma risk in minority children the GALA II and SAGE II studies. Am J Respir Crit Care Med 2013;188(3):309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Delfino RJ, Wu J, Tjoa T, Gullesserian SK, Nickerson B, Gillen DL. Effect modification by residential traffic-related air pollution. Epidemiology 2014;25(1):48–57. [DOI] [PubMed] [Google Scholar]
- 72.Alcala E, Brown P, Capitman JA, Gonzalez M, Cisneros R. Cumulative impact of environmental pollution and population vulnerability on pediatric asthma hospitalizations: A multilevel analysis of calenviroscreen. Int J Environ Res Public Health 2019;16(15):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morello-Frosch R, Lopez R. The riskscape and the color line: Examining the role of segregation in environmental health disparities. Environ Res 2006;102(2):181–96. [DOI] [PubMed] [Google Scholar]
- 74.Alexander D, Currie J. Is it who you are or where you live? Residential segregation and racial gaps in childhood asthma. J Heal Econ 2017;55:186–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gaskin DJ, Dinwiddie GY, Chan KS, McCleary RR. Residential Segregation and the Availability of Primary Care Physicians. Health Serv Res 2012;47(6):2353–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burke LM, Schwalbach J. Housing Redlining and Its Lingering Effects on Education Opportunity [Internet] 2021. cENTEr FOr EDUcaTION POLIcY. 2021. Available from: http://report.heritage.org/bg3594 [Google Scholar]
- 77.Williams DR, Collins C. Racial residential segregation: A fundamental cause of racial disparities in health. Public Health Rep 2001;116(5):404–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sullivan K, Thakur N. Structural and Social Determinants of Health in Asthma in Developed Economies: a Scoping Review of Literature Published Between 2014 and 2019. Curr Allergy Asthma Rep 2020;20(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thakur N, Martin M, Castellanos E, Oh SS, Roth LA, Eng C, et al. Socioeconomic status and asthma control in African American youth in SAGE II. J Asthma 2014;51(7):720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thakur N, Oh SS, Nguyen EA, Martin M, Roth LA, Galanter J, et al. Socioeconomic status and childhood asthma in urban minority youths: The GALA II and SAGE II studies. Am J Respir Crit Care Med 2013;188(10):1202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bacon SL, Bouchard A, Loucks EB, Lavoie KL. Individual-level socioeconomic status is associated with worse asthma morbidity in patients with asthma. Respir Res 2009;10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chung J, Simpson EL. The socioeconomics of atopic dermatitis. Ann Allergy, Asthma Immunol [Internet] 2019;122(4):360–6. Available from: 10.1016/j.anai.2018.12.017 [DOI] [PubMed] [Google Scholar]
- 83.Semega J, Kollar M, Creamer J, Mohanty A. Income and Poverty in the United Sates: 2018 United States Census Bur. 2019; [Google Scholar]
- 84.Wagner P, Kopf D. The Racial Geography of Mass Incarceration [Internet] Prison Policy Initiative. 2015. [cited 2021 Jul 7]. Available from: https://www.prisonpolicy.org/racialgeography/report.html [Google Scholar]
- 85.Race & Justice News: Native Americans in the Justice System [Internet] The Sentencing Project. 2016. [cited 2021 Jul 7]. Available from: https://www.sentencingproject.org/news/race-justice-news-native-americans-in-the-justice-system/ [Google Scholar]
- 86.Milner AN, George BJ, Allison DB. Black and Hispanic Men Perceived to Be Large Are at Increased Risk for Police Frisk, Search, and Force. PLoS One 2016;11(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cooper HLF. War on Drugs Policing and Police Brutality. Subst Use Misuse 2015;50(8–9):1188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang EA, Green J. Incarceration as a key variable in racial disparities of asthma prevalence. BMC Public Health 2010;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Frank JW, Hong CS, Subramanian SV., Wang EA. Neighborhood incarceration rate and asthma prevalence in New York city: A multilevel approach. Am J Public Health 2013;103(5):38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yonas, Michael A, Lange Nancy E, Celedon JC. Psychosocial stress and asthma morbidity. Curr Opin Allergy Clin Immunol 2012;12(2):202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wright RJ. Stress and atopic disorders. J Allergy Clin Immunol 2005;116(6):1301–6. [DOI] [PubMed] [Google Scholar]
- 92.Chen E, Hanson MD, Paterson LQ, Griffin MJ, Walker HA, Miller GE. Socioeconomic status and inflammatory processes in childhood asthma: The role of psychological stress. J Allergy Clin Immunol 2006;117(5):1014–20. [DOI] [PubMed] [Google Scholar]
- 93.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 1998;14(4):245–58. [DOI] [PubMed] [Google Scholar]
- 94.Shonkoff JP, Garner AS, Siegel BS, Dobbins MI, Earls MF, McGuinn L, et al. The lifelong effects of early childhood adversity and toxic stress. Pediatrics 2012;129(1). [DOI] [PubMed] [Google Scholar]
- 95.Lee RD, Fang X, Luo F. The impact of parental incarceration on the physical and mental health of young adults. Pediatrics 2013;131(4):1188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bhan N, Glymour MM, Kawachi I, Subramanian SV. Childhood adversity and asthma prevalence: Evidence from 10 US states (2009–2011). BMJ Open Respir Res 2014;1(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Remigio-Baker RA, Hayes DK, Reyes-Salvail F. Adverse Childhood Events are Related to the Prevalence of Asthma and Chronic Obstructive Pulmonary Disorder Among Adult Women in Hawaii. Lung 2015;193(6):885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wing R, Gjelsvik A, Nocera M, McQuaid EL. Association between adverse childhood experiences in the home and pediatric asthma. Ann Allergy, Asthma Immunol [Internet] 2015;114(5):379–84. Available from: 10.1016/j.anai.2015.02.019 [DOI] [PubMed] [Google Scholar]
- 99.Tiotiu AI, Novakova P, Nedeva D, Chong-Neto HJ, Novakova S, Steiropoulos P, et al. Impact of air pollution on asthma outcomes. Int J Environ Res Public Health 2020;17(17):1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kanchongkittiphon W, Mendell MJ, Gaffin JM, Wang G, Phipatanakul W. Indoor environmental exposures and exacerbation of asthma: An update to the 2000 review by the institute of medicine. Environ Health Perspect 2015;123(1):6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sanders VM. The beta2-adrenergic receptor on T and B lymphocytes: Do we understand it yet? Brain Behav Immun [Internet] 2012;26(2):195–200. Available from: 10.1016/j.bbi.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liberman AC, Budziñski ML, Sokn C, Gobbini RP, Steininger A, Arzt E. Regulatory and mechanistic actions of glucocorticoids on T and inflammatory cells. Front Endocrinol (Lausanne) 2018;9(MAY):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Meduri GU, Yates CR. Systemic Inflammation-Associated Glucocorticoid Resistance and Outcome of ARDS. Ann NY Acad Sci 2004;1024:24–53. [DOI] [PubMed] [Google Scholar]
- 104.Fahy JV Type 2 inflammation in asthma-present in most, absent in many. Nat Rev Immunol 2015;15(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pomes A, Mueller GA, Randall TA, Chapman MD, Karla A. New insights into cockroach allergens. Curr Allergy Asthma Rep 2017;17(4):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu L, Zhang M, Ma W, Jin S, Song W, He S. Cockroach allergen Bla g 7 promotes TIM4 expression in dendritic cells leading to Th2 polarization. Mediators Inflamm 2013;2013. [DOI] [PMC free article] [PubMed]
- 107.Goel C, Gaur SN, Bhati G, Arora N. DC type 2 polarization depends on both the allergic status of the individual and protease activity of Per a 10. Immunobiology [Internet] 2015;220(10):1113–21. Available from: 10.1016/j.imbio.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 108.Chen E, Miller GE. Stress and inflammation in exacerbations of asthma. Brain Behav Immun 2007;21(8):993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen E, Fisher E, Bacharier L, Strunk R. Socioeconomic Status, Stress, and Immune Markers in Adolescents With Asthma. Psychosom Med 2003;65(6):984–92. [DOI] [PubMed] [Google Scholar]
- 110.Chen E, Shalowitz MU, Story RE, Ehrlich KB, Levine CS, Hayen R, et al. Dimensions of socioeconomic status and childhood asthma outcomes: Evidence for distinct behavioral and biological associations. Psychosom Med 2016;78(9):1043–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ramirez F, Fowell D, Puklavec M, Simmonds S, Mason D. Glucocorticoids promote a TH2 cytokine response by CD4+ T cells in vitro. J Immunol 1996;156(7):2406–12. [PubMed] [Google Scholar]
- 112.Huang HW, Tang JL, Han XH, Peng YP QY. Lymphocyte-derived catecholamines induce a shift of Th1/Th2 balance toward Th2 polarization. Neuroimmunomodulation 2013;20(1):1–8. [DOI] [PubMed] [Google Scholar]
- 113.Ma L, Xue H, Guan X, Shu C, Wang F, Zhang J, et al. The Imbalance of Th17 cells and CD4+CD25highFoxp3+ Treg cells in patients with atopic dermatitis. J Eur Acad Dermatology Venereol 2013;28(8):1079–86. [DOI] [PubMed] [Google Scholar]
- 114.Miller GE, Chen E, Shalowitz MU, Story RE, Leigh AKK, Ham P, Arevalo JMG CS. Divergent transcriptional profiles in pediatric asthma patients of low and high socioeconomic status. Pediatr Pulmonol 2018;53(6):710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kroner J de C, Knoke K, Kofler DM, Steiger J, Fabri M. Glucocorticoids promote intrinsic human TH17 differentiation. J Allergy Clin Immunol 2018;142(5):1669–73. [DOI] [PubMed] [Google Scholar]
- 116.Xiang L, Marshall GD. Immunomodulatory effects of dexamethasone on gene expression of cytokine and stress hormone receptors in peripheral blood mononuclear cells. Int Immunopharmacol [Internet] 2013;17(3):556–60. Available from: 10.1016/j.intimp.2013.07.019 [DOI] [PubMed] [Google Scholar]
- 117.Purser JL, Kuchibhatla MN, Miranda ML, Blazer DG, Cohen HJ, Fillenbaum GG. Geographical segregation and IL-6: A marker of chronic inflammation in older adults. Biomark Med 2008;2(4):335–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ichiyama K, Yoshida H, Wakabayashi Y, Chinen T, Saeki K, Nakaya M, et al. Foxp3 inhibits RORγt-mediated IL-17A mRNA transcription through direct interaction with RORγt. J Biol Chem [Internet] 2008;283(25):17003–8. Available from: 10.1074/jbc.M801286200 [DOI] [PubMed] [Google Scholar]
- 119.Zijlmans MAC, Korpela K, Riksen-Walraven JM, de Vos WM, de Weerth C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology [Internet] 2015;53:233–45. Available from: 10.1016/j.psyneuen.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 120.McCauley K, Durack J, Valladares R, Fadrosh DW, Lin DL, Calatroni A, et al. Distinct nasal airway bacterial microbiotas differentially relate to exacerbation in pediatric patients with asthma. J Allergy Clin Immunol [Internet] 2019;144(5):1187–97. Available from: 10.1016/j.jaci.2019.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang YJ, Nariya S, Harris JM, Lynch SV., Choy DF, Arron JR, et al. The airway microbiome in patients with severe asthma: Associations with disease features and severity. J Allergy Clin Immunol [Internet] 2015;136(4):874–84. Available from: 10.1016/j.jaci.2015.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cardenas A, Sordillo JE, Rifas-Shiman SL, Chung W, Liang L, Coull BA, et al. The nasal methylome as a biomarker of asthma and airway inflammation in children. Nat Commun [Internet] 2019;10(1):1–10. Available from: 10.1038/s41467-019-11058-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rosenberg SL, Miller GE, Brehm JMCJ. Stress and Asthma: Novel Insights on Genetic, Epigenetic and Immunologic Mechanisms. J Allergy Clin Immunol 2014;134(5):1009–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen W, Boutaoui N, Brehm JM, Han YY, Schmitz C, Cressley A, et al. ADCYAP1R1 and asthma in Puerto Rican children. Am J Respir Crit Care Med 2013;187(6):584–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Harb H, Renz H. Update on epigenetics in allergic disease. J Allergy Clin Immunol [Internet] 2015;135(1):15–24. Available from: 10.1016/j.jaci.2014.11.009 [DOI] [PubMed] [Google Scholar]
- 126.Snow SJ, Henriquez AR, Costa DL, Kodavanti UP. Neuroendocrine regulation of air pollution health effects: Emerging insights. Toxicol Sci 2018;164(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim D, Chen Z, Zhou L-F, Huang S-X. Air pollutants and early origins of respiratory diseases. Chronic Dis Transl Med [Internet] 2018;4(2):75–94. Available from: 10.1016/j.cdtm.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]


