In the MOVe-OUT trial, molnupiravir showed a clinically meaningful reduction in the risk for hospitalization or death in adults with mild to moderate COVID-19 and risk factors for progression to severe disease. This secondary analysis of the MOVe-OUT trial sought to identify other potential clinical benefits of molnupiravir versus placebo.
Visual Abstract. Additional Clinical Benefits With Molnupiravir for COVID-19.
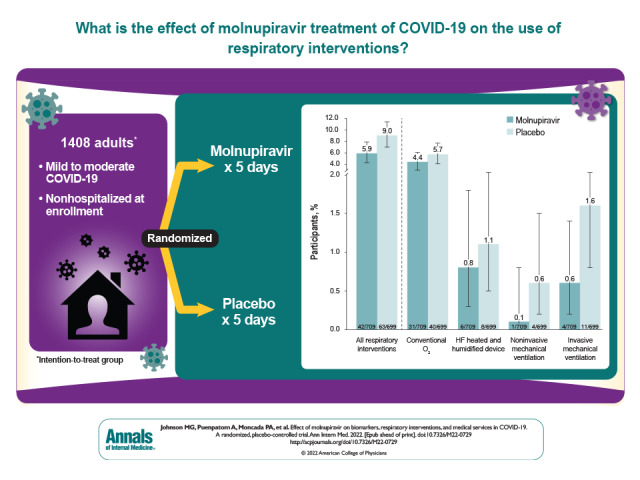
In the MOVe-OUT trial, molnupiravir showed a clinically meaningful reduction in the risk for hospitalization or death in adults with mild to moderate COVID-19 and risk factors for progression to severe disease. This secondary analysis of the MOVe-OUT trial sought to identify other potential clinical benefits of molnupiravir versus placebo.
Abstract
Background:
In the MOVe-OUT trial, molnupiravir showed a clinically meaningful reduction in the risk for hospitalization or death in adults with mild to moderate COVID-19 and risk factors for progression to severe disease.
Objective:
To identify other potential clinical benefits of molnupiravir versus placebo.
Design:
Secondary analysis of the randomized, double-blind, placebo-controlled phase 3 component of MOVe-OUT. (ClinicalTrials.gov: NCT04575597)
Setting:
107 sites globally.
Participants:
1433 nonhospitalized adults aged 18 years or older with mild to moderate COVID-19.
Intervention:
Molnupiravir, 800 mg, or placebo every 12 hours for 5 days.
Measurements:
Changes from baseline in C-reactive protein (CRP) concentration and oxygen saturation (Spo 2), need for respiratory interventions (including invasive mechanical ventilation), and need for medical services in all randomly assigned participants through day 29, and need for respiratory interventions and time to discharge in the subgroup of participants who were hospitalized after randomization.
Results:
Participants receiving molnupiravir showed faster normalization of CRP and Spo 2, with improvements observed on day 3 of therapy, compared with placebo. Molnupiravir-treated participants had a decreased need for respiratory interventions versus placebo-treated participants (relative risk reduction [RRR], 34.3% [95% CI, 4.3% to 54.9%]), with similar findings in participants who were hospitalized after randomization (RRR, 21.3% [CI, 0.2% to 38.0%]). Hospitalized participants who received molnupiravir were discharged a median of 3 days before those who received placebo. Acute care visits (7.2% vs. 10.6%; RRR, 32.1% [CI, 4.4% to 51.7%]) and COVID-19–related acute care visits (6.6% vs. 10.0%; RRR, 33.8% [CI, 5.6% to 53.6%]) were less frequent in molnupiravir- versus placebo-treated participants.
Limitations:
Some analyses were performed post hoc. Longer-term benefits of molnupiravir therapy were not evaluated. Participants were not immunized against SARS-CoV-2.
Conclusion:
The findings suggest there are additional important clinical benefits of molnupiravir beyond reduction in hospitalization or death.
Primary Funding Source:
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc.
SARS-CoV-2 is responsible for an unprecedented global pandemic that has resulted in 527 million cases and 6.3 million deaths worldwide as of 31 May 2022 (1, 2). The clinical presentation of SARS-CoV-2 infection varies; some people remain asymptomatic, whereas others develop COVID-19 that can range in severity from mild to critical illness resulting from a hyperinflammatory response to the virus (3, 4). Maintaining the availability of life-saving interventions, such as ventilatory and/or hemodynamic support, for all patients with severe or critical COVID-19 has been a major challenge throughout the pandemic, especially in regions with limited resources at baseline and during COVID-19 surges (5–8).
Immunization for COVID-19 decreases hospitalizations and progression to severe disease (9). However, many people remain unvaccinated due to lack of access or vaccine hesitancy (10, 11), breakthrough infections can occur, and vaccines may need to be modified in response to emerging SARS-CoV-2 variants (12, 13). Some monoclonal antibodies (mAbs) and antivirals have been shown to decrease the risk for hospitalization and/or death compared with placebo in nonhospitalized patients with COVID-19 who have risk factors for progression to severe disease (14–19). The effect of these therapies on other clinically relevant outcomes, such as changes in inflammatory markers, oxygen saturation (Spo 2), or ventilation requirements, in nonhospitalized patients or those requiring hospitalization after receiving these therapies has not been fully elucidated. The implementation of some of these therapies has been challenging (20), limiting their widespread availability and uptake globally. For instance, most mAbs and intravenous remdesivir must be administered in a medical setting (21–25), further burdening health care systems, limiting access for patients, and introducing infection control risks. Moreover, some mAbs are no longer recommended because they are ineffective against the Omicron (B.1.1.529) variant, which has been responsible for recent surges (3, 7, 26). The only other oral antiviral currently available, nirmatrelvir–ritonavir, requires careful assessment for drug–drug interactions before use (3, 27). As a result, there remains an unmet need for antiviral therapies that can be easily self-administered in the outpatient setting to not only improve outcomes for patients but also ease the burden of COVID-19 on the health care system.
Molnupiravir is an orally available small-molecule ribonucleoside prodrug of β-D-N4-hydroxycytidine (NHC) with potent, broad-spectrum in vitro activity against coronaviruses, including SARS-CoV-2 variants of concern, and a high barrier to the development of resistance (28–38). Molnupiravir is metabolized to NHC by esterases and then phosphorylated intracellularly to the active form, NHC-triphosphate, which is incorporated into the viral genome, ultimately resulting in replication-incompetent SARS-CoV-2 (39, 40). Molnupiravir does not require dose adjustment for renal or hepatic impairment, and in vitro studies indicate that molnupiravir and NHC are not substrates, inhibitors, or inducers of CYP3A4 enzymes (41).
The phase 3 component of the MOVe-OUT clinical trial established the efficacy and safety of molnupiravir in nonhospitalized adults with mild to moderate COVID-19 and risk factors for progression to severe disease. Molnupiravir (800 mg every 12 hours for 5 days, initiated within 5 days of the onset of signs or symptoms of COVID-19) was superior to placebo at the prespecified interim analysis for the primary efficacy end point of all-cause hospitalization or death by day 29 (7.3% vs. 14.1%; difference, −6.8 percentage points [95% CI, −11.3 to −2.4 percentage points]). In the final analysis of all randomly assigned participants, molnupiravir showed a clinically meaningful reduction in the risk for hospitalization or death (6.8% vs. 9.7%; difference, −3.0 percentage points [CI, −5.9 to −0.1 percentage points]), providing evidence of a substantial mortality benefit (89% decreased risk for death), a shorter time to resolution for most COVID-19 signs and symptoms, a greater reduction in mean viral load from baseline, and a lack of safety concerns compared with placebo through day 29 (19).
We conducted additional analyses to evaluate additional potential benefits of molnupiravir for the treatment of mild to moderate COVID-19 based on clinical markers and the need for respiratory interventions and medical services from the phase 3 component of MOVe-OUT. Changes in high-sensitivity C-reactive protein (CRP) concentration, Spo 2, the need for respiratory interventions, acute care visits, and COVID-19–related acute care visits were evaluated in all randomly assigned participants who received molnupiravir or placebo. Respiratory interventions plus time to discharge were assessed in the subgroup of participants who were hospitalized after randomization.
Methods
Design Overview, Setting, and Participants
MOVe-OUT (ClinicalTrials.gov: NCT04575597) was a phase 2/3 double-blind, parallel-group, randomized, placebo-controlled trial designed to evaluate the efficacy and safety of molnupiravir compared with placebo. Full details of the phase 2 and phase 3 components of MOVe-OUT have been published previously (19, 42). Here, we report on results from the phase 3 component.
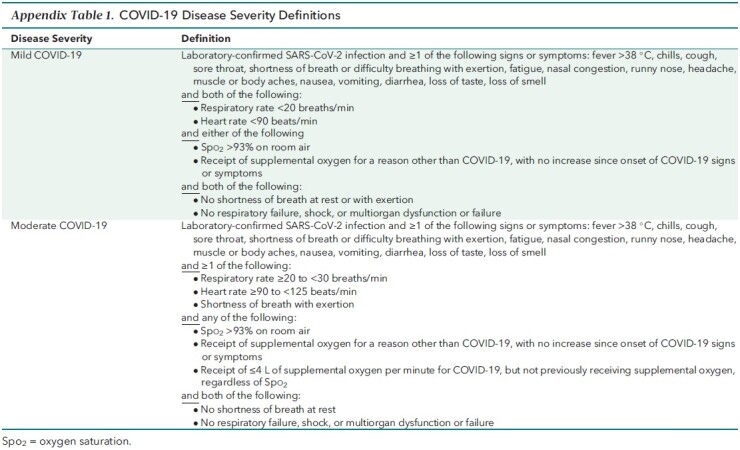
Nonhospitalized adults aged 18 years or older with laboratory-confirmed mild to moderate COVID-19 (Appendix Table 1) were included in the phase 3 component of MOVe-OUT. Participants had to have onset of signs or symptoms no more than 5 days prior and at least 1 risk factor for progression to severe disease. Prespecified risk factors for severe disease were age older than 60 years, active cancer, chronic kidney disease, chronic obstructive pulmonary disease, obesity (body mass index ≥30 kg/m2), serious heart conditions (heart failure, coronary artery disease, or cardiomyopathies), and diabetes mellitus. Key exclusion criteria included an anticipated need for inpatient treatment of COVID-19 within 48 hours, stage 4 or 5 chronic kidney disease or dialysis, pregnancy, and receipt of a SARS-CoV-2 vaccine.
Appendix Table 1.
COVID-19 Disease Severity Definitions
Participants were randomly assigned in a 1:1 ratio to receive molnupiravir, 800 mg, or matching placebo every 12 hours for 5 days, stratified by the time of onset of signs or symptoms (≤3 or >3 days), with the first dose of study drug administered within 24 hours of randomization. Participants could receive supportive care with antipyretics, anti-inflammatory agents, or glucocorticoids at the investigator's discretion, but therapies indicated specifically for COVID-19 (for example, mAbs, remdesivir) were prohibited through day 29. For participants who required hospitalization after randomization, all study procedures and study drug administration were to be continued if possible, and treatment with COVID-19–specific therapies was permitted.
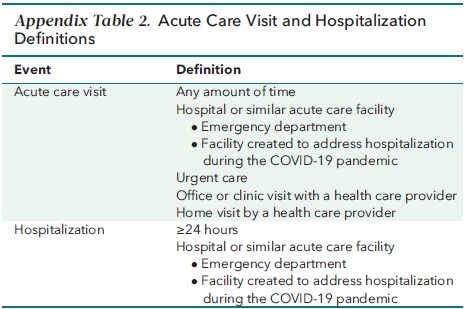
Study visits occurred at screening and on days 1 (baseline), 3, 5 (end of therapy), 10, 15, and 29. All assessments were prespecified in the study protocol (Supplement) and performed prospectively over the course of the trial. Spo 2 and the need for and type of respiratory intervention were reviewed at screening and at every study visit thereafter. At each study visit, Spo 2 was measured by pulse oximetry after participants rested for at least 5 minutes. Blood samples were collected on day 1 and at all postbaseline study visits for hematology, chemistry, and CRP laboratory measurements. Acute care visits, such as urgent care, office, or clinic visits, and hospitalization status (Appendix Table 2) were assessed through day 29.
Appendix Table 2.
Acute Care Visit and Hospitalization Definitions
Outcomes
Baseline demographic and clinical characteristics and mean changes in CRP concentration and Spo 2 from baseline through day 29 were evaluated in the safety population, which consisted of all participants who had undergone randomization and had received at least 1 dose of study drug. The use of any respiratory interventions (including conventional oxygen therapy, a high-flow heated and humidified device, noninvasive mechanical ventilation, and invasive mechanical ventilation), acute care visits, and COVID-19–related acute care visits were assessed in the modified intention-to-treat (MITT) population. The MITT population included all participants who were randomly assigned, received at least 1 dose of study drug, and were not hospitalized before the first dose of study drug. Participants hospitalized before the first dose were not included in the MITT population because they could not be assessed for the primary efficacy end point. Respiratory interventions along with time to hospital discharge were examined in participants in the MITT population who required hospitalization after randomization.
Statistical Analysis
Acute care visits and COVID-19–related acute care visits were prespecified exploratory end points in the MOVe-OUT study protocol; the analysis plan for these end points was also prespecified. The differences in the proportions for acute care visits and COVID-19–related acute care visits between treatment groups, and their corresponding 95% CIs, were estimated based on the Miettinen–Nurminen method, with stratification by randomization strata (43). The median time to hospital discharge among participants with observed hospitalization through day 29 was estimated post hoc using the product-limit (Kaplan–Meier) method for censored data. Death and early discontinuation were censored at the time of the event, and participants who completed day 29 with no discharge by that time were censored at day 29. All other analyses presented were performed post hoc, and descriptive statistics were provided for all other outcomes included in this article. Relative risk reductions (RRRs) and their corresponding 95% CIs were calculated as appropriate. All analyses were conducted using SAS, version 9.4 (SAS Institute).
Role of the Funding Source
The trial sponsor, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., was involved in the study design; collection, analysis, and interpretation of the data; and writing of the report. All authors had access to the study data and final responsibility for the decision to submit the manuscript for publication.
Results
In the phase 3 component of MOVe-OUT, 1433 participants were randomly assigned from 107 sites in Africa, the Asia-Pacific region, Europe, Latin America, and North America. Of these, 710 molnupiravir-treated participants and 701 placebo recipients were included in the safety population. One participant in the molnupiravir group and 2 in the placebo group were hospitalized before receiving the first dose of study drug and were excluded from the MITT population (Appendix Figure). Baseline demographic and clinical characteristics were generally similar between groups (Table). One hundred fifty-two participants in the molnupiravir group and 151 in the placebo group received systemic corticosteroids before or during the study treatment period in the safety population. Forty-eight of 709 (6.8%) molnupiravir-treated participants and 67 of 699 (9.6%) placebo recipients in the MITT population were hospitalized through day 29.
Appendix Figure. Participant flow chart.
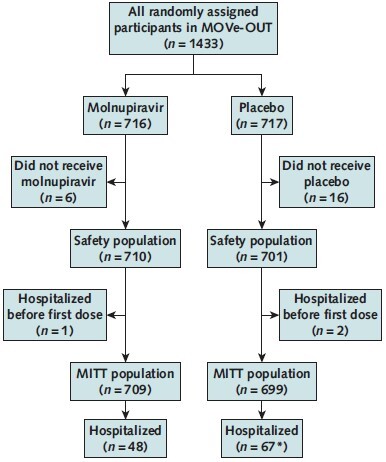
MITT = modified intention-to-treat.
* One placebo recipient was lost to follow-up and was thus not considered to be hospitalized for this analysis.
Table.
Baseline Demographic and Clinical Characteristics in the Safety Population
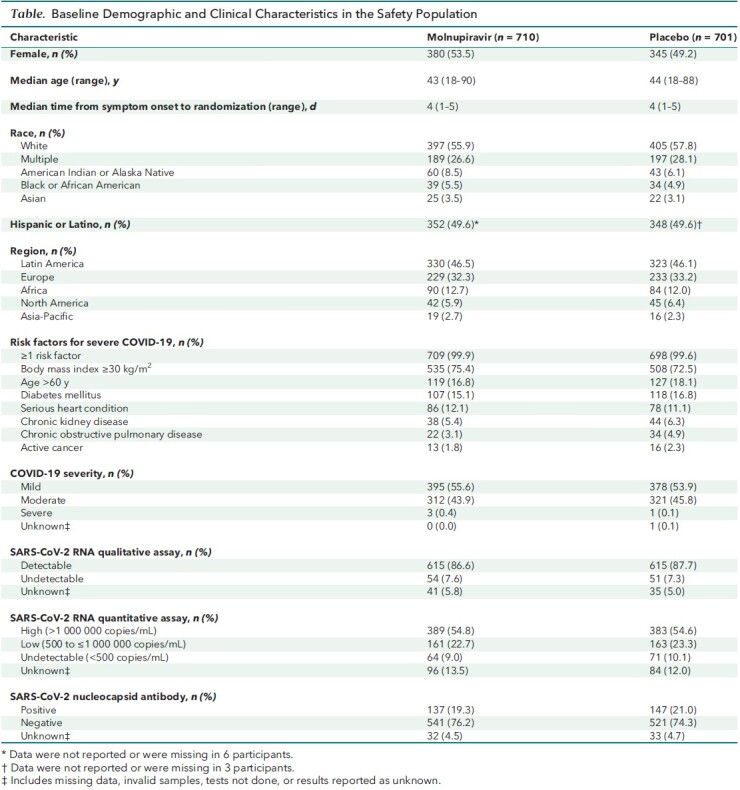
In the safety population, participants who received molnupiravir had earlier and larger reductions in mean change from baseline in CRP values at all postbaseline visits than those who received placebo. In the molnupiravir group, a reduction in mean CRP values was evident as early as day 3 (the first postbaseline visit) and continued through day 29, whereas in the placebo group, a reduction was not seen until day 10 (Figure 1, top; Appendix Table 3).
Figure 1. Mean change in CRP concentration (top) and Spo 2 (bottom) through day 29 (safety population).
Error bars represent 95% CIs. CRP = high-sensitivity C-reactive protein; EOT = end of therapy; Spo 2 = oxygen saturation.
Appendix Table 3.
Mean CRP Concentrations and Spo 2 Through Day 29 in the Safety Population
Similarly, molnupiravir-treated participants had earlier and larger improvements in mean change from baseline in Spo 2 values compared with placebo recipients at all postbaseline visits in the safety population. Improvement in mean Spo 2 was observed as early as day 3 and continued through day 29 in the molnupiravir group, whereas placebo recipients did not show an increase in mean Spo 2 until day 10 (Figure 1, bottom; Appendix Table 3).
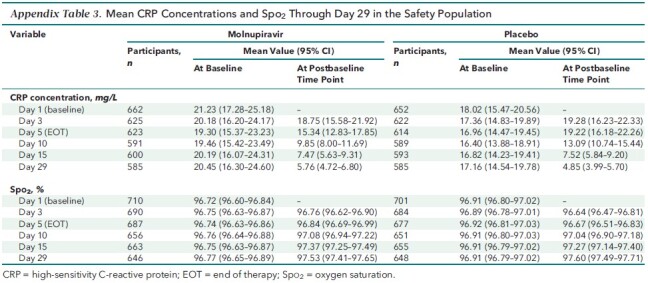
In the MITT population, fewer molnupiravir-treated participants required use of respiratory interventions (Figure 2, top), with an RRR of 34.3% (CI, 4.3% to 54.9%) for all respiratory interventions compared with placebo recipients. The RRRs were 23.6% (CI, −20.7% to 51.6%) for oxygen therapy with conventional oxygen, 26.1% (CI, −112% to 74.2%) for a high-flow heated and humidified device, 75.4% (CI, −120% to 97.2%) for noninvasive mechanical ventilation, and 64.1% (CI, −12.1% to 88.5%) for invasive mechanical ventilation. A reduction in the use of respiratory interventions was also observed in the subgroup of participants who required hospitalization after randomization (Figure 2, bottom). Compared with placebo recipients, this subgroup had RRRs of 21.3% (CI, 0.2% to 38.0%) for all respiratory interventions, 12.8% (CI, −32.5% to 42.6%) for conventional oxygen, −4.7% (CI, −182.2% to 61.2%) for a high-flow heated and humidified device, 65.1% (CI, −202.5% to 96.0%) for noninvasive mechanical ventilation, and 49.2% (CI, −49.9% to 82.8%) for invasive mechanical ventilation. In this subgroup of hospitalized participants, the median time to hospital discharge was 9 days (CI, 7 to 12 days) in the molnupiravir group and 12 days (CI, 9 to 14 days) in the placebo group.
Figure 2. Respiratory interventions through day 29 in the MITT population (top) and the hospitalized MITT population (bottom).
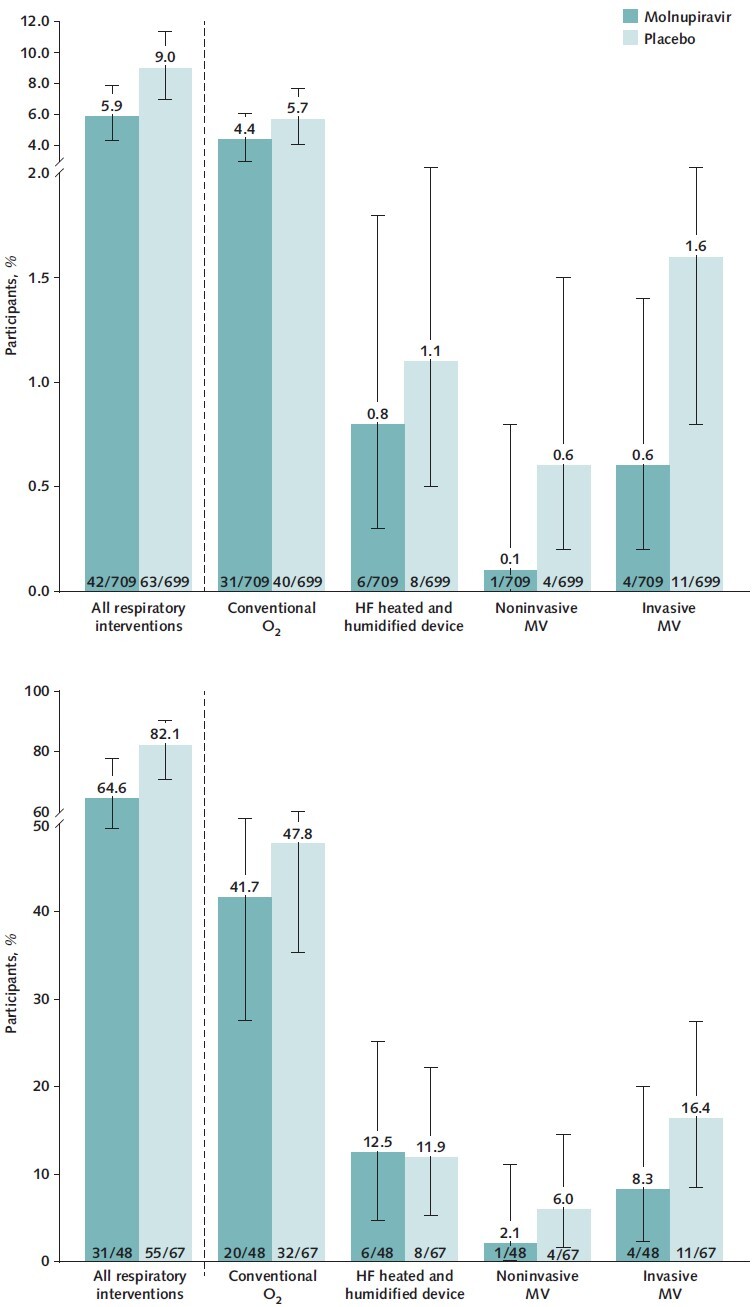
Error bars represent 95% CIs. Each participant was counted only once according to the highest level of O2 therapy needed. Respiratory interventions included conventional O2, HF heated and humidified device, noninvasive MV, and invasive MV. HF = high-flow; MITT = modified intention-to-treat; MV = mechanical ventilation; O2 = oxygen.
In the MITT population, prespecified analyses showed that the proportion of participants who had an acute care visit or a COVID-19–related acute care visit was lower in the molnupiravir group (7.2% and 6.6%, respectively) than in the placebo group (10.6% and 10.0%, respectively) (Figure 3), with RRRs of 32.1% (CI, 4.4% to 51.7%) and 33.8% (CI, 5.6% to 53.6%), respectively.
Figure 3. Acute care visits and COVID-19–related acute care visits through day 29 (MITT population).
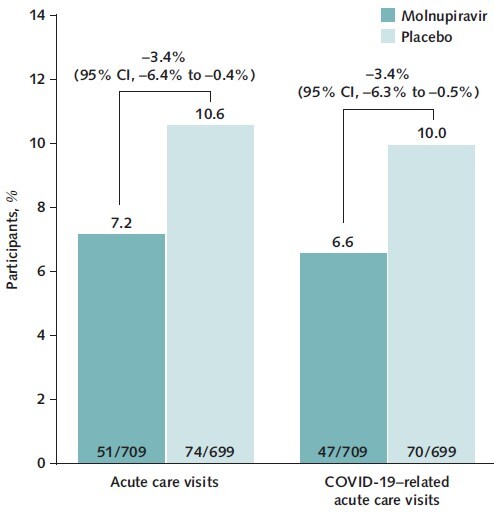
95% CIs were based on the Miettinen–Nurminen method, with stratification by randomization strata. MITT = modified intention-to-treat.
Discussion
In the phase 3 portion of the MOVe-OUT trial, participants receiving molnupiravir showed faster normalization of CRP and Spo 2 than placebo recipients. The improvements in these clinically relevant surrogate markers were noted early in the 5-day treatment period and continued through day 29. Molnupiravir-treated participants required fewer respiratory interventions, including invasive mechanical ventilation, than those receiving placebo. Even among the subgroup of hospitalized participants, molnupiravir was associated with a reduced need for any respiratory intervention as well as invasive mechanical ventilation. Furthermore, hospitalized participants who received molnupiravir were discharged earlier than those who received placebo. Finally, the need for acute care visits and COVID-19–related acute care visits was lower with molnupiravir versus placebo.
Excessive inflammation and respiratory decompensation are key drivers of COVID-19 progression, morbidity, and mortality (44–48). Higher CRP concentrations are associated with severe or critical illness, respiratory failure, and mortality in patients with COVID-19 (46–49), and CRP decreases as patients recover from SARS-CoV-2 infection (46, 49–52). Spo 2 can be easily and continuously measured with pulse oximetry, allowing providers to monitor respiratory function in patients with COVID-19 not only in acute care settings but also at home. The continuous decreases in CRP concentration and increases in Spo 2 with molnupiravir indicate subsiding systemic inflammation and recovering respiratory function. This correlates with the decreased need for respiratory interventions (including invasive mechanical ventilation) and acute care visits (including COVID-19–related acute care visits) and the shorter hospital stays in participants who received molnupiravir. The decreased need for invasive mechanical ventilation is particularly relevant because patients with COVID-19 who receive this respiratory intervention have mortality rates up to 50% (53–55).
Over the course of the pandemic, medical facilities have had to pause or limit routine, nonessential medical services and reallocate health care resources, including personal protective equipment, ventilators, and medications, to sustain the capacity to manage both patients with COVID-19 and those with other conditions requiring hospital care (5, 6, 8). Given that health care systems continue to be overburdened (7, 56), decreasing the need for invasive mechanical ventilation, the need for acute care visits, and the time to hospital discharge are particularly important to preserve limited health care resources. Participants treated with molnupiravir demonstrated earlier clinical improvement (shown by improvement in CRP concentration and Spo 2 on day 3) than placebo recipients, with a shorter length of stay in participants who were hospitalized, providing a possible opportunity for more efficient use of hospital beds.
A strength of this work is that MOVe-OUT is a large, global, prospective, double-blind, placebo-controlled trial that allowed for a carefully controlled evaluation of CRP and Spo 2 over time. We were able to objectively evaluate clinical improvement with these surrogate markers. An additional strength of this analysis was the prespecified evaluation of acute care visits, which included inpatient hospitalizations. This allowed us to include medically attended visits not captured strictly by hospitalizations. Ongoing real-world research will yield additional evidence on the use of molnupiravir therapy in the outpatient setting (57).
A key limitation of this work is that although the data were collected prospectively, most of these analyses were conducted retrospectively. In addition, although these analyses elucidate the immediate and shorter-term benefits of molnupiravir therapy for patients with COVID-19, potential longer-term benefits of molnupiravir therapy on postacute sequelae of COVID-19 were not studied in this trial. Another limitation is that participants in MOVe-OUT were not immunized against SARS-CoV-2; it is unclear whether vaccination or the time elapsed since vaccination would affect these findings. Finally, the potential financial impact associated with the observed decreases in respiratory interventions and medical services with molnupiravir was not included in this analysis; the cost-effectiveness of molnupiravir therapy has been evaluated separately (58).
Altogether, these findings suggest there is added clinical value of molnupiravir for the treatment of nonhospitalized adults with mild to moderate COVID-19. Meaningful benefits of molnupiravir to patients and health care systems may exceed the previously demonstrated benefits of reducing hospitalizations or death due to disease progression as well as alleviating symptoms in high-risk patients.
Supplementary Material
Footnotes
This article was published at Annals.org on 7 June 2022.
References
- 1. Cucinotta D , Vanelli M . WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157-60. [PMID: ] doi: 10.23750/abm.v91i1.9397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Accessed at https://covid19.who.int on 31 May 2022.
- 3. National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. 2022. Accessed at www.covid19treatmentguidelines.nih.gov on 17 February 2022. [PubMed]
- 4. Yuki K , Fujiogi M , Koutsogiannaki S . COVID-19 pathophysiology: a review. Clin Immunol. 2020;215:108427. [PMID: ] doi: 10.1016/j.clim.2020.108427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ranney ML , Griffeth V , Jha AK . Critical supply shortages – the need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med. 2020;382:e41. [PMID: ] doi: 10.1056/NEJMp2006141 [DOI] [PubMed] [Google Scholar]
- 6. Webb E , Hernández-Quevedo C , Williams G , et al. Providing health services effectively during the first wave of COVID-19: a cross-country comparison on planning services, managing cases, and maintaining essential services. Health Policy. 2021. [PMID: ] doi: 10.1016/j.healthpol.2021.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization. Enhancing response to Omicron SARS-CoV-2 variant: technical brief and priority actions for Member States. 2022.
- 8. Kanji S , Burry L , Williamson D , et al; Ontario COVID-19 ICU Drug Task Force (Appendix). Therapeutic alternatives and strategies for drug conservation in the intensive care unit during times of drug shortage: a report of the Ontario COVID-19 ICU Drug Task Force. Can J Anaesth. 2020;67:1405-16. [PMID: ] doi: 10.1007/s12630-020-01713-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fiolet T , Kherabi Y , MacDonald CJ , et al. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28:202-21. [PMID: ] doi: 10.1016/j.cmi.2021.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sheikh AB , Pal S , Javed N , et al. COVID-19 vaccination in developing nations: challenges and opportunities for innovation. Infect Dis Rep. 2021;13:429-36. [PMID: ] doi: 10.3390/idr13020041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Finney Rutten LJ , Zhu X , Leppin AL , et al. Evidence-based strategies for clinical organizations to address COVID-19 vaccine hesitancy. Mayo Clin Proc. 2021;96:699-707. [PMID: ] doi: 10.1016/j.mayocp.2020.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuhlmann C , Mayer CK , Claassen M , et al. Breakthrough infections with SARS-CoV-2 Omicron despite mRNA vaccine booster dose [Letter]. Lancet. 2022;399:625-6. [PMID: ] doi: 10.1016/S0140-6736(22)00090-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li X . Omicron: call for updated vaccines. J Med Virol. 2022;94:1261-3. [PMID: ] doi: 10.1002/jmv.27530 [DOI] [PubMed] [Google Scholar]
- 14. Hammond J , Leister-Tebbe H , Gardner A , et al; EPIC-HR Investigators. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397-408. [PMID: ] doi: 10.1056/NEJMoa2118542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gottlieb RL , Vaca CE , Paredes R , et al; GS-US-540-9012 (PINETREE) Investigators. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386:305-15. [PMID: ] doi: 10.1056/NEJMoa2116846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weinreich DM , Sivapalasingam S , Norton T , et al; Trial Investigators. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N Engl J Med. 2021;385:e81. [PMID: ] doi: 10.1056/NEJMoa2108163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta A , Gonzalez-Rojas Y , Juarez E , et al; COMET-ICE Investigators. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941-50. [PMID: ] doi: 10.1056/NEJMoa2107934 [DOI] [PubMed] [Google Scholar]
- 18. Dougan M , Nirula A , Azizad M , et al; BLAZE-1 Investigators. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N Engl J Med. 2021;385:1382-92. [PMID: ] doi: 10.1056/NEJMoa2102685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jayk Bernal A , Gomes da Silva MM , Musungaie DB , et al; MOVe-OUT Study Group. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386:509-20. [PMID: ] doi: 10.1056/NEJMoa2116044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lambrou AS , Redd JT , Stewart MA , et al. Implementation of SARS-CoV-2 monoclonal antibody infusion sites at three medical centers in the United States: strengths and challenges assessment to inform COVID-19 pandemic and future public health emergency use. Disaster Med Public Health Prep. 2022:1-32. [PMID: ] doi: 10.1017/dmp.2022.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eli Lilly and Company. Fact sheet for healthcare providers: emergency use authorization for bebtelovimab. 2022.
- 22. Gilead Sciences. VEKLURY (remdesivir) for injection. Prescribing information. 2022.
- 23. GlaxoSmithKline. Fact sheet for healthcare providers: emergency use authorization (EUA) of sotrovimab. 2021.
- 24. Eli Lilly and Company. Fact sheet for health care providers: emergency use authorization (EUA) of bamlanivimab and etesevimab. 2022.
- 25. Regeneron Pharmaceuticals. Fact sheet for health care providers: emergency use authorization (EUA) of REGEN-COV® (casirivimab and imdevimab). 2021.
- 26. Chen J , Wang R , Gilby NB , et al. Omicron variant (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. J Chem Inf Model. 2022;62:412-22. [PMID: ] doi: 10.1021/acs.jcim.1c01451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pfizer. Fact sheet for healthcare providers: emergency use authorization for nirmatrelvir/ritonavir. 2021.
- 28. Yoon JJ , Toots M , Lee S , et al. Orally efficacious broad-spectrum ribonucleoside analog inhibitor of influenza and respiratory syncytial viruses. Antimicrob Agents Chemother. 2018;62. [PMID: ] doi: 10.1128/AAC.00766-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cox RM , Wolf JD , Plemper RK . Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nat Microbiol. 2021;6:11-8. [PMID: ] doi: 10.1038/s41564-020-00835-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sheahan TP , Sims AC , Zhou S , et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020;12. [PMID: ] doi: 10.1126/scitranslmed.abb5883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wahl A , Gralinski LE , Johnson CE , et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature. 2021;591:451-7. [PMID: ] doi: 10.1038/s41586-021-03312-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abdelnabi R , Foo CS , De Jonghe S , et al. Molnupiravir inhibits replication of the emerging SARS-CoV-2 variants of concern in a hamster infection model. J Infect Dis. 2021;224:749-53. [PMID: ] doi: 10.1093/infdis/jiab361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Agostini ML , Pruijssers AJ , Chappell JD , et al. Small-molecule antiviral β-d-N 4-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. J Virol. 2019;93. [PMID: ] doi: 10.1128/JVI.01348-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Urakova N , Kuznetsova V , Crossman DK , et al. β-d-N 4-hydroxycytidine is a potent anti-alphavirus compound that induces a high level of mutations in the viral genome. J Virol. 2018;92. [PMID: ] doi: 10.1128/JVI.01965-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grobler J, Strizki J, Murgolo N, et al. Molnupiravir maintains antiviral activity against SARS-CoV-2 variants in vitro and in early clinical studies. Open Forum Infect Dis. 2021;8 (Suppl_1):S373. doi: 10.1093/ofid/ofab466.742 [DOI]
- 36. Vangeel L , Chiu W , De Jonghe S , et al. Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antiviral Res. 2022;198:105252. [PMID: ] doi: 10.1016/j.antiviral.2022.105252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li P , Wang Y , Lavrijsen M , et al. SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination [Letter]. Cell Res. 2022;32:322-4. [PMID: ] doi: 10.1038/s41422-022-00618-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meng B , Abdullahi A , Ferreira IATM , et al; CITIID-NIHR BioResource COVID-19 Collaboration. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature. 2022;603:706-14. [PMID: ] doi: 10.1038/s41586-022-04474-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kabinger F , Stiller C , Schmitzová J , et al. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol. 2021;28:740-6. [PMID: ] doi: 10.1038/s41594-021-00651-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gordon CJ , Tchesnokov EP , Schinazi RF , et al. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J Biol Chem. 2021;297:100770. [PMID: ] doi: 10.1016/j.jbc.2021.100770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Merck & Co., Inc. Fact sheet for healthcare providers: emergency use authorization for molnupiravir. 2021.
- 42. Caraco Y, Crofoot GE, Moncada PA, et al. Phase 2/3 trial of molnupiravir for treatment of Covid-19 in nonhospitalized adults. NEJM Evidence. 2022;1. doi: 10.1056/EVIDoa2100043 [DOI] [PubMed]
- 43. Miettinen O , Nurminen M . Comparative analysis of two rates. Stat Med. 1985;4:213-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 44. Machado C , González-Quevedo A . Hypoxemia and cytokine storm in COVID-19: clinical implications. MEDICC Rev. 2021;23:54-9. [PMID: ] doi: 10.37757/MR2021.V23.N3.10 [DOI] [PubMed] [Google Scholar]
- 45. Liu W , Yang C , Liao YG , et al. Risk factors for COVID-19 progression and mortality in hospitalized patients without pre-existing comorbidities. J Infect Public Health. 2022;15:13-20. [PMID: ] doi: 10.1016/j.jiph.2021.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smilowitz NR , Kunichoff D , Garshick M , et al. C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J. 2021;42:2270-9. [PMID: ] doi: 10.1093/eurheartj/ehaa1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Luo X , Zhou W , Yan X , et al. Prognostic value of C-reactive protein in patients with coronavirus 2019. Clin Infect Dis. 2020;71:2174-9. [PMID: ] doi: 10.1093/cid/ciaa641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Herold T , Jurinovic V , Arnreich C , et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146:128-136.e4. [PMID: ] doi: 10.1016/j.jaci.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li T , Wang X , Zhuang X , et al. Baseline characteristics and changes of biomarkers in disease course predict prognosis of patients with COVID-19. Intern Emerg Med. 2021;16:1165-72. [PMID: ] doi: 10.1007/s11739-020-02560-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lu G , Wang J . Dynamic changes in routine blood parameters of a severe COVID-19 case. Clin Chim Acta. 2020;508:98-102. [PMID: ] doi: 10.1016/j.cca.2020.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pinzón MA , Ortiz S , Holguín H , et al. Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia. PLoS One. 2021;16:e0252057. [PMID: ] doi: 10.1371/journal.pone.0252057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xu X , Han M , Li T , et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117:10970-5. [PMID: ] doi: 10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Auld SC , Caridi-Scheible M , Blum JM , et al; Emory COVID-19 Quality and Clinical Research Collaborative. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020;48:e799-e804. [PMID: ] doi: 10.1097/CCM.0000000000004457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. King CS , Sahjwani D , Brown AW , et al. Outcomes of mechanically ventilated patients with COVID-19 associated respiratory failure. PLoS One. 2020;15:e0242651. [PMID: ] doi: 10.1371/journal.pone.0242651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Domecq JP , Lal A , Sheldrick CR , et al; Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS): COVID-19 Registry Investigator Group. Outcomes of patients with coronavirus disease 2019 receiving organ support therapies: the International Viral Infection and Respiratory Illness Universal Study Registry. Crit Care Med. 2021;49:437-48. [PMID: ] doi: 10.1097/CCM.0000000000004879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Iuliano AD , Brunkard JM , Boehmer TK , et al. Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods—United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:146-52. [PMID: ] doi: 10.15585/mmwr.mm7104e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. University of Oxford. PANORAMIC (Platform Adaptive trial of NOvel antiviRals for eArly treatMent of COVID-19 In the Community). Accessed at www.panoramictrial.org on 18 February 2022.
- 58. Goswami H, Alsumali A, Jiang Y, et al. Cost-effectiveness analysis of molnupiravir versus best supportive care for the outpatient treatment of adults with COVID-19 [Abstract]. Presented at 32nd European Congress of Clinical Microbiology & Infectious Diseases, Lisbon, Portugal, 23–26 April 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


