Abstract
Background:
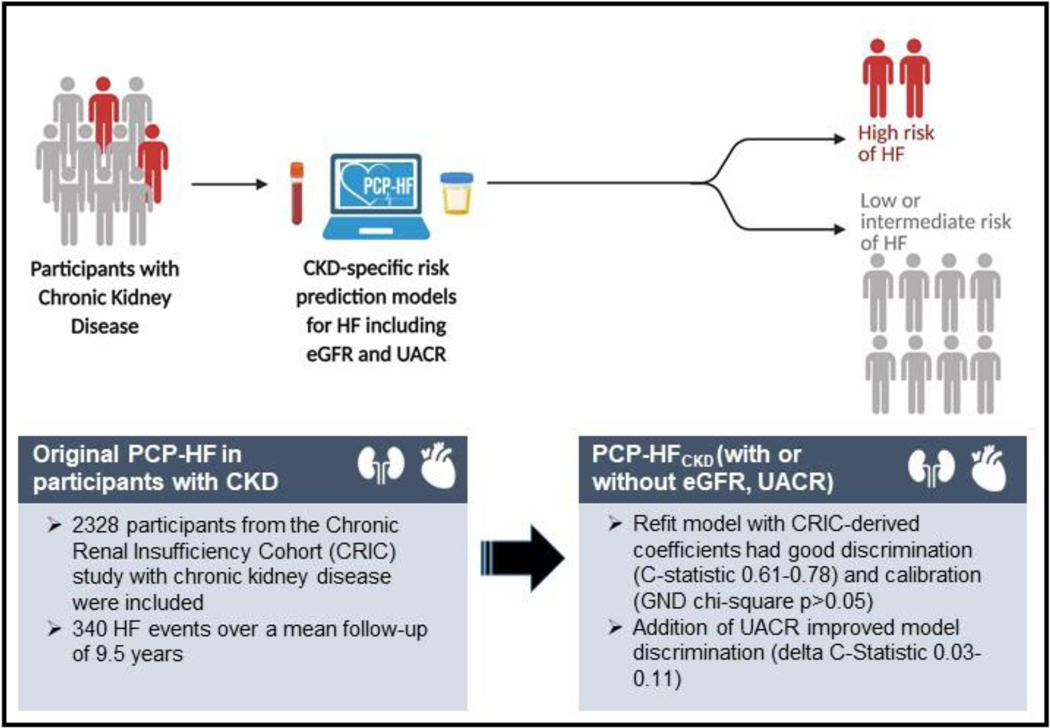
Heart failure (HF) is a leading contributor of cardiovascular morbidity and mortality in the chronic kidney disease (CKD) population. HF risk prediction tools that utilize readily available clinical parameters to risk stratify individuals with CKD are needed.
Methods:
We included Black and White participants aged 30 to 79 years with CKD stages 2–4 enrolled in the Chronic Renal Insufficiency Cohort (CRIC) Study and without self-reported cardiovascular disease. We assessed model performance of the Pooled Cohort Equations to Prevent Heart Failure (PCP-HF) to predict incident HF hospitalizations and refit the PCP-HF in the CKD population using CRIC data-derived coefficients and survival from CRIC Study participants in the CKD population (PCP-HFCKD). We investigated the improvement in HF prediction with inclusion of estimated glomerular filtration rate [eGFR] and urine albumin to creatinine ratio [UACR] into the PCP-HFCKD equations by change in C-statistic, net reclassification improvement (NRI) and integrated discrimination improvement index (IDI). We validated the PCP-HFCKD with and without eGFR and UACR in Multi-Ethnic Study of Atherosclerosis (MESA) participants with CKD.
Results:
Among 2328 CRIC Study participants, 340 incident HF hospitalizations occurred over a mean follow-up of 9.5 years. The PCP-HF equations did not perform well in most participants with CKD and had inadequate discrimination and insufficient calibration (C-statistic 0.64–0.71, GND chi-square statistic p value <0.05), with modest improvement and good calibration after being refit (PCP-HFCKD: C-statistic 0.61–0.78), GND chi-square p value >0.05). Addition of UACR, but not eGFR, to the refit PCP-HFCKD improved model performance in all race-sex groups (C-statistic [0.73–0.81], GND chi-square p value > 0.05, delta C-statistic ranging from 0.03–0.11 and NRI and IDI p values <0.01). External validation of the PCP-HFCKD in MESA demonstrated good discrimination and calibration.
Conclusions:
Routinely available clinical data that includes UACR in patients with CKD can reliably identify individuals at risk of HF hospitalizations.
Keywords: heart failure, chronic kidney disease, albuminuria, risk prediction
Lay Summary:
Patients with chronic kidney disease frequently develop heart failure; however, no tool exists to predict heart failure in patients with chronic kidney disease. This study investigated whether a previously developed tool that used routinely available clinical data could predict heart failure events in individuals with chronic kidney disease. Results demonstrated that this tool performed poorly in individuals with chronic kidney disease. However, when albuminuria, a measurement of protein in the urine that represents kidney dysfunction, was added to the risk prediction equation, the risk prediction tool was better able to predict heart failure events in this population. Implementation of this tool may be able to identify individuals with chronic kidney disease at high risk for heart failure hospitalization and improve clinical outcomes.
Introduction
In patients with chronic kidney disease (CKD), heart failure (HF) is a major manifestation of cardiovascular disease (CVD) that is associated with marked morbidity, mortality, and healthcare expenditures.(1, 2) Although there is substantial overlap in the risk factors for CKD and HF (e.g. hypertension, diabetes), patients with CKD have higher risk for the development of and adverse outcomes related to HF when compared with the general population who have similar risk factor levels. HF risk prediction tools that risk stratify individuals with CKD are needed to facilitate enhanced surveillance, intensification of risk factor modification and uptake of evidence-based therapies.
American College of Cardiology/American Heart Association (ACC/AHA) guidelines highlight the need to identify and stratify individuals at highest risk for HF.(3) Risk prediction equations, such as the Pooled Cohort Equations to Prevent Heart Failure (PCP-HF),(4) are available for use and have been validated in the general population.(4–8) The PCP-HF tool was developed to predict 10-year HF risk from 5 general population-based cohorts and validated in two general population-based cohorts in order to enhance personalized risk-based clinical care. The PCP-HF demonstrated good-to-excellent discrimination in the derivation and validation cohorts (C-Statistics ranged from 0.71–0.85 in validation).(4) Since then, additional HF risk prediction tools have been developed using machine learning algorithms.(9) However, the utility of any of these models in individuals with CKD remains unknown. Given the increased propensity of HF hospitalizations in individuals with CKD and known CKD-specific risk factors for the development of HF, understanding HF risk in CKD populations is highly relevant to the clinical cardiologist and HF practitioner. Additionally, the emergence of novel therapies, such as sodium glucose co-transporter 2 inhibitors (SGLT2i), that influence cardiac and kidney outcomes underscore the importance of understanding how HF risk prediction models perform in CKD populations.
In the current investigation, we 1) assessed the performance of the existing PCP-HF risk prediction model among a subset of Chronic Renal Insufficiency Cohort (CRIC) Study participants with CKD and without self-reported CVD, 2) refit the PCP-HF risk prediction model for the CKD population (PCP-HFCKD), 3) investigated the added value of including routinely assessed CKD-specific markers (estimated glomerular filtration rate [eGFR] and albuminuria) to the refit PCP-HFCKD risk prediction model, and 4) externally validated the models in the Multi-Ethnic Study of Atherosclerosis (MESA) participants with CKD.
Methods
Study Population
The design and implementation of the CRIC Study and MESA cohorts have been published previously.(10, 11) The CRIC Study is a multi-center prospective cohort study designed to investigate risk factors for CVD and CKD progression in individuals with mild to severe CKD.(10) Phase 1 of the CRIC Study was conducted between June 2003 - September 2008 and recruited 3939 individuals aged 29–74 with age-specific eGFR of 20–70 ml/min/1.73m2 from 7 clinical centers across the United States.(10) Main exclusion criteria included New York Heart Association (NYHA) class III and IV HF, renal cancer, multiple myeloma, recent chemotherapy or immunotherapy, cirrhosis, polycystic kidney disease, prior renal replacement therapy within 1 month or renal transplantation, pregnancy, institutionalization, or inability to consent. Black and Hispanic participants were oversampled.(10) In brief, MESA is a multi-center, prospective cohort study of 6814 men and women aged 45–84 years old without known CVD at baseline who were recruited across 6 cities in the United States.(11) Major exclusion criteria included history of clinical CVD, any CVD procedure, current atrial fibrillation, active cancer, pregnancy, and weight > 136 kg.(11) All CRIC Study and MESA participants provided written informed consent, and the respective protocols were approved by each clinical center’s Institutional Review Board.
We sought to refit and externally validate the PCP-HF risk prediction equation in a population with CKD but without prior CVD. We studied CRIC Study participants without prior CVD since patients with prevalent CVD require specific secondary prevention therapies, are already considered to be at higher risk for HF and are often recommended to receive specific therapies that can prevent progression to HF. We matched the inclusion criteria in the CRIC and MESA cohorts to those used in the derivation of the PCP-HF risk tool.(4) Of the total 3939 individuals enrolled in the CRIC Study, we excluded 1316 individuals with baseline self-reported history of CVD. Self-reported CVD included prior history of coronary revascularization, myocardial infarction, stroke, peripheral vascular disease, or heart failure.(12) We also excluded 186 individuals who self-reported race other than Black or White, were < 30 or > 79 years, or any participant without follow-up time. Additionally, 109 individuals were excluded for missing covariates including indices of eGFR and urine albumin-to-creatinine ratio (UACR) measured at baseline, yielding a total analytic population of 2328 individuals (Supplemental Figure 1). Of the full MESA cohort, which includes 6814 women and men aged 45 to 84 years, 522 self-identified Black and White individuals had a eGFR< 60ml/min/1.73m2 and were ≤ 79 years of age and were included in the external validation cohort.
Exposure Assessment to Calculate Predicted Heart Failure Risk (PCP-HF)
CRIC Study participant demographics, medication use and clinical data were obtained at the baseline visit. Participants self-identified as Black or White. Hypertension medication use included use of any of the following: angiotensin converting enzyme inhibitors, angiotensin receptor blockers, loop diuretics, thiazide diuretics, alpha 2 agonists, alpha blockers, vasodilators, beta-blockers, or calcium channel blockers. Resting blood pressure was measured via standardized protocols.(10) Diabetes medication use included any of the following: alphaglucosidase inhibitors, sulfonylureas, biguanides, meglitinides, thiazolidinediones, or oral dipeptidyl peptidase-4 inhibitors. A centralized laboratory measured serum creatinine and urine albumin and urine creatinine via standard assays at the baseline study visit.(10, 13) eGFR was calculated using the creatinine-based Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, which incorporates serum creatinine, age, sex and Black race, which is self-reported in our sample.(14)
Outcome Ascertainment of Incident Heart Failure
In the CRIC Study, acute HF hospitalization events were adjudicated by two independent physicians who reviewed hospitalization records and any differences were resolved by discussion.(12) HF events were classified as possible, probable or definite using a combination of symptoms and either physical examination, chest radiographs, or invasive hemodynamics or echocardiographic evidence. Symptoms included paroxysmal nocturnal dyspnea or dyspnea on exertion. Physical examination findings included at least two of the following: pulmonary rales, S3 gallop, jugular venous distention > 5 centimeters, or peripheral edema. Relevant chest radiographic findings included: pulmonary edema, vascular congestion or pleural effusion. Invasive hemodynamic or echocardiographic evidence included pulmonary capillary wedge pressure > 18 mmHg, cardiac index < 2.0 L/min/m2, or left ventricular ejection fraction ≤ 35%.(12) Incident HF in this report included only the first definite or probable event. Participants were followed until death, censored for withdrawal from study, loss to follow-up, or at administrative end of follow-up in March 2018. In MESA, HF events were adjudicated as probable or definite by two paired physicians using medical records obtained after biyearly study visits or telephone interviews conducted every 9–12 months.(15) Definite or probable HF was classified based on symptoms, including shortness of breath or edema. In addition, classification of probable HF required a physician diagnosis and receipt of medical treatment for HF. Definite HF required findings of pulmonary edema on chest x-ray, dilated ventricle or poor left ventricular function on echocardiography or ventriculography, or evidence of left ventricular diastolic dysfunction.(15) Incident HF in this report included only the first definite or probable event.
Statistical Analysis
Baseline characteristics of the overall and sex- and race-stratified CRIC Study population were described using means with standard deviations (SDs) or medians with interquartile ranges (IQR) for continuous variables and with proportions for categorical variables. Additionally, we compared baseline characteristics of our study population stratified by incident HF event occurrence.
In the CRIC Study, we investigated model performance of the original PCP-HF(4) to predict 10-year risk of HF hospitalizations. The PCP-HF model includes age, sex, race, current smoking, body mass index (BMI), systolic blood pressure (SBP) (treated or untreated), hypertension treatment, fasting glucose (treated or untreated), diabetes treatment, total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), and QRS duration. The HF risk estimates were developed from sex- and race-specific proportional hazards models from 5 population-based cohorts from the Cardiovascular Lifetime Risk Pooling Project.(4) The included covariates were chosen based on their known association with incident HF. Model fit was evaluated through Harrell C-statistic for discrimination(16) and the Greenwood-Nam-D’Agostino (GND) chi-squared statistic for calibration.(17) The distribution of the C statistic was defined a priori based on prior publications with less than 0.70, 0.70 to 0.80, and greater than 0.80 as inadequate, acceptable, and excellent discrimination levels, respectively. Model calibration was evaluated by the GND approach with adequate calibration defined a priori as X2<20, similar to prior publications, including the Pooled Cohort Equations for atherosclerotic cardiovascular disease (ASCVD).(18–20)
Since electrocardiograms are not routinely performed in all patients with CKD, we refit the PCP-HF model without the QRS duration in the original derivation cohort and noted minimal change to the coefficients of other covariates. (Supplemental Table 1). To enhance future dissemination and implementation efforts, we excluded QRS duration for all iterative models derived from the CRIC Study. Model performance was assessed with C-statistics (discrimination) and GND X2 (calibration) statistics. Discrimination refers to the ability of the model to assign a higher risk to individuals who develop the outcome of interest compared to those who remain free of disease. Calibration is a measure of the accuracy of the predicted risk. We then refit the PCP-HF model (PCP-HFCKD, Supplemental Table 2) using CRIC data-derived coefficients and survival from CRIC Study participants (PCP-HFCKD) using Cox proportional hazard models and assessed discrimination and calibration of the PCP-HFCKD model.
We next evaluated the performance of risk equations for predicting incident hospitalized HF with the inclusion of two additional CKD-specific laboratory measures (eGFR and UACR; Supplemental Table 3). We assessed the discrimination and calibration of the PCP-HFCKD as described above. We examined the ability of the CKD laboratory measures to reclassify participants based on PCP-HFCKD using a continuous net reclassification improvement (NRI) statistic.(21, 22) We also used the integrated discrimination improvement (IDI) index to test improvement in model performance with the addition of eGFR and UACR. The IDI estimate is explained as the amount of separation between the mean predicted probabilities for HF events and non-events.(23) We performed an external validation study of the refit PCP-HFCKD with and without eGFR and UACR in the MESA cohort and examined 10-year HF prediction using C-statistic, NRI, and IDI as described above. Lastly, in sensitivity analyses, we re-ran our refit PCP-HFCKD in CRIC Study participants after excluding 98 individuals with incident HF events after end stage renal disease (ESRD) onset. We confirmed that there was no violation of the proportional hazards assumption by testing the interaction between time and risk predictors (P>0.05 for all).
All statistical analyses were performed with the use of SAS statistical software version 9.4 (SAS institute) and R version 3.4. A statistically significant threshold of a P value less than 0.05 with 2-sided tests were used.
Results
Among the 2328 CRIC Study participants at baseline, the mean (SD) age was 56.8 (10.8) years and ~90% of the population were being treated for hypertension (Table 1). Mean (SD) eGFR was 46 (15) ml/min/1.73m2 and median UACR (interquartile range [IQR]) was 37 (7, 381) mg/g. In Black men and women, 92% and 94% and 25% and 29% were undergoing hypertension and diabetes treatment. In White men and women, 90% and 80% and 25% and 20% were undergoing hypertension and diabetes treatment (Table 1). Median UACR (IQR) in Black men and women was 86 (13, 452) mg/g and 32 (7, 352) mg/g, respectively. Median UACR (IQR) in White men and women was 50 (7, 467) mg/g and 17 (6, 199) mg/g, respectively. Mean (SD) eGFR in individuals with and without an incident HF hospitalization was 40 (14) and 47 (15), respectively. Median UACR (IQR) in individuals with and without an incident HF hospitalization was 288 (33, 1358) mg /g and 27 (6, 278) mg/g, respectively. (Supplemental Table 4).
Table 1.
Baseline demographics of the eligible participants from the Chronic Renal Insufficiency Cohort Study, overall and stratified by sex-race
| Overall | Black | White | |||
|---|---|---|---|---|---|
| N=2328 | Men N=442 | Women N=491 | Men N=761 | Women N=634 | |
| Mean age, years (SD) | 56.8 (10.8) | 56.4 (10.7) | 57.5 (10.2) | 56.8 (10.8) | 56.6 (11.2) |
| Diabetes treatment, n (%) | 566 (24) | 109 (25) | 142 (29) | 188 (25) | 127 (20) |
| Mean fasting glucose, mg/dL (SD) | 111 (49) | 115 (59) | 113 (50) | 111 (45) | 109 (46) |
| Current smoking, n (%) | 281 (12) | 77 (17) | 87 (18) | 68 (9) | 49 (8) |
| Mean systolic blood pressure, mm Hg (SD) | 127 (21) | 130 (21) | 131 (21) | 126 (20) | 123 (20) |
| Hypertension treatment, n (%) | 2049 (89) | 406 (92) | 460 (94) | 682 (90) | 501 (80) |
| Mean total cholesterol, mg/dL (SD) | 188 (43) | 183 (43) | 193 (42) | 181 (43) | 196 (44) |
| Mean HDL cholesterol, mg/dL (SD) | 49 (16) | 46 (14) | 54 (18) | 42 (12) | 55 (17) |
| Mean BMI, kg/m2 (SD) | 31.9 (7.8) | 31.5 (6.2) | 35.1 (9.5) | 30.5 (6.0) | 31.5 (8.6) |
| Mean eGFR CKD-EPI, ml/min/1.73m2 (SD) | 46 (15) | 46 (15) | 45 (15) | 46 (15) | 47 (17) |
| Median UACR, mg/g (IQR) | 37 (7, 381) | 86 (13, 452) | 32 (7, 352) | 50 (7, 467) | 17 (6, 199) |
Abbreviations: SD, standard deviation; HDL, high density lipoprotein; BMI, body mass index; eGFR, estimated glomerular filtration rate; UACR, urine albumin-to-creatinine ratio; IQR, interquartile range
PCP-HF in CKD population
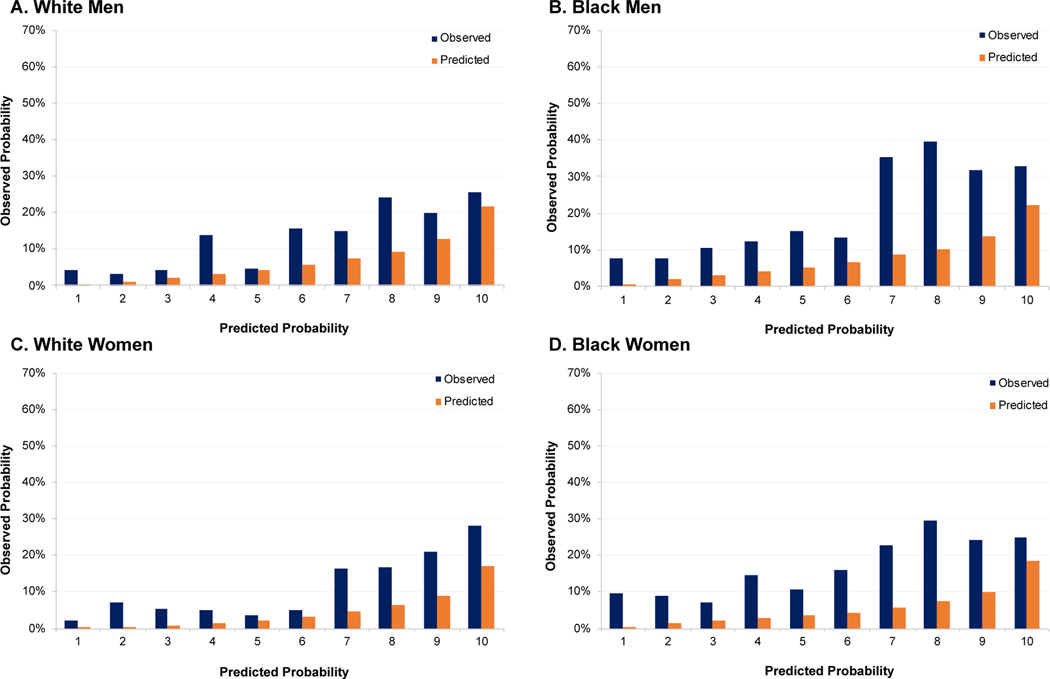
Over a mean follow-up of 9.5 years, 340 incident hospitalized HF events occurred in CRIC Study participants, of which 98 occurred after the onset of end-stage renal disease. The PCP-HF model had generally inadequate discrimination and calibration for HF risk prediction in the CRIC Study sample (Table 2, Figure 1). Among Black men and women, and White men and women in the CRIC Study population, the C-statistics (95% Confidence Interval [CI]) in each sex-race group were 0.66 (95% CI: 0.61 to 0.72), 0.64 (95% CI: 0.58 to 0.70), 0.67 (95% CI: 0.62 to 0.73), and 0.71 (95% CI: 0.64 to 0.78), respectively. There was consistent under-estimation of event rates, as expected in this high-risk sample, and the GND chi-square statistic p-value was < 0.05 for all, indicating insufficient calibration.
Table 2.
Numbers of events, discrimination and calibration statistics of the original and refit 10-year PCP-HF models in the Chronic Renal Insufficiency Cohort Study
| Black | White | |||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| Total N | 442 | 491 | 761 | 634 |
| Events | 88 | 85 | 97 | 70 |
| PCP-HF Model | ||||
| C statistics (95% CI) | 0.66 (0.61, 0.72) | 0.64 (0.58, 0.70) | 0.67 (0.62, 0.73) | 0.71 (0.64, 0.78) |
| GND Chi-square, P value | 39.9 (p=0.001) | 40.3 (p=0.001) | 29.5 (p=0.001) | 26.7 (p=0.001) |
| Refit PCP-HFCKD Model | ||||
| C statistics (95% CI) | 0.76 (0.71, 0.81) | 0.61 (0.54, 0.68) | 0.75 (0.70, 0.80) | 0.78 (0.73, 0.83) |
| GND Chi-square, P-value | 11.5 (p=0.12) | 4.7 (p=0.86) | 7.7 (p=0.46) | 8.2 (p=0.31) |
Abbreviations: PCP-HF, Pooled Cohort equations to Prevent Heart Failure; PCP-HFCKD, Pooled Cohort equations to Prevent Heart Failure Chronic Kidney Disease; CI, confidence interval
Figure 1.
Sex- and race specific observed (blue) and predicted (orange) mean 10-year predicted risk of incident HF hospitalizations by decile of predicted risk applying the original PCP-HFCKD without QRS duration in the Chronic Renal Insufficiency Cohort Study
Abbreviations: HF, heart failure: PCP-HFCKD, Pooled Cohort equations to Prevent Heart Failure Chronic Kidney Disease
Development of PCP-HFCKD Model
The refit PCP-HFCKD had acceptable discrimination, except in Black women. The C-statistics (95% CI) among Black men and women and White men and women for the PCP-HFCKD model were 0.76 (95% CI: 0.71 to 0.81), 0.61 (95% CI: 0.54 to 0.68), 0.75 (95% CI: 0.70 to 0.80), and 0.78 (95% CI: 0.73 to 0.83), respectively (Table 2). The PCP-HFCKD risk equation had good calibration for all sex-race subgroups in the CRIC Study sample as assessed by the GND chi-square statistic (p > 0.05 for all, Table 2).
PCP-HFCKD with the addition of eGFR and UACR
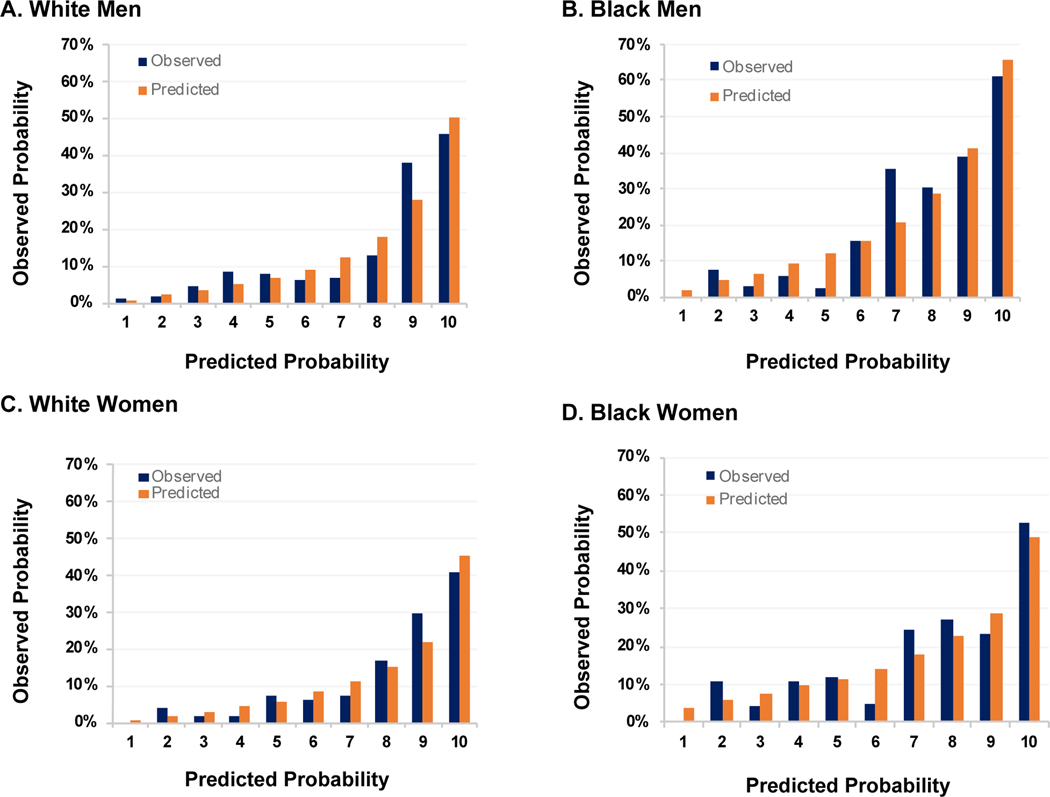
When UACR was added to the PCP-HFCKD model, the model performance improved significantly in all groups. The PCP-HFCKD + UACR model had acceptable to excellent discrimination and calibration (Table 3, Figure 2). The C-statistic ranged from 0.73 – 0.81 with GND Chi-square p values >0.05 for all sex and race groups (Table 3). The change in C-statistic (95% CI) among Black men and women, and White men and women for the PCP-HFCKD + UACR model were 0.04 (p value 0.02) and 0.11 (p value = 0.02), and 0.04 (p value = 0.01), 0.03 (p value = 0.11), respectively (Table 3). The continuous NRI ranged from 0.44 – 0.61 with p values <0.01 across all groups. The IDI was also significant across all groups (p value < 0.01) (Table 3). The addition of eGFR to the PCP-HFCKD also improved model performance for some, but not all performance measurements (Table 3). The addition of both UACR and eGFR to the PCP-HFCKD did not provide substantive improvement over the addition of UACR alone (Table 3).
Table 3.
C-statistic change, NRI, and IDI of data-derived 10-year PCP-HFCKD equation with addition of eGFR and UACR in the Chronic Renal Insufficiency Cohort Study
| C-statistic PCP-HFCKD (95% CI) | C-statistics PCP-HFCKD + renal marker (95% CI) | Delta C-Statistic (p-value) | NRI (p-value) | IDI (p-value) | |
|---|---|---|---|---|---|
| UACR | |||||
| Black Men | 0.76 (0.71, 0.81) | 0.80 (0.75, 0.85) | 0.04 (p = 0.02) | 0.44 (p <0.01) | 0.04 (p <0.01) |
| Black Women | 0.61 (0.54, 0.68) | 0.73 (0.67, 0.79) | 0.11 (p = 0.02) | 0.51 (p < 0.01) | 0.07 (p <0.01) |
| White Men | 0.75 (0.70, 0.80) | 0.79 (0.74, 0.84) | 0.04 (p = 0.01) | 0.61 (p < 0.01) | 0.07 (p <0.01) |
| White Women | 0.78 (0.73, 0.83) | 0.81 (0.76, 0.86) | 0.03 (p = 0.11) | 0.44 (p < 0.01) | 0.04 (p < 0.01) |
| eGFR | |||||
| Black Men | 0.76 (0.71, 0.81) | 0.77 (0.72, 0.83) | 0.01 (p = 0.30) | 0.27 (p = 0.03) | 0.02 (p = 0.09) |
| Black Women | 0.61 (0.54, 0.68) | 0.72 (0.66, 0.78) | 0.11 (p = 0.02) | 0.35 (p < 0.01) | 0.05 (p < 0.01) |
| White Men | 0.75 (0.70, 0.80) | 0.77 (0.72, 0.81) | 0.02 (p = 0.15) | 0.31 (p < .01) | 0.02 (p = 0.03) |
| White Women | 0.78 (0.73, 0.83) | 0.79 (0.75, 0.84) | 0.01 (p = 0.35) | 0.36 (p < 0.01) | 0.01 (p = 0.14) |
| UACR + eGFR | |||||
| Black Men | 0.76 (0.71, 0.81) | 0.80 (0.75, 0.85) | 0.04 (P = 0.02) | 0.35 (p < 0.01) | 0.04 (p < 0.01) |
| Black Women | 0.61 (0.54, 0.68) | 0.75 (0.69, 0.81) | 0.14 (P <0.01) | 0.48 (p < 0.01) | 0.07 (p < 0.01) |
| White Men | 0.75 (0.70, 0.80) | 0.80 (0.75, 0.84) | 0.05 (P < 0.01) | 0.64 (p < 0.01) | 0.07 (p < 0.01) |
| White Women | 0.78 (0.73, 0.83) | 0.81 (0.77, 0.86) | 0.03 (P = 0.11) | 0.46 (p < 0 .01) | 0.04 (p < 0.01) |
Abbreviations: PCP-HFCKD, Pooled Cohort equations to Prevent Heart Failure Chronic Kidney Disease; UACR, urine albumin-to-creatinine ratio; eGFR, estimated glomerular filtration rate; CI, confidence interval
Figure 2.
Sex- and race specific observed (blue) and predicted (orange) mean 10-year predicted risk of incident HF hospitalizations by decile of predicted risk applying the refit PCP-HFCKD incorporating urine albumin-to-creatinine ratio in the Chronic Renal Insufficiency Cohort Study
Abbreviations: HF, heart failure: PCP-HFCKD, Pooled Cohort equations to Prevent Heart Failure Chronic Kidney Disease
External Validation of the PCP-HFCKD in MESA
Over mean follow-up of 15.6 years, 56 incident hospitalized HF events occurred in MESA study participants. Baseline characteristics of the MESA participants with CKD are shown in Supplemental Table 5. External validation of the PCP-HFCKD to predict 10-year risk of heart failure with and without eGFR and UACR demonstrated good discrimination (C-statistic 0.73–0.76) and calibration (GND chi-square statistic (p > 0.05 for all; Supplemental Table 6, Supplemental Figure 2).
Sensitivity Analyses
In sensitivity analyses, we re-ran our refit PCP-HFCKD after excluding individuals with HF events after ESRD onset. Over a mean follow-up of 9.6 years, 242 incident hospitalized HF events occurred. Results remained qualitatively similar. After exclusion, the C-statistics (95% CI) among Black men and women and White men and women for the PCP-HFCKD model were 0.78 (95% CI: 0.73 to 0.84), 0.62 (95% CI: 0.54 to 0.71), 0.77 (95% CI: 0.71 to 0.83), and 0.79 (95% CI: 0.73 to 0.85), respectively (Supplemental Table 7). The PCP-HFCKD risk equation remained well calibrated for all sex-race subgroups (GND chi-square statistic p > 0.05 for all, Supplemental Table 7).
Discussion
In over 2300 participants with moderate to severe CKD, we investigated the performance of the PCP-HF risk prediction tool, which includes routinely available clinical variables, and was derived and previously validated in general populations without CKD. Whereas the original PCP-HF equations did not perform well in a CKD-specific population, as expected, the refit PCP-HFCKD model performance improved except in Black women. Importantly, the addition specifically of UACR to the PCP-HFCKD model significantly improved performance in all sex-race groups and performed well in an external validation cohort of participants with CKD from MESA. These data suggest that commonly available clinical data, which should include measurement of albuminuria, can reliably identify individuals at highest risk for HF hospitalizations in patients with CKD.
There are multiple explanations for the poor performance of the original PCP-HF risk prediction model in the CRIC Study cohort. The original PCP-HF model was derived largely in community-based study samples, whereas the CRIC Study recruited a majority referred population undergoing subspecialty care specifically for management of CKD. Application of general population-derived risk scores to higher risk samples frequently demonstrates poorer discrimination and especially poorer calibration with systemic under-estimation of event rates.(24) There are multiple unique mechanisms known to place patients with CKD at high risk for CVD, particularly HF.(1, 2, 25–27) Pathophysiologic mechanisms that include hemodynamic alterations, upregulation of the renin-angiotensin-aldosterone system, chronic systemic inflammation, disordered mineral metabolism, and disturbances in the iron-anemia axis in CKD can all contribute to structural and functional myocardial changes that precede HF development.(1, 28–32) Although the PCP-HF equation took into account hypertension and diabetes, two important risk factors for CKD development and progression, other variables that characterize the severity of CKD, such as eGFR and UACR, were not represented in the original PCP-HF model. Taken together, the risk-enhancing features of CKD for HF in a real world sample led to under-estimation of HF hospitalization risk by the general PCP-HF equations.
Although levels of albuminuria were relatively low at baseline in the CRIC Study, inclusion of UACR added significant value to the refit PCP-HFCKD model that had already been optimized for the CKD population of CRIC Study. Numerous prior studies show that UACR is strongly associated with CVD in patients with CKD, even more so than eGFR.(33–37) UACR is consistently shown to be associated with CVD and all-cause mortality, even for differences within levels below 30–300 mg/g of albuminuria.(34) These data are consistent with the observed improvement in performance of the refit PCP-HFCKD model when UACR was added to the risk equation. The pathophysiologic mechanisms that result in the development of albuminuria may be the similar to the pathways that contribute to HF development. For example, systemic vascular endothelial dysfunction may result in increased glomerular permeability and albuminuria, as well as myocardial remodeling and HF.(38, 39) Chronic systemic inflammation in CKD may also result in albuminuria and myocardial dysfunction, predisposing patients to HF. UACR may also be simply be a biomarker for more severe underlying glomerular pathology. However, the absence of consistent improvement in model performance with the addition of eGFR makes this as the sole explanation less likely and may reflect that lower eGFR and higher UACR may represent different physiologic processes. Recent data suggest underutilization of UACR testing, despite guideline recommendations.(40) Our results signify the importance of measuring albuminuria in patients with CKD, who are at a significant risk for HF events. Early CKD may not present to nephrologists, but other providers. Given the impact of new therapies on CKD and HF outcomes, recognizing the propensity to develop heart failure in the presence of underlying kidney disease, even if UACR is minimal or kidney disease is not severe, remains of clinical importance to all providers.
Although we were able to refit and validate the previously derived and validated sex- and race-specific PCP-HF risk prediction model in a well-established cohort of individuals with CKD with well-adjudicated outcomes, we acknowledge certain limitations. The use of IDI and NRI to assess model performance have inherent limitations.(41) However, our re-fit model was well calibrated and we used multiple methods to test for model performance with the addition of kidney-specific markers. Analyses were restricted to individuals self-identified as Black and White and required the availability of all variables included in the risk prediction model, which limits the generalizability of the modified PCP-HFCKD risk prediction model to this patient population. Race and sex stratification were done to compare our results to the original PCP-HF derivation and validation studies that also used race and sex stratification based on self-report.(4) However, we acknowledge that self-reported race is a social construct and race-specific models were derived instead of using race as a covariate. Future models that better delineate social determinants of health that race is reflecting (e.g., structural and systemic racism) in risk prediction are needed. Despite excluding individuals with known clinical CVD at baseline, we did not exclude individuals with subclinical CVD in the CRIC cohort (structural cardiac or vascular changes [Stage B] who do not yet have overt clinical HF [Stage C]). Although a limitation, this makes our findings more applicable to real-world scenarios where providers may not have assessment of cardiac structure. Using HF risk equations may identify high-risk individuals who would benefit from further risk stratification with echocardiography. We defined HF events as both definite and probable events in both the CRIC Study and MESA. We also did not delineate subtypes of HF such as preserved versus mid-range or reduced ejection fraction HF, but predicting risk of each subtype may not have additive clinical value as current preventive measures are similar for both subtypes.(42) We also likely underestimated HF risk given that we only included adjudicated hospitalized events and many patients with CKD may have been diagnosed with HF as outpatients without hospitalization, or may have had subclinical heart failure with preserved ejection fraction.(43) Finally, our validation cohort in MESA among those with CKD was a relatively small sample and validation in larger CKD cohorts should be completed in the future.
Conclusions
We demonstrate that the refit and modified PCP-HFCKD risk prediction equations can reliably predict incident HF hospitalization events in patients with CKD. Risk stratification of individuals with CKD for HF may lead to improvement in patient outcomes by identifying patients appropriate for more frequent monitoring, additional risk stratification with non-invasive imaging, and earlier therapeutic interventions. HF risk stratification would also allow enrichment of clinical trials to include individuals with CKD at highest risk for HF who may benefit most from emerging therapeutics. Implementation of the modified PCP-HFCKD risk prediction equation, which includes UACR, to identify individuals with CKD at highest risk for HF hospitalization has the potential to change both clinical and research practices and improve outcomes in the CKD population.
Supplementary Material
Central Illustration.
10 Year Risk Prediction Equations for Incident Heart Failure Hospitalization in Chronic Kidney Disease.
Highlights.
Previously developed risk prediction equations for heart failure that use routinely available clinical parameters in the general population perform poorly in a population with chronic kidney disease stages 2–4 who are at high risk for heart failure.
Addition of albuminuria, but not estimated glomerular filtration rate, to risk prediction equations improve model performance in patients with chronic kidney disease as assessed by discrimination and calibration statistics.
Routinely available clinical data that includes albuminuria in patients with chronic kidney disease can reliably identify individuals at risk of HF hospitalizations.
Acknowledgements
The authors thank the participants, investigators, and staff of the CRIC Study and MESA for their time and commitment. CRIC Study Investigators include Lawrence J. Appel, MD, MPH, Harold I. Feldman, MD, MSCE, Alan S. Go, MD, Jiang He, MD, PhD, Robert G. Nelson, MD, PhD, MS, Panduranga S. Rao, MD, Vallabh O Shah, PhD, MS, Mark L. Unruh, MD, MS. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding
This study was supported by grants P30DK114857, R01DK110087 (TI), K24HL150235 (TI), K23DK120811 (AS), K23HL150236 (RM), and the American Heart Association grant 9TPA34890060 (SSK). Research was also supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number KL2TR001424 (SSK and RM). Research reported in this publication was also supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422 (SSK, RM, and DLJ). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1TR000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131. MESA was supported by contracts 75N92020D00001, HHSN268201500003I, N01- HC- 95159, 75N92020D00005, N01- HC- 95160, 75N92020D00002, N01- HC- 95161, 75N92020D00003, N01- HC- 95162, 75N92020D00006, N01- HC- 95163, 75N92020D00004, N01- HC- 95164, 75N92020D00007, N01- HC- 95165, N01- HC- 95166, N01- HC- 95167, N01- HC- 95168, and N01- HC- 95169 from the NHLBI, and by grants UL1- TR- 000040, UL1- TR- 001079, and UL1- TR- 001420 from NCATS
Abbreviations
- CKD
chronic kidney disease
- HF
heart failure
- CVD
cardiovascular disease
- ACC/AHA
American College of Cardiology/American Heart Association
- PCP-HF
Pooled Cohort Equations to Prevent Heart Failure
- SGLT2i
sodium glucose co-transporter 2 inhibitors
- CRIC
Chronic Renal Insufficiency Cohort
- eGFR
estimated glomerular filtration rate
- MESA
Multi-Ethnic Study of Atherosclerosis
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- SD
standard deviation
- IQR
interquartile ranges
- TC
total cholesterol
- GND
Greenwood-Nam-D’Agostino
- NRI
net reclassification improvement
- IDI
integrated discrimination improvement
- ESRD
end stage renal disease
- BMI
body mass index
- CI
confidence interval
Footnotes
Disclosures
RM has interest in AbbVie, Inc. and Teva Pharmaceuticals Industries Ltd and consultant/honoraria fees from Akebia/Oksuba and AstraZeneca. TI has received honoraria from Akebia Therapeutics, Inc. AS received honoraria from Horizon Pharma, PLC and AstraZeneca, and consulting fees from CVS Caremark. SS has received research funding from Roche Diagnostics, Inc and is the co-owner of a US patent “Methods for Assessing Differential Risk for Developing Heart Failure” (Patent No 10,509,044).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tuegel C, Bansal N. Heart failure in patients with kidney disease. Heart. 2017;103(23):1848–53. [DOI] [PubMed] [Google Scholar]
- 2.Bansal N, Zelnick L, Bhat Z, Dobre M, He J, Lash J, et al. Burden and Outcomes of Heart Failure Hospitalizations in Adults With Chronic Kidney Disease. Journal of the American College of Cardiology. 2019;73(21):2691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136(6):e137–e61. [DOI] [PubMed] [Google Scholar]
- 4.Khan SS, Ning H, Shah SJ, Yancy CW, Carnethon M, Berry JD, et al. 10-Year Risk Equations for Incident Heart Failure in the General Population. Journal of the American College of Cardiology. 2019;73(19):2388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segar MW, Jaeger BC, Patel KV, Nambi V, Ndumele CE, Correa A, et al. Development and Validation of Machine Learning-Based Race-Specific Models to Predict 10-Year Risk of Heart Failure: A Multi-Cohort Analysis. Circulation. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, Garcia M, et al. Incident heart failure prediction in the elderly: the health ABC heart failure score. Circulation Heart failure. 2008;1(2):125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal SK, Chambless LE, Ballantyne CM, Astor B, Bertoni AG, Chang PP, et al. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation Heart failure. 2012;5(4):422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bavishi A, Bruce M, Ning H, Freaney PM, Glynn P, Ahmad FS, et al. Predictive Accuracy of Heart Failure-Specific Risk Equations in an Electronic Health Record-Based Cohort. Circulation Heart failure. 2020;13(11):e007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adler ED, Voors AA, Klein L, Macheret F, Braun OO, Urey MA, et al. Improving risk prediction in heart failure using machine learning. European journal of heart failure. 2020;22(1):139–47. [DOI] [PubMed] [Google Scholar]
- 10.Feldman HI. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. Journal of the American Society of Nephrology : JASN. 2003;14(90002):148S–53. [DOI] [PubMed] [Google Scholar]
- 11.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. American journal of epidemiology. 2002;156(9):871–81. [DOI] [PubMed] [Google Scholar]
- 12.Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, et al. Fibroblast growth factor-23 and cardiovascular events in CKD. Journal of the American Society of Nephrology : JASN. 2014;25(2):349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clinical journal of the American Society of Nephrology : CJASN. 2009;4(8):1302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–12. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chahal H, Bluemke DA, Wu CO, McClelland R, Liu K, Shea SJ, et al. Heart failure risk prediction in the Multi-Ethnic Study of Atherosclerosis. Heart. 2015;101(1):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Statistics in medicine. 2011;30(10):1105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Agostino RB, Nam BH. Evaluation of the performance of survival analysis models: discrimination and calibration measures. In: Balakrishnan N, editor. Handbook of Statistics. New York, NY: Elsevier; 2004. p. 1–25. [Google Scholar]
- 18.Demler OV, Paynter NP, Cook NR. Tests of calibration and goodness-of-fit in the survival setting. Statistics in medicine. 2015;34(10):1659–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–73. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd-Jones DM. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation. 2010;121(15):1768–77. [DOI] [PubMed] [Google Scholar]
- 21.Pencina MJ, D’Agostino RB, D’Agostino RB Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in medicine. 2008;27(2):157–72; discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 22.Pencina MJ, D’Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pencina MJ, D’Agostino RB, Pencina KM, Janssens AC, Greenland P. Interpreting incremental value of markers added to risk prediction models. American journal of epidemiology. 2012;176(6):473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lloyd-Jones DM, Braun LT, Ndumele CE, Smith SC Jr., Sperling LS, Virani SS, et al. Use of Risk Assessment Tools to Guide Decision-Making in the Primary Prevention of Atherosclerotic Cardiovascular Disease: A Special Report From the American Heart Association and American College of Cardiology. Journal of the American College of Cardiology. 2019;73(24):3153–67. [DOI] [PubMed] [Google Scholar]
- 25.Colantonio LD, Baber U, Banach M, Tanner RM, Warnock DG, Gutierrez OM, et al. Contrasting Cholesterol Management Guidelines for Adults with CKD. J Am Soc Nephrol. 2015;26(5):1173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305. [DOI] [PubMed] [Google Scholar]
- 27.Bansal N, Katz R, Robinson-Cohen C, Odden MC, Dalrymple L, Shlipak MG, et al. Absolute Rates of Heart Failure, Coronary Heart Disease, and Stroke in Chronic Kidney Disease: An Analysis of 3 Community-Based Cohort Studies. JAMA cardiology. 2017;2(3):314–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segall L, Nistor I, Covic A. Heart failure in patients with chronic kidney disease: a systematic integrative review. BioMed research international. 2014;2014:937398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004;109(8):1004–9. [DOI] [PubMed] [Google Scholar]
- 30.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol. 2012;60(3):200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fishbane S, Miyawaki N. Anemia treatment in chronic kidney disease accompanied by diabetes mellitus or congestive heart failure. Kidney Int. 2010;77(3):175–7. [DOI] [PubMed] [Google Scholar]
- 32.Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, Yanucil C, et al. Activation of Cardiac Fibroblast Growth Factor Receptor 4 Causes Left Ventricular Hypertrophy. Cell metabolism. 2015;22(6):1020–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA : the journal of the American Medical Association. 2001;286(4):421–6. [DOI] [PubMed] [Google Scholar]
- 34.Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, Jensen G, Clausen P, Scharling H, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110(1):32–5. [DOI] [PubMed] [Google Scholar]
- 35.Arnlöv J, Evans JC, Meigs JB, Wang TJ, Fox CS, Levy D, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112(7):969–75. [DOI] [PubMed] [Google Scholar]
- 36.Patel RB, Colangelo LA, Reis JP, Lima JAC, Shah SJ, Lloyd-Jones DM. Association of Longitudinal Trajectory of Albuminuria in Young Adulthood With Myocardial Structure and Function in Later Life: Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAMA Cardiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandsmark DK, Messe SR, Zhang X, Roy J, Nessel L, Lee Hamm L, et al. Proteinuria, but Not eGFR, Predicts Stroke Risk in Chronic Kidney Disease: Chronic Renal Insufficiency Cohort Study. Stroke; a journal of cerebral circulation. 2015;46(8):2075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weir MR. Microalbuminuria and cardiovascular disease. Clin J Am Soc Nephro. 2007;2(3):581–90. [DOI] [PubMed] [Google Scholar]
- 39.Patel RB, Colangelo LA, Reis JP, Lima JAC, Shah SJ, Lloyd-Jones DM. Association of Longitudinal Trajectory of Albuminuria in Young Adulthood With Myocardial Structure and Function in Later Life: Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAMA cardiology. 2020;5(2):184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Folkerts K, Petruski-Ivleva N, Comerford E, Blankenburg M, Evers T, Gay A, et al. Adherence to Chronic Kidney Disease Screening Guidelines Among Patients With Type 2 Diabetes in a US Administrative Claims Database. Mayo Clinic proceedings. 2021;96(4):975–86. [DOI] [PubMed] [Google Scholar]
- 41.Hilden J, Gerds TA. A note on the evaluation of novel biomarkers: do not rely on integrated discrimination improvement and net reclassification index. Statistics in medicine. 2014;33(19):3405–14. [DOI] [PubMed] [Google Scholar]
- 42.Schocken DD, Benjamin EJ, Fonarow GC, Krumholz HM, Levy D, Mensah GA, et al. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117(19):2544–65. [DOI] [PubMed] [Google Scholar]
- 43.Goyal A, Norton CR, Thomas TN, Davis RL, Butler J, Ashok V, et al. Predictors of incident heart failure in a large insured population: a one million person-year follow-up study. Circulation Heart failure. 2010;3(6):698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.