Abstract
The sperm chromatin structure assay (SCSA) is crucial for assessing male fertility. However, the predictive value of the SCSA parameters, including the DNA fragment indices (DFI) and the percentages of high DNA stainability (HDS), for outcomes of artificial insemination by husband (AIH) remains controversial. This study aims to evaluate the correlations between SCSA parameters and male aging as well as other routine semen parameters, and explore their prognostic powers on AIH outcomes of the Chinese infertile couples. A total of 809 AIH cycles were retrospectively analyzed. The results showed that DFI in the age groups < 35 years were significantly lower than that in the age groups ≥ 35 years (P < 0.001). Meanwhile, there was no statistical difference in HDS between the age groups (P = 0.063). DFI and HDS are negatively correlated with most routine semen parameters (all P < 0.05). The chi-square and generalized linear model tests indicated that neither DFI nor HDS influenced the clinical pregnancy rate of AIH. In summary, this study found that aging is a critical factor leading to increased sperm DFI but not HDS. DFI and HDS are negatively correlated with most semen parameters but do not significantly influence AIH outcomes.
Keywords: sperm chromatin structure assay, spermatozoon DNA fragmentation index, high DNA stainability, male age, artificial insemination by husband
INTRODUCTION
Sperm DNA is the carrier of paternal genetic materials, and its integrity is associated with sperm fertilization capacity and embryo development potential [1, 2]. The sperm DNA integrity could be gauged by DNA fragmentation index (DFI) as well as high DNA stainability (HDS) via sperm chromatin structure assay (SCSA). However, the correlation between SCSA parameters and routine sperm parameters is controversial. Some studies demonstrated that the routine sperm parameters and DNA damage are complementary, rather than strongly linked [3]. In contrast, some studies noted no clear correlation between DNA fragments and some routine semen parameters [4, 5]. Interestingly, increasing evidence indicated that male age harms sperm DNA integrity [6–8].
The value of SCSA parameters in predicting assisted reproduction treatments (ART) outcomes also remains controversial. On one hand, some studies indicated that high sperm DFI could lead to a reduced clinical pregnancy rate (CPR) of intrauterine insemination (IUI) [9]. Nevertheless, some studies suggested that the sperm DFI had no significant predictive value for ART outcomes [10, 11]. For cases with excessively high sperm DFI, doctors often have concerns about the harmful effects induced by high DFI, such as miscarriages, and suggest treatments of in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) other than IUI [12].
To address these issues, this study retrospectively analyzed the correlation between SCSA parameters and male age as well as routine sperm parameters of 809 cycles receiving artificial insemination by husband (AIH), and explore its influence and predictive power on the AIH outcomes.
RESULTS
Comparison of SCSA parameters among different male age groups
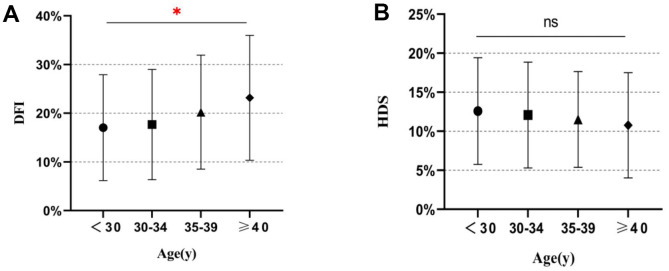
Correlation analysis by Spearman correlation coefficients showed that the Total DFI, High DFI, and Low DFI were positively correlated with male age, while HDS has no such correlation (Table 1 and Figure 1). Furthermore, Total DFI and Low DFI in the two groups of age < 35 years were lower than those in the groups of age ≥ 35 years significantly (all P < 0.001). Besides, the High DFI of the group of age ≥ 40 years was significantly higher than those of other age groups, except the group of age 35-39 years (P < 0.001). However, regarding HDS, there was no statistically significant variation among the age groups (P = 0.063).
Table 1. Comparison of SCSA parameters in different male age groups.
| <30 | 30-34 | 35-39 | ≥40 | p-value | |
| Total DFI | 17.08±10.88 | 17.71±11.33 | 20.27±11.72 | 23.34±12.85 | <0.001 |
| High DFI | 5.76±7.30 | 6.01±5.53 | 6.42±4.60 | 7.79±5.20 | <0.001 |
| Low DFI | 11.82±6.31 | 11.70±7.09 | 13.85±8.79 | 15.55±8.79 | <0.001 |
| HDS | 12.63±6.84 | 12.11±6.78 | 11.55±6.13 | 10.83±6.78 | 0.063 |
Abbreviations: DFI, DNA fragmentation index; HDS, high DNA stainability; Values are presented as mean ± standard deviation.
Figure 1.
Distribution of SCSA parameters in different age groups. (A) DNA fragment index (DFI) values for different age groups. (B) High DNA stainability (HDS) values for different age groups. *, P < 0.05; ns, not significant.
Correlation of SCSA parameters with the other semen parameters
Spearman correlation coefficients analysis between SCSA parameters and semen parameters showed that DFI parameters, but not HDS, were positively associated with semen volume and negatively correlated with semen pH. SCSA parameters are negatively correlated with most routine semen parameters pre- and post-processing, and sperm motility and kinetic parameters (Tables 2, 3). Regarding sperm morphology, the SCSA parameters showed positive correlations with most sperm morphological parameters, including sperm deformity index (SDI), teratozoospermia index (TZI), sperm headpiece deformity rate (H%), sperm middle piece deformity rate (M%), sperm principal piece deformity (P%), sperm abnormal form rate and sperm head area. The sperm head elongation was only relevant to High DFI (Table 4).
Table 2. Correlation between SCSA parameters and semen parameters pre- and post-processing.
| Variables | Total DFI | High DFI | Low DFI | HDS |
| Volume | 0.095** | 0.082* | 0.089*** | NS |
| pH | -0.193*** | -0.13*** | -0.203*** | NS |
| Pre-TMSC | -0.162*** | -0.243*** | -0.098** | -0.237*** |
| Pre-concentration | -0.101** | -0.186*** | NS | -0.264*** |
| Pre-motility | -0.401*** | -0.44*** | -0.327*** | -0.21*** |
| Pre-PR | -0.402*** | -0.432*** | -0.335*** | -0.188*** |
| Post-TMSC | -0.204*** | -0.275*** | -0.140*** | -0.222*** |
| Post-concentration | -0.187*** | -0.259*** | -0.126*** | -0.222*** |
| Post-motility | -0.331*** | -0.363*** | -0.266*** | -0.136*** |
| Post-PR | -0.318*** | -0.345*** | -0.259*** | -0.122*** |
Abbreviations: DFI, DNA fragmentation index; HDS, high DNA stainability; TMSC, total motile sperm count; PR, progressive motility. NS, not significant; * P < 0.05, ** P < 0.01, *** P < 0.001.
Table 3. Correlation between SCSA parameters and sperm motility parameters.
| Variables | Total DFI | High DFI | Low DFI | HDS |
| ALH | -0.343*** | -0.377*** | -0.278*** | -0.168*** |
| VCL | -0.331*** | -0.366*** | -0.266*** | -0.162*** |
| VSL | -0.353*** | -0.383*** | -0.290*** | -0.149*** |
| VAP | -0.357*** | -0.389*** | -0.292*** | -0.166*** |
| LIN | -0.396*** | -0.426*** | -0.328*** | -0.180*** |
| STR | -0.377*** | -0.408*** | -0.310*** | -0.179*** |
| BCF | -0.384*** | -0.433*** | -0.304*** | -0.225*** |
Abbreviations: DFI, DNA fragmentation index; HDS, high DNA stainability; TPMSC, total progressed motile sperm count; VCL, curvilinear velocity; VSL, straight-line velocity; VAP, average pathway velocity; LIN, linearity of movement; STR, straightness; BCF, beat cross frequency; ALH, amplitude of lateral head displacement. NS, not significant; * P < 0.05, ** P<0.01, *** P < 0.001.
Table 4. Correlation between SCSA parameters and sperm morphology parameters.
| Variables | Total DFI | High DFI | Low DFI | HDS |
| SDI | 0.272*** | 0.311*** | 0.209*** | 0.204*** |
| TZI | 0.153*** | 0.178*** | 0.117*** | 0.113** |
| Abnormal forms | 0.314*** | 0.368*** | 0.234*** | 0.261*** |
| H | 0.304*** | 0.356*** | 0.224*** | 0.243*** |
| M | 0.188*** | 0.198*** | 0.154*** | 0.114*** |
| P | 0.230*** | 0.242*** | 0.186*** | 0.123*** |
| C | NS | NS | NS | NS |
| sperm head area | 0.224*** | 0.306*** | 0.136*** | 0.264*** |
| elongation | NS | 0.083* | NS | NS |
Abbreviations: DFI, DNA fragmentation index; HDS, high DNA stainability; SDI, sperm deformity index; TZI, teratozoospermia index; H, sperm headpiece deformity; M, sperm middle piece deformity; P, sperm principal piece deformity; C, sperm cytoplasm deformity. NS, not significant; * P < 0.05, ** P<0.01, *** P < 0.001.
Male age is significantly different between DFI and HDS subgroups
Also, we have applied the chi-square test to compare the clinical and demographic characteristics of the couples receiving AIH treatment among different DFI and HDS subgroups. The results showed that only male age had statistical differences between different DFI and HDS subgroups (P = 0.007 and P = 0.018, Tables 5, 6).
Table 5. Comparison of the clinical and demographic characteristics of the couples receiving AIH treatment among DFI and HDS subgroups.
| DFI<30% | DFI≥30% | p | HDS<15% | HDS≥15% | p | |
| Cycle treatment options | ||||||
| Natural cycle | 246(35.24%) | 39(35.14%) | 0.982 | 221(34.99%) | 64(32.82%) | 0.419 |
| Stimulated cycle | 452(64.76%) | 72(64.86%) | 393(64.01%) | 131(68.18%) | ||
| The number of IUI cycle | ||||||
| 1 | 435(62.32%) | 69(62.16%) | 0.667 | 382(62.21%) | 122(62.56%) | 0.654 |
| 2 | 196(28.08%) | 34(30.63%) | 172(28.01%) | 58(29.74%) | ||
| ≥3 | 67(9.60%) | 8(7.21%) | 60(9.77%) | 15(7.69%) | ||
| Single/double IUI | ||||||
| single | 639(91.55%) | 99(89.19%) | 0.415 | 564(91.86%) | 174(89.23%) | 0.259 |
| double | 59(8.45%) | 12(10.81%) | 50(8.14%) | 21(10.77) | ||
| Type of infertility | ||||||
| Primary | 433(62.03%) | 72(64.86%) | 0.567 | 388(63.19%) | 117(60.00%) | 0.423 |
| Secondary | 265(37.97%) | 39(35.14%) | 226(36.81%) | 78(40.00%) | ||
| pregnancy rate | ||||||
| Pregnant | 64(9.17%) | 11(9.91%) | 0.860 | 61(9.93%) | 14(7.18%) | 0.262 |
| Non-pregnant | 634(90.83%) | 100(90.09%) | 553(90.07%) | 181(92.82%) |
Abbreviations: DFI, DNA fragmentation index; HDS, high DNA stainability.
Table 6. Comparison of the age characteristics of the couples receiving AIH treatment among DFI and HDS subgroups.
| DFI<30% | DFI≥30% | p | HDS<15% | HDS≥15% | p | |
| female age groups (years) | ||||||
| <30 | 258(36.96%) | 32(28.83%) | 0.129 | 209(34.04%) | 81(41.54%) | 0.156 |
| 30-34 | 298(42.69%) | 50(45.05%) | 276(44.95%) | 72(36.92%) | ||
| 35-39 | 130(18.62%) | 25(22.52%) | 116(18.89%) | 39(20.00%) | ||
| ≥40 | 12(1.72%) | 4(3.60%) | 13(2.12%) | 3(1.54%) | ||
| male age groups (years) | ||||||
| <30 | 172(24.64%) | 17(15.32%) | 0.007 | 129(21.01%) | 60(30.77%) | 0.018 |
| 30-34 | 313(44.84) | 44(39.64%) | 272(44.30%) | 85(43.59%) | ||
| 35-39 | 163(23.35%) | 34(30.63%) | 160(26.06%) | 37(18.97%) | ||
| ≥40 | 50(7.16%) | 16(14.41%) | 53(8.63%) | 13(6.67%) | ||
SCSA parameters and AIH pregnancy outcomes
Furthermore, the chi-square test results showed no statistical difference between AIH clinical pregnancy rates with both DFI ≥ 30% and < 30% groups (χ2 = 0.062, P = 0.860). The AIH clinical pregnancy rate in the HDS ≥ 15% group (7.18%) was lower than that in the HDS < 15% group (9.93%), but not statistically significant (χ2 = 1.336, P = 0.262). Similarly, the generalized linear model test results showed that DFI and HDS did not influence AIH clinical pregnancy rates (Table 7).
Table 7. Comparison of AIH pregnancy rate among DFI and HDS subgroups.
| Total cycle | Pregnant cycle | χ2 | p-value | ||
| DFI | |||||
| <30% | 698 | 64 (9.17%) | 0.062 | 0.860 | |
| ≥30% | 111 | 11 (9.91%) | |||
| HDS | |||||
| <15% | 614 | 61 (9.93%) | 1.336 | 0.262 | |
| ≥15% | 195 | 14 (7.18%) | |||
| generalized linear model | Wald Chi-Squared Test | ||||
| Dependent variable. Pregnant/Non-pregnant | χ2 | p-value | |||
| DFI | 0.01 | 0.942 | |||
| HDS | 1.50 | 0.221 | |||
| DFI & HDS | 0.35 | 0.554 | |||
Abbreviations: DFI, DNA fragmentation index; HDS, high DNA stainability.
DISCUSSION
A strong correlation between sperm DFI/HDS and male age was shown previously. For examples, Guo et al. pointed out that aging, not routine semen parameters, was an essential influencing factor for sperm DNA integrity [13]. Das et al. reported more than twice the odds ratio for sperm DNA instability in aging males compared to young males [6]. Evenson et al. proved that the percentages of sperm HDS in males were positively correlated with age, averagely from 12.2% at age 20-25 years to 7.9% at age 60-65 years [14]. Similarly, another study indicated that men with ages > 45 years had lower sperm HDS [15]. As expected, our results showed that male aging is the leading influencing factor for increased sperm DFI, which was consistent with other reports [16–19]. And our results indicated the proportion of sperm HDS decreased with increasing age, but there was no statistical difference between men's age groups.
Recently, it was reported by Mohammadi et al. that unlike DFI, HDS was hardly associated with the classical conditions of male infertility [20]. However, some studies showed that inflammation on the male genital tract led to high DFI, which is correlated with disturbed sperm DNA integrity, which may hamper successful fertilization and induction of pregnancy [21, 22]. In addition, considerable research efforts have been devoted to revealing that both DFI and HDS were positively associated with semen volume and sperm H%, but negatively associated with sperm concentration, motility and normal form percentage [18, 23–25]. Likewise, our data exhibited that DFI and HDS were significantly associated with most semen parameters, especially sperm motility and normal form percentage, implicating sperm DNA damage as an essential cause for decreased semen quality.
The influence of DFI and HDS on AIH pregnancy outcome and its predictive value were controversial in previous studies. It was reported that men with DFI < 27% and HDS < 10% had significantly higher successful rate of pregnancy following AIH [12]. Another study suggested that DFI could serve as an independent predictor for successful pregnancy following AIH [9]. Similarly, there were reports that sperm DFI and HDS could predict men's fertility capacity, assist therapeutic decision and assess risk of congenital diseases to the newborns [14, 26]. Besides, several studies have linked DFI to miscarriage. For human fertilization and embryo development, the sperm chromatin stability is the intrinsic factor of DNA damage during fertilization pronucleus formation, in which the percentage of sperm DNA damage > 30% would likely cause male infertility [27–29]. By contrast, some scholars found that sperm DFI does not affect the AIH outcomes [10, 30]. Best et al. pointed out that neither DFI nor HDS predicted pregnancy rate as assessed by SCSA [31]. Also, a meta-analysis consisted 30 studies suggested that sperm DNA fragmentation assays could not predict ART outcomes [32]. Moreover, the American Society for Reproductive Medicine stated a lack of evidence for the association of sperm DNA damage and ART failures [33]. An interesting discovery indicated that men underwent IUI might have sperm chromatin instability, even though most of these are normozoospermic [23]. In addition, Sugihara et al. found that a therapeutic algorithm integrating sperm DNA integrity could help couples with unexplained infertility decide the treatment method and elevate the chances of successful pregnancy following IUI [34]. Our previous studies proved that cycle treatment options, single/double IUI, female age, and certain sperm parameters could predict AIH outcomes in China [35, 36]. In the current investigation, we did not identify any predictive value of sperm DFI and HDS for the AIH outcomes, which might be due to the optimized semen treatment that probably removed differences between the sperm DFI and HDS.
In summary, our study indicated that aging is a critical factor leading to increased sperm DFI in Chinese males, while it has an insignificant impact on HDS. Moreover, sperm DNA damage does cause decreased semen quality, but not significantly influence AIH outcomes in Chinese infertile couples.
MATERIALS AND METHODS
Patient selection
This was a retrospective cohort study enrolling patients that received AIH treatment at the fertility clinic of the Third Affiliated Hospital of Guangzhou Medical University between August 2019 and August 2020. All pregnancies were confirmed with serum positive of beta-human chorionic gonadotropin (β-hCG) on day 14 after the AIH treatment. We extracted demographic data such as the couple's age, duration of infertility, semen parameters before and after sperm processing, and the pregnancy outcomes from the patients’ records. Patients with ovarian cyst detected in the ultrasound examination, uterine lesions such as submucosal leiomyoma, and a previous diagnosis of moderate to severe pelvic endometriosis were excluded. Records with incomplete or missing data were also excluded. As a result, a total of 809 AIH cycles from 504 infertile Chinese couples were analyzed (Table 8).
Table 8. The clinical and demographic characteristics of the couples receiving AIH treatment among different pregnancy outcome subgroups.
| Pregnant (n=75) | Non-pregnant (n=734) | P | |
| Female age | 31.02±3.74 | 31.37±4.05 | 0.133 |
| Male age | 32.93±4.36 | 33.29±4.75 | 0.402 |
| Total DFI | 18.71±11.33 | 18.67±11.72 | 0.873 |
| High DFI | 6.56±5.53 | 6.42±8.60 | 0.899 |
| Low DFI | 12.15±7.09 | 12.25±6.79 | 0.829 |
| HDS | 11.76±7.06 | 12.01±6.61 | 0.715 |
| Volume | 2.93±1.32 | 2.86±1.23 | 0.915 |
| pH | 7.36±0.18 | 7.37±0.17 | 0.567 |
| Post-TMSC | 32.88±26.29 | 33.18±26.90 | 0.923 |
| Post-concentration | 70.86±53.92 | 71.28±55.02 | 0.89 |
| Post-motility | 93.58±7.70 | 93.44±7.98 | 0.894 |
| Post-PR | 87.85±12.84 | 89.81±10.23 | 0.811 |
| Cycle treatment options | <0.001 | ||
| Natural cycle | 15(20.0%) | 200(27.2%) | |
| Stimulated cycle | 60(80.0%) | 534(72.8%) | |
| The number of IUI cycle | 0.049 | ||
| 1 | 42(56.1%) | 399(54.2%) | |
| 2 | 23(30.6%) | 260(35.4%) | |
| ≥3 | 10(13.3%) | 75(10.2%) | |
| Single/double IUI | <0.001 | ||
| single | 49(65.3%) | 570(77.7%) | |
| double | 26(34.7%) | 164(22.3%) | |
| Type of infertility | 0.988 | ||
| Primary | 46(61.4%) | 450(61.4%) | |
| Secondary | 29(38.6%) | 284(38.6%) |
Semen sample collection and analysis
Semen samples were collected by masturbation on the day of ovum-pick-up or AIH. The semen specimens were kept at 37° C and were examined within half an hour after collection. After complete liquefaction, all tests were conducted according to the World Health Organization (WHO) Laboratory Manual for the Examination and Processing of Human Semen (Fifth Edition) [37]. Semen specimens were treated by density gradient centrifugation.
Sperm chromatin structure assay
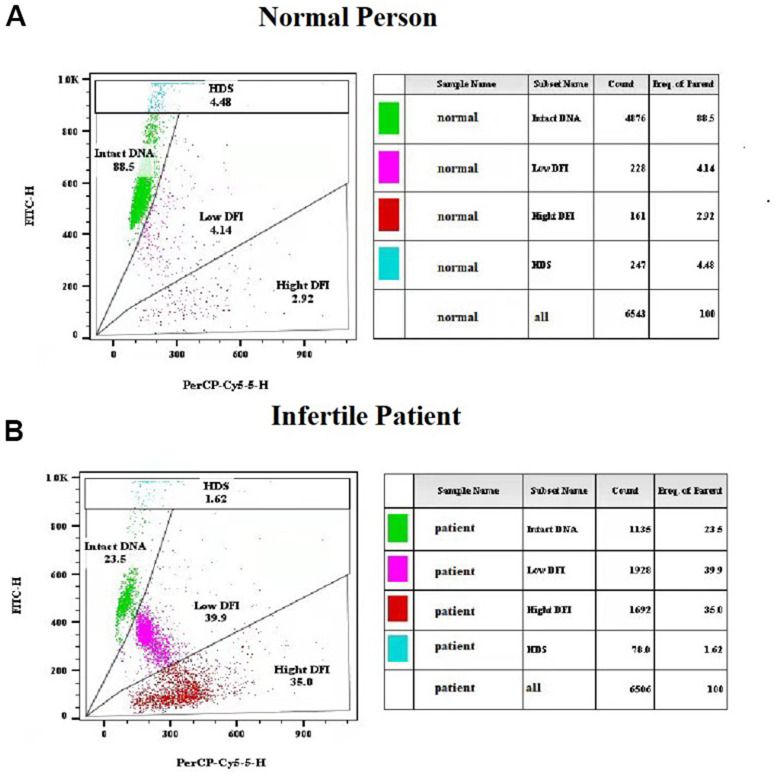
Freshly liquefied semen collected after 2-7 days of abstinence were used for sperm chromatin structure assay (SCSA). All assays were completed within two months before AIH treatment following a protocol based on the previous description [38]. In brief, the sperms were stained with acridine orange solution (pH 6.0) and then analyzed with a flow cytometer (BD FACS Canto II). The sperm DNA fragment index (DFI) was calculated with the ratio of single- and double-stranded DNA, which are quantified by the red (denatured, single-stranded DNA) and green (native, double-stranded DNA) fluorescent intensities of acridine orange. DFI < 30% was defined as normal human sperm cells that contains mostly intact DNA (Figure 2A), while DFI ≥ 30% as infertile patient sperm cells that has less intact DNA and a significant number of DNA fragments based on previous reports (Figure 2B) [9, 12, 39]. Following acid exposure which caused denaturation of double-stranded DNA in immature sperms with incomplete chromatin condensation, the percentage of sperm with high DNA stainability (HDS) was quantified by the flow cytometry measurements of the metachromatic shift from green to red fluorescence.
Figure 2.
Representative results of sperm chromatin structure assay (SCSA) using flow cytometry. (A) SCSA parameters of a normal person. (B) SCSA parameters of an infertile patient.
Intrauterine insemination
After emptying the bladder, the patient was in the bladder lithotomy position, washed the vulva with normal saline, and wiped the vagina, cervix, and fornix with a large cotton swab. A 1-mL syringe and an artificial insemination tube were connected to the uterine cavity. The catheter containing 0.5 mL of the husband’s sperm suspension is slowly placed in the uterine cavity through the cervix and about 1cm above the uterine cavity. After the semen is slowly injected into the uterus for 3 to 5 s, it is carefully withdrawn from the artificial insemination tube and speculum in the uterine cavity of the husband, and the patient is kept in the position of lowering the head and hips for about 30 min, and then can leave the operating room.
Statistical analysis
The SPSS v22.0 was used to analyze all data. The correlations between SCSA parameters and categorical variables were evaluated by chi-square analysis. The correlations between SCSA parameters and continuous parameters were evaluated by Spearman correlation coefficients. Differences in pregnancy rates between DFI and HDS groups were evaluated by a generalized linear model. A P value < 0.05 was considered statistically significant.
Footnotes
AUTHOR CONTRIBUTIONS: XX and DZ conceived and designed the study; YL, SW, HZ, JY, YZ, YY, MZ, QL, and XS collected the samples, performed the assays, and acquired the data; YL, SW, XX, and DZ analyzed the data; YL, SW, XX, and DZ wrote the manuscript. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest.
FUNDING: This research was funded by the National Natural Science Foundation of China (82002774), Guangdong Province Natural Science Foundation (2020A1515010065), Guangzhou City Science, Technology and Innovation Commission (201804010340, 202002030077) and Guangdong Province Outstanding Youth Medical Talent Program (110217110).
REFERENCES
- 1.Osman A, Alsomait H, Seshadri S, El-Toukhy T, Khalaf Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod Biomed Online. 2015; 30:120–7. 10.1016/j.rbmo.2014.10.018 [DOI] [PubMed] [Google Scholar]
- 2.Gomes M, Gonçalves A, Rocha E, Sá R, Alves A, Silva J, Barros A, Pereira ML, Sousa M. Effect of in vitro exposure to lead chloride on semen quality and sperm DNA fragmentation. Zygote. 2015; 23:384–93. 10.1017/S0967199413000671 [DOI] [PubMed] [Google Scholar]
- 3.Cohen-Bacrie P, Belloc S, Ménézo YJ, Clement P, Hamidi J, Benkhalifa M. Correlation between DNA damage and sperm parameters: a prospective study of 1,633 patients. Fertil Steril. 2009; 91:1801–5. 10.1016/j.fertnstert.2008.01.086 [DOI] [PubMed] [Google Scholar]
- 4.Berkovitz A, Eltes F, Yaari S, Katz N, Barr I, Fishman A, Bartoov B. The morphological normalcy of the sperm nucleus and pregnancy rate of intracytoplasmic injection with morphologically selected sperm. Hum Reprod. 2005; 20:185–90. 10.1093/humrep/deh545 [DOI] [PubMed] [Google Scholar]
- 5.Hazout A, Dumont-Hassan M, Junca AM, Cohen Bacrie P, Tesarik J. High-magnification ICSI overcomes paternal effect resistant to conventional ICSI. Reprod Biomed Online. 2006; 12:19–25. 10.1016/s1472-6483(10)60975-3 [DOI] [PubMed] [Google Scholar]
- 6.Rosiak-Gill A, Gill K, Jakubik J, Fraczek M, Patorski L, Gaczarzewicz D, Kurzawa R, Kurpisz M, Piasecka M. Age-related changes in human sperm DNA integrity. Aging (Albany NY). 2019; 11:5399–411. 10.18632/aging.102120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu R, Chen X, Yu W, Jiang F, Zhou X, Xu Y, Wang F. Analysis of age-associated alternation of SCSA sperm DNA fragmentation index and semen characteristics of 1790 subfertile males in China. J Clin Lab Anal. 2020; 34:e23548. 10.1002/jcla.23548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albani E, Castellano S, Gurrieri B, Arruzzolo L, Negri L, Borroni EM, Levi-Setti PE. Male age: negative impact on sperm DNA fragmentation. Aging (Albany NY). 2019; 11:2749–61. 10.18632/aging.101946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, Erenpreiss J, Giwercman A. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007; 22:174–9. 10.1093/humrep/del326 [DOI] [PubMed] [Google Scholar]
- 10.Siddhartha N, Reddy NS, Pandurangi M, Muthusamy T, Vembu R, Kasinathan K. The Effect of Sperm DNA Fragmentation Index on the Outcome of Intrauterine Insemination and Intracytoplasmic Sperm Injection. J Hum Reprod Sci. 2019; 12:189–98. 10.4103/jhrs.JHRS_22_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun TC, Zhang Y, Li HT, Liu XM, Yi DX, Tian L, Liu YX. Sperm DNA fragmentation index, as measured by sperm chromatin dispersion, might not predict assisted reproductive outcome. Taiwan J Obstet Gynecol. 2018; 57:493–8. 10.1016/j.tjog.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 12.Bungum M, Humaidan P, Spano M, Jepson K, Bungum L, Giwercman A. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod. 2004; 19:1401–8. 10.1093/humrep/deh280 [DOI] [PubMed] [Google Scholar]
- 13.Guo LY, Zhou H, Liu M, Li Q, Sun XF. Male age is more critical to sperm DNA integrity than routine semen parameters in Chinese infertile males. Andrologia. 2020; 52:e13449. 10.1111/and.13449 [DOI] [PubMed] [Google Scholar]
- 14.Evenson DP, Djira G, Kasperson K, Christianson J. Relationships between the age of 25,445 men attending infertility clinics and sperm chromatin structure assay (SCSA®) defined sperm DNA and chromatin integrity. Fertil Steril. 2020; 114:311–20. 10.1016/j.fertnstert.2020.03.028 [DOI] [PubMed] [Google Scholar]
- 15.Deenadayal Mettler A, Govindarajan M, Srinivas S, Mithraprabhu S, Evenson D, Mahendran T. Male age is associated with sperm DNA/chromatin integrity. Aging Male. 2020; 23:822–9. 10.1080/13685538.2019.1600496 [DOI] [PubMed] [Google Scholar]
- 16.Bojar I, Witczak M, Wdowiak A. Biological and environmental conditionings for a sperm DNA fragmentation. Ann Agric Environ Med. 2013; 20:865–8. [PubMed] [Google Scholar]
- 17.Das M, Al-Hathal N, San-Gabriel M, Phillips S, Kadoch IJ, Bissonnette F, Holzer H, Zini A. High prevalence of isolated sperm DNA damage in infertile men with advanced paternal age. J Assist Reprod Genet. 2013; 30:843–8. 10.1007/s10815-013-0015-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu JC, Jing J, Chen L, Ge YF, Feng RX, Liang YJ, Yao B. Analysis of human sperm DNA fragmentation index (DFI) related factors: a report of 1010 subfertile men in China. Reprod Biol Endocrinol. 2018; 16:23. 10.1186/s12958-018-0345-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Håkonsen LB, Spano M, Bonde JP, Olsen J, Thulstrup AM, Ernst E, Ramlau-Hansen CH. Exposures that may affect sperm DNA integrity: two decades of follow-up in a pregnancy cohort. Reprod Toxicol. 2012; 33:316–21. 10.1016/j.reprotox.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 20.Mohammadi Z, Tavalaee M, Gharagozloo P, Drevet JR, Nasr-Esfahani MH. Could high DNA stainability (HDS) be a valuable indicator of sperm nuclear integrity? Basic Clin Androl. 2020; 30:12. 10.1186/s12610-020-00110-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallegos G, Ramos B, Santiso R, Goyanes V, Gosálvez J, Fernández JL. Sperm DNA fragmentation in infertile men with genitourinary infection by Chlamydia trachomatis and Mycoplasma. Fertil Steril. 2008; 90:328–34. 10.1016/j.fertnstert.2007.06.035 [DOI] [PubMed] [Google Scholar]
- 22.Moazenchi M, Totonchi M, Salman Yazdi R, Hratian K, Mohseni Meybodi MA, Ahmadi Panah M, Chehrazi M, Mohseni Meybodi A. The impact of Chlamydia trachomatis infection on sperm parameters and male fertility: A comprehensive study. Int J STD AIDS. 2018; 29:466–73. 10.1177/0956462417735245 [DOI] [PubMed] [Google Scholar]
- 23.Alkhayal A, San Gabriel M, Zeidan K, Alrabeeah K, Noel D, McGraw R, Bissonnette F, Kadoch IJ, Zini A. Sperm DNA and chromatin integrity in semen samples used for intrauterine insemination. J Assist Reprod Genet. 2013; 30:1519–24. 10.1007/s10815-013-0101-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venkatesh S, Singh A, Shamsi MB, Thilagavathi J, Kumar R, Mitra DK, Dada R. Clinical significance of sperm DNA damage threshold value in the assessment of male infertility. Reprod Sci. 2011; 18:1005–13. 10.1177/1933719111401662 [DOI] [PubMed] [Google Scholar]
- 25.Al Omrani B, Al Eisa N, Javed M, Al Ghedan M, Al Matrafi H, Al Sufyan H. Associations of sperm DNA fragmentation with lifestyle factors and semen parameters of Saudi men and its impact on ICSI outcome. Reprod Biol Endocrinol. 2018; 16:49. 10.1186/s12958-018-0369-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue X, Wang WS, Shi JZ, Zhang SL, Zhao WQ, Shi WH, Guo BZ, Qin Z. Efficacy of swim-up versus density gradient centrifugation in improving sperm deformity rate and DNA fragmentation index in semen samples from teratozoospermic patients. J Assist Reprod Genet. 2014; 31:1161–6. 10.1007/s10815-014-0287-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evgeni E, Charalabopoulos K, Asimakopoulos B. Human sperm DNA fragmentation and its correlation with conventional semen parameters. J Reprod Infertil. 2014; 15:2–14. [PMC free article] [PubMed] [Google Scholar]
- 28.Evenson DP. The Sperm Chromatin Structure Assay (SCSA(®)) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim Reprod Sci. 2016; 169:56–75. 10.1016/j.anireprosci.2016.01.017 [DOI] [PubMed] [Google Scholar]
- 29.Evenson DP, Wixon R. Environmental toxicants cause sperm DNA fragmentation as detected by the Sperm Chromatin Structure Assay (SCSA). Toxicol Appl Pharmacol. 2005; 207:532–7. 10.1016/j.taap.2005.03.021 [DOI] [PubMed] [Google Scholar]
- 30.Yang H, Li G, Jin H, Guo Y, Sun Y. The effect of sperm DNA fragmentation index on assisted reproductive technology outcomes and its relationship with semen parameters and lifestyle. Transl Androl Urol. 2019; 8:356–65. 10.21037/tau.2019.06.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Best JC, Kohn T, Patel P, Blachman-Braun R, de Quadros E, Beyhan Z, Jacobs M, Ramasamy R. Elevated sperm DNA fragmentation does not predict recurrent implantation failure. Andrologia. 2021; 53:e14094. 10.1111/and.14094 [DOI] [PubMed] [Google Scholar]
- 32.Cissen M, Wely MV, Scholten I, Mansell S, Bruin JP, Mol BW, Braat D, Repping S, Hamer G. Measuring Sperm DNA Fragmentation and Clinical Outcomes of Medically Assisted Reproduction: A Systematic Review and Meta-Analysis. PLoS One. 2016; 11:e0165125. 10.1371/journal.pone.0165125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Practice Committee of the American Society for Reproductive Medicine. The clinical utility of sperm DNA integrity testing: a guideline. Fertil Steril. 2013; 99:673–7. 10.1016/j.fertnstert.2012.12.049 [DOI] [PubMed] [Google Scholar]
- 34.O’Neill CL, Parrella A, Keating D, Cheung S, Rosenwaks Z, Palermo GD. A treatment algorithm for couples with unexplained infertility based on sperm chromatin assessment. J Assist Reprod Genet. 2018; 35:1911–7. 10.1007/s10815-018-1270-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo Y, Wu S, Yuan J, Zhou H, Zhong Y, Zhang M, Li Q, Xu X, Sun X, Zhu D. Evaluation of Prognostic Factors for Clinical Pregnancy Rate Following Artificial Insemination by Husband in the Chinese Population. Front Med (Lausanne). 2021; 8:638560. 10.3389/fmed.2021.638560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou H, Wu S, Tang X, Zhou G, Yuan J, Li Q, Chen Y, Xu X, Sun X, Zhu D, Luo Y. Chlamydia trachomatis infection in the genital tract is associated with inflammation and hypospermia in the infertile male of China. Asian J Androl. 2022; 24:56–61. 10.4103/aja.aja_54_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barratt CL, Björndahl L, Menkveld R, Mortimer D. ESHRE special interest group for andrology basic semen analysis course: a continued focus on accuracy, quality, efficiency and clinical relevance. Hum Reprod. 2011; 26:3207–12. 10.1093/humrep/der312 [DOI] [PubMed] [Google Scholar]
- 38.Evenson DP. Sperm chromatin structure assay (SCSA®). Methods Mol Biol. 2013; 927:147–64. 10.1007/978-1-62703-038-0_14 [DOI] [PubMed] [Google Scholar]
- 39.Boe-Hansen GB, Fedder J, Ersbøll AK, Christensen P. The sperm chromatin structure assay as a diagnostic tool in the human fertility clinic. Hum Reprod. 2006; 21:1576–82. 10.1093/humrep/del019 [DOI] [PubMed] [Google Scholar]