Abstract
With the increasing demand for organ transplantation, organ procurement from a deceased donor is an essential step for deceased donor organ transplantation. A proper surgical technique for the procurement of an organ graft from a deceased donor must be carried out to avoid any damage to it. Moreover, how to manage deceased donors until they enter the operating room in a stable condition is a critical point to be considered. The establishment of a surgical technique and preoperative management for organ procurement is encouraged to achieve a nationwide standard and consistency for organ graft sharing among the transplant units.
Keywords: Transplantation, Organ harvesting, Brain death
| HIGHLIGHTS |
|---|
|
INTRODUCTION
Organ transplantation is the optimal treatment for end-stage organ disease. Successful organ transplantation is completely dependent on a well-preserved and properly functioning organ. In Korea, 400–500 deceased organ donors are noted every year, and approximately 1,800 transplant surgeries are performed. However, like other countries, Korea has significantly fewer organ donors than patients waiting for transplantation. Although it is important to discover even one brain-dead donor, it can be said that maintaining the brain-dead donor in the most ideal state and obtaining organs through a proper technique should also be considered basically [1]. The surgical technique for organ procurement is based on the anatomical feature and has been evolving as a result of the increased demand for organ transplantation. In this review, we intended to supplement the previously published guidelines for deceased donor organ recovery from the Korean Society of Transplantation in 2018 with the addition of donor management. Furthermore, the surgical technique is described with several images inserted to facilitate the understanding of organ procurement.
RECOMMENDATIONS IN DECEASED ORGAN DONOR MANAGEMENT
Brain death is associated with changes in the hemodynamic, hormonal, pulmonary, metabolic, and inflammatory processing. These lead to impaired graft function and increased immunogenicity and are associated with impaired patient as well as graft survival rates in organ transplantation from a deceased donor [1-3]. The optimal management of a potential organ donor achieves not only a greater number of transplantable organs but also a higher quality of these organs to ensure their optimal function after transplantation. Although the progress of deceased donor management has been made, specific therapeutic approaches remain largely consensus-based because of a lack of substantial high-quality evidence [2,4]. This review presents brain death-related systemic changes and proper management to preserve the function of donor organs. The important points for each system are summarized in Table 1.
Table 1.
Recommendation of deceased organ donor management
| System | Value | Target |
|---|---|---|
| Cardiovascular | Mean arterial pressure | 60–90 mmHg |
| Heart rate | 60–120 beats/min | |
| Central venous pressure | 4–12 mmHg | |
| Respiratory | Mechanical ventilation | |
| Tidal volume | 6–8 mL/kg | |
| Peak pressure | <35 mmHg | |
| Peak end-expiratory pressure | 5–10 cmH2O | |
| Arterial blood gas analysis | ||
| pH | 7.35–7.45 | |
| PaCO2 | 30–45 mmHg | |
| PaO2 | ≥80 mmHg | |
| O2 saturation | ≥95% | |
| Diabetes insipidus with polyuria | Urine output | 1–3 mL/kg/hr |
| Plasma sodium | 140–155 mEq/L | |
| Endocrine | Plasma glucose | 90–180 mg/dL |
| Hormonal therapy and doses in adults | ||
| Triiodothyronine | 4 μg IV bolus, followed by infusion of 3 μg/hr | |
| Thyroxine | 20 μg IV bolus, followed by infusion of 10 μg/hr | |
| Hydrocortisone | 50 mg IV bolus every 6 hours | |
| Methylprednisolone | 15 mg/kg single IV bolus | |
| General | Core body temperature | >34°C |
| Hemoglobin | >7 g/dL |
IV, intravenous.
Cardiovascular Management
The first step in managing a hypotensive donor is to preserve an adequate intravascular volume. The choice of fluid and infusion rate should result from previous therapy, incidence of diabetes insipidus (DI), and consideration of the effects of excessive fluids on the respiratory system. To replace the intravascular volume, an isotonic crystalloid is recommended [1,2,5]. Either lactated ringers or 0.9% isotonic saline is used. However, hyperchloremic metabolic acidosis may lead to favoring the lactated ringers.
After correction of the intravenous (IV) volume, the persistence of hypotension requires the use of vasoactive drugs. Dopamine or norepinephrine is the drug recommended in several other guidelines for organ donor management [1,2,5]. In a donor with severe cardiogenic shock, dobutamine or epinephrine can be considered. The administration of a high dose of vasoactive drugs should be avoided whenever possible. The high dose of vasoactive drugs is deleterious to the lung and heart, especially for the heart, and can reduce renal and hepatic perfusion [6]. At that time, vasopressin administration may reduce the dose of other vasoactive drugs required for maintenance [3,6]. There are conflicts in the hemodynamic criteria depending on the transplanted organ management during aggressive fluid replacement strategy. In more potential lung donors, the goal central venous pressure is not exceeding 8 mmHg without renal dysfunction [3]. Excess fluid replacement should be avoided to prevent pulmonary edema. Catecholamine storm (CS) is characterized by hypertension, tachycardia, and increased cardiac output and myocardial oxygen consumption. CS induces a serious myocardial oxygen imbalance and neurogenic pulmonary edema [5]. It should be treated with drugs of short half-life such as esmolol, nitroprusside, or nicardipine [2,3,5].
In the assessment of the heart for donation, ventricular dysfunction (VD) prior to cardiac procurement is one of the most important factors associated with graft failure. VD in brain death may be potentially reversible due to the causes of stunned myocardium. Echocardiography should be performed once hemodynamic stabilization of the donor has been achieved. If the first echocardiography shows VD, a second echocardiography can be performed hours later [3]. Besides the evaluation of the cardiac function before cardiac procurement, an initial echocardiography should identify significant structural heart disease, such as left ventricular hypertrophy (wall thickness >13 mm), regional wall motion abnormality, valvular dysfunction, infective endocarditis, and congenital heart disease.
Respiratory Management
Lung injury of deceased donors is similar to the injury seen in acute lung injury and acute respiratory distress syndrome. Protective ventilator management, including a low tidal volume (TV), positive end-expiratory pressure (PEEP), apnea test performed using continuous positive airway pressure, and closed circuit for airway suction, increases the number of potential transplantable lungs compared with a conventional management [4]. High inspired oxygen concentrations should be avoided to decrease the risk of bronchiolitis obliterans syndrome in lung recipients. Moreover, aerosolized α-agonists (e.g., albuterol) should not be used because these do not lead to improved oxygenation or lung utilization and induce tachycardia as a side effect [1,6].
Management of DI with Polyuria
DI (urine output >3 mL/kg/hr, urine specific gravity <1.005) caused by vasopressin deficit occurs in 80% of deceased donors [7]. For suspected DI with polyuria of >3 mL/kg/hr and/or hypernatremia, 4 μg (range, 2–6 μg) of desmopressin is administered by IV bolus, and this is repeated every 6 to 8 hours as required [1,7]. Hypovolemia and hypernatremia are corrected with infusion of 5% glucose or half saline solution. For hypotensive DI, arginine vasopressin is started by infusion at a rate of 0.5–2.4 units/hr [7].
Hyperglycemia Management
Brain death may lead to insulin resistance and glucogenesis that result in hyperglycemia. For hyperglycemia management, 50 units of short-acting insulin is mixed with 50 mL of normal saline, and then, it is administered at a rate of 2–3 mL/hr using an infusion pump. The plasma glucose level is checked every 6 hours [5].
Endocrine Management
Thyroid hormone treatment is based on low-level evidence. However, thyroid hormone administration may be justified in that there is little evidence that thyroid hormone causes harm. Thyroid hormone administration may provide benefit in donors who are hemodynamically unstable or potential heart donors who have borderline cardiac function despite other optimized managements [2,7]. Thyroxine may be administered enterally if the IV formulation is not available [7]. However, in Korea, there is no available IV thyroid hormone.
There is uncertainty as to the benefit in steroid replacement to the deceased donors [2]. The rationales for steroid administration are as follows: steroid deficiency as a result of hypothalamic–pituitary–adrenal dysfunction, functional or relative adrenal insufficiency, or immunomodulatory and anti-inflammatory effects of steroids [3,6]. Physiologic stress dose (hydrocortisone, 50 mg IV bolus every 6 hours) may improve the blood pressure and reduce the dose of vasoactive drugs. A high dose of steroid (methylprednisolone, 15 mg/kg single IV bolus) may reduce the harmful effects of the inflammatory cascade in brain death [7]. Blood for tissue typing should be collected before high-dose steroid treatment because steroids may suppress human leukocyte antigen expression [5].
General Management
The core body temperature is maintained above 34°C. A recent study shows a lower incidence of delayed graft function (DGF) in the kidneys from the deceased donors with hypothermia (34°C–35°C) [8]. This benefit increases in the kidneys from a donor with expanded criteria. With regard to the transfusion of blood products, optimal thresholds are unknown in the potential organ donor population, but adhering to a target of more than 7 g/dL and replacement with products as needed to manage coagulopathy have been suggested [5]. Interestingly, a prospective observational study of renal transplant recipients noted blood transfusion in brain-dead donors to be associated with a protective 23% decrease in DGF of these recipients [8].
Unnecessary drugs (e.g., anticonvulsants, osmotics) should be withdrawn, while other drugs such as antibiotics and prophylactics of deep vein thrombosis or gastrointestinal bleeding are continued. Moreover, enteral nutrition should be withdrawn, and parenteral nutrition is maintained if already in place. Loss of vagal tone in brain death is likely to disrupt intestinal motility and nutrient absorption [1,5].
HEART AND LUNG PROCUREMENT
General Consideration and Preparation
During organ transplant surgery, transplant surgeons inevitably have a tendency to pay attention to the implantation procedure. However, successful transplantation depends on the safe procurement of the organs. Procurement must be performed in an accurate and timely manner according to the progress of implantation surgery. In particular, since the heart and lungs are adjacent organs, a cardiac and thoracic surgeon must ensure thorough discussion and cooperation prior to the procurement procedure. Organs harvested by a surgeon who lacks an understanding of the importance of procurement may result in serious posttransplant lung or cardiac dysfunction or failure.
Upon arrival to the operating room for procurement, surgeons and the team for procurement must identify themselves to the center coordinator and be escorted. Then, they should discuss the donor’s condition, fluid management, and inotropic drug use with the anesthesiologist. The anesthesiologist may tend to overhydrate to maintain the blood pressure, but a vasoactive medication is more recommended because overhydration may lead to pulmonary edema. The ventilator must be set at a TV <10 mL/kg and FiO2 of 0.5. PEEP should also be maintained at 5–8 cmH2O [9]. The anesthesiologist must be asked to keep the ventilator on after heart procurement and stay in place until the end of the lung procurement.
The procurement surgeons should confirm that the procurement hospital has the surgical instruments that they want to use prior to the operation. Instruments that are not commonly used must be prepared by each institute. Before the commencement of the surgery, all surgeons who procure each organ should discuss the order, duration, and procedure of procurement. Once an estimated time of procurement is obtained, the implantation hospital must be contacted to proceed with the recipient’s surgery accordingly.
Although the procedure may vary depending on the situation of both the procurement and recipient’s hospitals, the general procedure is as follows: the donor is transported to the operating room. Once anesthesia is complete, the donor is prepped and draped with the chest and abdomen exposed. The chin must be appropriately exposed during draping.
Incision and Exposure
The abdominal surgeon first makes an incision in the abdomen and performs liver biopsy. While the pathologist analyzes the results of liver biopsy, the cardiac surgeon (or thoracic surgeon if there is no heart procurement) performs sternotomy and inspects the heart, while the thoracic surgeon inspects the lungs. The midline laparotomy incision is extended cephalad to perform mid-sternotomy. The skin incision must be extended sufficiently above the manubrium so that the sternum can be sufficiently spread a part and the organ can be extracted without being damaged by the sharp sternal edge. When spreading the sternum, the exposed liver must be covered by a surgical towel to prevent damage. Sternal bleeding should be controlled by bone wax and electrocautery. When spreading the sternum with a sternal retractor, the handle should be faced toward the head side to prevent liver damage or hindrance of the abdominal organ procurement.
The pericardium is opened vertically from the diaphragm to the reflection of the arch and ascending aorta. Next, 5–6 silk retention sutures are hung in the pericardium to spread it apart and expose the heart sufficiently. The heart surgeon should observe visually whether there is any cardiac abnormality or contusion and palpate the heart to examine for chamber distension and coronary artery calcification. When touching or lifting the heart by hand, the anesthesiologist should be notified in advance, as vital signs can change rapidly. Usually, the thoracic surgeon inspects the lungs after the cardiac surgeon examines the heart, but the cardiac surgeon may proceed with tissue dissection for vascular cannulation upon consensus with the thoracic surgeon.
To inspect the lungs, the area below the sternum is dissected bilaterally to locate the parietal pleura and open it. When opening the pleura, care must be taken to avoid lung injury or laceration due to electrocautery or instruments. The lungs are then inspected for edema, pleural adhesion, lung atelectasis, or contusion and palpated for any masses. The anesthesiologist is requested to inflate the lungs sufficiently and smooth out areas of severe atelectasis manually [10]. Usually, atelectasis is most severe in the bilateral lower lobes. When inspecting for such a case by lifting the lungs, care should be taken to preserve the blood pressure and heart rate. After lung examination, tissue dissection of the heart or abdominal organs may proceed.
Dissection and Cannulation
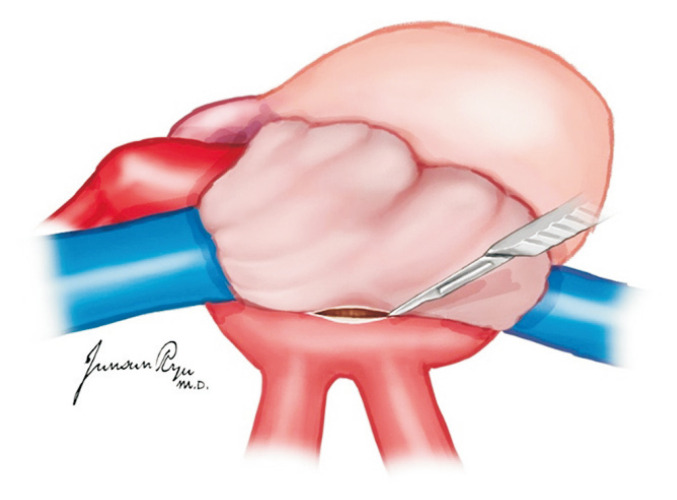
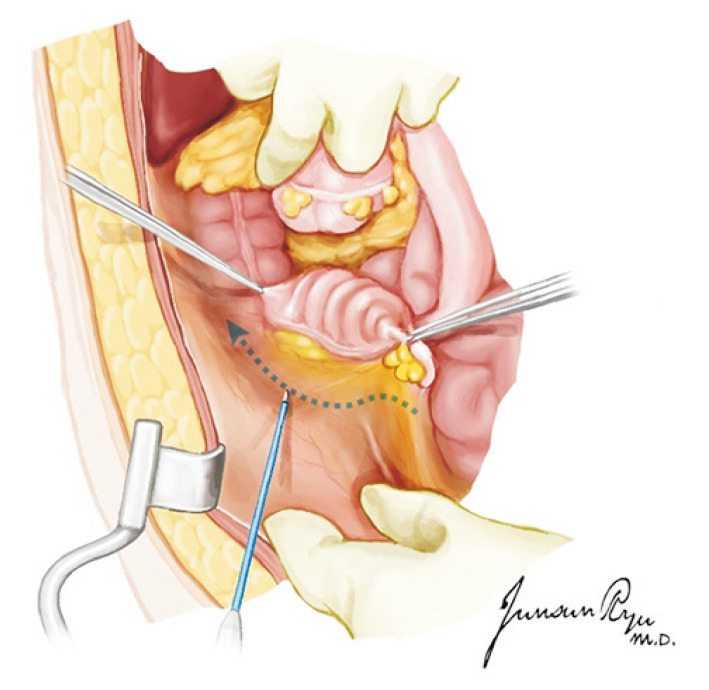
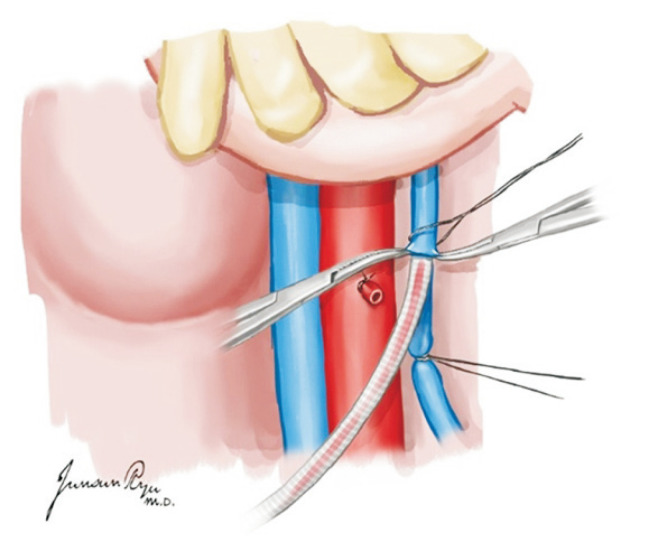
For the heart, dissection between the main pulmonary artery (PA) and aorta is the first step. The posterior section of the ascending aorta should be dissected sufficiently away from the right PA. Care must be taken not to dissect too proximally to the root of the aorta and cause damage to the coronary artery. The anterior portion of the aorta is then exposed along with the arch and great vessels. The exposure of the great vessels can be maximized by dissecting the brachiocephalic vein away from the aorta. After the exposure of the arch, the main PA should be mobilized from the bifurcation of the bilateral PA. The superior vena cava (SVC) is held, instead of the periauricular tissue of the right atrium, and pushed laterally to prevent damage to the sinoatrial node. The incision is made from the reflection of the right PA and moved up toward the brachiocephalic vein bifurcation. The azygos vein should then be located, and the medial and posterior tissues of the SVC are carefully dissected to mobilize it from the right atrium to the azygos vein. A suture is hung for ligation in the dissected SVC. A purse-string suture is performed in the anterior ascending aorta for cannulation as well as in the distal portion of the main PA (Fig. 1). Placing this too distally should be avoided for the cannula tip not to enter either the right or left PA side. The azygos vein can be ligated at this stage or clamped with the SVC after the aorta cross-clamp (ACC).
Fig. 1.
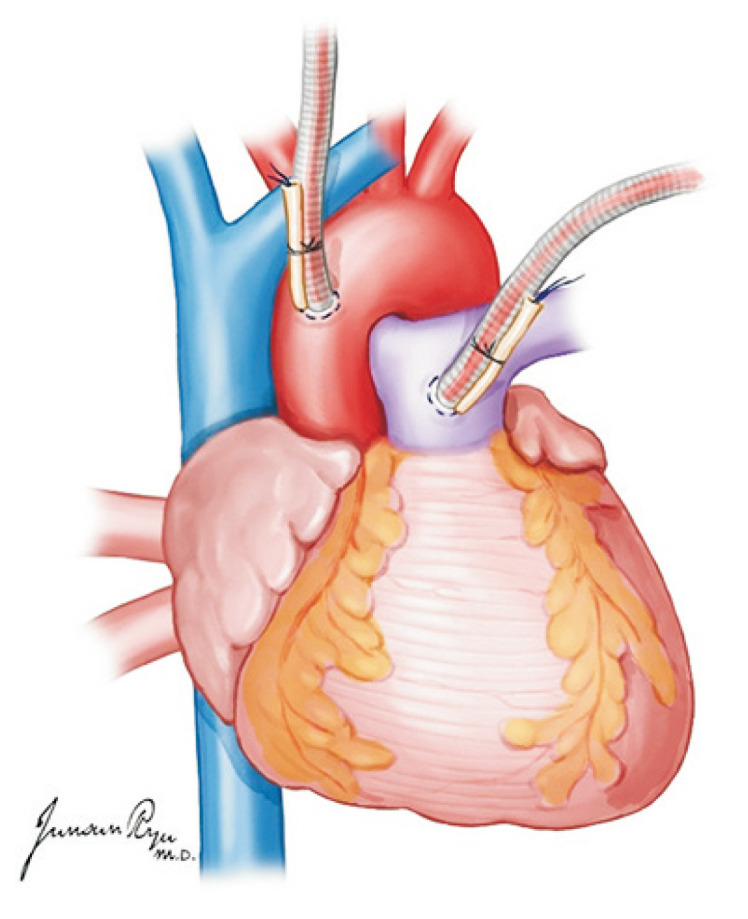
An aortic cannula is inserted in the anterior ascending aorta, and a pulmonary artery (PA) is in the distal portion of the main PA. Placing the PA cannula too distally should be avoided because the cannula tip can enter either the right or left PA side.
The abdominal surgeon can then proceed with dissection for procurement. It does not matter which organs are cannulated first, but the order can be decided through discussion with the abdominal and cardiothoracic surgeons preoperatively. After the dissection of all organs and prior to cannulation, the anesthesiologist should administer heparin. For an average adult donor, 20,000–25,000 IU is injected [11]. Aortic line is inserted into the ascending aorta with air removal, and the line is connected to the cardioplegic solution. The PA cannula is then inserted into the distal portion of the main PA with the purse-string suture, air is removed, and the cannula is fixed. The lung preservation solution line can then be connected. The abdomen is cannulated and checked for the preparation of perfusion. Upon discussion with all surgeons, the dissection site is marked on the right side of the left atrium (Waterston’s groove), which marks the border between the heart and lung, as well as inferior vena cava (IVC), which marks the border between the heart and liver, before the administration of prostaglandin E1 (PGE1). Care should be taken not to lift the heart up too far for visualization, as the margins of the left atrium and abdominal IVC can be much shorter than expected.
After the aforementioned preparation, PGE1 (500 μg) is administered. The route of administration differs between institutions. Some inject through a syringe in the main PA or via the central line by an anesthesiologist, while others inject half the dose on the table through the PA and the other half by an anesthesiologist [12]. A sudden drop in the blood pressure may occur during the injection, so the anesthesiologist must always be notified beforehand. The rest of the procedure should be performed as fast as possible since ischemia begins to occur at this point.
Aorta Clamping and Perfusion
After notifying the anesthesiologist, the ACC is performed with the aorta clamp after PGE1 administration. If the azygos vein is not ligated, the SVC and azygos vein should then be clamped. Some institutions ligate the SVC as well. Next, incision of the pre-marked area on the left atrium is carried out to drain the left atrial blood. Too small length of an incision prevents the perfusate from draining and can cause heart distension, so the minimum size should be over 2 cm. If the institution of heart procurement allows it, the left atrial appendage may also be partially dissected. Then, an incision is made on the pre-marked area of the IVC to drain the blood. It is recommended that the left atrium be opened first since dissecting the IVC first may interfere with the visualization of the left atrium due to blood pooling in the pericardium. The histidine–tryptophan–ketoglutarate (HTK) solution should be administered at 20 mL/kg or more (generally 2 L) over sufficient time (generally 7 minutes). An infusion pump for constant pressure or gravity is used to infuse the solution. At the same time, the lung preservation solution is infused through a PA cannula. In cases where Perfadex solution (XVIVO Perfusion, Göteborg, Sweden) is used, which is most commonly used nowadays, the amount of 70 mL/kg is administered by lifting the solution bag above 30 cm from the operating table to prevent high-pressure injury. Slush ice is then placed in the pericardium and thoracic cavity right after ACC. Care must be taken not to damage the organs with sharp ice chips. During cardioplegic infusion, the proximal pressure of the aorta should be checked with the hands to ensure that the solution is infused properly and the aortic valve function is normal. A check of whether there is any extension of the left atrium and how fast the electric activity of the heart dissipates should be carried out. If the electric activity persists, there may be coronary artery occlusion disease or left ventricular hypertrophy. In such cases, the cardioplegic solution should be administered over a longer duration. When injecting the lung preservation solution, the anesthesiologist should be asked to perform the lung recruitment maneuver from time to time to prevent lung atelectasis.
Procurement
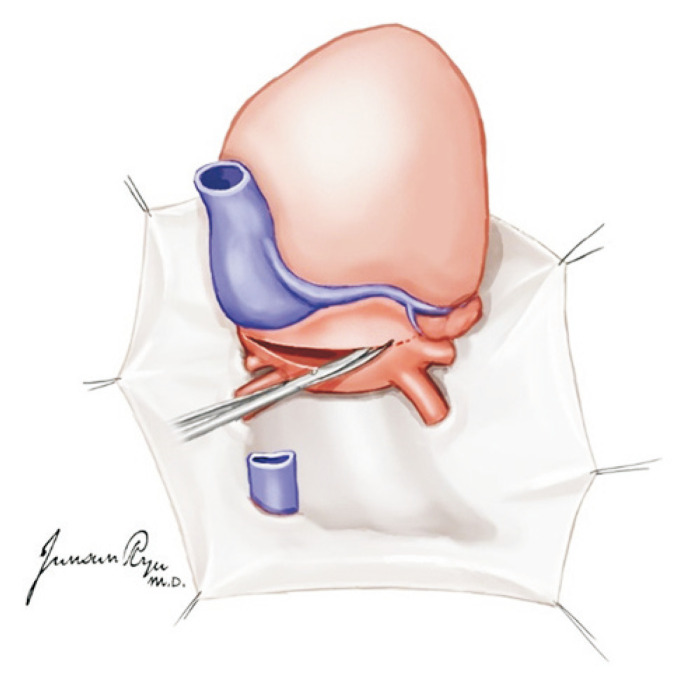
Subsequently, after the removal of the cannula, heart procurement is started. The SVC is divided below the area of ligation while making sure there is no injury to the right PA. The tissue of the leftover portions in the IVC dissection site should be divided completely. The aorta is then divided from all head vessels and cut around the ligamentum arteriosus from the descending aorta. The PA is divided in the site where the cannula was inserted in the distal main PA while ensuring the left and right PA are not separated (Fig. 2).
Fig. 2.
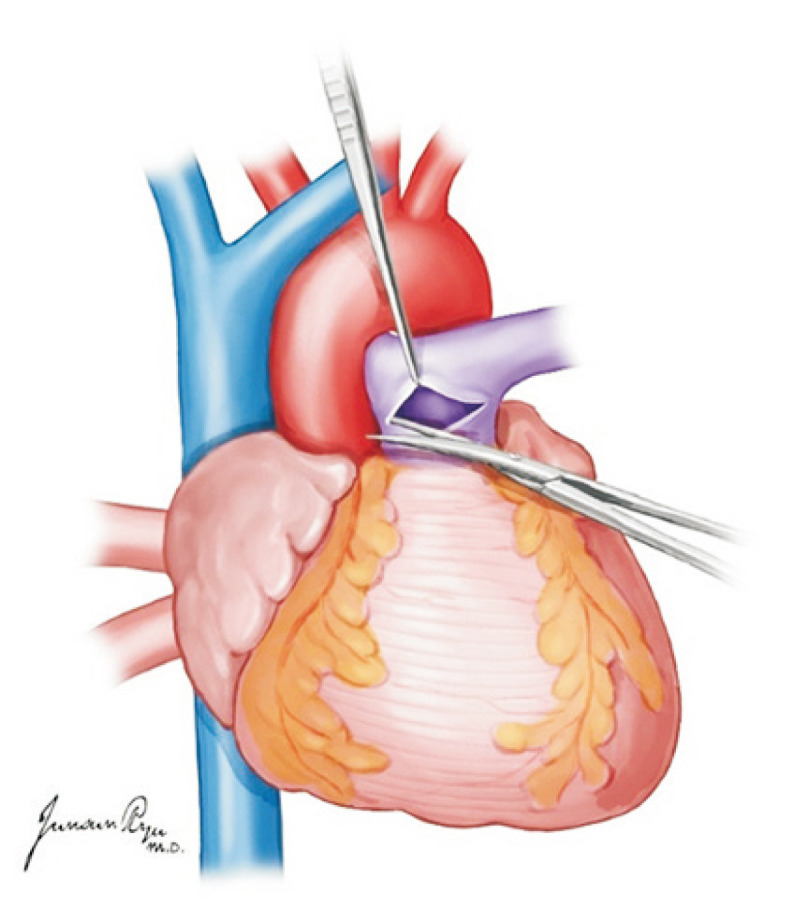
The pulmonary artery (PA) is divided in the site where the cannula was inserted in the distal main PA while ensuring the left and right PA are not separated.
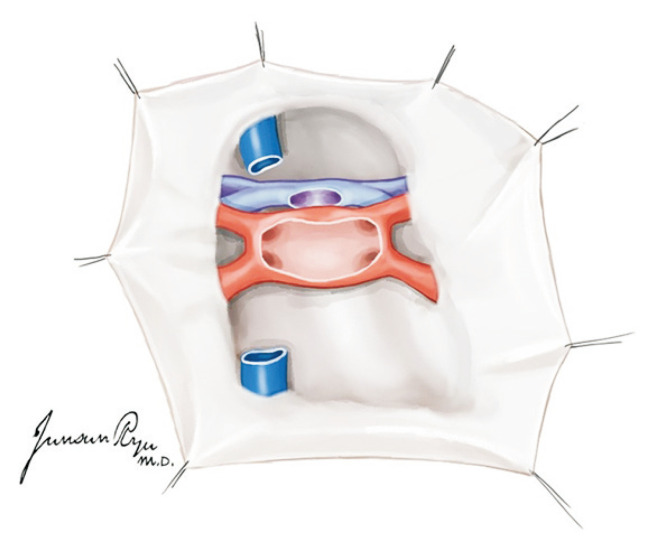
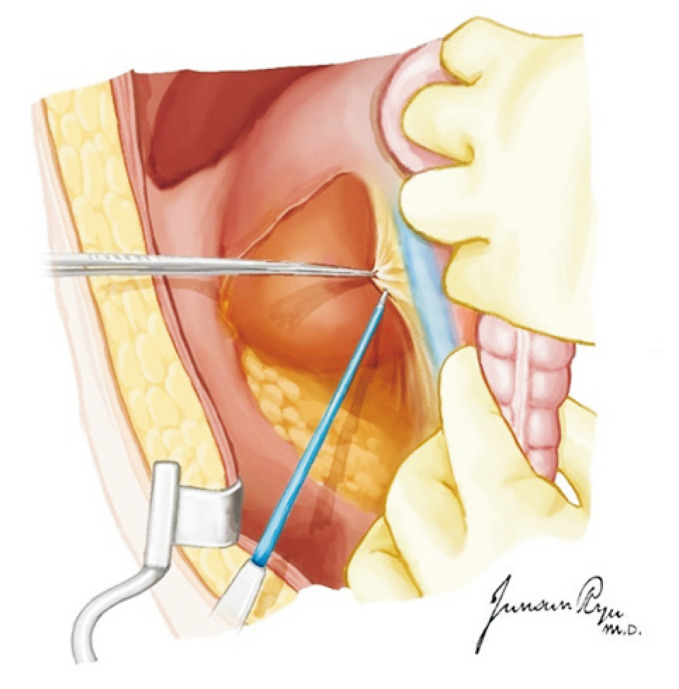
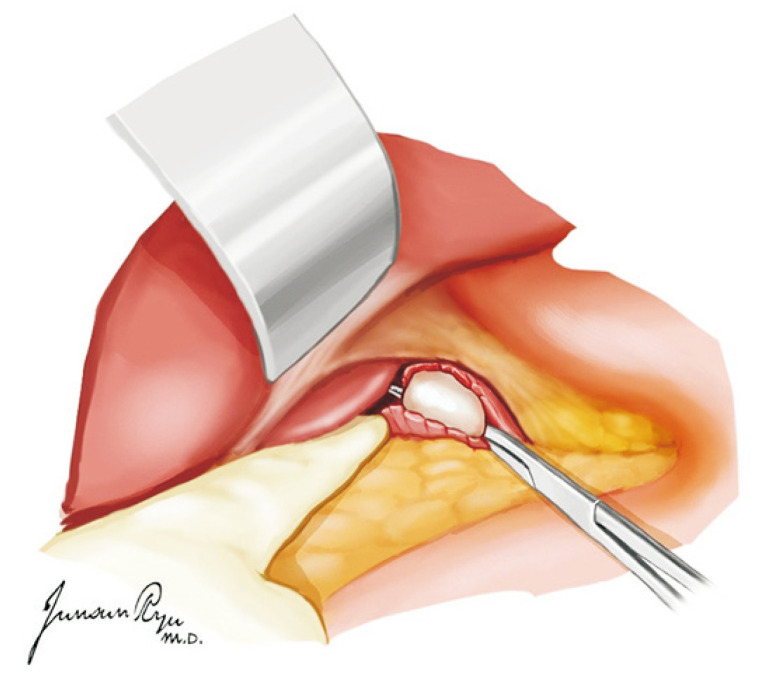
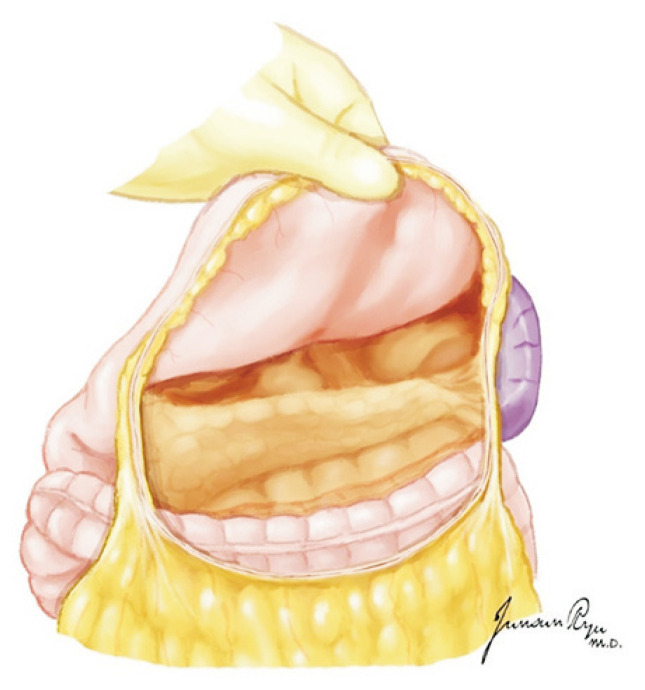
The heart should then be pushed to the left to expose Waterston’s groove that was incised for drainage (Fig. 3). This is a very important time for the cardiac and thoracic surgeon. They should always discuss about the dissection border of the left atrium while preserving sufficient muscular cuff around the opening of the pulmonary vein. This cuff is most important when implanting the lung into the recipient [13]. The heart is lifted toward the head and the left atrium incised in a transverse manner (Fig. 4). The heart should then be moved to the right, and the incision be extended to dissect the left atrium. The left pulmonary veins should form a whole cuff. The borders of the right and left incisions of the left atrium are then connected transversely, and the heart procurement is completed (Fig. 5).
Fig. 3.
When Waterston’s groove is incised, sufficient muscular cuff of the left atrium should be preserved. This cuff is most important when implanting the lung to the recipient.
Fig. 4.
After the incision of Waterston’s groove, the heart is lifted toward the head, and the left atrium is incised in a transverse manner. The heart should then be moved to the right, and the incision should be extended to dissect the left atrium.
Fig. 5.
After left atrium division, the left pulmonary veins should form a whole cuff.
Next, the heart is moved to the back table and checked to ensure that all structures, including the valves, are anatomically normal. The heart is wrapped for transportation. After the heart procurement, the anesthesiologist is asked to perform Ambu-bagging to expand the collapsed lungs. Using the Foley catheter, retrograde perfusion of the Perfadex solution through the pulmonary vein is carried out. Perfusion should be conducted with 500 mL per vein, totaling 2 L [14]. During perfusion, checks should be performed through the dissected PA to ensure the solution is draining well. The left lung is lifted from the thoracic cavity and pushed to the right, and then, dissection of the inferior pulmonary ligament (IPL) can be performed. The dissection of the anterior esophageal tissue from the IPL upward is the next step, followed by the dissection of the descending aorta from the bifurcation of the left subclavian artery. After the left lung is placed back in the thoracic cavity, the right lung is lifted and pushed to the left to dissect the IPL with the anterior esophageal tissue to the azygos vein. The right lung then is put down, and all tissues around the upper trachea are dissected using a finger. When manipulating the trachea, it should be noted that the membranous portion must not be damaged. A finger is placed in the left and right spaces next to the dissected trachea to dissect toward the left and right thoracic cavity. After this procedure, the lungs are attached to the donor only by the trachea. Ambu-bagging should be performed by the anesthesiologist until atelectasis completely resolves. The endotracheal tube can then be pulled out sufficiently so that the tube is not caught in the division area, and the trachea can be divided using staplers (usually transverse anastomosis stapler [TA] 30) [15]. During this procedure, the anesthesiologist should be asked to perform the Valsalva maneuver to maintain lung inflation with the proper pressure, with FiO2 fixed at 0.5. With the lungs inflated, the trachea is divided at the highest level possible. After the first use of the stapler, mechanical ventilation should be stopped. Usually, the stapler is used three times, and the trachea is divided between the top and middle staples. The divided tracheal margin is disinfected before the extraction of the lungs. Care should be taken here to prevent lung laceration by the sternum.
A check should then be carried out to see if there is anything notable in the procured lungs, such as bleeding, air leakage, or laceration during procurement. All parts (e.g., ventricular cuff) should be well dissected. Three plastic bags should be used for maintaining hypothermia and safety. One bag is filled with cold lung preservation solution, and the other two are filled with cold normal saline and ice chips. The lungs are then stored in an ice box filled with ice, taking care not to press on the lungs.
LIVER PROCUREMENT
Liver transplantation (LT) is the optimal treatment for patients with hepatic failure or hepatocellular carcinoma. Successful LT depends on a well-preserved functioning liver; thus, meticulous procedure is an essential step for deceased donor liver transplantation [16]. The surgical technique for liver procurement is divided into warm and cold dissections according to the tissue perfusion time. Warm dissection has the advantage of perfusion after confirming the vascular structures. Cold dissection is able to reduce operative time and organ damage with rapid organ procurement [17].
Warm Dissection
Incision
A longitudinal incision is made from the suprasternal notch to the symphysis pubis. The abdominal cavity is entered first, and a Balfour retractor is placed to have an adequate exposure of the abdomen (Fig. 6). The round ligament of the liver is divided with tie ligation, and the falciform ligament of the liver is dissected. The exploration of intra-abdominal organs should be performed to exclude potential malignant disease. An assessment of the overall quality of the liver should be made at this time, and liver biopsy should be taken if any consideration is raised. The biopsy is taken by liver wedge resection from the left or right lobe for the frozen section for pathologic examination. Then, the soft tissue over the sternum is opened by electrocautery along the line for sternotomy. A long Metzenbaum scissors or capped suction tip is used for tunneling behind the sternum to ensure that the posterior aspect of the sternum is clean from soft tissue attachment. The sternum is divided by electric sawing with bone wax applied to the cut surface. Then, a sternal retractor is applied to open the chest wall. The pleura and pericardium should be remained intact at this stage. A surgical pack applied over the chest wound may allow to give the cardiothoracic team for clean surgical field. Now, the concentration is directed to the dissection of the abdominal organs.
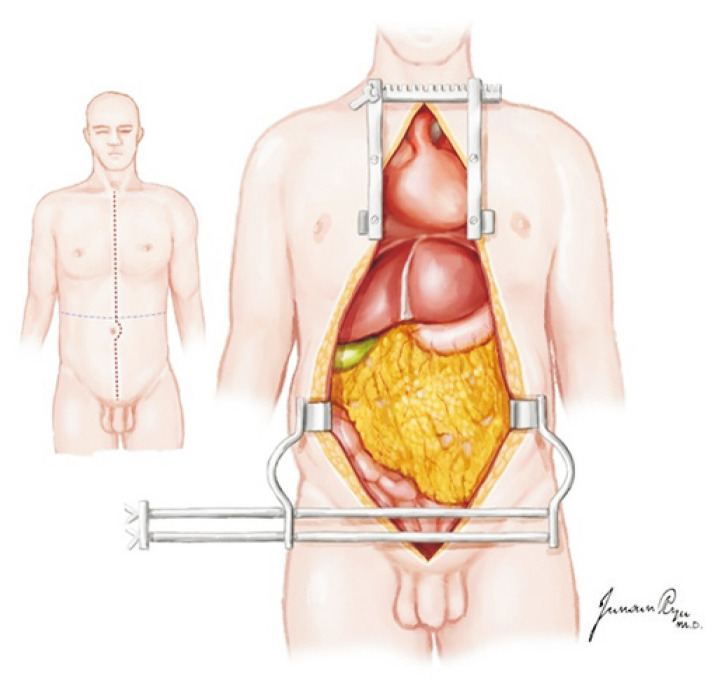
Fig. 6.
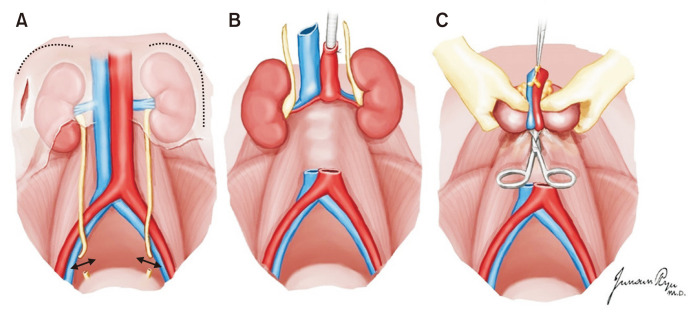
An incision extending from the sternal notch to the pubis, which is cruciate at the level of the umbilicus, provides maximal exposure for multi-organ procurements.
Identification of the distal aorta
The retroperitoneum is incised along the white line of Toldt from the cecum to the hepatic flexure (Fig. 7). The dissection is continued superiorly and medially along the retroperitoneal avascular plane to mobilize and retract the ascending colon medially. The right ureter is easily identified and preserved with the surrounding tissue. The dissection is continued by the Cattell–Braasch and Kocher maneuvers (Fig. 8). The inferior duodenal fold division allows the broad exposure of the IVC and aorta. This procedure leads to left renal vein visualization and superior mesenteric artery (SMA) palpation at its origin from the aorta. The distal part of the abdominal aorta is dissected, and the inferior mesenteric artery can be ligated to expose the aorta adequately. A Dacron tie or umbilical tape is placed around the distal aortic bifurcation of the common iliac arteries, which is used for ligation of the distal aorta prior to cold perfusion. Another tie is placed around the aorta proximally, which is used for fixing the cannula to the aorta. The inferior mesenteric vein (IMV) is readily exposed along the edge of the dissected mesentery of the sigmoid colon lateral to the proximal jejunum. If portal system perfusion is required, then the IMV can be isolated, and a tie is encircled for cannula placement [16].
Fig. 7.
A Cattell–Braasch maneuver extending across the midline, with complete mobilization of the distal small bowel, right colon, and duodenum, allows for the identification of the distal aorta, iliac bifurcation, and distal inferior vena cava. Dotted line: initial dissection plane of Cattell-Braasch maneuver.
Fig. 8.
Kocherization with the mobilization of the infrahepatic inferior vena cava and upper margin of both renal veins.
Dissection of the liver
The left triangle ligament is divided to mobilize the left lobe of the liver. The hepato-gastric ligament division should be proceeded with checking the left accessory hepatic artery that usually arises from the left gastric artery. It should be preserved if present. The common bile duct is dissected and transected at the level to the upper margin of the duodenum. The portal vein is visualized in a meticulous manner. In this time, it should be checked whether there is accessory or replacement of the right hepatic artery posteriorly to the portal vein by palpating the hepato-duodenal ligament via the foramen of Winslow [16,17]. It usually arises from the right side of the SMA. The right gastric artery and veins are tied and divided. The proper hepatic artery as well as the gastroduodenal artery (GDA) is dissected carefully toward the celiac trunk. As the dissection proceeds, the splenic and left gastric arteries are seen, and a short segment is dissected, respectively, for transection after cold perfusion. The left gastric vein may be encountered, which is ligated and divided. The liver dissection is now completed.
Dissection of the aorta around the diaphragm
The hepato-esophageal ligament is divided, and the esophagus is retracted left laterally. The aorta pulse is palpated, and the overlayer crus is divided to expose the aorta. A thin fascia layer is incised, and the aorta is freed from the surrounding attachment (Fig. 9). A nylon type is encircled over this segment of the aorta that is subsequently tied when the cold perfusion is commenced to limit the perfusion to the abdominal organs. The dissection must be performed to prevent the injury of the aorta, especially the posterior part of the aorta for bringing through the nylon type. This procedure can be performed later with a high risk of bleeding. Alternatively, proximal aorta clamping can be used by the segment of the thoracic aorta in the left chest, which is dissected through the pleural cavity access. The gallbladder is incised open and flushed with room temperature normal saline until the outflow at the common bile duct becomes clear. This procedure could prevent autolysis by bile acid. The cardiothoracic team is informed for scrubbing and proceeds to heart–lung dissection.
Fig. 9.
After the mobilization of the left hepatic lobe, the supraceliac aorta should be exposed with movement of the esophagus toward the left and blunt dissection of the periaortic soft tissue.
Insertion of the perfusion cannula and perfusion
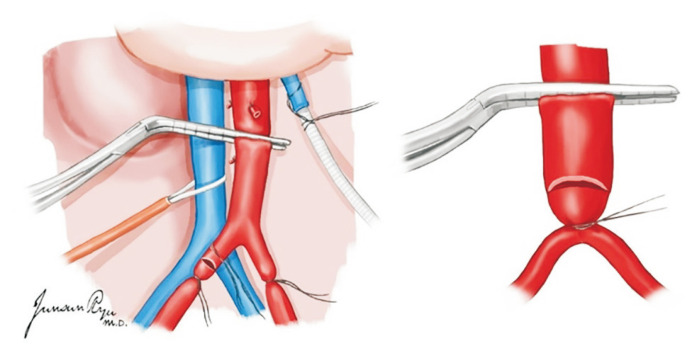
The line for perfusion should be checked for whether air bubbles are removed. After heart–lung dissection, 25,000–30,000 IU of unfractionated heparin is administered intravenously. The distal abdominal aorta is ligated with an umbilical tape tie at the level of the common iliac artery bifurcation. A vascular clamp is placed onto the aorta approximately 5 cm proximally to this tie to block the blood flow to this segment of the aorta where an arteriotomy is made (Fig. 10). A 20–24-Fr cannula is then inserted via arteriotomy into the aorta and secured with a heavy tie or umbilical tape. The cannula should be secured, and the vascular clamp removed. If the surgeon chooses to use the dual-perfusion technique, the IMV is exposed and used for venous cannulation and portal flush using a 14-Fr or smaller cannula (Fig. 11). At this point, cold perfusion is commenced simultaneously with cardiothoracic perfusion. The IVC is divided just below the right atrium for blood and perfusion fluid drainage. Attention is paid to leave adequate length of the IVC with the liver at the suprahepatic end. Alternatively, IVC incision for venting the blood is made usually at the lower part just proximal to the level of the confluence of the common iliac veins. A suction tube tip can be placed into the IVC for adequate perfusion fluid drainage. At least three sets of suction lines are used to properly evacuate the blood and fluid during the perfusion period. At the same time, slush ice is poured into the abdominal cavity around the liver, pancreas, kidney, and intestine for immediate cooling of the organs. Usually, 10–20 L of HTK preservation solution is used for flushing. The organs are checked in the meantime to ensure that the progression of perfusion is adequate.
Fig. 10.
A vascular clamp is placed onto the aorta approximately 5 cm proximally to this tie to block the blood flow to this segment of the aorta where an arteriotomy is made. After systemic heparinization (300 units/kg) is performed, the distal aorta is ligated, and a 24-Fr aortic cannula is placed at this site.
Fig. 11.
The inferior mesenteric vein is ligated, and a 14-Fr perfusion cannula is placed.
Cold Dissection
Liver procurement
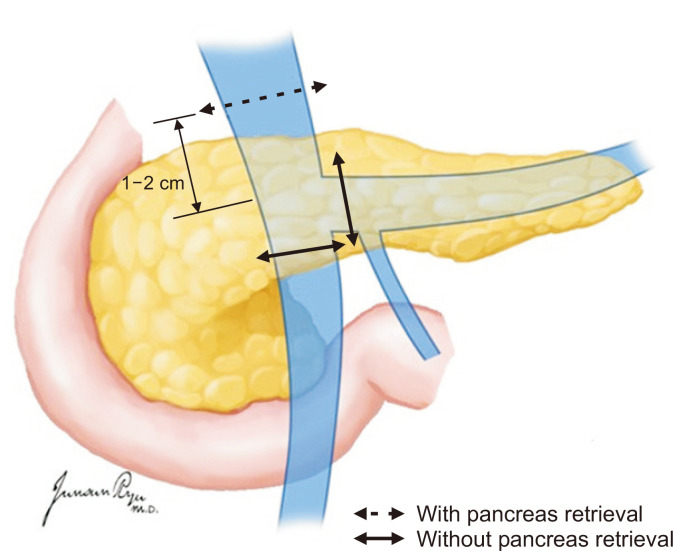
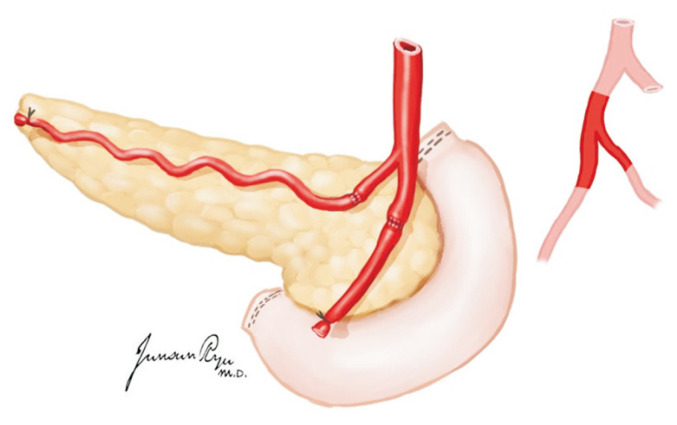
In general, liver procurement is performed after heart and lung procurement by the cardiothoracic team, and if the small intestine is not for procurement, the liver is taken for the first time among abdominal organs. The GDA is ligated and divided. The portal vein division depends on whether the pancreas is for procurement (Fig. 12). With pancreatic procurement, the portal vein is divided at the level of 1–2 cm proximal to the junction of the splenic vein and superior mesenteric vein (SMV) [18]. Without pancreatic procurement, the division may be at the level distal to the confluence of the splenic vein and SMV. The splenic and left gastric arteries are divided 0.5–1.0 cm from their origin arising from the celiac trunk. However, the left gastric artery is preserved on the celiac trunk if the left accessory hepatic artery is present arising from the left gastric artery. Along with celiac trunk dissection, the lymphatic tissues and nerve complex are divided at the left side of the aorta, and the aorta is exposed. The division of the celiac trunk is included with the aortic wall in the ostium for the aortic patch. The division of the SMA is included in the aortic patch if the right accessory or replacement hepatic artery is present. If the pancreas is for procurement, the SMA is divided just above the level where the accessory hepatic artery arises. The anterior wall of the IVC is divided transversely above the level of the renal vein. After all, the close cooperation of the liver and pancreas teams enables suitable transplantation without jeopardizing its vasculature.
Fig. 12.
The portal vein is divided at the level of 1–2 cm proximal to the junction of the splenic vein and superior mesenteric vein (SMV) if the pancreas is retrieved for transplantation. Otherwise, the division can be at the level of the confluence of the splenic vein and SMV.
Procurement of the iliac vessels
The iliac arteries and veins are harvested as long as possible toward the distal external iliac vessels. The harvest of the iliac vessels is usually conducted together as one bundle. It should be performed carefully with caution of over-pulling on the vessels. The harvested vessels are immersed in a jar with HTK solution and packed with two more layers of sterile bags for transportation like procured organs. The iliac arteries and veins are good vascular conduit for artery or vein reconstruction during liver and pancreas transplantation [19]. Generally, one set of iliac vessels is taken with the liver, and another set is taken with the pancreas.
Organ Package and Transportation in Liver Graft
The liver is inspected in the basin. The first sterile bag is filled with the liver graft and 700–1,000 mL of HTK solution (4°C), and the bag is secured with a tie. It is then placed into the second sterile bag filled with 1 L of cold normal saline or slush ice and tied. The second bag is placed into the third bag and tied. The liver in the three-layered bag is then placed in the heat preservation container box filled with ice blocks for transportation [16,17].
SMALL BOWEL PROCUREMENT
The small bowel procurement procedure consists of warm organ dissection with intact donor circulation, aortic perfusion, and post-procurement bench surgery [20].
Organ Warm Dissection
It begins with the standard techniques of abdominal organ procurement. A thorough abdominal exploration is quickly performed to identify the small bowel conditions and contraindications for transplant. The extension of the Cattell–Braasch maneuver with the mobilization of the right colon and duodenum exposes the distal abdominal aorta and IVC from the iliac bifurcation up to the SMA. In isolated small bowel procurement, proximal intestinal dissection is initiated at the ligament of Treitz. The highest jejunal arcades are separated close to the jejunal wall, and the proximal jejunum is divided 5–10 cm distal to the ligament of Treitz with a linear stapler. The gastrocolic omentum is divided from the pylorus up to the splenic flexure of the colon, and then, the left colon is mobilized.
The preparation of the intestinal graft continues with the division of the terminal ileum closely proximal the ileocecal valve (Fig. 13), following the dissection laterally and distally along the mesocolon outside of the ileocolic SMA arcade. It is emphasized that careful dissection should be performed to avoid the disruption of any vascular arcade with maximal preservation of the terminal ileum. If the right colon retrieval is needed together, the colon resection line should be distal to the shedding area of the middle colic artery. The distal colon has been free from the splenic flexure and retracted out of the surgical field. The duodenum should be preserved for pancreatic procurement. According to the medial visceral rotation, it allows that the tail of the pancreas with the spleen, left side of the aorta, and origin of the mesenteric vessels are exposed. In the standard simultaneous procurement of the small bowel, pancreas, and liver, the GDA is ligated during the retrieval of the liver, and the pancreas will be deprived of the superior pancreaticoduodenal artery, which is the terminal branch of the GDA. Therefore, it is emphasized that the inferior pancreaticoduodenal artery should be intact with the pancreas to avoid the devascularization of the pancreatic head and uncinate process.
Fig. 13.
Level of the transection for small bowel procurement.
In situ flushing
The remaining dissection is identical via abdominal solid organs cold perfusion. At the time of cold perfusion, the intestine should not be in direct contact with slush ice using an isolated laparotomy pad to prevent subserosal hematoma. Intestinal perfusion is limited to approximately 500–1,000 mL by manual compression of the SMA to avoid hyperperfusion [21]. In addition, the use of the IMV for flushing during intestinal procurement is discouraged, as high flow and pressure in the IMV flush decrease the intestinal outflow through the SMV [22]. After cold perfusion, the liver is removed first, and the mesenteric root at the bisected ligament of Treitz is dissected medially to isolate the SMA and SMV immediately below the takeoff of the middle colic vessels. Great care must be taken to maintain the vascular integrity of the pancreas through the preservation of the inferior pancreaticoduodenal arcades. Therefore, pancreas and intestine procurement should be performed simultaneously with discussion between the two teams. The intestinal allograft remains attached by its vascular pedicle and is separated using a TA 60 stapling device just distal to the middle colic vessels, followed by sharp transection to create an intestinal allograft vascular cuff (Fig. 14). Gently separating the SMA and SMV 2–3 cm below the uncinate process of the pancreas is safe. The anterior side of the mesenteric root should be marked with a polypropylene suture to avoid rotated implantation. The totally procured intestinal allograft is wrapped in a surgical pad before removal en bloc, leaving the pancreas free for isolated procurement [23].
Fig. 14.
The surgical stapling device is placed immediately below the middle colic vessels.
Back-table procedure
Isolated small bowel grafts require little revision during the bench procedure. However, in most cases, the vascular conduits are required to lengthen the graft arterial supply or venous drainage. Usually, either a short portion of the external iliac or superficial femoral artery and vein is of suitable caliber for anastomosis to the superior mesenteric vessels in an end-to-end fashion. The procured donor isolated small bowel is ready for transplantation.
PANCREATIC PROCUREMENT
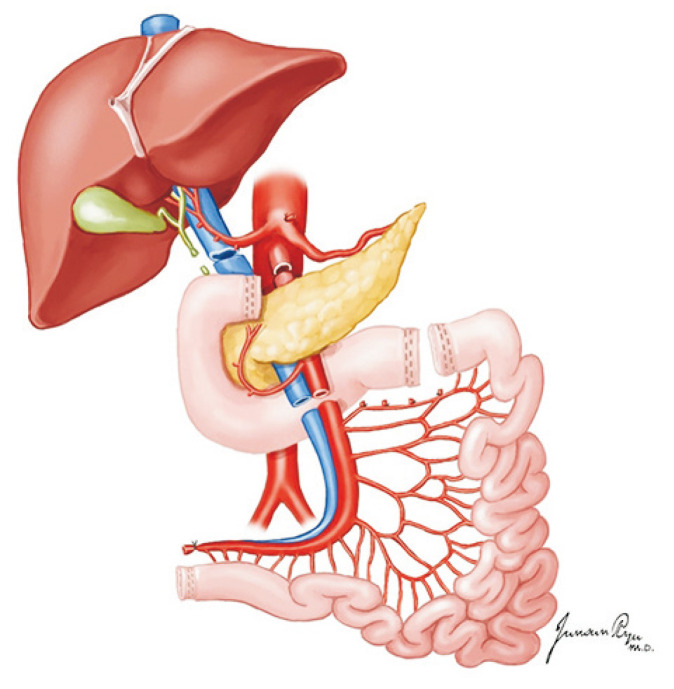
The recovery of the pancreas from a deceased donor is a part of standardized multi-organ recovery [24]. The recovery process starts with the observation and palpation of the pancreas to decide whether the pancreas is suitable for transplantation, and the most important method for deciding the appropriateness of the pancreas for transplantation is a direct visual inspection at the time of recovery. First, the portions of the head and body are exposed after the dissection of the hepatogastric ligament. Then, the greater omentum is separated from the transverse colon to open the lesser sac, and the whole pancreas is exposed for evaluation (Fig. 15). Most surgeons give up pancreas recovery in cases of significant calcification, fibrosis, fat infiltration, and edema in the pancreas or severe atherosclerosis in the feeding arteries.
Fig. 15.
The greater omentum is separated from the transverse colon to open the lesser sac, and the whole pancreas is exposed for evaluation.
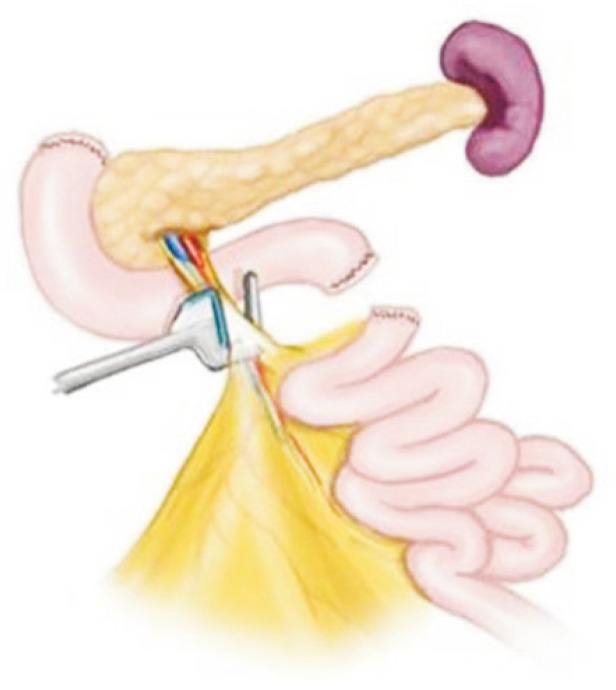
When the pancreas is considered suitable for recovery, the dissection of the pancreas and duodenum is initiated. First, the pancreatic head, aorta, and IVC are exposed after dissection with the Kocher maneuver. The anterior and posterior pancreaticoduodenal arteries are ligated to apply the gastrointestinal anastomosis (GIA) stapler. The right gastric artery is ligated as well as the supraduodenal arteries. Caution is needed to avoid injuring atypical right hepatic arteries originating from the SMA. After the ligation of the supraduodenal arteries, the GDA from the common hepatic artery is exposed. For tagging with 6–0 polypropylene at the time of recovery, the GDA is encircled with a vessel loop. Dissection should progress from the GDA to the celiac trunk to identify the origin of the splenic artery that should be encircled with a vessel loop. The IMV at the lower border of the pancreas should be identified and encircled with a vessel loop. If the surgeon in charge of liver recovery considers carrying out portal perfusion through the IMV, it is important not to insert the cannula deep into the pancreas.
A nasogastric tube is lowered to the ligament of Treitz, and the proximal jejunum is clamped for duodenal irrigation with a mixture of antibiotics and antifungals and normal saline. After irrigation, the nasogastric tube is repositioned up to the stomach. The proximal duodenum is separated from the pylorus with a GIA 60 stapler (Fig. 16).
Fig. 16.
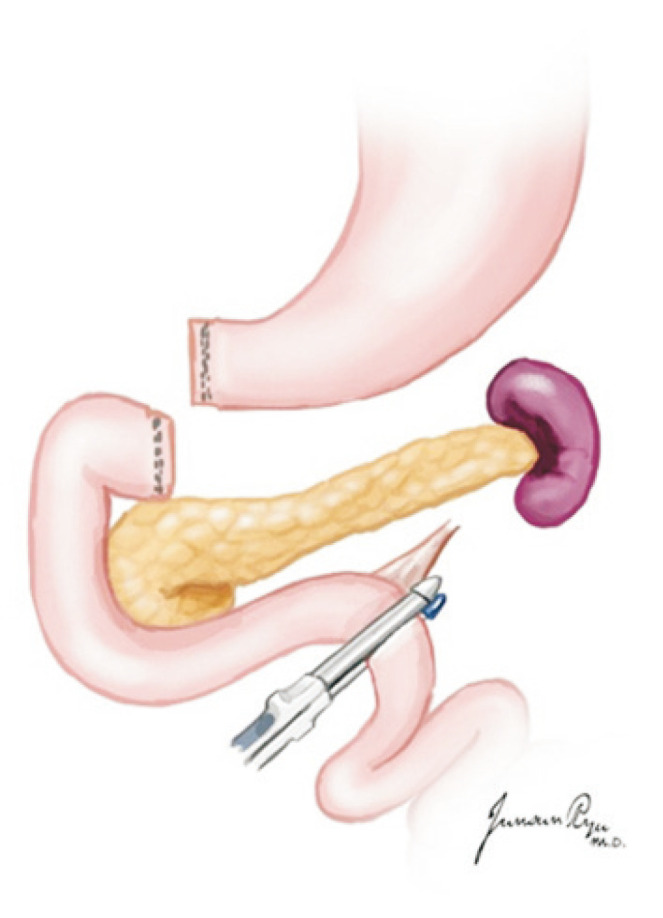
The first portion of the duodenum is circumferentially dissected, and a mechanical stapler is used to divide the duodenum from the stomach just distal to the pylorus. The stapler is also used to divide the duodenum just distal to the ligament of Treitz.
After perfusion with the HTK solution, the pancreas and liver is usually separated in situ. The splenic artery is separated from the celiac trunk at the origin, and the GDA is divided from the common hepatic artery at the origin; both arteries should be tagged with 6–0 polypropylene before separation. The portal vein should be divided at an appropriate point to secure a proper length. After finishing liver recovery, the SMA is separated at its origin from the aorta (Fig. 17). During the separation of the SMA, caution should be taken not to injure the renal arteries. The IMV is ligated at the lower border of the pancreas. The mesenteric root below the uncinate process is divided with a TA 90 stapler. The spleen is separated from the stomach by dividing the short gastric arteries. While the spleen is held in the supporting hand, the distal pancreas and spleen are separated from adjacent tissues (Fig. 18). After recovery of both kidneys, the en bloc dissection of the common, external, and internal iliac arteries is performed for Y-graft interposition at the time of back-table procedures (Fig. 19) [25].
Fig. 17.
The superior mesenteric artery (SMA) is separated at its origin from the aorta. During the separation of the SMA, caution should be taken not to injure the renal arteries.
Fig. 18.
While the spleen is held in the supporting hand, the distal pancreas and spleen are separated from adjacent tissues.
Fig. 19.
The procurement of the common, external, and internal iliac arteries is performed for Y-graft interposition at the time of back-table procedures.
KIDNEY PROCUREMENT
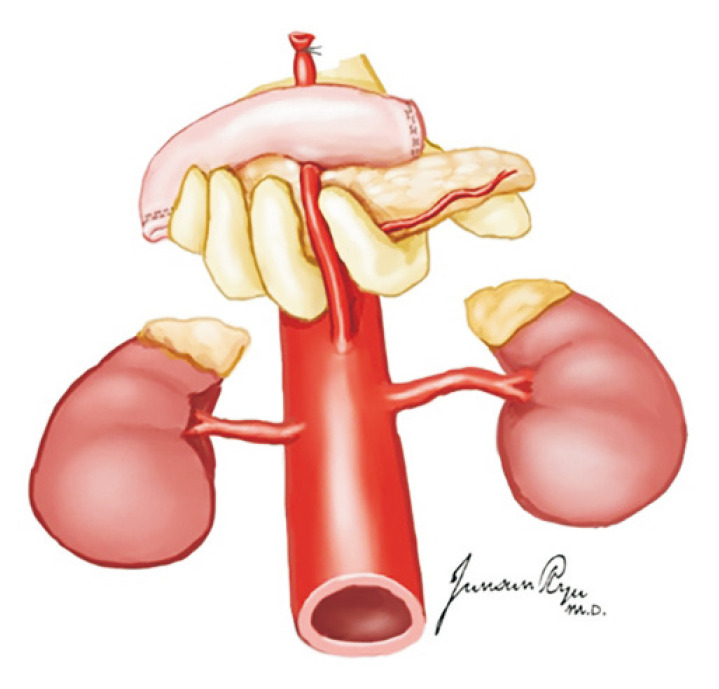
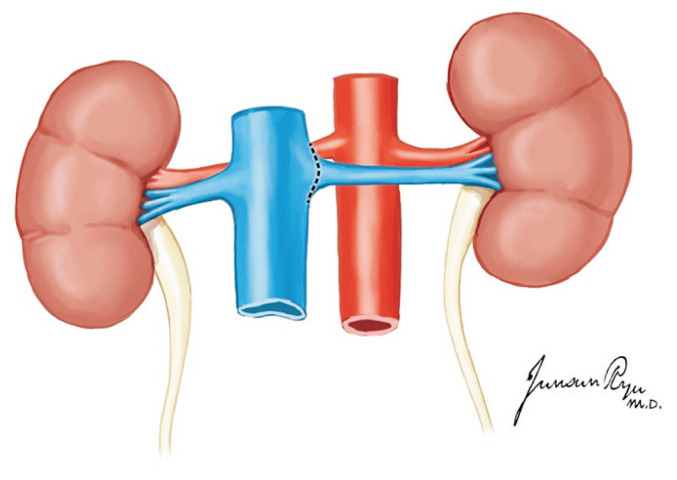
There are no significant differences in the long-term allograft function between multi-organ and kidney-only donors [26]. If the pancreas is not to be recovered, the duodenum and pancreas are mobilized to the left side to expose the IVC, aorta, right kidney, and right ureter [26]. When the pancreas is recovered, the retroperitoneal organs may be more easily exposed. The right ureter is visible at the level of the right common iliac artery and dissected and transected near the bladder [16]. The tip of the right ureter is held with mosquito forceps. In the same manner as in the right ureter, the left ureter is dissected after the mobilization of the descending and sigmoid colons. Thereafter, both ureters are proximally dissected while preserving the peri-ureteric vessels and tissues. Of note, the lower polar artery should be preserved to prevent ureteric complications such as stricture and leakage [27]. After ureter dissection, the aorta and IVC are transected at the iliac bifurcation level and are pulled upward with mosquito forceps to separate them from the lumbar spines. Afterward, the proximal ends of the aorta and IVC are transected and separated from the lumbar spines. The en bloc kidneys are recovered after the dissection of the retroperitoneal neurofibrous tissues (Fig. 20).
Fig. 20.
The two kidneys are removed en bloc with the cava and aorta. The lateral attachments of the kidney is divided, and the ureters are traced caudally and transected distally near the bladder junction (A). The aorta and inferior vena cava are divided at their bifurcations. The ureters and these vessels are retracted cephalad and anteriorly, along with the two kidneys (B), and dissection proceeds along their posterior aspects, anterior to the surface of the vertebral bodies and psoas muscle (C).
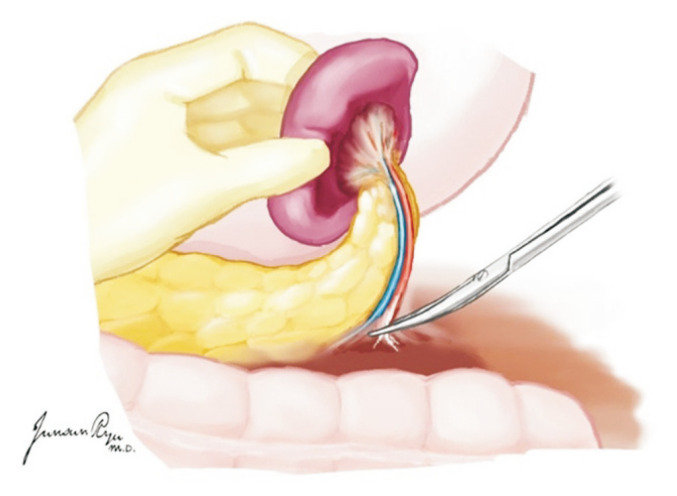
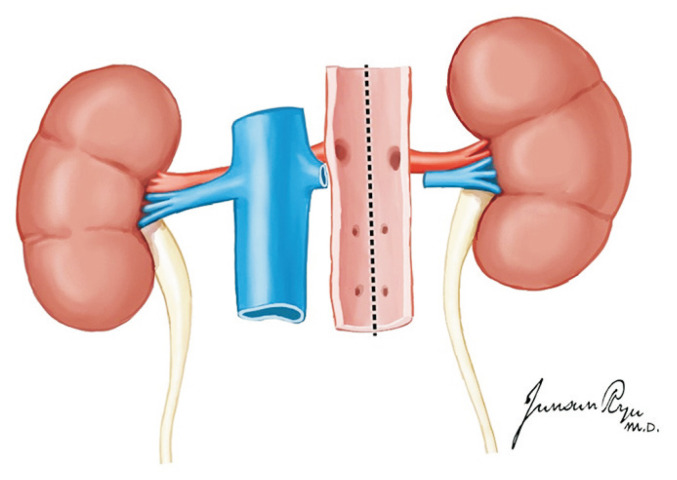
The kidneys are placed en bloc in a cold preservation solution and separated on the back table. The left renal vein is dissected at its junction with the IVC (Fig. 21). The aortic wall is then divided longitudinally with the inspection of the renal artery orifices from within the aortic lumen (Fig. 22). The posterior aortic wall is then divided between the renal artery orifices. This completes the division of the right and left kidneys.
Fig. 21.
The left renal vein is identified and divided at its junction with the inferior vena cava.
Fig. 22.
The aortic wall is divided longitudinally down its center aspect, which allows for inspection of the renal artery orifices.
CONCLUSION
The current shortage of suitable donor organs underlines the need for optimization of deceased donor management. Treatment strategies aim at reducing the detrimental effects of a cascade of hemodynamic, inflammatory, and immunologic events that affect the outcome of transplanted organs. The optimal management of the potential organ donor can help increase the supply of organs for transplantation.
Organ procurement from a deceased donor should be planned in advance because the procedure affects the outcomes of transplantation. The importance of having a firm grasp on the surgical technique of both the harvest and organ transplantation cannot be overemphasized.
ACKNOWLEDGMENTS
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding/Support
All figures of this article are sourced from the Brain Death Organ Procurement Guidelines of Korean Society for Transplantation.
Author Contributions
Conceptualization: HCY. Data curation: HJA. Formal analysis: DJJ. Funding acquisition: HCY. Methodology: SYH. Project administration: HCY. Visualization: JMK. Writing–original draft: SL, SJH, JMK, JKH, SS. Writing–review & editing: HPH.
Additional Contributions
This article was produced by the Education Committee of the Korean Society for Transplantation. The authors appreciate the support of the society and committee.
REFERENCES
- 1.Dare AJ, Bartlett AS, Fraser JF. Critical care of the potential organ donor. Curr Neurol Neurosci Rep. 2012;12:456–65. doi: 10.1007/s11910-012-0272-9. [DOI] [PubMed] [Google Scholar]
- 2.Patel MS, Abt PL. Current practices in deceased organ donor management. Curr Opin Organ Transplant. 2019;24:343–50. doi: 10.1097/MOT.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 3.Souter MJ, Eidbo E, Findlay JY, Lebovitz DJ, Moguilevitch M, Neidlinger NA, et al. Organ donor management: part 1. toward a consensus to guide anesthesia services during donation after brain death. Semin Cardiothorac Vasc Anesth. 2018;22:211–22. doi: 10.1177/1089253217749053. [DOI] [PubMed] [Google Scholar]
- 4.van Erp AC, van Dullemen LF, Ploeg RJ, Leuvenink HG. Systematic review on the treatment of deceased organ donors. Transplant Rev (Orlando) 2018;32:194–206. doi: 10.1016/j.trre.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Chamorro-Jambrina C, Muñoz-Ramírez MR, Martínez- Melgar JL, Pérez-Cornejo MS. Organ donor management: eight common recommendations and actions that deserve reflection. Med Intensiva. 2017;41:559–68. doi: 10.1016/j.medin.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Chudoba P, Krajewski W, Wojciechowska J, Kamińska D. Brain death-associated pathological events and therapeutic options. Adv Clin Exp Med. 2017;26:1457–64. doi: 10.17219/acem/65068. [DOI] [PubMed] [Google Scholar]
- 7.Opdam HI. Hormonal therapy in organ donors. Crit Care Clin. 2019;35:389–405. doi: 10.1016/j.ccc.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Berthelsen PG, Marik PE. Therapeutic hypothermia in deceased organ donors and kidney-graft function. N Engl J Med. 2015;373:2686. doi: 10.1056/NEJMc1511744. [DOI] [PubMed] [Google Scholar]
- 9.Miñambres E, Pérez-Villares JM, Chico-Fernández M, Zabalegui A, Dueñas-Jurado JM, Misis M, et al. Lung donor treatment protocol in brain dead-donors: a multicenter study. J Heart Lung Transplant. 2015;34:773–80. doi: 10.1016/j.healun.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen DC, Loor G, Carrott P, Shafii A. Review of donor and recipient surgical procedures in lung transplantation. J Thorac Dis. 2019;11(Suppl 14):S1810–6. doi: 10.21037/jtd.2019.06.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inci I. Donors after cardiocirculatory death and lung transplantation. J Thorac Dis. 2017;9:2660–9. doi: 10.21037/jtd.2017.07.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Perrot M, Fischer S, Liu M, Jin R, Bai XH, Waddell TK, et al. Prostaglandin E1 protects lung transplants from ischemia-reperfusion injury: a shift from pro- to anti-inflammatory cytokines. Transplantation. 2001;72:1505–12. doi: 10.1097/00007890-200111150-00006. [DOI] [PubMed] [Google Scholar]
- 13.Oto T, Rabinov M, Negri J, Marasco S, Rowland M, Pick A, et al. Techniques of reconstruction for inadequate donor left atrial cuff in lung transplantation. Ann Thorac Surg. 2006;81:1199–204. doi: 10.1016/j.athoracsur.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 14.Slama A, Schillab L, Barta M, Benedek A, Mitterbauer A, Hoetzenecker K, et al. Standard donor lung procurement with normothermic ex vivo lung perfusion: a prospective randomized clinical trial. J Heart Lung Transplant. 2017;36:744–53. doi: 10.1016/j.healun.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Huddleston CB, Richey SR. Heart-lung transplantation. J Thorac Dis. 2014;6:1150–8. doi: 10.3109/9781420019285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu HC, Cho BH. How to do I make an organ procurement in deceased donor? J Korean Soc Transplant. 2006;20:14–24. [Google Scholar]
- 17.Choi YR, Lee KW. Liver procurement. J Korean Soc Transplant. 2015;29:109–17. doi: 10.4285/jkstn.2015.29.3.109. [DOI] [Google Scholar]
- 18.Keutgen XM, Petrowsky H. Procurement for visceral organ transplantation: where to cannulate and how to perfuse? Curr Opin Organ Transplant. 2014;19:92–9. doi: 10.1097/MOT.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 19.Makowka L, Stieber AC, Sher L, Kahn D, Mieles L, Bowman J, et al. Surgical technique of orthotopic liver transplantation. Gastroenterol Clin North Am. 1988;17:33–51. [PMC free article] [PubMed] [Google Scholar]
- 20.Nickkholgh A, Contin P, Abu-Elmagd K, Golriz M, Gotthardt D, Morath C, et al. Intestinal transplantation: review of operative techniques. Clin Transplant. 2013;27 Suppl 25:56–65. doi: 10.1111/ctr.12190. [DOI] [PubMed] [Google Scholar]
- 21.Wunderlich H, Brockmann JG, Voigt R, Rauchfuss F, Pascher A, Brose S, et al. DTG procurement guidelines in heart beating donors. Transpl Int. 2011;24:733–57. doi: 10.1111/j.1432-2277.2011.01266.x. [DOI] [PubMed] [Google Scholar]
- 22.Humar A, Payne WD, Matas AJ. Atlas of organ transplantation. Springer; London, UK: 2006. [DOI] [Google Scholar]
- 23.Yersiz H, Renz JF, Hisatake GM, Gordon S, Saggi BH, Feduska NJ, Jr, et al. Multivisceral and isolated intestinal procurement techniques. Liver Transpl. 2003;9:881–6. doi: 10.1053/jlts.2003.50155. [DOI] [PubMed] [Google Scholar]
- 24.Hakim NS SR, Gray D, Friend P, Colman A. Pancreas, islet and stem cell transplantation for diabetes. 2nd ed. Oxford University Press; Oxford, UK: 2010. [DOI] [Google Scholar]
- 25.Yang HC, Gifford RR, Dafoe DC, Neumyer MM, Thiele BL. Arterial reconstruction of the pancreatic allograft for transplantation. Am J Surg. 1991;162:262–4. doi: 10.1016/0002-9610(91)90083-P. [DOI] [PubMed] [Google Scholar]
- 26.PM, author. Techniques of multiple organ harvesting. In: Morris PJ, Tilney NL, editors. Progress in transplantation. Edinburgh. Churchill Livingstone; burgh, UK: 1984. [Google Scholar]
- 27.Knechtle S, Morris P. Kidney transplantation. 6th ed. Saunders Elsevier; Philadelphia, PA: 2008. [Google Scholar]