Abstract
Amylovorin L471 is a small, heat-stable, and hydrophobic bacteriocin produced by Lactobacillus amylovorus DCE 471. The nutritional requirements for amylovorin L471 production were studied with fed-batch fermentations. A twofold increase in bacteriocin titer was obtained when substrate addition was controlled by the acidification rate of the culture, compared with the titers reached with constant substrate addition or pH-controlled batch cultures carried out under the same conditions. An interesting feature of fed-batch cultures observed under certain culture conditions (constant feed rate) is the apparent stabilization of bacteriocin activity after obtaining maximum production. Finally, a mathematical model was set up to simulate cell growth, glucose and complex nitrogen source consumption, and lactic acid and bacteriocin production kinetics. The model showed that bacterial growth was dependent on both the energy and the complex nitrogen source. Bacteriocin production was growth associated, with a simultaneous bacteriocin adsorption on the producer cells dependent on the lactic acid accumulated and hence the viability of the cells. Both bacteriocin production and adsorption were inhibited by high concentrations of the complex nitrogen source.
Lactic acid bacteria are known to have an antagonistic activity toward a variety of microorganisms. Bacteriocin production is one of the properties responsible for the antibacterial activity against closely related species and possibly gram-positive food spoilers and pathogens (10, 18). Bacteriocins produced by lactic acid bacteria are either small thermostable peptides or large thermolabile proteins. Large numbers of bacteriocin producers have been found among different genera of the lactic acid bacteria (10, 11, 13, 14, 29). Bacteriocins are of interest for potential application in the food industry because of their antimicrobial activity and their technologically favorable properties (10, 30).
Optimal bacteriocin production in batch fermentation usually requires complex media and well-controlled physical conditions, such as temperature and pH (3, 4, 9, 17, 20, 23, 27, 28, 34). Bacteriocin production is correlated with bacterial growth, implying that the volumetric bacteriocin production is dependent on the total biomass formation (6, 9, 23). After reaching a maximal bacteriocin activity in the fermentation medium during the active growth phase, often a drastic decrease in soluble bacteriocin activity occurs. This disappearance of bacteriocin activity was ascribed to proteolytic inactivation (9, 16), protein aggregation (6, 7), and adsorption of the bacteriocin molecules to the cell surface of the producer cells (6, 26, 27, 33). Mørtvedt-Abildgaard et al. (23) proposed adding ethanol to the fermentation medium to prevent aggregation. Matsusaki et al. (21) found that the use of calcium in the fermentation medium prevented the adsorption of nisin Z to the producer cells. Fed-batch fermentation technology allows the obtaining of high cell densities through the continuous supply of fresh medium. The growth rate can be controlled by the application of growth-limiting feeding strategies. Bacteriocin production in fed-batch fermentation to prevent substrate inhibition has already been described for nisin (8) and epidermin and gallidermin (32). Bacteriocin production was modeled in a few cases only (19, 20, 26–28). The models were set up with a term for growth-associated bacteriocin production and a term for bacteriocin degradation or adsorption.
Lactobacillus amylovorus DCE 471 produces a small, heat-stable, and strongly hydrophobic bacteriocin, named amylovorin L471, with a narrow inhibitory spectrum (7). The effects of different physical and chemical factors on the production of amylovorin L471 in batch fermentation have already been studied (6, 19). A decrease in the activity of soluble amylovorin L471 after the active growth phase was due to adsorption of the bacteriocin molecules to the producer cells. In this paper, fed-batch fermentations were studied to examine the nutritional requirements of bacteriocin production. In addition, it is shown that amylovorin L471 production could be improved and stabilized by adequate nutrient feeding. A mathematical model was set up to simulate L. amylovorus DCE 471 cell growth and bacteriocin production during fed-batch fermentation.
MATERIALS AND METHODS
Bacterial strains, maintenance, inoculum preparation, and media.
L. amylovorus DCE 471 was used as the producer strain of the bacteriocin amylovorin L471. Lactobacillus delbrueckii subsp. bulgaricus LMG 6901T was used as a sensitive indicator organism for detection of bacteriocin activity. The strains were stored at −80°C in MRS medium (Oxoid, Basingstoke, United Kingdom) containing 25% (vol/vol) glycerol. Cultures were propagated twice in MRS medium (Oxoid; initial pH 6.5, 12 h, 37°C) prior to use as the inoculum (1% [vol/vol]) for the fermentation experiments. The transfer inoculum was 1% (vol/vol). Fermentations were carried out in modified MRS medium adjusted to pH 5.0 (i.e., MRS medium containing different concentrations of glucose and complex nitrogen source [CNS; see below]). The CNS consisted of the following (in grams per gram of CNS): Lab Lemco powder (Oxoid), 0.36; yeast extract (E. Merck, Darmstadt, Germany), 0.18; and bacteriological peptone (Oxoid), 0.46. All media were sterilized by being heated at 121°C for 20 min. Glucose was sterilized separately and added aseptically to the medium. Bottom and overlay agar media were prepared by addition of, respectively, 15 and 7 g of granulated agar (Oxoid) to 1 liter of MRS medium (Oxoid).
Fermentation experiments.
A 15-liter stainless steel Biostat C fermentor (B. Braun Biotech International, Melsungen, Germany) that was in situ sterilizable was used throughout this study. The software program Micro-MFCS for Windows NT (B. Braun Biotech International) was used to control the fermentation process. Fermentations were carried out, without aeration, at a controlled temperature of 37°C and at a controlled constant pH of 5.0 through the automatic addition of 10 N NaOH. Slow agitation (50 rpm) was maintained to keep the medium homogeneous. Fermentations were carried out in duplicate.
For constant fed-batch fermentations (Table 1), the fermentor was filled with 8 liters of modified MRS medium containing 2 g (each) of glucose and CNS per liter (fermentations 1, 4, and 5). From the start of the fermentation, 4 liters of concentrated glucose (120 g liter−1) and CNS (264 g liter−1) was added to the fermentation medium at constant feed rates of 0.13, 0.36, and 0.06 liters per h for fermentations 1, 4, and 5, respectively (Table 1). This would result in a final added concentration of 40 g of glucose per liter and 88 g of CNS per liter in the end volume of 12 liters. This proportion of the energy source to the CNS was chosen because it was optimal for cell growth and bacteriocin production in batch fermentation (6). In fermentations 2 and 3, the initial CNS and glucose concentration of the modified MRS medium were 112 and 57 g liter−1, respectively (Table 1). During fermentation, concentrated glucose and CNS were added, respectively, which would again result in a final added concentration of 40 g of glucose per liter and 88 g of CNS per liter in the end volume of 12 liters.
TABLE 1.
Medium composition used for the different fed-batch fermentationsa
| Fermentation no., feeding rate, and feeding time | Initial concn in fermentation medium (g liter−1)
|
Concn in feeding solution (g liter−1)b
|
||
|---|---|---|---|---|
| Glucose | CNSc | Glucose | CNSc | |
| 1, constant at 0.13 liters/h for 30.5 h | 2 | 2 | 120 | 264 |
| 2, constant at 0.15 liters/h for 27.0 h | 2 | 112 | 120 | |
| 3, constant at 0.14 liters/h for 28.5 h | 57 | 2 | 264 | |
| 4, constant at 0.36 liters/h for 11.0 h | 2 | 2 | 120 | 264 |
| 5, constant at 0.06 liters/h for 72.0 h | 2 | 2 | 120 | 264 |
| 6, acidification-controlled for 12 h | 11 | 25 | 120 | 264 |
Fermentations were started with an initial volume of 8 liters of modified MRS medium; an additional 4 liters of a concentrated solution of glucose and/or CNS was added during fed-batch fermentation.
This feeding would result in a medium with final concentrations of 40 g of glucose per liter and 88 g of CNS per liter in a volume of 12 liters.
CNS contained the following (in grams per gram): Lab Lemco powder (Oxoid), 0.36; yeast extract (Merck), 0.18; bacteriological peptone (Oxoid), 0.46.
For acidification-controlled fed-batch fermentations (Table 1), the starting volume consisted of 8 liters of modified MRS medium with 11 g of glucose per liter and 25 g of CNS per liter (fermentation 6). The fermentor was fed with concentrated glucose and the CNS (see above) at an automatically controlled feeding rate according to the amount of alkali (10 N NaOH) consumed at each time. Because of the homofermentative character of L. amylovorus DCE 471, glucose was completely converted to lactic acid. The amount of alkali consumed to neutralize the lactic acid formed was hence proportional to the amount of glucose converted to lactic acid and thus was a measure of glucose consumption.
Samples were withdrawn aseptically from the fermentation medium at regular time intervals and analyzed for cell growth and bacteriocin production.
Analysis of cell growth.
Cell dry mass (CDM) determinations were performed by filtrating 50 ml of fermentation medium through 0.45-μm-pore-size filters (type HA; Millipore Corporation, Bedford, Mass.) and subsequent drying at 105°C for 24 h. Standard deviations were 0.11 g of CDM per liter. Viable cell numbers were enumerated by plating on MRS agar and expressed as CFU per milliliter.
Quantitative determination of bacteriocin titers.
Amylovorin L471 activity was measured by an adaptation of the critical dilution method used for the assay of bacteriocins (7). Serial twofold dilutions of cell-free culture supernatant containing bacteriocin were spotted (10 μl) onto fresh indicator lawns of L. delbrueckii subsp. bulgaricus LMG 6901T. These lawns were prepared by propagating fresh cultures to an optical density at 600 nm of 0.45 and adding 100 μl of the cell suspension to 3.5 ml of the overlaid agar. Overlaid agar plates were incubated at 37°C for at least 24 h. Bacteriocin activity is expressed in millions of arbitrary units (MAU). One arbitrary unit is defined as the reciprocal of the highest dilution displaying a clear zone of inhibition. When a turbid zone followed a clear zone, the critical dilution was taken as the average of the two dilutions.
Glucose and lactate concentration determination.
Glucose consumption and lactate production were determined by high-performance liquid chromatography (Waters Corporation, Milford, Mass.). Samples were pretreated with 20% (wt/vol) trichloroacetic acid to precipitate proteins. A prepacked column, RT 300-7,8 Polyspher OA KC (Merck), and a differential refraction detector (Waters) were used. As the mobile phase, a 0.005 N H2SO4 solution was used at a fixed flow rate of 0.4 ml min−1. Standard deviations were 0.04 g of glucose per liter and 0.03 g of lactic acid per liter.
Model development.
Bacterial growth and bacteriocin production with L. amylovorus DCE 471 in batch fermentation have been modeled by Lejeune et al. (19). However, the logistic equation used for cell growth was not suitable for the simulation of fed-batch fermentation processes (unpublished data). The dynamic model developed in this work is based on Monod growth kinetics.
Changes in volume as a result of nutrient feeding (Fin) and sampling (Fout) are given by the following equation:
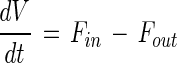 |
1 |
where V is the volume of the fermentation medium (in liters) and Fin and Fout are the inflow and outflow, respectively (in liters per hour).
Medium compounds are divided into two parts: an energy source (glucose) and a CNS that provides the necessary building blocks (e.g., amino acids, peptides, vitamins) for cell synthesis. Both compounds are necessary for bacterial growth and bacteriocin production (see below). On the other hand, high glucose concentrations and lactic acid inhibit cell growth. Biomass formation is hence given by the following equation:
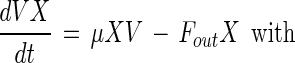 |
2 |
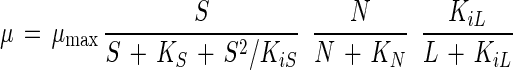 |
where X is the biomass (in grams of CDM per liter); μ and μmax are the specific growth rate and the maximal specific growth rate, respectively (per hour); S is the concentration of the energy source (in grams of glucose per liter); N is the concentration of the CNS (in grams of CNS per liter); and KS and KN are the corresponding Monod constants for glucose (in grams of glucose per liter) and CNS (in grams of CNS per liter), respectively; L is the lactic acid concentration (in grams of lactic acid per liter); and KiS and KiL are the inhibition constants for glucose (in grams of glucose per liter) and lactic acid (in grams of lactic acid per liter), respectively.
Substrate (glucose and CNS) consumption is described by the linear equations
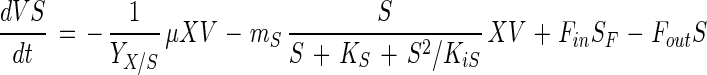 |
3 |
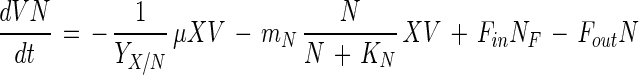 |
4 |
where YX/S and YX/N are the yield coefficients of biomass for glucose (in grams of CDM per gram of glucose) and CNS (in grams of CDM per gram of CNS), respectively; mS and mN are the maintenance coefficients for glucose (in grams of glucose per gram of CDM per hour) and CNS (in grams of CNS per gram of CDM per hour), respectively; and SF and NF are the concentrations of glucose (in grams of glucose per liter) and CNS (in grams of CNS per liter) in the feed, respectively. Because the maintenance metabolism of lactic acid bacteria seems to be strongly dependent on growth rate, a similar limitation by glucose and CNS was introduced (19, 25).
Since glucose is homofermentatively converted to lactic acid by L. amylovorus, formation of lactic acid is described by the equation
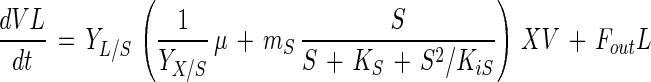 |
5 |
where YL/S is the yield coefficient of lactic acid for glucose (in grams of lactic acid per gram of glucose).
Bacteriocin production is described as growth associated and is inhibited by high concentrations of the CNS. Bacteriocin adsorption is proportional to the bacteriocin titer, is dependent on the physiological state of the cells and in particular on the lactic acid concentration, and is inhibited by the CNS. These observations were also made by Meghrous et al. (22) and Parente et al. (26). Consequently, soluble bacteriocin activity in the fermentation medium is given by the equation
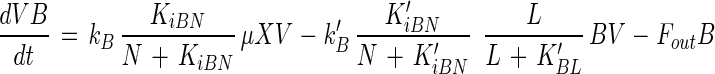 |
6 |
where B is the bacteriocin titer (in MAU per liter), kB is the specific bacteriocin production (in MAU per gram of CDM), KiBN is the CNS inhibition constant for bacteriocin production (in grams of CNS per liter), k′B is the specific bacteriocin adsorption rate (per hour), K′iBN is the CNS inhibition constant for bacteriocin adsorption (in grams of CNS per liter), and K′BL is the lactic acid constant for bacteriocin adsorption (in grams of lactic acid per liter).
Determination of biokinetic parameters.
The Euler integration method was used to simulate fermentation runs according to the model equations. Model parameters were determined by nonlinear regression. The sum of the squared differences between simulated and experimental values was minimized by varying the values of the model parameters. The growth parameters μmax, YL/S, and YX/S were obtained from simulation of batch fermentations run at 37°C and controlled pH of 5.0 in modified MRS medium with initial concentrations of 40 g of glucose per liter and 88 g of CNS per liter (6). The parameters KS, KiL, and mS were obtained from simulations of fed-batch fermentations with a high initial CNS concentration and feeding of concentrated glucose. The parameters KN, YX/N, mN, and KiS were obtained from simulations of fed-batch fermentations with a high initial glucose concentration and feeding of the concentrated CNS.
Calculation of Fin and Fout.
The inflow (Fin) was calculated as the sum of alkali, glucose, and CNS supplies. These experimental values were monitored online. For the simulation of the fermentation profiles, the supply of alkali was calculated from the simulated amount of lactic acid produced (equation 5). One mole of lactic acid was neutralized with 1 mol of NaOH. For the simulation of acidification-controlled fed-batch fermentations, the supplies of glucose and CNS were calculated from the simulated supply of alkali. Simulated values were compared to the experimental values (see Results). The outflow (Fout) was calculated from the experimental sample volumes during discrete time intervals (see Results).
RESULTS
Influence of a constant addition of glucose and/or CNS.
Three fed-batch fermentations were carried out with constant feeding of glucose and/or CNS during approximately 30 h from the start of the fermentation (Fig. 1, 2, and 3). Fermentations started with a low initial concentration of glucose and/or CNS. Feeding of concentrated glucose and/or CNS would result in a medium with a final concentration of 40 g of glucose per liter and 88 g of CNS per liter in the final volume of 12 liters (Table 1). This experimental design enabled us to find out the effect of glucose and CNS limitation on cell growth and bacteriocin production.
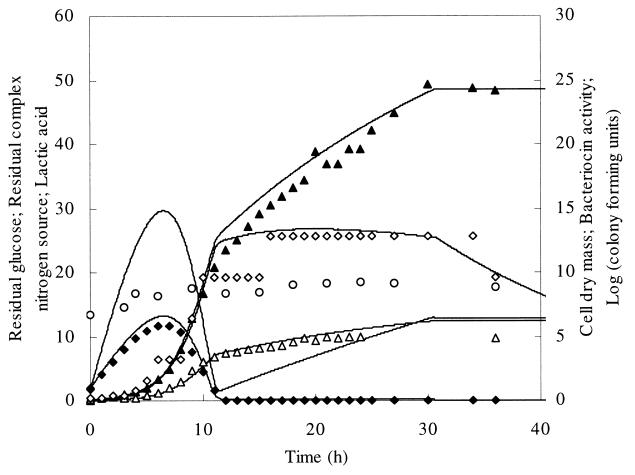
FIG. 1.
Fed-batch fermentation of L. amylovorus DCE 471 with constant addition of glucose and CNS from the start of the fermentation during 30.5 h. ▵, biomass (X; grams of CDM per liter); ⧫, residual glucose (S; grams of glucose per liter); ▴, lactic acid (L; grams of lactic acid per liter); ◊, bacteriocin activity (B; MAU per liter); ○, CFU (log CFU per milliliter). The corresponding lines represent simulated values. The line without experimental points represents the residual CNS (N; grams of CNS per liter).
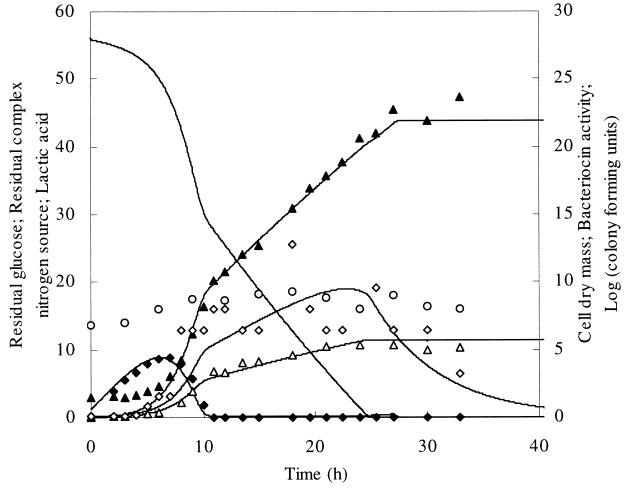
FIG. 2.
Fed-batch fermentation of L. amylovorus DCE 471 with constant addition of glucose from the start of the fermentation during 27.0 h. Symbols and lines are similar to those in Fig. 1, except that for clarity, the residual CNS is expressed in grams of CNS per liter ×2−1. The values of the CNS on the left y axis have to be multiplied by 2.
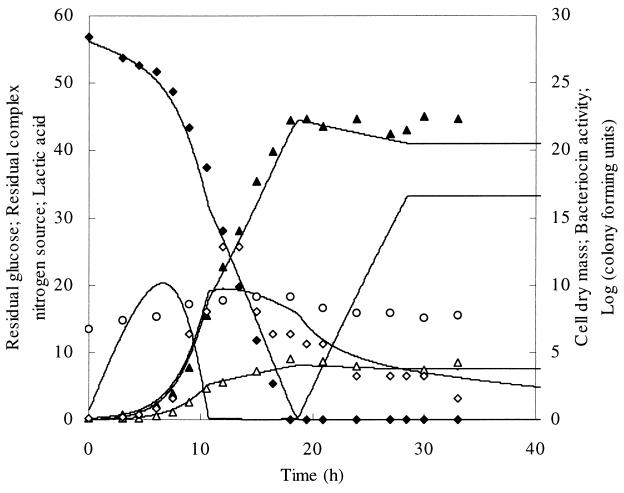
FIG. 3.
Fed-batch fermentation of L. amylovorus DCE 471 with constant addition of CNS from the start of the fermentation during 28.5 h. Symbols and lines are similar to those in Fig. 1.
In the first fed-batch fermentation experiment, glucose and the CNS were added to the fermentation medium at a constant rate of 0.13 liters per h (Fig. 1). An exponential growth phase of 8 h was followed by a decrease in the growth rate and finally a linear growth phase after 12 h of fermentation, when the glucose concentration became growth limiting. Bacteriocin production started at the beginning of the exponential growth phase and reached a maximum of 6.4 MAU per liter in this phase. In the beginning of the linear growth phase, when glucose was growth limiting, the amylovorin titer increased to 12.8 MAU per liter and remained constant afterwards. A final maximal biomass of 5.0 g of CDM per liter was reached. The maximal viable cell number was 1.5 × 109 CFU per ml and remained constant upon prolonged fermentation.
In a second fed-batch experiment, only glucose was fed to the fermentation medium at a constant feeding rate of 0.15 liters per h (Fig. 2). A profile similar to that of the first fermentation was obtained. Due to limitation of glucose, a linear growth phase of about 20 h followed the exponential growth phase of 9 h. A bacteriocin activity of 6.4 MAU per liter was reached at the end of exponential growth. The bacteriocin titer varied between 6.4 and 12.8 MAU per liter during linear growth. After the linear growth phase, at 30 h of fermentation, the bacteriocin activity decreased. The maximal biomass obtained was 5.0 g of CDM per liter. The maximal viable cell number was 1.9 × 109 CFU per ml and decreased slowly upon prolonged fermentation.
In a third fed-batch experiment, only CNS was fed to the fermentation medium at a constant feeding rate of 0.14 liters per hour (Fig. 3). Again, a linear growth phase followed an exponential growth phase of approximately 9 h. Glucose was still sufficiently available, indicating that the CNS was growth limiting. The activity obtained in the exponential growth phase was 6.4 MAU per liter and increased further to a maximal activity of 12.8 MAU per liter. Thereafter, the soluble bacteriocin activity decreased sharply. The maximal viable cell number was 1.5 × 109 CFU per ml and decreased along with the bacteriocin activity. A maximal biomass of 4.6 g of CDM per liter was reached when glucose was completely consumed.
Influence of time of addition of glucose and/or CNS.
In the fermentations described above, bacteriocin production could be improved by increasing the availability of nutrients. In the fourth and fifth constant fed-batch fermentation experiments, glucose and CNS were added much more slowly and much more quickly, respectively (Table 1).
Slow, constant addition of glucose and CNS (i.e., addition within 72 h of fermentation) resulted in low growth rates. Amylovorin L471 was again mainly produced during the exponential growth phase of approximately 9 h. At the end of this phase, the medium reached a maximal activity of 4.8 MAU per liter. From 9 h to the end of the fermentation, growth was restricted by the slow addition of glucose. The bacteriocin titer decreased slowly to a value of 0.4 MAU per liter after 60 h of fermentation. The presence of small measurable amounts of glucose in the fermentation medium at the end of the fermentation (0.02 and 0.05 g of glucose per liter after, respectively, 30 and 60 h of fermentation) indicated that another factor (e.g., a compound supplied with the CNS) became growth limiting or that growth was severely inhibited by lactic acid or bacteriocin molecules adsorbed to the cells. The maximal biomass production (4.4 g of CDM per liter) was lower than those of the other fed-batch fermentation runs due to the stronger nutrient limitation. The viable cell numbers reached a maximum of 9.7 × 108 CFU per ml after 9 h of fermentation and decreased slowly upon further fermentation.
Fast addition of glucose and CNS (i.e., addition within 11 h of fermentation) resulted again in an exponential growth phase of 8 h. A bacteriocin titer of 8.0 MAU per liter was obtained in this phase, and it increased further to a maximal value of 12.8 MAU per liter. A high biomass production was obtained with a maximum of 5.9 g of CDM per liter. The maximal viable cell number was 2.1 × 109 CFU per ml. After 14 h of fermentation, glucose was completely consumed, resulting in a drop in viable cell number and a corresponding decrease in bacteriocin activity.
Acidification-controlled addition of the substrates.
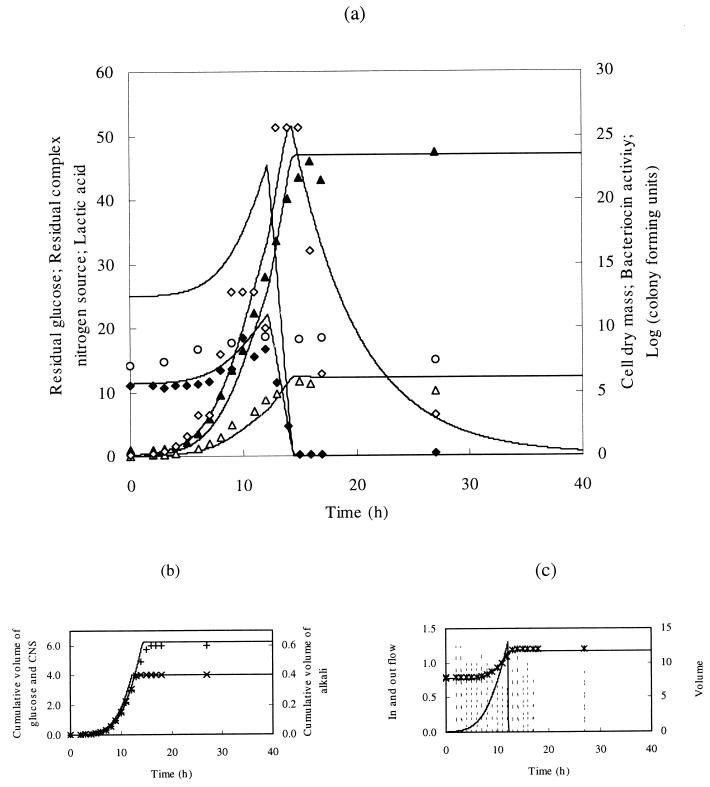
In order to prolong the exponential growth phase and hence the concomitant bacteriocin production phase, substrate was added to the fermentation medium in an acidification-controlled manner (Fig. 4). The biomass increase was exponential during feeding, since glucose and CNS were present in excess. The glucose concentration increased slowly until 12 h, when feeding stopped; apparently, the ratio of glucose to alkali in the feed was not well adjusted to keep the glucose concentration constant in the medium. At 15 h, the growth phase stopped due to a depletion of glucose. Biomass production was similar, as compared to a fast, constant fed-batch fermentation (addition within 11 h of fermentation). A maximal biomass of 5.8 g of CDM per liter was reached. The maximal bacteriocin titer averaged 25.6 MAU per liter, which is twice the amount obtained during constant fed-batch fermentation. This titer decreased strongly when glucose was completely consumed. At this time, the amount of viable cells, which had reached a maximum of 2.1 × 109 CFU per ml, decreased drastically too.
FIG. 4.
(a) Fed-batch fermentation of L. amylovorus DCE 471 with an acidification-controlled addition of glucose and CNS from the start of the fermentation during 12 h. Symbols and lines are similar to those in Fig. 1. (b) Addition of glucose, CNS, and alkali. ×, cumulative volume of glucose and CNS (liters); +, cumulative volume of alkali (liters [10 N NaOH]). (c) Volume and flow rate in and out of the fermentor. Solid line, inflow (Fin; liters per hour); broken line, outflow (Fout; liters per hour); atyp0250;, volume (liters).
Determination of cell growth and bacteriocin production model parameters.
Biokinetic cell growth parameters were determined by simulating the profiles of both batch and constant fed-batch fermentation runs according to the model described above (Table 2). The maximal specific growth rate (μmax), the yield coefficient of biomass for glucose (YX/S), the cell maintenance coefficient for glucose (mS), and the yield coefficient of lactic acid for glucose (YL/S) were comparable to those obtained for other batch fermentations (19).
TABLE 2.
Biokinetic cell growth parameters for L. amylovorus DCE 471 determined by simulation of batch and fed-batch fermentation profiles
| Parameter | Value |
|---|---|
| Maximal specific growth rate (μmax) | 0.64a h−1 |
| Monod constant for glucose (KS) | 0.70b g of glucose liter−1 |
| Yield coefficient, biomass for glucose (YX/S) | 0.20a g of CDM g of glucose−1 |
| Maintenance coefficient for glucose (mS) | 0.90b g of glucose g of CDM−1 h−1 |
| Monod constant for CNS (KN) | 0.30c g of CNS liter−1 |
| Yield coefficient, biomass for CNS (YX/N) | 0.07c g of CDM g of CNS−1 |
| Maintenance coefficient for CNS (mN) | 0.50c g of CNS g of CDM−1 h−1 |
| Yield coefficient, glucose for lactic acid (YL/S) | 1.00a g of lactic acid g of glucose−1 |
| Inhibition constant of lactic acid (KiL) | 30.0b g of lactic acid liter−1 |
| Inhibition constant of glucose (KiS) | 300.0c g of glucose liter−1 |
Obtained from a batch fermentation with initial concentrations of 40 g of glucose per liter and 88 g of CNS per liter (6).
Obtained from a fed-batch fermentation with a high initial CNS concentration and feeding of glucose.
Obtained from a fed-batch fermentation with a high initial glucose concentration and feeding of the CNS.
Bacteriocin production was clearly growth associated. The specific bacteriocin production obtained from simulation of the different fermentation runs was approximately 4.7 MAU per g of CDM (Table 3). When the fermentation was started with a high initial concentration of the CNS, similar to that in batch fermentations, a bacteriocin activity lower than expected was obtained (Fig. 2). Consequently, the specific bacteriocin production had to be adjusted to 2.4 MAU per g of CDM, similar to that in a comparable batch fermentation in modified MRS medium (40 g of glucose per liter, 88 g of CNS per liter, pH 5.0, 37°C) (results not shown). The maximal volumetric bacteriocin titer in the latter fermentation was 11.2 MAU per liter and corresponded to a maximal biomass of 5.3 g of CDM per liter (6). The introduction of a term of inhibition of the bacteriocin production by the CNS was able to demonstrate the resulting reduction in specific bacteriocin production (equation 6).
TABLE 3.
Biokinetic parameters for bacteriocin production determined by simulation of fed-batch fermentation profiles
| Parameter | Value |
|---|---|
| Specific bacteriocin production constant (kB) | 4.70 MAU g of CDM−1 |
| Bacteriocin production inhibition constant for CNS (KiBN) | 50.0 g of CNS liter−1 |
| Bacteriocin adsorption constant (k′B) | 0.50 h−1 |
| Bacteriocin adsorption inhibition constant for CNS (K′iBN) | 10.0 g of CNS liter−1 |
| Lactic acid constant for bacteriocin adsorption (K′BL) | 90.0 g of lactic acid liter−1 |
The decrease in bacteriocin activity accompanied a decrease in viable cell numbers (Fig. 3 and 4). In contrast, bacteriocin activity remained stable when no decrease in viable cells was observed (Fig. 1). Bacteriocin adsorption seemed to be dependent on the viability of the producer cells, since a higher lactic acid concentration coincided with a lower number of viable cells and hence a larger amount of bacteriocin molecules adsorbed to the cells (see equation 6). A value of 90 g of lactic acid per liter was found for the lactic acid constant for bacteriocin adsorption (Table 3). In addition, the CNS concentration seemed to have an inhibitory effect on bacteriocin adsorption (see equation 6).
Finally, the model was validated by simulation of both a slow and a fast constant fed-batch fermentation (results not shown) and an acidification-controlled fed-batch fermentation (Fig. 4).
DISCUSSION
Amylovorin L471 is produced during the active growth phase, implying that the volumetric bacteriocin production is dependent on the total biomass formation. Hence, conditions that provide high cell densities favor a high bacteriocin production. Indeed, an increased nutrient supply in batch fermentation results in an increased biomass and volumetric bacteriocin production. However, the specific bacteriocin production decreases under these conditions (6, 19). Furthermore, during batch fermentation of L. amylovorus DCE 471, a rapid decline in bacteriocin activity is observed after the growth-associated bacteriocin production phase (6, 7, 19). This decline is concomitant with the depletion of the energy source, glucose, at the end of the fermentation. It is due to adsorption of the bacteriocin molecules to the producer cells (6).
In this paper, bacteriocin production was improved by fed-batch fermentation and could be stabilized, provided an appropriate feeding strategy was applied. This cultivation technique allowed a controlled growth through limitation of glucose or the CNS. In addition, the high nutrient supply did not inhibit growth or bacteriocin production. Slow growth due to limitation of the energy or CNS was not beneficial for amylovorin L471 production. Limitation of the CNS resulted in a stagnation of amylovorin L471 production and a decrease in soluble bacteriocin activity in the fermentation medium. Under glucose-limiting conditions in a constant fed-batch fermentation, no amylovorin L471 production could be observed. Stronger limitation of the energy source resulted in a decrease in the viable cell number and a concomitant decrease in the soluble amylovorin L471 titer. Hence, for optimal bacteriocin production, the energy source and other nutrients should be sufficiently available.
When substrates were sufficiently available during a fast, constant fed-batch fermentation or an acidification-controlled fed-batch fermentation of L. amylovorus DCE 471, bacterial growth was optimal. A maximal specific bacteriocin production and volumetric bacteriocin titer seemed to have been achieved. Growth limitation by lactic acid is probably the major reason for the restricted bacteriocin production.
Slow growth upon prolonged fermentation resulted in weak or no amylovorin L471 production. A minimal growth rate seemed to be necessary for bacteriocin production. Although some bacteriocins are mainly produced in the stationary growth phase (2, 3, 15), generally bacteriocin production occurs only in the active growth phase (9, 23, 28). In contrast, Ten Brink et al. (31) reported on acidocin B production by nongrowing L. acidophilus M46 cells.
Usually, during batch fermentation experiments, the amylovorin L471 titer decreases drastically after reaching a peak of activity in the active growth phase. A drop in CFU is also observed, apparently indicating cell death. This phenomenon was shown to be due to adsorption of amylovorin L471 to the producer cells (6). The apparent loss of bacteriocin could be avoided during fed-batch fermentation. Compared to batch fermentations, a less drastic decrease or no decrease in the amylovorin L471 titer was observed after reaching maximal activity. Constant addition of glucose and CNS at an appropriate rate could stabilize the bacteriocin titer. Similarly, acidophilicin LA-1 activity decreased sharply in batch fermentation of L. acidophilus LA-1 when the death phase started. However, bacteriocin activity was stabilized by adding concentrated medium (5). Ferreira and Gilliland (12) explained the drastic drop in CFU by demonstrating the presence of a mixed culture of L. acidophilus NCFM cells containing a major fraction of sensitive nonproducing strains and a minor fraction of resistant bacteriocin-producing strains. Resistant bacteriocin-producing cells formed large and small colonies on MRS agar. Two morphologically different colonies were also observed for L. amylovorus DCE 471 plated on MRS agar (7). A limited immunity of the producing cells to their own bacteriocin may explain this phenomenon. For several bacteriocin producers, it is already well documented that genes for bacteriocin production and immunity are regulated and transcribed simultaneously (1, 24). This implies that bacteriocin nonproducing cells are sensitive to their own bacteriocin. When bacteriocin production stops during fermentation, cells become sensitive because they do not further produce immunity proteins. During fed-batch fermentation, certain nutrient compounds necessary for bacteriocin production may become limited or exhausted, resulting in more sensitive bacteria and causing a decrease in viable cell numbers. Constant feeding of nutrients enables continuous growth of at least part of the biomass. As a consequence, amylovorin L471 was still produced by this part of the L. amylovorus population due to the growth-associated bacteriocin production. The other part of the culture, nonproducers and sensitive cells, mainly adsorbed the bacteriocin molecules to their cell surfaces. While the first fraction, the growing cells, were able to form colonies on MRS agar, the second fraction, the nongrowing cells, were not. Hence, stabilization of the bacteriocin titer could be explained by assuming that bacteriocin production compensated for the loss of bacteriocin in the medium by adsorption. Once the energy or CNS was insufficient, the production/adsorption equilibrium was lost, resulting in a decrease in bacteriocin activity. In addition, the CNS possibly interfered with bacteriocin adsorption. The latter was also shown for nisin adsorption on Lactococcus lactis cells (22). However, these hypotheses require additional experimental data.
In this work, an unstructured mathematical model was set up that was able to describe the effect of nutrient limitations on growth and bacteriocin production or adsorption of L. amylovorus DCE 471 in fed-batch fermentation. The model was validated under different fed-batch fermentation conditions. From this model, the following conclusions can be extrapolated. Bacteriocin production and adsorption were considered to occur simultaneously. Whereas at a low growth rate, bacteriocin adsorption dominated, at a high growth rate, bacteriocin production dominated. In the exponential growth phase, bacteriocin adsorption was low, resulting in a high specific bacteriocin production. Subsequent reduction of the growth rate upon prolonged fermentation resulted in increased bacteriocin adsorption. Adequate nutrient feeding stabilized the bacteriocin titer by equilibrating the bacteriocin production and adsorption. However, too strong nutrient limitation resulted in a decrease in bacteriocin activity; furthermore, the CNS inhibited both bacteriocin production and adsorption. Finally, the specific amylovorin L471 production seemed to have attained a natural maximum during acidification-controlled fed-batch fermentation.
The presented results obtained by fed-batch fermentation are interesting regarding the development of continuous fermentation processes. During continuous processes, the concentrations of nutrients can be controlled in order to improve and stabilize the bacteriocin production. In addition, the continuous removal of inhibitory metabolites such as lactic acid or the bacteriocin itself should maximize volumetric bacteriocin production, especially at dilution rates that are high enough to provide the necessary nutrients.
ACKNOWLEDGMENTS
The research presented in this paper was financially supported by the Research Council of the Vrije Universiteit Brussel, the Fund for Scientific Research—Flanders, and the Biotechnology Programme of the Commission of the European Community (grants BIO2-CT943055 and ERB-CIPACT-940160).
REFERENCES
- 1.Abee T. Pore-forming bacteriocins of Gram-positive bacteria and self-protection mechanisms of producer organisms. FEMS Microbiol Lett. 1995;129:1–9. doi: 10.1016/0378-1097(95)00137-T. [DOI] [PubMed] [Google Scholar]
- 2.Bárcena J M B, Siñeriz F, González de Llano D, Rodríguez A, Suárez J E. Chemostat production of plantaricin C by Lactobacillus plantarum LL441. Appl Environ Microbiol. 1998;64:3512–3514. doi: 10.1128/aem.64.9.3512-3514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswas S R, Ray P, Johnson M C, Ray B. Influence of growth conditions on the production of a bacteriocin, pediocin AcH, by Pediococcus acidilactici H. Appl Environ Microbiol. 1991;57:1265–1267. doi: 10.1128/aem.57.4.1265-1267.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daba H, Lacroix C, Huang J, Simard R E. Influence of growth conditions on production and activity of mesenterocin 5 by a strain of Leuconostoc mesenteroides. Appl Microbiol Biotechnol. 1993;39:166–173. [Google Scholar]
- 5.Dave R I, Shah N P. Characteristics of bacteriocin produced by Lactobacillus acidophilus LA-1. Int Dairy J. 1998;7:707–715. [Google Scholar]
- 6.De Vuyst L, Callewaert R, Crabbé K. Primary metabolite kinetics of bacteriocin biosynthesis by Lactobacillus amylovorus and evidence for stimulation of bacteriocin production under unfavourable growth conditions. Microbiology. 1996;142:817–827. doi: 10.1099/00221287-142-4-817. [DOI] [PubMed] [Google Scholar]
- 7.De Vuyst L, Callewaert R, Pot B. Characterization of the antagonistic activity of Lactobacillus amylovorus DCE 471 and large scale isolation of its bacteriocin amylovorin L471. System Appl Microbiol. 1996;19:9–20. [Google Scholar]
- 8.De Vuyst L, Vandamme E J. Microbial manipulation of nisin biosynthesis and fermentation. In: Jung G, Sahl H-G, editors. Nisin and novel lantibiotics. Leiden, The Netherlands: ESCOM Science Publishers; 1991. pp. 397–409. [Google Scholar]
- 9.De Vuyst L, Vandamme E J. Influence of the carbon source on nisin production in Lactococcus lactis subsp. lactis batch fermentations. J Gen Microbiol. 1992;138:571–578. doi: 10.1099/00221287-138-3-571. [DOI] [PubMed] [Google Scholar]
- 10.De Vuyst L, Vandamme E J. Bacteriocins of lactic acid bacteria: microbiology, genetics and applications. London, United Kingdom: Blackie Academic & Professional; 1994. [Google Scholar]
- 11.Dodd H M, Gasson M J. Bacteriocins of lactic acid bacteria. In: Gasson M J, De Vos W M, editors. Genetics and biotechnology of lactic acid bacteria. London, United Kingdom: Blackie Academic & Professional; 1994. pp. 211–251. [Google Scholar]
- 12.Ferreira C L, Gilliland S E. Bacteriocin involved in premature death of Lactobacillus acidophilus NCFM during growth at pH 6. J Dairy Sci. 1988;71:306–315. doi: 10.3168/jds.S0022-0302(88)79559-4. [DOI] [PubMed] [Google Scholar]
- 13.Hoover D, Steenson L. Bacteriocins of lactic acid bacteria. New York, N.Y: Academic Press; 1993. [Google Scholar]
- 14.Jack R W, Tagg J R, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiménez-Díaz R, Rios-Sánchez R M, Desmazeaud M, Ruiz-Barba J L, Piard J-C. Plantaricins S and T, two new bacteriocins produced by Lactobacillus plantarum LPCO10 isolated from a green olive fermentation. Appl Environ Microbiol. 1993;59:1416–1424. doi: 10.1128/aem.59.5.1416-1424.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joerger M C, Klaenhammer T R. Characterization and purification of helveticin J and evidence for a chromosomally determined bacteriocin produced by Lactobacillus helveticus 481. J Bacteriol. 1986;167:439–446. doi: 10.1128/jb.167.2.439-446.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaiser A L, Montville T J. The influence of pH and growth rate on production of the bacteriocin, bavaricin MN, in batch and continuous fermentations. J Appl Bacteriol. 1993;75:536–540. [Google Scholar]
- 18.Klaenhammer T R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 1993;12:39–85. doi: 10.1111/j.1574-6976.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 19.Lejeune R, Callewaert R, Crabbé K, De Vuyst L. Modeling the growth and bacteriocin production by Lactobacillus amylovorus DCE 471 in batch cultivation. J Appl Microbiol. 1998;84:159–168. [Google Scholar]
- 20.Leroy F, De Vuyst L. Temperature and pH conditions that prevail during fermentation of sausages are optimal for production of the antilisterial bacteriocin sakacin K. Appl Environ Microbiol. 1999;65:974–981. doi: 10.1128/aem.65.3.974-981.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsusaki H, Endo N, Sonomoto K, Ishizaki A. Lantibiotic nisin-Z fermentative production by Lactococcus lactis IO-1—relationship between production of the lantibiotic and lactate and cell-growth. Appl Microbiol Biotechnol. 1996;45:36–40. doi: 10.1007/s002530050645. [DOI] [PubMed] [Google Scholar]
- 22.Meghrous J, Huot E, Quittelier M, Petitdemange H. Regulation of nisin biosynthesis by continuous cultures and by resting cells of Lactococcus lactis subsp. lactis. Res Microbiol. 1992;143:879–890. doi: 10.1016/0923-2508(92)90075-y. [DOI] [PubMed] [Google Scholar]
- 23.Møortvedt-Abildgaard C I, Nissen-Meyer J, Jelle B, Grenov B, Skaugen M, Nes I F. Production and pH-dependent bactericidal activity of lactocin S, a lantibiotic from Lactobacillus sake L45. Appl Environ Microbiol. 1995;61:175–179. doi: 10.1128/aem.61.1.175-179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nes I F, Diep D B, Håvarstein L S, Brurberg M B, Eijsink V, Holo H. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Leeuwenhoek Int Gen Mol Microbiol. 1996;70:113–128. doi: 10.1007/BF00395929. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen J, Nikolajsen K, Villadsen J. Structured modelling of a microbial system. II. Experimental verification of a structured lactic acid fermentation model. Biotechnol Bioeng. 1991;38:11–23. doi: 10.1002/bit.260380103. [DOI] [PubMed] [Google Scholar]
- 26.Parente E, Brienza C, Ricciardi A, Addario G. Growth and bacteriocin production by Enterococcus faecium DPC1146 in batch and continuous culture. J Ind Microbiol Biotechnol. 1997;18:62–67. doi: 10.1038/sj.jim.2900368. [DOI] [PubMed] [Google Scholar]
- 27.Parente E, Ricciardi A. Influence of pH on the production of enterocin 1146 during batch fermentation. Lett Appl Microbiol. 1994;19:12–15. doi: 10.1111/j.1472-765x.1994.tb00891.x. [DOI] [PubMed] [Google Scholar]
- 28.Parente E, Ricciardi A, Addario G. Influence of pH on growth and bacteriocin production by Lactococcus lactis subsp. lactis 140NWC during batch fermentation. Appl Microbiol Biotechnol. 1994;41:388–394. [Google Scholar]
- 29.Piard J C, Desmazeaud M J. Inhibiting factors produced by lactic acid bacteria. 2. Antibacterial substances and bacteriocins. Lait. 1992;72:113–142. [Google Scholar]
- 30.Ray B, Daeschel M. Food biopreservatives of microbial origin. Boca Raton, Fla: CRC Press; 1992. [Google Scholar]
- 31.Ten Brink B, Minekus M, Van der Vossen J M B M, Leer R J, Huis in't Veld J H J. Antimicrobial activity of lactobacilli: preliminary characterization and optimization of production of acidocin B, a novel bacteriocin produced by Lactobacillus acidophilus M46. J Appl Bacteriol. 1994;77:140–148. doi: 10.1111/j.1365-2672.1994.tb03057.x. [DOI] [PubMed] [Google Scholar]
- 32.Ungermann V, Goeke K, Fiedler H-P, Zähner H. Optimization of fermentation and purification of gallidermin and epidermin. In: Jung G, Sahl H-G, editors. Nisin and novel lantibiotics. Leiden, The Netherlands: ESCOM Science Publishers; 1991. pp. 410–421. [Google Scholar]
- 33.Yang R, Johnson M C, Ray B. Novel method to extract large amounts of bacteriocins from lactic acid bacteria. Appl Environ Microbiol. 1992;58:3355–3359. doi: 10.1128/aem.58.10.3355-3359.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang R, Ray B. Factors influencing production of bacteriocins by lactic acid bacteria. Food Microbiol. 1994;11:281–291. [Google Scholar]