Abstract
Spores of Bacillus subtilis possess a thick protein coat that consists of an electron-dense outer coat layer and a lamellalike inner coat layer. The spore coat has been shown to confer resistance to lysozyme and other sporicidal substances. In this study, spore coat-defective mutants of B. subtilis (containing the gerE36 and/or cotE::cat mutation) were used to study the relative contributions of spore coat layers to spore resistance to hydrogen peroxide (H2O2) and various artificial and solar UV treatments. Spores of strains carrying mutations in gerE and/or cotE were very sensitive to lysozyme and to 5% H2O2, as were chemically decoated spores of the wild-type parental strain. Spores of all coat-defective strains were as resistant to 254-nm UV-C radiation as wild-type spores were. Spores possessing the gerE36 mutation were significantly more sensitive to artificial UV-B and solar UV radiation than wild-type spores were. In contrast, spores of strains possessing the cotE::cat mutation were significantly more resistant to all of the UV treatments used than wild-type spores were. Spores of strains carrying both the gerE36 and cotE::cat mutations behaved like gerE36 mutant spores. Our results indicate that the spore coat, particularly the inner coat layer, plays a role in spore resistance to environmentally relevant UV wavelengths.
Dormant bacterial endospores are highly resistant to a number of physical and chemical treatments which are normally considered germicidal (reviewed in reference 37). Spores owe some of their resistance to the presence of an outer proteinaceous layer termed the spore coat (reviewed in references 1, 6, 9, and 28). The question of how the spore coat contributes to spore resistance is currently a subject of considerable investigation (reviewed in reference 6). Experimental evidence indicates that the coat protects a dormant spore from enzymes, such as lysozyme (11), and from mechanical disruption (1, 9). The spore coat also protects the spore from some chemicals, such as hydrogen peroxide (H2O2) (11), but not others, such as organic solvents (19). Spore resistance to organic solvents and heat seems to be a function of the peptidoglycan cortex which underlies the coat (19, 29, 30), and protection of spore DNA from 254-nm UV radiation and from free radical damage is associated with binding of spore DNA by small, acid-soluble spore proteins in the spore core (reviewed in reference 37). Much of the information regarding the role of the spore coat in spore resistance is derived from studies in which workers examine spore resistance after the coat protein layers are chemically removed by treatment with reducing and protein-denaturing agents (24, 36, 41). Although it is not known how such harsh chemical treatments alter other spore components, such as the cortex, membranes, or core, there is some evidence which suggests that chemical decoating may also affect these protective structures (3, 46).
The synthesis and substructure of the spore coat were examined initially by electron microscopy and biochemical characterization of spore coat proteins (1) and more recently by molecular biological techniques (6, 49). Electron microscope studies have revealed that the spore coat of Bacillus subtilis is actually an ordered structure consisting of the following three morphologically distinct layers: an electron-dense outer coat, a thinner lamellalike inner coat, and an electron-diffuse undercoat (1, 6, 33a). The molecular events that underlie the control of coat protein synthesis and morphogenesis of the coat layers during B. subtilis sporulation have been the subject of recent intensive studies. All coat structural and morphogenetic proteins are synthesized in the mother cell compartment in a defined temporal sequence and are assembled in an ordered fashion on the surface of the developing spore (6). Two proteins produced in the mother cell, GerE and CotE, play major roles in the synthesis of spore coat proteins and in spore coat morphogenesis, respectively. GerE is a small DNA-binding protein (15) which appears to either positively or negatively regulate expression of several of the cot genes encoding spore coat structural proteins (55, 56). The gerE36 mutation, which was originally isolated on the basis of an impaired germination phenotype (22), was later shown to be a nonsense mutation (4a) which behaves like a null allele (57). Spores of gerE36 strains appear to be completely devoid of the lamellalike inner coat layers, but there is a somewhat misassembled outer coat that is loosely associated with each spore (6, 21). It has been shown that the CotE protein forms a shell around the developing forespore and that internal and external to this shell the inner and outer coat layers, respectively, assemble (7). cotE::cat insertion mutants produce mature spores which lack an outer coat, and the inner coat of each mature spore appears to be loosely associated with the cortex (6). A mutant strain carrying both gerE36 and cotE::cat produces mature spores that lack both the outer and inner coat layers (7).
Of recent interest in our laboratory has been the exploration of the factors which confer B. subtilis spore resistance to environmentally relevant extreme conditions, particularly solar UV radiation (reviewed in reference 23). The current molecular models of spore UV resistance mechanisms have been developed mainly from laboratory experiments performed with monochromatic 254-nm (UV-C) radiation, which is absent from the solar UV spectrum on the earth's surface (45a). We have found that B. subtilis spores exposed to solar UV radiation, which consists of UV-B and UV-A wavelengths ranging from 290 to 400 nm, exhibit quite different DNA photochemistry (40) and DNA repair responses (52) than do spores exposed to monochromatic 254-nm UV-C radiation in the laboratory. It has been reported that the spore coat layers do not contribute to spore resistance to 254-nm UV-C radiation (41). However, do the spore coats contribute to spore resistance to environmentally relevant solar UV wavelengths? Using strains carrying the gerE36 and/or cotE::cat mutation that affects spore coat synthesis and/or assembly, in this study we investigated the contributions of the inner and outer spore coat layers to the resistance of B. subtilis spores to sporicidal conditions both in the laboratory and in the environment.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
All of the B. subtilis and Escherichia coli strains and plasmids used in this study are listed in Table 1. All of the B. subtilis strains used are isogenic derivatives of wild-type strain PY79 (54). Luria-Bertani medium (20) and Spizizen's minimal medium (42) containing the appropriate antibiotic(s) and/or growth requirements were used for routine maintenance and cultivation of B. subtilis and E. coli strains. When appropriate, selective antibiotics were added to media at the following final concentrations: chloramphenicol, 3 μg/ml; ampicillin, 50 μg/ml; and a combination of erythromycin (1 μg/ml) and lincomycin (25 μg/ml) (MLS). Liquid cultures were incubated with vigorous aeration, and all cultivations were performed at 37°C. Spores of B. subtilis strains were produced by cultivating the strains for 3 days at 37°C on solid agar plates containing nutrient broth sporulation medium (NSM) (32); this procedure resulted in more free spores available for purification than did cultivation in liquid media. Due to the lysozyme-sensitive nature of mutant spores lacking coats, all spore preparations were purified by repeated centrifugation and water washing as previously described (24) until more than 99% free spores were obtained, as assessed by phase-contrast microscopy. Purified spores were suspended in phosphate-buffered saline (PBS) (pH 7.4) (24), heat shocked (80°C, 10 min), and stored in the dark at 4°C until they were used. In all experiments in which spore resistance was measured, the plates were incubated at 37°C for at least 48 h prior to scoring to ensure that all survivors had formed visible colonies.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype and phenotype | Source | Reference |
|---|---|---|---|
| B. subtilis strains | |||
| PY79 | Prototroph | 54 | |
| AD17 | gerE36 | A. Driks | |
| AD28 | cotE::cat, Cmr | A. Driks | 7 |
| AD142 | gerE36 cotE::cat, Cmr | A. Driks | |
| WN512 | Same as AD17, amyE::cat, Cmr | ptrpBG1→AD17a | |
| WN515 | Same as PY79, amyE::cat::erm, MLSr | pECE72→WN511a | |
| E. coli strains | |||
| JM83 | ara Δ(lac-proAB) rpsL φ80lacZΔM15 | Lab stock | 53 |
| JM103 | [F′ traD36 laclq Δ(lacZ)M15 proA+ B+] endA1 supE sbcBC thi-1 rpsL (Strr) Δ(lac-pro) (P1) (rK+mK+rP1+mP1+) | Lab stock | |
| Plasmids | |||
| ptrpBG1 | amyE::[PtrptrpE-lacZcat], amyE insertion vector | 38 | |
| pECE72 | Cmr::MLSr in B. subtilis-E. coli shuttle plasmid pIC177 | BGSCb | 43 |
Transformation.
BGSC, Bacillus Genetic Stock Center.
Chemical decoating of spores.
Spores were suspended in decoating solution (50 mM Tris base [pH 10], 8 M urea, 50 mM dithiothreitol, 1% [wt/vol] sodium dodecyl sulfate) and incubated at 60°C for 90 min with vigorous vortexing at 10-min intervals. The treated spores were washed three times by resuspending them in STE buffer (150 mM NaCl, 10 mM Tris-HCl [pH 8], 1 mM EDTA) and centrifugating them (10,000 × g, 10 min), and then they were resuspended in PBS.
Assay for spore lysozyme resistance.
Spores were diluted to a concentration of ca. 108 CFU/ml in PBS and were titrated on NSM before and after treatment with lysozyme (final concentration, 0.5 mg/ml; Sigma) at 37°C for 10 min.
Assay for spore H2O2 resistance.
Spores were diluted to a concentration of approximately 108 CFU/ml in PBS, and 933 μl of the spore suspension was placed in a 1.7-ml microcentrifuge tube. After 100 μl was removed in order to determine the titer of viable spores in the remaining preparation (833 μl), 167 μl of 30% H2O2 (Mallinkrodt) was added to the spore suspension. The final H2O2 concentration was 5%. The suspension was incubated at room temperature (∼25°C) with continuous gentle mixing, and 100-μl samples were removed at various times and immediately diluted 1:10 with a solution of bovine catalase (100 μg/ml in PBS; Sigma) that previously had been filter sterilized with a 0.45-μm-pore-size filter. Serial 1:10 dilutions of the catalase-treated spore suspension were then plated onto NSM and incubated in order to determine the number of viable colonies.
Assays for spore UV resistance.
Spores were exposed to artificial UV-C, UV-B, and solar radiation and levels of spore survival were determined as described in detail previously (52). Artificial UV-C radiation was provided by a commercial low-pressure mercury arc lamp (model UVGL-25; UV Products, San Gabriel, Calif.) which emitted essentially monochromatic 254-nm UV radiation. Artificial UV-B radiation was provided by a commercial medium-pressure mercury arc lamp (model UVM-57; UV Products) which emitted a spectrum of UV wavelengths from 280 to 320 nm, with peak emission at 302 nm. The UV-B lamp was further modified to block UV wavelengths of less than 290 nm; this was done by using a polystyrene filter fashioned from a petri dish lid (LifeLINE Dishes; product no. LS-6601; Life Science Products, Denver, Colo.). For each artificial UV-B trial, the UV-B lamp was fitted with a fresh polystyrene filter. For solar UV radiation experiments, spore samples were exposed to sunlight on the roof of Building 90 at the University of Arizona during the daily period of maximal solar intensity, from 2 h before local noon to 2 h after local noon; local noon was calculated for the longitude of Tucson, Ariz. (111° 02′ W), by using the Voyager II computer program (Carina Software, San Leandro, Calif.). Spore samples were exposed to full-spectrum sunlight by using a single layer of Saran Wrap (Dow Brands, Indianapolis, Ind.) as a UV-transparent covering (52). Spore samples were exposed to sunlight in which the UV-B portion had been filtered out (referred to as solar UV-A radiation below) by utilizing a 1.25-cm (0.5-in.)-thick glass plate as a solar UV-B filter which blocked UV wavelengths shorter than 325 nm (52). The UV doses produced by the artificial UV and solar UV radiation sources were measured by using a model UVX radiometer (UV Products) and the appropriate calibrated probes for UV-C radiation (model UVX-25), UV-B radiation (model UVX-31), and UV-A radiation (model UVX-36). UV doses are reported below in Joules per square meter.
Resistance to full-spectrum sunlight (solar UV-B + UV-A radiation) and sunlight from which the UV-B component had been filtered out (solar UV-A radiation) was determined essentially as described previously (52). Suspensions containing 106 spores were spotted in triplicate on sterile microscope slides and allowed to air dry. The slides were exposed to solar radiation by using the appropriate filter, and slides were removed at hourly intervals during the exposure period. Spore spots were removed from each slide with 10% polyvinyl alcohol as described previously (18, 52). Each spore population in a sample spot consisted of spores of wild-type strain WN515 (i.e., PY79 carrying an MLSr marker) and spores of the spore coat mutant strains carrying a Cmr marker (strain AD28, AD142, or WN512, which is a Cmr derivative of AD17) (Table 1) at a 1:1 ratio. The presence of two different antibiotic resistance markers greatly reduced variability, as both of the strains in a spot were subjected to identical variations in solar flux and efficiency of recovery (52). In all solar UV experiments, heat controls consisting of samples that were prepared as described above but were wrapped in aluminum foil were exposed in parallel in order to correct for the lethal effect of environmental heating. Viable spores were enumerated by serial dilution and plate counting on NSM containing the appropriate antibiotic.
Statistical analysis.
Below, 90% lethal doses (LD90) determined in experiments in which we examined spore resistance to hydrogen peroxide and to artificial UV treatments are expressed as averages ± standard deviations. LD90 determined in experiments in which we examined spore resistance to solar UV radiation are expressed as averages ± standard errors because triplicate samples were studied at each time point in each independent trial. The significance of differences in LD90 was determined by analysis of variance (ANOVA) by using Minitab software, version 10.5. Values were analyzed in multigroup pairwise combinations, and differences with P values of ≤0.05 were considered statistically significant.
RESULTS AND DISCUSSION
It has been suggested that the spore coat plays a major role as a barrier to harmful sporicidal conditions that a spore might encounter during dormancy (reviewed in reference 6). In previous studies in which this issue was examined, researchers used a combination of chemical decoating procedures and spore coat-defective mutants to elucidate how the spore coat contributes to a spore's resistance to various sporicidal agents (1, 3, 10–12, 17, 21, 25, 41, 47, 50, 51). In this study, we used both chemical removal of the spore coat and mutants which produce incomplete and aberrant coats to examine the contributions of the inner and outer spore coat layers to resistance of spores to the common oxidant H2O2, to laboratory-generated UV-C and UV-B radiation, and to solar UV-B and UV-A radiation.
Spore resistance to lysozyme.
Resistance to lysozyme is the traditional method used to assess the integrity of the spore coat layers (6, 21, 56); therefore, we tested the lysozyme resistance of spores of the strains used in this study. Under our lysozyme treatment conditions (0.5 mg of lysozyme per ml, 37°C, 10 min), 95% of wild-type PY79 spores survived; in contrast, chemically decoated PY79 spores became sensitized to lysozyme, and 4% of these spores survived. Spores of strains AD17, AD28, and AD142, which lacked the inner coat, the outer coat, and both coat layers, respectively, all were extremely sensitive to lysozyme (levels of survival, <0.006%), indicating that these strains contained severe defects in spore coat integrity.
Spore resistance to hydrogen peroxide.
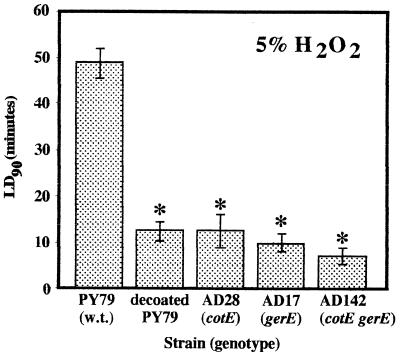
The levels of resistance of spores of wild-type strain PY79 and coat-defective derivatives to 5% H2O2 were determined. Semilogarithmic plots of the percentage of spores that survived versus time yielded the dose (in minutes) required to kill 90% of the spore population (LD90), and the average LD90 after at least three separate treatments were compared (Fig. 1). The LD90 for PY79 (wild-type) spores was 49 ± 3 min; spores of PY79 whose spore coats had been chemically removed became sensitized to 5% H2O2, and the LD90 was 12 ± 2 min (Fig. 1). Mutant derivatives of PY79 which made defective spore coats also were very sensitive to 5% H2O2. The LD90 for strains AD28 (cotE::cat), AD17 (gerE36), and AD142 (cotE::cat gerE36) were 12 ± 4, 10 ± 2, and 7 ± 2 min, respectively (Fig. 1). The differences between the LD90 for PY79 and the LD90 for all of the other strains were highly significant, as determined by ANOVA (P < 0.001), while the LD90 for strain AD28, AD17, and AD142 spores and chemically decoated PY79 spores were not significantly different, as determined by ANOVA. The results indicated that alterations in the spore coat resulting from either chemical removal or mutation increased the sensitivity of the endospores to 5% H2O2 approximately four- to sevenfold.
FIG. 1.
Spore resistance to 5% H2O2. The strains were assayed for H2O2 resistance as described in the text. LD90 are expressed as averages ± standard deviations (n ≥ 3). The asterisks indicate LD90 that were significantly different than the LD90 for wild-type PY79 spores, as determined by ANOVA (P ≤ 0.05). w.t., wild type.
How do the spore coat layers protect spores from the lethal effects of H2O2? Several enzymes which directly or indirectly inactivate H2O2 do not appear to be involved, as spore H2O2 resistance was not affected in B. subtilis mutants lacking the three major catalases (KatA, KatB, and KatX), the single superoxide dismutase (SodA), the DNA-protective MrgA protein, or the alkylhydroperoxide reductase Ahp (4). It is possible that H2O2 is actively destroyed by an additional catalase(s) that resides in the spore coats themselves. For example, SodA has been detected in spore coat extracts, and it has been proposed that this enzyme acts in concert with an unidentified catalase(s) to cross-link coat proteins (14). Some possible candidates for these putative spore coat catalases are the CotJC and CotE proteins based on their levels of amino acid sequence similarity to non-heme- and heme-containing peroxidases, respectively (8, 13, 14, 33). However, direct evidence that these proteins exhibit catalase or peroxidase activity in the mature spore coat has not been obtained to date. Interestingly, in spores of sodA mutants there are aberrations in coat structure and organization (specifically a reduced inner coat and a diffuse outer coat) (14), but such ultrastructural coat alterations apparently do not lead to H2O2 sensitivity (4). Indeed, removal of the spore coat and degradation of the cortex resulting from prolonged exposure to H2O2 do not necessarily lead to spore death (2, 16, 39). The specific target(s) of H2O2 which results in death of wild-type spores is not known with certainly at this time but apparently is not DNA due to the protective effects on spore DNA mediated through binding by small, acid-soluble spore proteins (30, 34). Palop et al. (26, 27) have suggested that the targets of H2O2 that result in death are enzymes required for germination and outgrowth contained in the spore core. Regardless of the target of H2O2 in the spore core, in the absence of hard data which show that catalase and peroxidase activities are present in the dormant spore coat it appears that either the spore coat layers serve as a diffusion barrier to H2O2 or spore coat proteins act as oxidation targets which decrease the effective H2O2 concentration before H2O2 reaches the target(s) in the spore core.
Spore resistance to artificial UV-C radiation.
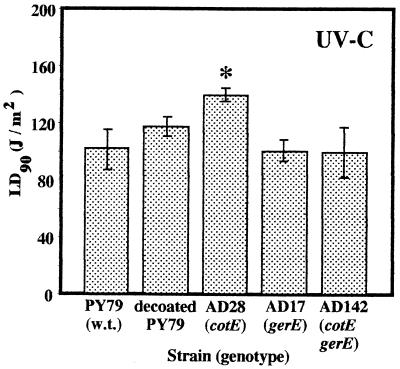
Early experiments which indicated that the spore coat layers are not important determinants of spore UV resistance (41, 44, 45) were based on studies in which monochromatic 254-nm UV-C radiation was used. Resistance of spores to 254-nm UV-C radiation was assayed by determining the average LD90 for each strain in three (strains AD17 and AD28 and chemically decoated strain PY79) or seven (strains PY79 and AD142) independent trials (Fig. 2). The LD90 for spores of PY79, chemically decoated PY79, AD17 (gerE36), and coatless mutant AD142 (cotE::cat gerE36) were 102 ± 14, 118 ± 6, 101 ± 8, and 100 ± 18 J/m2, respectively (Fig. 2). The LD90 for these four strains were not significantly different, as determined by ANOVA, which strongly implied that removal of both spore coat layers by chemical or mutational methods did not affect spore resistance to 254-nm UV-C radiation; this conclusion is consistent with historical observations that chemical coat removal did not affect spore UV-C resistance (41, 44, 45). Interestingly, spores of mutant strain AD28 (cotE::cat), which lacks the outer spore coat, were more resistant to UV-C than were spores of all of the other strains; the LD90 for AD28 spores was 140 ± 5 J/m2 (Fig. 2). The difference, while not dramatic, was nonetheless statistically significant, as determined by ANOVA (P = 0.004).
FIG. 2.
Spore resistance to 254-nm UV-C radiation. The strains were irradiated and levels of survival were determined as described in the text. LD90 are expressed as averages ± standard deviations (n = 3 for AD17, AD28, and chemically decoated PY79; n = 7 for PY79 and AD142). The asterisk indicates an LD90 that was significantly different than the LD90 for wild-type spores, as determined by ANOVA (P ≤ 0.05). w.t., wild type.
Spore resistance to artificial UV-B radiation.
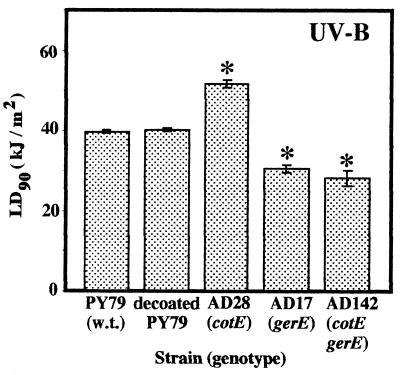
While UV-C radiation is a convenient germicidal treatment and relevant to sterilization procedures, results obtained by using 254-nm UV-C radiation are not truly representative of the results obtained by using the UV wavelengths that endospores encounter in their natural environments (45a). Therefore, we examined the contribution which the spore coats made to spore resistance to artificial UV-B radiation (wavelengths, 290 to 320 nm), which includes wavelengths found in the UV-B portion of sunlight. Surprisingly, we found that although chemical decoating of PY79 spores did not affect the UV-B resistance of the spores, spores of coatless mutant strain AD142 (gerE36 cotE::cat) and gerE36 strain AD17 were significantly more UV-B sensitive than wild-type spores. Spore resistance to artificial UV-B radiation (290 to 320 nm) was assayed by determining the average LD90 for each strain based on a minimum of three trials (Fig. 3). The LD90 obtained for spores of wild-type strain PY79, chemically decoated strain PY79, AD28 (cotE::cat), AD17 (gerE36), and AD142 (gerE36 cotE::cat) were 39.8 ± 0.8, 41.9 ± 0.3, 52.1 ± 1.3, 31.3 ± 1.3, and 28.0 ± 2.3 kJ/m2, respectively. The LD90 for decoated PY79 spores was not significantly different than the LD90 for PY79 spores (Fig. 3), indicating that chemical spore coat removal did not affect spore resistance to UV-B radiation. However, the LD90 for spores of coat mutant strains AD17 (gerE36) and AD142 (gerE36 cotE::cat) were significantly lower than the LD90 for wild-type PY79 spores (Fig. 3), indicating that, in contrast to the results obtained with artificial UV-C radiation, spores that had defects in their coats resulting from the gerE36 mutation were more sensitive to UV-B wavelengths. Again, as observed with UV-C radiation (Fig. 2), spores of strain AD28 (cotE::cat) were significantly more resistant to artificial UV-B radiation than spores of wild-type strain PY79 (Fig. 3).
FIG. 3.
Spore resistance to UV-B radiation. The strains were irradiated and levels of survival were determined as described in the text. LD90 are expressed as averages ± standard deviations (n ≥ 3). The asterisk indicate LD90 that were significantly different than the LD90 for wild-type PY79, as determined by ANOVA (P ≤ 0.05). w.t., wild type.
Spore resistance to full-spectrum solar radiation.
In order to assess the contribution of the spore coat to solar radiation resistance in the field, we utilized an exposure system described in detail previously (52). In field trials we noted that exposure to solar radiation resulted in considerable heating of spore samples (temperatures greater than 70°C were not uncommon) (40) and that chemically decoated spores of PY79 survived field exposure poorly. As a result of further investigation of this phenomenon, we observed that our chemical decoating procedure did not affect PY79 spore viability but resulted in sensitization of PY79 spores to 90°C wet heat; the LD90 for these spores decreased from 11 ± 3 to 2 ± 0.3 min. While sensitization of chemically decoated PY79 spores to lysozyme and H2O2 (Fig. 1) is consistent with results obtained previously with spores of several different Bacillus and Clostridium species (2, 11, 12, 16), the reduction in the heat resistance of PY79 spores exposed to our decoating procedure may have been due to additional weakening of the spore integument. In support of this hypothesis, it has been observed that chemical decoating causes the release of dipicolinic acid (DPA) and hexosamine from spores, which indicates that the cortex integrity may also be compromised (3, 46). Therefore, we were not able to assess the effect of solar UV radiation on chemically decoated spores. In contrast to chemically decoated spores, spores of mutant strains carrying the gerE36 and/or cotE::cat mutation were very sensitive to 5% H2O2 (Fig. 1) but were as heat resistant as wild-type spores (data not shown), indicating that the absence of coat layers due to the gerE36 and cotE::cat mutations probably does not affect cortex integrity and/or core dehydration.
To assess spore resistance to solar radiation, some modifications were made to the experimental system. First, in order to control for variations in sample recovery and solar UV flux, wild-type and mutant strains were paired within the same sample by using constructed derivatives carrying different antibiotic resistance markers (48, 52) (Table 1). Second, as an additional control for killing of spores due to exposure to solar heating alone, a set of slides containing dried spore films wrapped in aluminum foil were exposed in parallel to each test condition. At the end of each time course trial, spores were recovered from the foil-wrapped slides, and the average titer for the heat controls was compared to the average initial titers of viable spores. None of the types of wild-type or coat mutant spores were killed to a significant extent by solar heating in the control samples (data not shown).
Spores were exposed to full-spectrum solar radiation, and we constructed semilogarithmic plots of the percentage of survival versus dose, as measured with the UV-B radiation probe. The LD90 was calculated for each strain in at least three independent trials. The LD90 for wild-type strain WN515 (the MLSr derivative of PY79 used in this experiment) was 31.3 ± 1.9 kJ/m2, whereas coat mutant strains WN512 (gerE36) and AD142 (gerE36 cotE::cat) were significantly more sensitive to full-spectrum solar radiation; the LD90 for the latter two strains were 19.2 ± 1.3 and 18.9 ± 0.1 kJ/m2, respectively (Fig. 4). Again, as observed with artificial UV-C radiation (Fig. 2) and artificial UV-B radiation (Fig. 3), spores of strain AD28 (cotE::cat) were significantly more resistant to full-spectrum solar radiation than the wild-type spores, and the LD90 for AD28 spores was 51.2 ± 1.5 kJ/m2 (Fig. 4).
FIG. 4.
Spore resistance to full-spectrum solar radiation. The strains were irradiated and levels of survival were determined as described in the text. LD90 are expressed as averages ± standard errors (n ≥ 3). The asterisks indicate LD90 that were significantly different than the LD90 for wild-type WN515 spores, as determined by ANOVA (P ≤ 0.05). w.t., wild type.
Spore resistance to solar UV-A radiation.
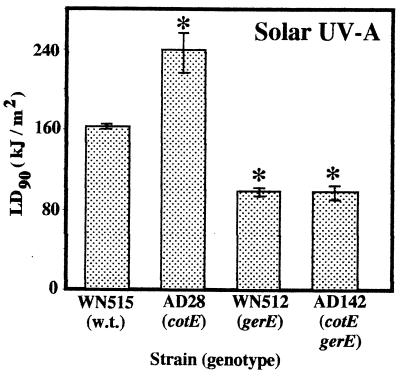
Spores were exposed to solar radiation from which the UV-B portion was filtered by plate glass, and we constructed semilogarithmic plots of the percentage of survival versus dose, as measured with the UV-A radiation probe. The LD90 was calculated for each strain in at least three independent trials. Killing of spores by UV-A sunlight was much less efficient and required longer exposure times. The LD90 of UV-A sunlight for wild-type strain WN515 spores was 164.9 ± 3.2 kJ/m2, whereas spores of coat mutant strains WN512 (gerE36) and AD142 (gerE36 cotE::cat) were significantly more sensitive to UV-A solar radiation; the LD90 for the latter two strains were 101.0 ± 5.0 and 100.0 ± 7.5 kJ/m2, respectively (Fig. 5). Again, as observed with artificial UV-C radiation (Fig. 2), UV-B radiation (Fig. 3), and full-spectrum solar radiation (Fig. 4), spores of strain AD28 (cotE::cat) were significantly more resistant to full-spectrum solar radiation than wild-type spores, and the LD90 for AD28 spores was 240.0 ± 20.2 kJ/m2 (Fig. 5).
FIG. 5.
Spore resistance to solar UV-A radiation. The strains were exposed and levels of survival were determined as described in the text. LD90 are expressed as averages ± standard errors (n ≥ 3). The asterisks indicate LD90 that were significantly different than the LD90 for wild-type WN515 spores, as determined by ANOVA (P ≤ 0.05). w.t., wild type.
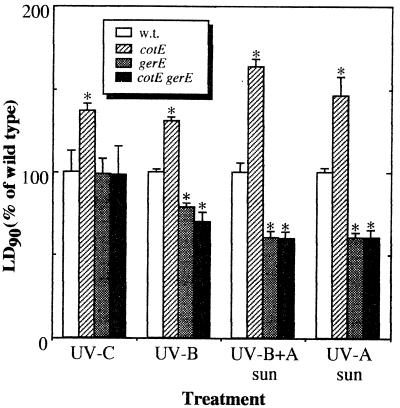
A normalized summary of the results of UV treatments is presented in Fig. 6. It appears that the gerE36 mutation alone is responsible for the decreased resistance of spores to artificial UV-B and solar UV wavelengths, as the LD90 for spores of strains AD17 (gerE36) and AD142 (cotE::cat gerE36) were both significantly lower than the LD90 for wild-type spores (Fig. 6) but were not significantly different from one another, as determined by ANOVA. In addition, it appears that the gerE gene product is involved either directly or indirectly in the resistance of spores to both the UV-B and UV-A components of sunlight, as the resistance of spores of strains harboring the gerE36 mutation to artificial UV-B radiation was 20 to 30% lower and the resistance of these spores to full-spectrum solar radiation (UV-B radiation plus UV-A radiation) or to sunlight containing only UV-A wavelengths was 40% lower (Fig. 6). It has been reported that spores of gerE36 mutants possess a severely misarranged outer coat and are completely devoid of the inner coat layers (6, 21); therefore, it appears that resistance of spores to solar UV radiation may be attributable to the inner coat and that the residual outer coat layer in gerE36 mutant spores does not significantly shield spores from environmentally relevant UV wavelengths.
FIG. 6.
Summary of the UV resistance of spores of coat mutant strains normalized to the resistance of the wild-type strain. The asterisks indicate LD90 that were significantly different than the LD90 for wild-type spores within a treatment group, as determined by ANOVA (P ≤ 0.05). w.t., wild type.
Alternative possibilities can also be envisioned. For example, it is possible that the loosely attached outer coat of gerE36 mutant spores became dissociated from the spores during the mild, yet lengthy, spore purification process which we used and that the purified gerE36 mutant spores in fact lacked all coat material. This possibility could be tested by performing an electron microscope examination of gerE36 mutant spores purified by the centrifugation and water washing technique used in this study. It is also possible that the gerE36 mutation may affect the UV resistance of spores through pleiotropic effects on factors that are not related to spore coat structure. One such factor could be DPA. DPA resides in the spore core and can act as an agent that photosensitizes spore DNA to 254-nm UV-C radiation because it increases the quantum efficiency of formation of the major spore photoproduct 5-thyminyl-5,6-dihydrothymine (35). DPA is produced by DPA synthetase in the mother cell and is transported into the core of the developing prespore. Expression of DPA synthetase in the mother cell is ςK dependent and is negatively regulated by the gerE gene product (5). Although the notion that gerE exerts effects on spore core components is attractive as a general hypothesis, our observation that gerE36 mutant spores were no more sensitive to 254-nm UV-C radiation than wild-type spores (Fig. 2 and 6) tends to rule out the possibility that DPA itself is the factor involved in this phenomenon, at least for 254-nm UV-C radiation. However, at present the role of DPA in spore resistance to solar radiation has not been assessed.
In striking contrast to gerE36 mutant spores, spores of strains carrying the cotE::cat mutation were found to be significantly more resistant than wild-type spores to all wavelengths of UV radiation tested (Fig. 6), even though cotE::cat mutant spores definitely have defective coats, as assessed by their sensitivity to H2O2 (Fig. 1) and lysozyme (6, 21; this study). Spores of cotE::cat mutants lack an outer coat but have a somewhat misarranged, partially swollen inner coat which is apparently only loosely associated with the cortex (6). How this morphological change in the arrangement of the coat results in enhanced spore resistance to all UV wavelengths from 254 to 400 mn is frankly puzzling if the CotE protein simply acts as part of a matrix upon which the inner and outer coat layers are assembled and perhaps cross-linked (6) (see above). Expression of the cotE gene is under the control of ςE in the mother cell compartment and precedes by several hours expression of most other cot genes; indeed, CotE is localized in the mother cell face of the spore septum even before prespore engulfment (7, 31). Perhaps in addition to its role as a coat morphogenetic protein, CotE also participates in an intercompartmental communication system that couples spore envelope morphogenesis to UV resistance factors residing in the spore core. Whether such a system exists or not, it appears that the effect of the gerE gene is dominant to the effect of cotE, since the spores of gerE36 single mutants exhibit a level of UV resistance characteristic of coatless gerE36 cotE::cat double mutant spores (Fig. 6).
Collectively, our data indicate that in addition to lysozyme and H2O2 resistance, the intact spore coat contributes to the resistance of B. subtilis spores to artificial UV-B radiation and solar UV-B and UV-A radiation but not to the resistance of the spores to 254-nm UV-C radiation. The increased sensitivity of spores lacking a functional gerE gene product to UV-B and solar UV radiation suggests that resistance of spores to solar UV radiation may be a function of an intact inner spore coat layer.
ACKNOWLEDGMENTS
We thank Adam Driks and Patricia Fajardo-Cavazos for generous donations of strains, for helpful discussions, and for critically reading the manuscript and Ulricke Philippar and Oscar Ho for technical assistance.
This work was supported by grant GM47461 from the National Institutes of Health and by grant USDA-HATCH ARZT-136753-02-H-116 from the Arizona Agricultural Experimental Station to W.L.N.
REFERENCES
- 1.Aronson A I, Fitz-James P C. Structure and morphogenesis of the bacterial spore coat. Bacteriol Rev. 1976;40:360–402. doi: 10.1128/br.40.2.360-402.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayliss C E, Waites W M. The effect of hydrogen peroxide on spores of Clostridium bifermentans. J Gen Microbiol. 1976;96:401–407. doi: 10.1099/00221287-96-2-401. [DOI] [PubMed] [Google Scholar]
- 3.Bloomfield S F, Megid R. Interaction of iodine with Bacillus subtilis spores and spore forms. J Appl Bacteriol. 1994;76:492–499. doi: 10.1111/j.1365-2672.1994.tb01107.x. [DOI] [PubMed] [Google Scholar]
- 4.Casillas-Martinez L, Setlow P. Alkyl hydroperoxide reductase, catalase, MrgA, and superoxide dismutase are not involved in resistance of Bacillus subtilis spores to heat or oxidizing agents. J Bacteriol. 1997;179:7420–7425. doi: 10.1128/jb.179.23.7420-7425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Cutting S, Panzer S, Losick R. Regulatory studies on the promoter for a gene governing synthesis and assembly of the spore coat in Bacillus subtilis. J Mol Biol. 1989;207:393–404. doi: 10.1016/0022-2836(89)90262-3. [DOI] [PubMed] [Google Scholar]
- 5.Daniel R A, Errington J. Cloning, DNA sequence, functional analysis and transcriptional regulation of the genes encoding dipcolinic acid synthetase required for sporulation in Bacillus subtilis. J Mol Biol. 1993;232:468–483. doi: 10.1006/jmbi.1993.1403. [DOI] [PubMed] [Google Scholar]
- 6.Driks A. Bacillus subtilis spore coat. Microbiol Mol Biol Rev. 1999;63:1–20. doi: 10.1128/mmbr.63.1.1-20.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Driks A, Roels S, Beall B, Moran C P, Jr, Losick R. Subcellular localization of proteins involved in the assembly of the spore coat of Bacillus subtilis. Genes Dev. 1994;8:234–244. doi: 10.1101/gad.8.2.234. [DOI] [PubMed] [Google Scholar]
- 8.Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993;57:1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gould G W. Mechanisms of resistance and dormancy. In: Hurst A, Gould G W, editors. The bacterial spore. Vol. 2. London, United Kingdom: Academic Press; 1983. pp. 173–209. [Google Scholar]
- 10.Gould G W, Dring G J. Role of an expanded cortex in resistance of bacterial endospores. In: Gerhardt P, Costilow R N, Sadoff H L, editors. Spores VI. Washington, D.C.: American Society for Microbiology; 1975. pp. 541–546. [Google Scholar]
- 11.Gould G W, Hitchins A D. Sensitization of bacterial spores to lysozyme and to hydrogen peroxide with agents which rupture disulfide bonds. J Gen Microbiol. 1963;33:413–423. doi: 10.1099/00221287-33-3-413. [DOI] [PubMed] [Google Scholar]
- 12.Gould G W, Stubbs J M, King W L. Structure and composition of resistant layers in bacterial spore coats. J Gen Microbiol. 1970;60:347–355. doi: 10.1099/00221287-60-3-347. [DOI] [PubMed] [Google Scholar]
- 13.Henriques A O, Beall B W, Roland K, Moran C P., Jr Characterization of cotJ, a ςE-controlled operon affecting the polypeptide composition of the coat of Bacillus subtilis spores. J Bacteriol. 1995;177:3394–3406. doi: 10.1128/jb.177.12.3394-3406.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henriques A O, Melsen L R, Moran C P., Jr Involvement of superoxide dismutase in spore coat assembly in Bacillus subtilis. J Bacteriol. 1998;180:2285–2291. doi: 10.1128/jb.180.9.2285-2291.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holland S K, Cutting S, Mandelstam J. The possible DNA-binding nature of the regulatory proteins, encoded by spoIID and gerE, involved in the sporulation of Bacillus subtilis. J Gen Microbiol. 1987;133:2381–2391. doi: 10.1099/00221287-133-9-2381. [DOI] [PubMed] [Google Scholar]
- 16.King W L, Gould G W. Lysis of bacterial spores with hydrogen peroxide. J Appl Bacteriol. 1969;32:481–490. doi: 10.1111/j.1365-2672.1969.tb01002.x. [DOI] [PubMed] [Google Scholar]
- 17.Koshikawa T, Beaman T C, Pankratz H S, Nakashino S, Corner T R, Gerhardt P. Resistance, germination, and permeability correlates of Bacillus megaterium spores successively divested of integument layers. J Bacteriol. 1984;159:624–632. doi: 10.1128/jb.159.2.624-632.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindberg C, Horneck G. Action spectra for survival and spore photoproduct formation of Bacillus subtilis irradiated with short wavelength (200–300 nm) UV at atmospheric pressure in vacuo. J Photochem Photobiol B Biol. 1991;11:69–80. doi: 10.1016/1011-1344(91)80269-n. [DOI] [PubMed] [Google Scholar]
- 19.Milhaud P, Balassa G. Biochemical genetics of bacterial sporulation. IV. Sequential development of resistance to chemical and physical agents during sporulation of Bacillus subtilis. Mol Gen Genet. 1973;125:241–250. doi: 10.1007/BF00270746. [DOI] [PubMed] [Google Scholar]
- 20.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 21.Moir A. Germination properties of a spore coat-defective mutant of Bacillus subtilis. J Bacteriol. 1981;146:1106–1116. doi: 10.1128/jb.146.3.1106-1116.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moir A, Lafferty E, Smith D A. Genetic analysis of spore germination mutants of Bacillus subtilis 168: the correlation of phenotype with map location. J Gen Microbiol. 1979;111:165–180. doi: 10.1099/00221287-111-1-165. [DOI] [PubMed] [Google Scholar]
- 23.Nicholson W L, Fajardo-Cavazos P. DNA repair and the ultraviolet radiation resistance of bacterial spores: from the laboratory to the environment. Recent Res Dev Microbiol. 1997;1:125–140. [Google Scholar]
- 24.Nicholson W L, Setlow P. Sporulation, germination and outgrowth. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Sussex, England: John Wiley and Sons; 1990. pp. 391–450. [Google Scholar]
- 25.Nishihara T, Takubo Y, Kawamata E, Koshikawa T, Ogaki J, Kondo M. Role of outer coat in resistance of Bacillus megaterium spore. J Biochem. 1989;106:270–273. doi: 10.1093/oxfordjournals.jbchem.a122843. [DOI] [PubMed] [Google Scholar]
- 26.Palop A, Rutherford G C, Marquis R E. Hydroperoxide inactivation of enzymes within spores of Bacillus megaterium ATCC 19213. FEMS Microbiol Lett. 1996;142:283–287. [PubMed] [Google Scholar]
- 27.Palop A, Rutherford G C, Marquis R E. Inactivation of enzymes within spores of Bacillus megaterium ATCC 19213 by hydroperoxides. Can J Microbiol. 1998;44:465–470. [PubMed] [Google Scholar]
- 28.Pandey N K, Aronson A I. Properties of the Bacillus subtilis spore coat. J Bacteriol. 1979;137:1208–1218. doi: 10.1128/jb.137.3.1208-1218.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popham D L, Helin J, Costello C E, Setlow P. Muramic acid lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc Natl Acad Sci USA. 1996;93:15405–15410. doi: 10.1073/pnas.93.26.15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popham D L, Sengupta S, Setlow P. Heat, hydrogen peroxide and UV resistance of Bacillus subtilis spores with increased core water content and with or without major DNA-binding proteins. Appl Environ Microbiol. 1995;61:3633–3638. doi: 10.1128/aem.61.10.3633-3638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price K D, Losick R. A four-dimensional view of assembly of a morphogenetic protein during sporulation in Bacillus subtilis. J Bacteriol. 1999;181:781–790. doi: 10.1128/jb.181.3.781-790.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaeffer P, Millet J, Aubert J-P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci USA. 1965;54:704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selyer R W, Henriques A O, Ozin A, Moran C P., Jr Assembly and interactions of cotJ-encoded protein, constituents of the inner layers of the Bacillus subtilis spore coat. Mol Microbiol. 1997;25:955–966. doi: 10.1111/j.1365-2958.1997.mmi532.x. [DOI] [PubMed] [Google Scholar]
- 33a.Serrano M, Zilhão R, Ricca E, Ozin A J, Moran C P, Jr, Henriques A O. A Bacillus subtilis secreted protein with a role in endospore coat assembly and function. J Bacteriol. 1999;181:3632–3643. doi: 10.1128/jb.181.12.3632-3643.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Setlow B, Setlow P. Binding of small, acid-soluble spore proteins to DNA plays a significant role in the resistance of Bacillus subtilis spores to hydrogen peroxide. Appl Environ Microbiol. 1993;59:3418–3423. doi: 10.1128/aem.59.10.3418-3423.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Setlow B, Setlow P. Dipicolinic acid greatly enhances production of spore photoproduct in bacterial spores upon UV irradiation. Appl Environ Microbiol. 1993;59:640–643. doi: 10.1128/aem.59.2.640-643.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Setlow P. Spore structural proteins. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C.: American Society for Microbiology; 1993. pp. 801–809. [Google Scholar]
- 37.Setlow P. Mechanisms for the prevention of damage to DNA in spores of Bacillus species. Annu Rev Microbiol. 1995;49:29–54. doi: 10.1146/annurev.mi.49.100195.000333. [DOI] [PubMed] [Google Scholar]
- 38.Shimotsu H, Henner D J. Construction of a single-copy integration vector and its use in analysis of regulation of the trp operon of Bacillus subtilis. Gene. 1986;43:85–94. doi: 10.1016/0378-1119(86)90011-9. [DOI] [PubMed] [Google Scholar]
- 39.Shin S-Y, Calvisi E G, Beaman T C, Pankratz H S, Marquis R E. Microscopic and thermal characterization of hydrogen peroxide killing and lysis of spore and protection by transition metal ions, chelators, and antioxidants. Appl Environ Microbiol. 1994;60:3192–3197. doi: 10.1128/aem.60.9.3192-3197.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slieman T A, Nicholson W L. Artificial and solar UV radiation induces strand breaks and cyclobutane pyrimidine dimers in Bacillus subtilis spore DNA. Appl Environ Microbiol. 2000;66:199–205. doi: 10.1128/aem.66.1.199-205.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Somerville H J, Delafield F P, Rittenberg S C. Urea-mercaptoethanol-soluble protein from spores of Bacillus thuringiensis and other species. J Bacteriol. 1970;101:551–560. doi: 10.1128/jb.101.2.551-560.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spizizen J. Transformation of biochemically-deficient strains of Bacillus subtilis by deoxyribonucleate. Proc Natl Acad Sci USA. 1958;44:1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinmetz M, Richter R. Plasmids designed to alter the antibiotic resistance by insertion mutations in Bacillus subtilis, through in vivo recombination. Gene. 1994;142:79–83. doi: 10.1016/0378-1119(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 44.Tanooka H. Ultraviolet resistance of DNA in spore spheroplast of Bacillus subtilis as measured by the transforming activity. Biochim Biophys Acta. 1968;166:581–583. doi: 10.1016/0005-2787(68)90248-7. [DOI] [PubMed] [Google Scholar]
- 45.Tanooka H, Sakakibara Y. Radioresistant nature of the transforming activity in bacterial spores. Appl Microbiol. 1968;26:592–597. [Google Scholar]
- 45a.Urbach F. Introduction. In: Urbach F, editor. Biological responses to ultraviolet-A radiation. Overland Park, Kans: Valdenmar Publishing Co.; 1992. pp. 1–6. [Google Scholar]
- 46.Vary J C. Germination of Bacillus megaterium spores after various extraction procedures. J Bacteriol. 1973;116:797–802. doi: 10.1128/jb.116.2.797-802.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waites W M, Bayliss C E, King N R. The effect of sporulation medium on spores of Clostridium bifermentans. J Gen Microbiol. 1980;116:271–276. [Google Scholar]
- 48.Wang T-C V. A simple convenient biological dosimeter for monitoring solar UV-B radiation. Biochem Biophys Res Commun. 1991;177:48–53. doi: 10.1016/0006-291x(91)91946-a. [DOI] [PubMed] [Google Scholar]
- 49.Webb C D, Decatur A, Teleman A, Losdick R. Use of green fluorescent protein for visualization of cell-specific gene expression and subcellular protein localization during sporulation in Bacillus subtilis. J Bacteriol. 1995;177:5906–5911. doi: 10.1128/jb.177.20.5906-5911.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wood D A. Sporulation in Bacillus subtilis. Biochem J. 1972;130:505–514. doi: 10.1042/bj1300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wyatt L R, Waites W M. The effect of chlorine on spores of Clostridium bifermentans, Bacillus subtilis, and Bacillus cereus. J Gen Microbiol. 1975;89:337–344. doi: 10.1099/00221287-89-2-337. [DOI] [PubMed] [Google Scholar]
- 52.Xue Y, Nicholson W L. The two major spore DNA repair pathways, nucleotide excision repair and spore photoproduct lyase, are sufficient for the resistance of Bacillus subtilis spores to artificial UV-C and UV-B but not to solar radiation. Appl Environ Microbiol. 1996;62:2221–2227. doi: 10.1128/aem.62.7.2221-2227.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequence of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 54.Youngman P, Perkins J B, Losick R. Construction of a cloning site near one end of Tn917 into which foreign DNA may be inserted without affecting transposition in Bacillus subtilis or expression of the transposon-borne erm gene. Plasmid. 1984;12:1–9. doi: 10.1016/0147-619x(84)90061-1. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J, Ichikawa H, Halberg R, Kroos L, Aronson A I. Regulation of the transcription of a cluster of Bacillus subtilis spore coat genes. J Mol Biol. 1994;240:405–415. doi: 10.1006/jmbi.1994.1456. [DOI] [PubMed] [Google Scholar]
- 56.Zheng L, Halberg R, Roels S, Ichikawa H, Kroos L, Losick R. Sporulation regulatory protein GerE from Bacillus subtilis binds to and can activate or repress transcription from promoters for mother-cell-specific genes. J Mol Biol. 1992;226:1037–1050. doi: 10.1016/0022-2836(92)91051-p. [DOI] [PubMed] [Google Scholar]
- 57.Zheng L B, Losick R. Cascade regulation of spore coat gene expression in Bacillus subtilis. J Mol Biol. 1990;212:645–660. doi: 10.1016/0022-2836(90)90227-d. [DOI] [PubMed] [Google Scholar]