Abstract
Purpose:
Because the Hedgehog and Notch pathways are often overexpressed in mesenchymal malignancies, we evaluated the efficacy of concurrent inhibition of Notch and Hedgehog signaling using the gamma secretase inhibitor (GSI) RO4929097 and the smoothened antagonist vismodegib in unresectable or metastatic sarcoma.
Patients and Methods:
In this investigator-initiated trial, phase 1b employed standard 3+3 dose-escalation in which patients first received vismodegib once daily for 21 days, followed by the combination of RO4929097 concurrently with vismodegib in 21-day cycles. In phase II, patients were randomized to RO4929097 alone or in combination with vismodegib.
Results:
Nine patients were treated in phase Ib with no dose-limiting toxicities. RO4929097 at 15 mg daily in combination with 150 mg daily of vismodegib was declared the recommended phase 2 dose. Most adverse events (AEs) were grade ≤ 2. In phase II (closed early due to discontinuation of RO4929097 evaluation), 34 patients were randomized to RO4929097 alone and 33 to RO4929097 plus vismodegib. RO4929097 did not interfere with the steady-state concentration of vismodegib, while vismodegib reduced the plasma concentration of RO492909. No patients had an objective response. Neither progression-free nor overall survival differed significantly between treatment arms. Paired tumor biopsies from a subset of patients demonstrated inhibition of cleaved Notch.
Conclusions:
The combination of RO4929097 plus vismodegib was generally well tolerated. Although accrual to this study was not completed, vismodegib did not meaningfully enhance the clinical efficacy of RO4929097 in an unplanned analysis. GSIs and GSIs plus vismodegib can inhibit intratumoral Notch and downstream pAkt signaling.
INTRODUCTION
Sarcomas are rare mesenchymal malignancies with an annual incidence of approximately 15,000 cases per year in the US.(1) Despite primary combined modality therapy, 30–80% of patients develop recurrent or metastatic disease, depending on the stage and sarcoma subtype. The standard of care front-line chemotherapy, typically anthracycline-based, has a median PFS of approximately 6 months.(2) Second-line therapies and beyond have modest clinical benefit,(3–5) highlighting the need for novel approaches to treat these diseases.
Sarcomas, like many cancers, often co-overexpress or co-downregulate the Hedgehog and Notch pathways, which share common downstream targets, including Akt-mTOR.(6,7) The Hedgehog and Notch pathways are evolutionarily conserved and crucial for the normal development of multiple organ systems, including mesenchymal cells. Hedgehog signaling leads to downstream activation of the glioma-associated family of transcription factors involved in proliferation, survival, and angiogenesis.(8) The Notch family of proteins are cell surface receptors involved in transmitting growth and proliferation signals, and require cleavage by the gamma-secretase complex for translocation into the nucleus to complete signal transduction. Inappropriate activation of the notch is seen in a host of malignancies(9–14), and inhibition of the Notch pathway with a gamma-secretase inhibitor (GSI) leads to growth suppression, apoptosis, and increased sensitivity to cytotoxic chemotherapy in multiple sarcomas.(15,16)
We hypothesized that dual inhibition of gamma-secretase and Hedgehog would have a synergistic antitumor effect leading to activity in advanced sarcomas. Vismodegib (formerly GDC-049) is a small-molecule inhibitor of Smoothened, a transmembrane protein involved in Hedgehog signaling, and RO4929097 is a GSI. Vismodegib is currently FDA-approved for advanced basal cell carcinoma at 150 mg daily. A phase I clinical trial of RO4929097 did not identify a maximum tolerated dose (MTD), and clinical activity was seen in multiple cancers, including sarcoma.(17) We conducted a phase Ib study to evaluate the pharmacokinetics (PK), pharmacodynamics (PD), and safety of combining vismodegib and R0492097. Following this, we conducted a randomized, phase II study of R04929097 alone or in combination with vismodegib in patients with advanced, metastatic soft tissue and bone sarcomas.
PATIENTS AND METHODS
Patient Selection
Eligible patients were ≥ 18 years of age, with histologically confirmed sarcoma, measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1, ECOG performance status of ≤ 2, and normal organ and bone marrow function. In both study phases, ≥ 1 prior line of therapy was required; in phase Ib, any greater number of prior therapies were allowed, while in phase II, a maximum of 5 lines of prior therapies were permitted. Patients with well/de-differentiated liposarcoma, clear cell sarcoma, chondrosarcoma, alveolar soft part sarcoma, or chordoma were eligible to enroll with no prior treatment due to a lack of known effective treatments in these disease subtypes at the time of this study. Patients with gastrointestinal stromal tumor (GIST) must have progressed or have not tolerated imatinib and sunitinib. Full inclusion and exclusion criteria are included in Supplementary Methods. RO4929097 and vismodegib were supplied by the Division of Cancer Treatment and Diagnosis, National Cancer Institute (NCI), under collaborative agreements with Hoffmann-La Roche (Basel, Switzerland) and Genentech (South San Francisco, CA). The study was conducted per FDA regulations and Good Clinical Practice guidelines. The study protocol (ClinicalTrials.gov identifier: NCT01154452) was approved by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute and conducted at Memorial Sloan Kettering Cancer Center (MSK) under an NCI-held investigational new drug application. The protocol was approved by the MSK Institutional Review Board and conducted in accordance with Declaration of Helsinki guidelines. Written informed consent was obtained from all participating patients. The trial was registered with clinialtrials.gov: NCT01154452.
Study Design, Treatment, and Endpoints
The primary objective of the phase Ib study portion was to determine the MTD and the recommended phase II dose (RP2D) of vismodegib in combination with RO4929097 (Figure 1). Secondary objectives were to describe the tolerability and adverse event (AE) profile, assess the overall response rate (ORR; complete response [CR] or partial response [PR]) by RECIST 1.1, describe the PK, and conduct PD analysis on tumor tissue specimens from patients receiving the combination of vismodegib and RO4929097.
Figure 1. Study design.
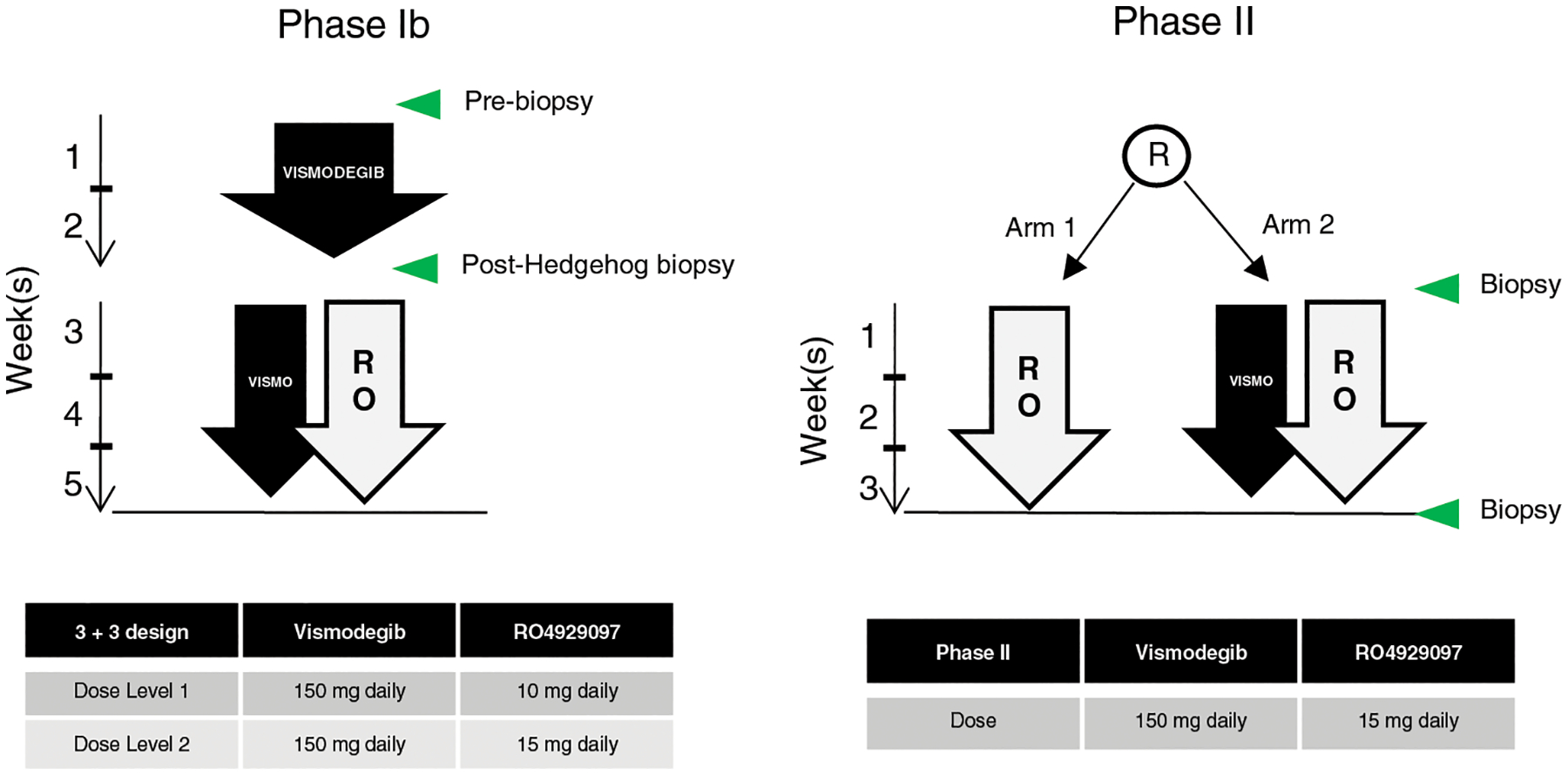
Left, phase Ib: after two weeks of vismodegib lead-in, patients received vismodegib in combination with RO429097 at the indicated dose levels according to standard 3+3 design. Right, phase II: patients were randomized to receive either RO429097 alone or in combination with vismodegib at the RP2D. Green arrows indicate biopsy time points.
The phase Ib portion of this study utilized a standard 3+3 dose-escalation design. Based on the time to reach a steady state, patients received vismodegib 150 mg daily by mouth for a lead-in period of 21 consecutive days. The vismodegib dose was based on the RP2D from completed clinical trials.(18) After the 21-day lead-in, vismodegib was continued at 150 mg daily, and oral daily RO4929097 was started concomitantly at a dose defined by the pre-specified dose escalation scheme: the first dose level of RO4929097 was 10 mg daily, and the second was 15 mg daily. An additional dose level of 5 mg daily of RO4929097 was planned if the first dose level was not tolerated. Pre-treatment and on-treatment biopsies, collected at days 15 and 21 of the lead-in period, were required in phase Ib.
All AEs were graded based on the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. During phase Ib, the dose-limiting toxicity (DLT) window was during the first cycle of combination therapy. DLTs were defined as Grade 4 hematologic toxicity (except lymphopenia or lymphocytosis) or Grade ≥ 3 non-hematologic toxicity, including diarrhea despite the use of antidiarrheal prophylaxis or glucocorticoids, nausea or vomiting despite the use of maximal antiemetics, or electrolyte abnormalities such as hyponatremia, hypophosphatemia, or hypomagnesemia that did not resolve within 72 h with supportive therapy or electrolyte abnormalities associated with new EKG changes.
Each cycle length was 21 days, and tumor responses were assessed by RECIST 1.1 every 6 weeks. Patients remained on the study as long as they did not develop RECIST or clinical progression or unacceptable toxicity or withdraw consent. Patients who discontinued one drug due to toxicity were permitted to continue the other agent. Vismodegib was held for clearly attributable toxicity until toxicities resolved to grade ≤ 1; no dose modification to vismodegib was allowed. Dose modifications of RO4929097 were permitted once AEs resolved to grade ≤ 1.
The primary objective of the phase II study portion was to assess progression-free survival (PFS) of patients treated with the combination of RO4929097 with and without vismodegib. PFS was defined as the time from randomization to either first evidence of disease progression or death from any cause. Secondary objectives were to assess the overall response rate per RECIST 1.1 (CR plus PR) and overall survival (OS) and further describe the PK and PD of combination treatment.
The phase II portion randomized patients 1:1 to receive RO4929097 monotherapy or RO4929097 in combination with vismodegib. Patients were stratified by sarcoma subtype (liposarcoma vs. non-liposarcoma) and performance status (ECOG 0 or 1 vs. 2). In the absence of treatment delay due to AEs, treatment continued until disease progression, intercurrent illness preventing further drug administration, or unacceptable toxicity. Pre-treatment and on-treatment biopsies (collected during cycle 1 on days 15 and 21) were optional in the phase II study.
Pharmacokinetics
PK analyses were performed on heparinized plasma samples obtained on Day 1 at the following time points: pre-treatment, 0.5, 1, 1.5, 2, 4, 6, and 24 h after the initiation of RO4929097. Procedures for plasma preparation and isolation and measurement of RO4929097 are described in Supplementary Methods.
Pharmacodynamics
In frozen biopsy samples, expression of NOXA, phosphorylation of Akt, and cleavage of Notch-1 were assessed by Western blotting and expression of GLI-1 by RT-PCR as described in the Supplementary Methods.
Statistical Analyses
A total of 90 patients were planned for the Phase 2 component of this study, with 45 patients on each arm. Randomization was accomplished by the method of random permuted block, and patients will be stratified by sarcoma type (liposarcoma vs. non-liposarcoma), and performance status (ECOG 0–1 vs. 2). With a one-sided type 1 error of 0.1, this sample size would detect a 75% improvement in median PFS from 6 weeks in the one-drug arm to 10.5 weeks in the two-drug arm with a power of 0.90; and a 60% improvement in median PFS, from 6 weeks in the one-drug arm to 9.6 weeks for the two-drug arm, with a power of 0.81. Stratified log-rank test was used to compare PFS between the two arms. For each arm, response rate and clinical benefit rate were estimated with the confidence interval provided, and overall survival probabilities were estimated using Kaplan-Meier curves. Statistical analyses utilized the intent-to-treat principle. All patients who enrolled in the phase II study and received drugs were included in the efficacy analysis. An interim futility analysis was planned after 50% of the anticipated events occurred.
Data Availability
De-identified data are available from the authors upon reasonable request.
RESULTS
Patient Characteristics
Seventy-six patients were enrolled and treated in this study (Table 1). Of these patients 9 were treated in the phase Ib portion and 67 on phase II, of which 34 patients were randomized to receive RO4929097 monotherapy and 33 to RO4929097 plus vismodegib. The study was closed prematurely after 67 of the planned 90 patients were enrolled on phase II due to manufacturer discontinuation of further RO4929097 development. Patient characteristics are detailed in Table 1 and balanced between the phase II study arms. The most common histologic subtypes in the monotherapy arm were well- or dedifferentiated liposarcoma, sarcoma not otherwise specified, and leiomyosarcoma. In the combination arm, well- or dedifferentiated liposarcoma, desmoid fibromatosis, and sarcoma not otherwise specified were most frequent (Supplementary Table 1). Patients on the monotherapy arm received a median of 2 cycles of therapy (range 1–24), while those in the combination arm received a median of 3 cycles (range 1–14).
Table 1.
Patient demographics and tumor characteristics.
| Phase Ib | Phase II | ||
|---|---|---|---|
| RO4929097 plus vismodegib | RO4929097 | RO4929097 plus vismodegib | |
| Total n | 9 | 34 | 33 |
| Age (median) | 60 | 56 | 46 |
| Male sex, n (%) | 5 (55.6%) | 20 (58.8%) | 18 (54.5%) |
| Histology, n (%) | |||
| Other* | 8 (66.7%) | 25 (73.5%) | 23 (69.7%) |
| ECOG performance status, n (%) | |||
| 2 | 0 (0) | 0 (0) | 1 (3.0) |
| Number of prior therapies (median) | 4 | 2 | 3 |
Pharmacokinetics
The mean plasma concentrations of vismodegib were 10,849 ng/mL and 13,996 ng/mL on days 8 and 15 of the vismodegib monotherapy phase I lead-in, respectively. Vismodegib plasma concentrations remained stable after the introduction of RO4929097 on cycle 1 day 1 after the 21-day lead-in, indicating that RO4929097 did not interfere with the pharmacokinetics of vismodegib. The mean concentration-time profile of total plasma vismodegib (Figure 2A) is similar to previous reports.(18,19) The mean Cmax of RO4929097 in the phase Ib was 125 ng/mL, with a mean AUC0–∞ of 1626.9 ng/mL*h. The mean fraction of unbound RO4929097 after the first dose, measured on cycle 1 day 1, was 1.9%, similar to the fraction unbound previously reported for RO4929097 monotherapy.(20)
Figure 2. Pharmacokinetics of each agent.
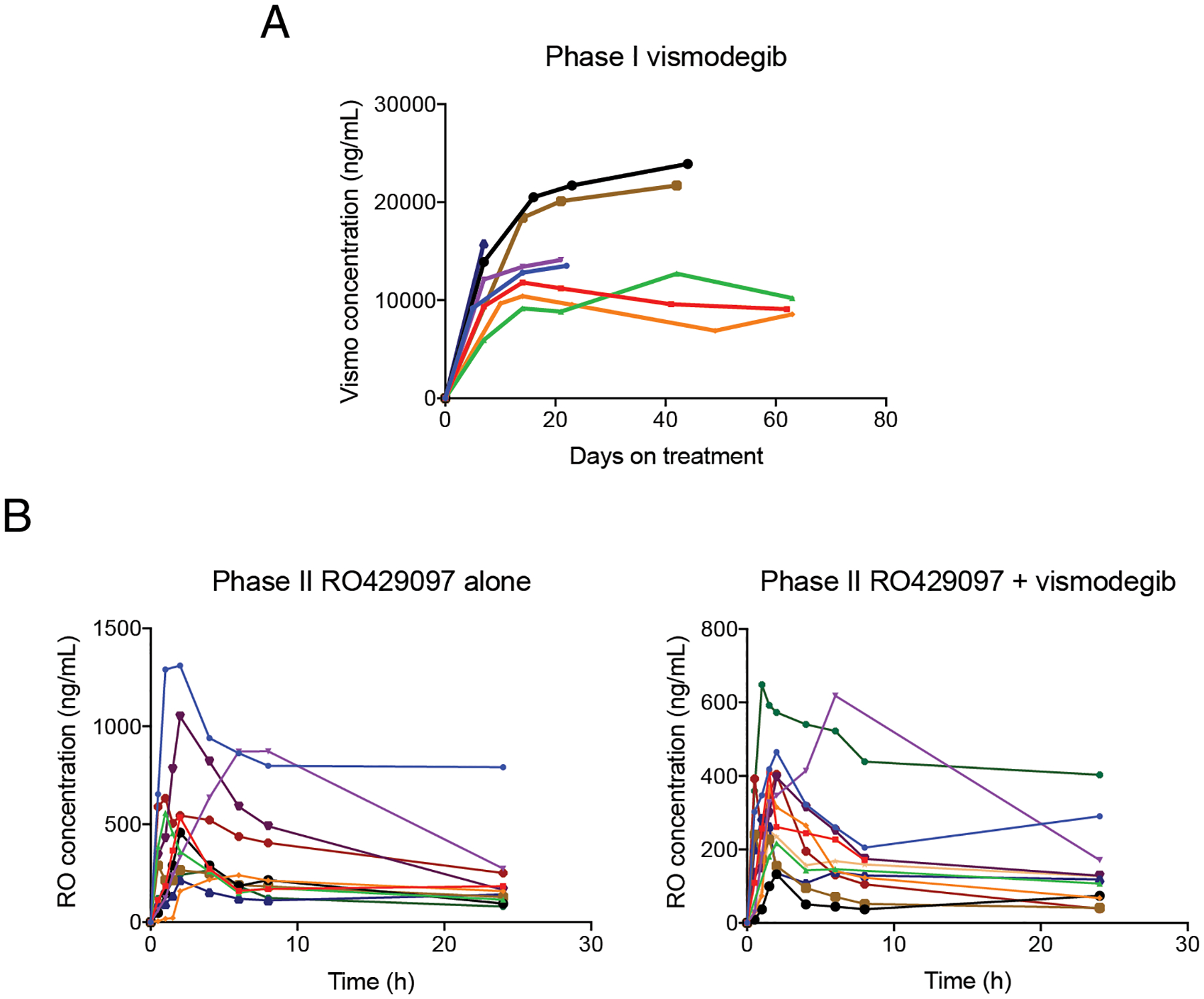
A, Plasma concentrations of total vismodegib in 8 patients in Phase Ib. B-C, plasma concentrations of total RO4929097 on the monotherapy arm (B) and combination therapy arm (C), in 10 and 12 patients, respectively, in Phase II. Each line represents an individual patient.
In phase II, the mean Cmax and AUC0–∞ of RO4929097 on the monotherapy arm were 584.5 ng/mL and 25314.4 ng/mL*h, compared to 369.6 ng/mL and 4306.7 ng/mL*h on the combination arm (Figure 2B). Despite the lower values on the combination arm, the mean percent of unbound RO4929097 remained comparable between both treatment arms (2.0% and 2.8%, respectively).(21)
Safety
There were no DLTs in the dose-escalation phase, and combination treatment was well tolerated. Three patients experienced progression of disease before the DLT window was complete and were therefore replaced. RO4929097 at 15 mg per day in combination with 150 mg per day of vismodegib was declared the RP2D. Most adverse events were grade ≤ 2. The most frequent TRAE were nausea, elevated aspartate aminotransferase, hyponatremia, fatigue, and hyperglycemia (Supplementary Table 2). The only grade ≥ 3 TRAEs were hypophosphatemia and lymphopenia (both grade 3).
In the phase II portion, grade ≥ 3 TRAEs were infrequent (Table 2). The most common TRAEs of any grade in the monotherapy arm were hypophosphatemia (38% of patients), nausea (29%), fatigue (29%), hyperglycemia (24%), and elevated alanine aminotransferase (ALT; 21%). Grade 3 TRAEs were hypophosphatemia (15% of patients), elevated ALT (6%), elevated alkaline phosphatase (6%), and lymphopenia (3%). The only grade 4 TRAE was neutropenia in one patient.
Table 2.
Treatment-related toxicities in the phase II study of RO4929097 with or without vismodegib occurring in ≥ 5% of patients. Values represent number of patients (%) experiencing each toxicity.
| RO4929097 (n = 33) | RO4929097 plus vismodegib (n = 33) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total |
| Alanine aminotransferase increased | 4 (12%) | 1 (3%) | 2 (6%) | 0 (0%) | 11 (33%) | 3 (9%) | 0 (0%) | 0 (0%) | 21 (31%) |
| Nausea | 8 (24%) | 2 (6%) | 0 (0%) | 0 (0%) | 7 (21%) | 3 (9%) | 0 (0%) | 0 (0%) | 20 (30%) |
| Hypophosphatemia | 1 (3%) | 7 (21%) | 5 (15%) | 0 (0%) | 0 (0%) | 6 (18%) | 1 (3%) | 0 (0%) | 20 (30%) |
| Fatigue | 8 (24%) | 2 (6%) | 0 (0%) | 0 (0%) | 6 (18%) | 2 (6%) | 0 (0%) | 0 (0%) | 18 (27%) |
| Hyperglycemia | 6 (18%) | 2 (6%) | 0 (0%) | 0 (0%) | 7 (21%) | 0 (0%) | 0 (0%) | 0 (0%) | 15 (22%) |
| Hypoalbuminemia | 4 (12%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 (21%) | 2 (6%) | 0 (0%) | 0 (0%) | 13 (19%) |
| White blood cells decreased | 2 (6%) | 3 (9%) | 0 (0%) | 0 (0%) | 5 (15%) | 2 (6%) | 1 (3%) | 0 (0%) | 13 (19%) |
| Hyponatremia | 3 (9%) | 0 (0%) | 0 (0%) | 0 (0%) | 8 (24%) | 0 (0%) | 1 (3%) | 0 (0%) | 12 (18%) |
| Alkaline phosphatase increased | 4 (12%) | 1 (3%) | 1 (3%) | 0 (0%) | 6 (18%) | 0 (0%) | 0 (0%) | 0 (0%) | 12 (18%) |
| Aspartate aminotransferase increased | 1 (3%) | 1 (3%) | 1 (3%) | 0 (0%) | 6 (18%) | 0 (0%) | 0 (0%) | 0 (0%) | 9 (13%) |
| Musculoskeletal and connective tissue disorders | 3 (9%) | 1 (3%) | 0 (0%) | 0 (0%) | 3 (9%) | 2 (6%) | 0 (0%) | 0 (0%) | 9 (13%) |
| Anemia | 1 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 (18%) | 0 (0%) | 1 (3%) | 0 (0%) | 8 (12%) |
| Dysgeusia | 5 (15%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (3%) | 2 (6%) | 0 (0%) | 0 (0%) | 8 (12%) |
| Constipation | 3 (9%) | 1 (3%) | 0 (0%) | 0 (0%) | 2 (6%) | 1 (3%) | 0 (0%) | 0 (0%) | 7 (10%) |
| Neutrophil count decreased | 0 (0%) | 4 (12%) | 0 (0%) | 1 (3%) | 0 (0%) | 1 (3%) | 1 (3%) | 0 (0%) | 7 (10%) |
| Vomiting | 5 (15%) | 1 (3%) | 0 (0%) | 0 (0%) | 1 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 (10%) |
| Platelet count decreased | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (15%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (7%) |
| Lymphocyte count decreased | 0 (0%) | 0 (0%) | 1 (3%) | 0 (0%) | 2 (6%) | 0 (0%) | 2 (6%) | 0 (0%) | 5 (7%) |
| Anorexia | 2 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (6%) | 1 (3%) | 0 (0%) | 0 (0%) | 5 (7%) |
| Myalgia | 2 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (9%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (7%) |
| Skin and subcutaneous tissue disorders | 2 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (9%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (7%) |
| Creatine phosphokinase increased | 1 (3%) | 2 (6%) | 0 (0%) | 0 (0%) | 2 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (7%) |
| Alopecia | 2 (6%) | 2 (6%) | 0 (0%) | 0 (0%) | 1 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (7%) |
| Oral mucositis | 4 (12%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (6%) |
In the combination arm, the most common TRAEs of any grade were elevated ALT (41%), nausea (29%), hypoalbuminemia (26%), hyponatremia (26%), fatigue (24%), and decreased white blood cells (24%). The only grade 3 TRAEs were lymphopenia (6%), hyponatremia (3%), decreased white blood cells (3%), hypophosphatemia (3%), anemia (3%), and neutropenia (3%). There was no grade 4 TRAE on this arm. Five patients withdrew consent due to adverse events: 4 in the combination arm (amenorrhea, alopecia and muscle spasms, dysgeusia and anorexia, and nausea and alopecia, respectively) and one in the monotherapy arm (lethargy).
Efficacy
There were no complete or partial responses in either treatment arm (Figure 3A–B). Stable disease was seen in 34 patients, 17 on each treatment arm. The most significant degrees of tumor reduction per RECIST was −20% in a patient with high-grade spindle cell sarcoma (on the monotherapy arm),–16% in a patient with dedifferentiated liposarcoma (monotherapy), −14% in a patient with GIST (monotherapy), and −14% in a patient with clear cell sarcoma (on the combination arm). In the RO4929097 monotherapy arm, 5 patients (15%) remained on treatment for ≥ 24 weeks: 3 with well- or dedifferentiated liposarcoma, one with desmoid fibromatosis, and one with epithelioid sarcoma. In the combination treatment arm, 6 patients (18%) remained on treatment for ≥ 24 weeks: 2 patients with desmoid fibromatosis, 2 with well/de-differentiated liposarcoma, clear cell sarcoma, and clear cell chondrosarcoma.
Figure 3.

Waterfall plots depicting best radiographic responses (RECIST 1.1) of each patient to A, R04929097; B, R04929097 and vismodegib. Numbers above each bar indicate time on treatment in weeks.
The median PFS was 8.9 weeks (95% CI 5.9–18.3) on the monotherapy arm and 12.0 weeks (95% CI 6.0–NR) on the combination therapy arm (P = 0.300) (Figure 4A). The median overall survival was 11.9 months (95% CI 9.6–25.4) in the monotherapy arm and 21.5 months (95% CI 11.6–33.1) in the combination arm (P = 0.400) (Figure 4B). The median follow-up for survivors was 42.8 months.
Figure 4.
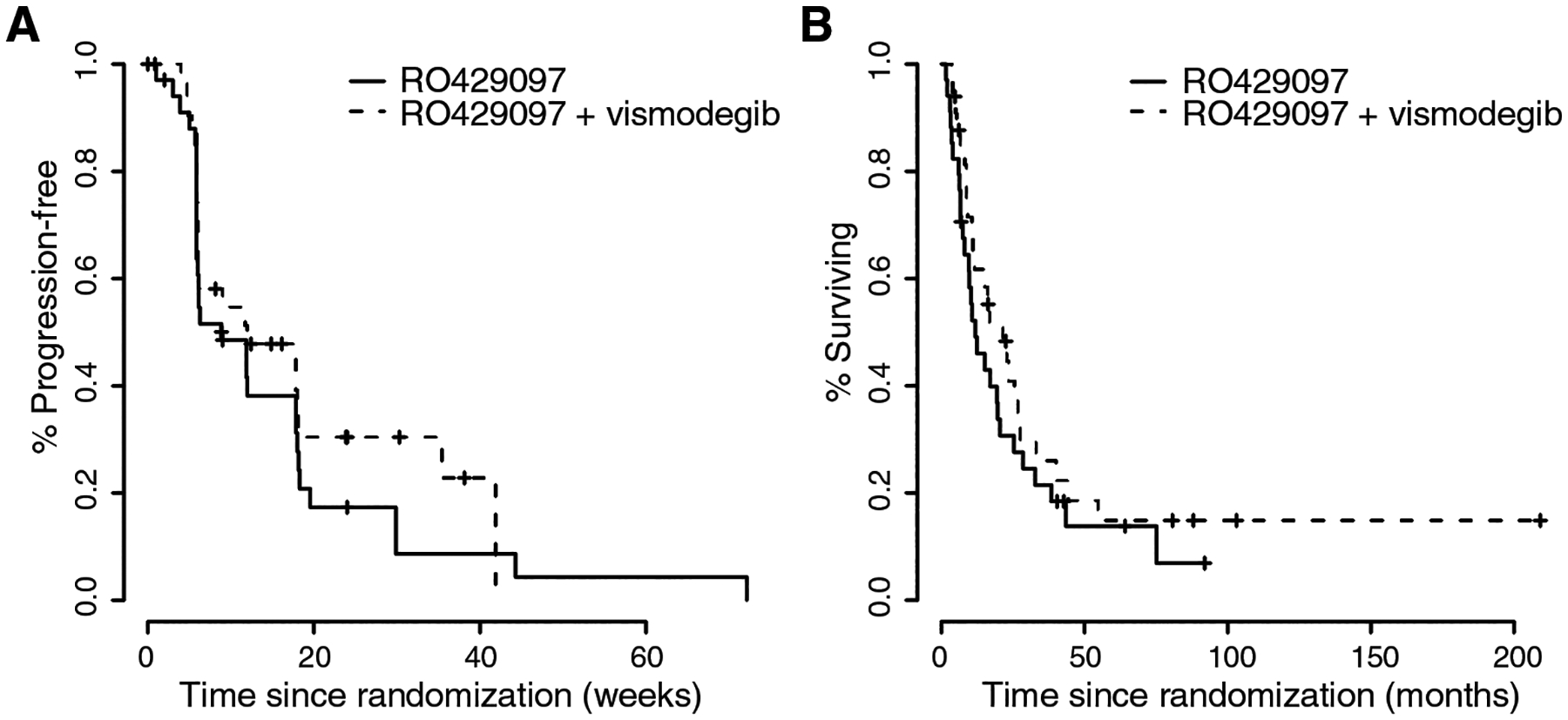
Kaplan-Meier curves of progression-free (A) and overall survival (B) of patients on the randomized phase II study portion.
Pharmacodynamics
Paired tumor biopsies were obtained in 6/10 (phase 1) and 22/67 patients (phase 2). Tumor tissue met quality control for further analysis in 4/6 (phase 1) and 18/22 (phase 2) tumor tissue. In phase Ib patients demonstrated upregulation of the pro-apoptotic protein NOXA on western blot after treatment with vismodegib, suggesting that Hedgehog pathway inhibition can induce upregulation of proapoptotic proteins (Figure 5A). Two of 10 patients in phase II with specimens available for RT-PCR analysis had a decrease in GLI1 expression (Figure 5B), confirming Hedgehog pathway inhibition. One of these patients had clear cell sarcoma, was randomized to the combination arm, and remained on treatment for 35.4 weeks with tumor shrinkage (–14.3% by RECIST 1.1); the other had chordoma, received RO4929097 monotherapy, and had tumor progression (+11% at 9 weeks). Subset of patients in both arms of phase II had decreased expression of cleaved Notch and decreased phosphorylated Akt (pAkt), suggesting successful inhibition of the gamma-secretase enzyme leading to downregulation of Notch signaling (Figure 5C–D).
Figure 5. Pharmacodynamic indicators of vismodegib.
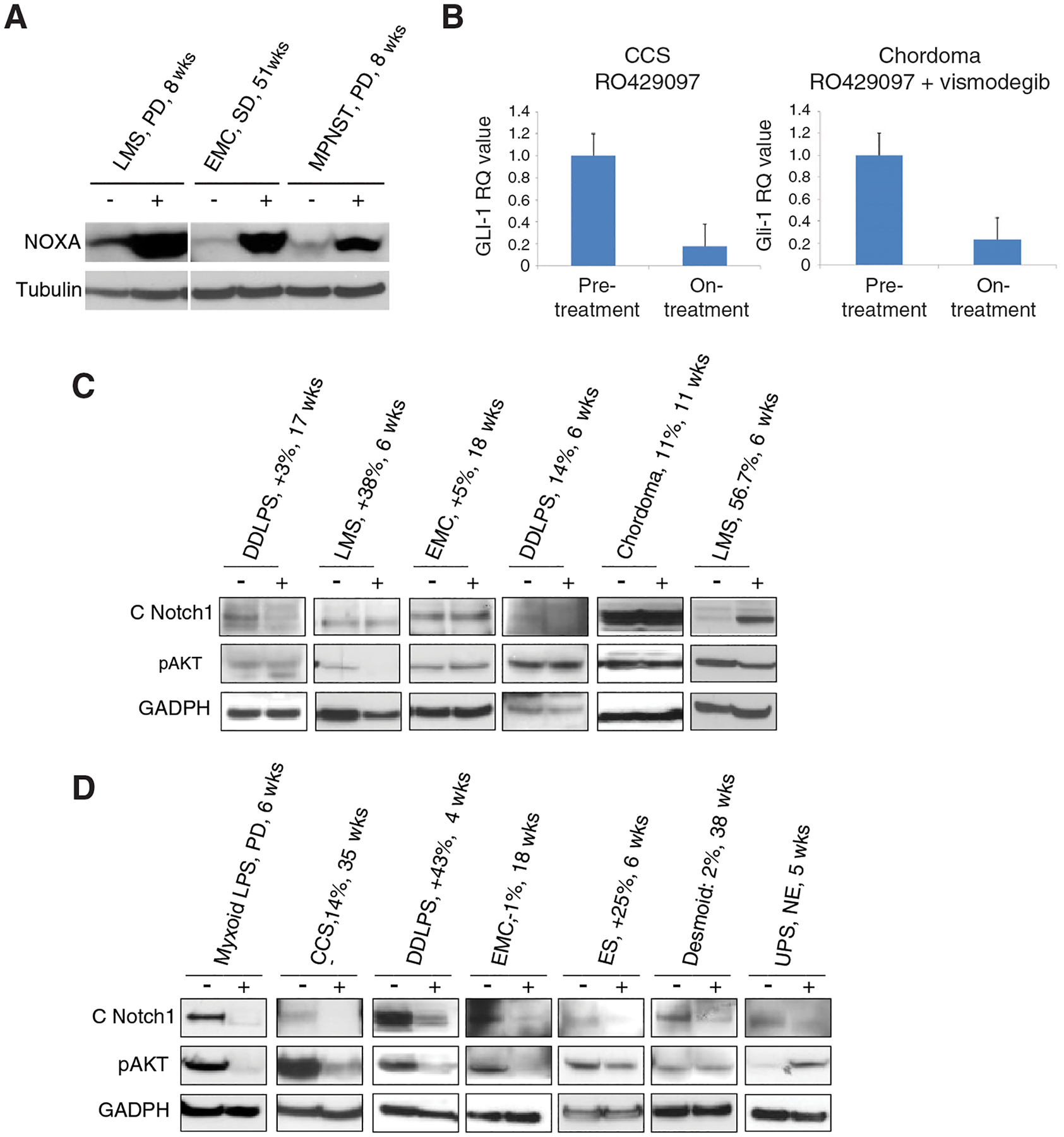
A, Western blot demonstrating induction of the pro-apoptotic protein NOXA in select paired biopsies treated with vismodegib. B, RT-PCR of GLI1 in paired tumor tissue from patients with clear cell sarcoma on the combination therapy arm (left) and chordoma on the RO4929097 monotherapy arm (right). C-D, Western blot indicating changes in cleaved Notch1 (C Notch1) and phosphorylated Akt (pAkt) in select paired biopsies on the RO4929097 (C) and combination (D) arms of the randomized phase II study portion. “-“ denotes baseline and “+” denotes on-treatment biopsy. GAPDH and tubulin are shown to confirm relative equal protein loading. Patients’ sarcoma type, best response of target lesion by RECIST 1.1, and time on study indicated above each pair of lanes. DDLPS, de-differentiated liposarcoma; LMS, leiomyosarcoma; EMC, extraskeletal myxoid chondrosarcoma; LPS, liposarcoma; CCS, clear cell sarcoma; UPS, undifferentiated pleomorphic sarcoma; MPNST, malignant peripheral nerve sheath tumor.
DISCUSSION
Motivated by dysregulation of Notch and Hedgehog signaling in sarcomas, as well as the significant cross-talk between these two pathways,(22,23) we conducted a Phase Ib/II trial of the combination of the gamma secretase inhibitor (GSI) RO4929097 and the smoothened antagonist vismodegib in unresectable or metastatic sarcoma. The study was prematurely terminated due to manufacturer discontinuation of development of RO4929097, and exploratory survival analysis in the 67 enrolled patients did not show a significant difference in the primary endpoint of PFS between the two study arms. Both arms had better PFS than anticipated single agent (8.9 vs. 6 weeks) and combination (12 vs. 9.6 weeks). Ultimately, we doubt that we would have seen a meaningful difference between the two arms if the accrual had been completed as originally planned. Although there were no objective responses seen in either treatment arm, select patients (n = 4) had regression of their tumors by more than 10% by RECIST 1.1, and others had prolonged disease stability ≥ 24 weeks (n = 11). This clinical finding indicates that study treatment likely had some degree of activity in select patients, many of whom were heavily pre-treated.
In paired tumor biopsies, we demonstrate that GSIs can inhibit downstream targets of the Notch pathway. While others have shown inhibition of the transcription factor hairy and enhancer of split 1 (HES-1) in hair follicles by GSIs (17), this is the first study, to our knowledge, demonstrating reduction of cleaved Notch in patients. In select, paired tumor tissue, we also demonstrate that the GSI RO4929097 inhibits expression of the Hedgehog pathway transcription factor GLI1, upregulates the pro-apoptotic protein NOXA, and reduces phospho-Akt. This PD evidence of target pathway inhibition did not consistently correspond to tumor shrinkage or duration of study treatment. Any robust conclusions regarding the degree of Notch and Hedgehog pathway inhibition are limited by the small sample size of available and evaluable paired tumor tissue; some biopsies yielded necrotic tissue.
Because of past reports of auto-inhibition by RO4929097 at higher doses, and based on the PK analysis in phase 1b indicating that vismodegib decreases systemic exposure to RO4929097, we measured free or unbound RO4929097. This analysis confirmed that the active drug concentration likely remained at pharmacologically active levels in both trial arms. This result was validated by the PD analyses, which demonstrated inhibition of cleaved Notch in both study arms. Our findings underscore the importance of PD endpoints with paired tumor tissue in early-phase drug development, especially in trials of agents with complicated PK.
Early-phase studies of these agents in sarcoma have also found hints of clinical efficacy. A phase II trial of vismodegib monotherapy in chondrosarcoma reported that 25.6% of patients achieved clinical benefit.(24) Ten patients with grade I-II conventional chondrosarcoma, who had documented progression within 6 months of enrollment, achieved stable disease for ≥ 6 months. In a phase I study of RO4929097 in patients with solid tumors, 4 of 12 patients with sarcoma achieved clinical benefit, including a patient with widely metastatic epithelioid sarcoma who achieved 12% tumor reduction; the specific histology of the remaining 3 sarcoma patients was not reported (17). In desmoid tumors, several phase 1 and 2 studies have shown objective responses and prolonged stable disease with GSIs, which are now being evaluated in a prospective phase 3 study. The exact mechanism of action of GSIs in desmoid tumors is unknown, and several different mechanisms have been postulated. In contrast, in our study, 5 evaluable patients with desmoid tumors were enrolled, none of whom had objective responses. However, 3 of 4 benefited from treatment, displaying prolonged stabilization of disease.
As the aim of this study was to broadly assess the activity of combined Notch and Hedhgehog pathway inhibition in sarcoma, the eligibility criteria were not restricted to specific subtypes or molecular profiles. Because sarcomas are a heterogeneous group of diseases with unique histologic and molecular profiles, the field has recently prioritized a histotype-tailored approach (25), which could reveal efficacy of this therapeutic strategy in selected sarcoma histo- or molecular subtypes. In the chondrosarcoma study cited above, among patients with tumor expression data available, 65% had Hedgehog ligand overexpression, including all who had stable disease for ≥ 6 months (24), suggesting that this feature could serve as a molecular biomarker of efficacy of GSIs.
Our study highlights the challenges of trial design in sarcoma especially regarding pre-trial assumptions for endpoints such as PFS in a heterogenous group of diseases with varying natural history and biology. PFS is used increasingly as a metric for comparison of chemotherapeutic agents in sarcomas, given that responses rates are relatively low, at least in part due to the biology of chemotherapy responses of sarcomas, which result more often from senescence than from apoptosis. Our pre-trial assumptions were based on a European Organisation for Research and Treatment of Cancer (EORTC) meta-analysis of 12 clinical trials of various agents, of which only two (ifosfamide and dacarbazine) yielded significant response rates.(26) From these data, the study summarized PFS for the 10 inactive agents (in which trials patients who had not received prior therapy) vs. the two active agents (in which trials patients had received prior anthracyclines) at 21% vs. 39% at 3 months an 8% vs. 14% at 6 months. Our trial did not require RECIST progression at time of enrollment, which may explain the better than expected PFS in both arms, as some patients may have had indolent disease.
This study has several strengths, including its randomized nature, the relatively large cohort for a phase II single-center trial in a rare disease, and the inclusion of PK and PD analyses. However, the study did not have a biomarker to select patients, an issue that continues to plague current trials of GSIs. We acknowledge that gamma secretase enzymes play critical roles in dozens of cell signaling pathways, but only a subset are likely to be essential in a given cellular context. For example, gamma secretases cleave B cell maturation antigen (BCMA) (27), the target of several therapies in multiple myeloma, leading to resistance, so gamma secretase inhibitors are being evaluated as a means of delaying resistance to BCMA-directed therapies (28). We suggest that studies utilizing GSIs evaluate the myriad of roles this enzyme plays in pathophysiology.
In summary, this trial confirmed the safety of RO4929097 plus vismodegib combination treatment and found that it did not significantly improve progression-free survival in patients with advanced sarcoma. Further studies targeting these pathways in sarcoma and other cancers should focus on histologies with clear evidence of pathway dysregulation at baseline and incorporate PD biomarkers to establish the relationship between the study drug and response.
Supplementary Material
Statement of translational relevance:
Because the Hedgehog and Notch pathways are often overexpressed in sarcomas, we conducted a phase Ib/II randomized trial of the gamma secretase inhibitor RO4929097 with or without the smoothened antagonist vismodegib in patients with unresectable or metastatic sarcoma. In the phase Ib portion (9 patients), there were no dose-limiting toxicities and most adverse events (AEs) were grade ≤ 2. In phase II, 34 patients were randomized to RO4929097 alone and 33 to RO4929097 plus vismodegib prior to premature closing of the study due to discontinuation of RO4929097. Vismodegib reduced the plasma concentration of RO492909. No patients had an objective response by RECIST 1.1. Neither median progression-free survival nor median overall survival differed significantly between the monotherapy arm and the combination arm. Paired tumor biopsies demonstrated inhibition of cleaved Notch. These results suggest that vismodegib does not meaningfully enhance the clinical efficacy of RO4929097.
Acknowledgements
This research was supported by Roche Pharmaceuticals, Gateway for Cancer Research, the Siskind Family Fund, Cycle for Survival, the Draper Family Fund, and the US National Cancer Institute through grants N01 CM62206, U01 CA069856, and P30 CA008748 to the institution. The authors thank Jessica Moore, MS (Department of Surgery, MSK), for editorial support.
Disclosures:
M.M. Gounder has served on advisory boards for Athenex, Ayala, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Epizyme, Karyopharm, Rain Therapeutics, SpringWorks Therapeutics, Tracon, and TYME Technologies; provides consulting services through Guidepoint, GLG Pharma, Third Bridge, and Flatiron Health; has received speaking honoraria from Medscape, More Health, Physicians Education Resource and touchIME; receives publishing royalties from Wolters Kluwer; holds a patent for a patient-reported outcome tool licensed through the institution; and has performed research without compensation in collaboration with Foundation Medicine. N. Wu is an employee of LipoSeuticals, which has no interest in this study. M.A. Dickson has received institutional research funding from Aadi Bioscience and Eli Lilly. S.P. D’Angelo has received institutional research funding from Amgen, Bristol Meyers Squibb, Deciphera, EMD Serono, Incyte, Merck, and Nektar Therapeutics, has served as a consultant or on advisory boards for Adaptimmune, Amgen, EMD Serono, GlaxoSmithKline, Immune Design, Immunocore, Incyte, Merck, and Nektar Therapeutics, and has served on data safety monitoring boards for Adaptimmune, GlaxoSmithKline, Merck, and Nektar Therapeutics. P. Chi has served on advisory boards for Deciphera, Exelixis, and Zai Lab Ltd., consulted for Novartis, and received research funding via the institution from Pfizer/Array, Deciphera, and Ningbo NewBay. A.M. Crago has served on an advisory board for SpringWorks Therapeutics. W.D. Tap has served on advisory boards for Agios Pharmaceuticals, Bayer, Blueprint Medicines, C4 Therapeutics, Certis Oncology Solutions (in which he also owns stock), Daiichi Sankyo, Deciphera, EMD Serono, Epizyme, Innova Therapeutics, Medpacto, Mundipharma, and NanoCarrier, has provided consulting services for Adcendo, Ayala Pharmaceuticals, Cogent Biosciences, Kowa, and Servier, holds two patents for biomarkers of CDK4 inhibitor efficacy in cancer, and is a co-founder of and owns stock in Atropos Therapeutics. All disclosed relationships are unrelated to the present study. No other authors have financial or other interests to disclose.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70(1):7–30 [DOI] [PubMed] [Google Scholar]
- 2.Tanaka K, Kawano M, Iwasaki T, Itonaga I, Tsumura H. A meta-analysis of randomized controlled trials that compare standard doxorubicin with other first-line chemotherapies for advanced/metastatic soft tissue sarcomas. PLoS One 2019;14(1):e0210671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Graaf WTA, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379(9829):1879––86 [DOI] [PubMed] [Google Scholar]
- 4.Demetri GD, von Mehren M, Jones RL, Hensley ML, Schuetze SM, Staddon A, et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J Clin Oncol 2016;34(8):786–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoffski P, Chawla S, Maki RG, Italiano A, Gelderblom H, Choy E, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet 2016;387(10028):1629–37 [DOI] [PubMed] [Google Scholar]
- 6.Francis P, Namlos HM, Muller C, Eden P, Fernebro J, Berner JM, et al. Diagnostic and prognostic gene expression signatures in 177 soft tissue sarcomas: hypoxia-induced transcription profile signifies metastatic potential. BMC Genomics 2007;8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dakubo GD, Mazerolle CJ, Wallace VA. Expression of Notch and Wnt pathway components and activation of Notch signaling in medulloblastomas from heterozygous patched mice. J Neurooncol 2006;79(3):221–7 [DOI] [PubMed] [Google Scholar]
- 8.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev 2001;15(23):3059–87 [DOI] [PubMed] [Google Scholar]
- 9.Nefedova Y, Cheng P, Alsina M, Dalton WS, Gabrilovich DI. Involvement of Notch-1 signaling in bone marrow stroma-mediated de novo drug resistance of myeloma and other malignant lymphoid cell lines. Blood 2004;103(9):3503–10 [DOI] [PubMed] [Google Scholar]
- 10.Dickson BC, Mulligan AM, Zhang H, Lockwood G, O’Malley FP, Egan SE, et al. High-level JAG1 mRNA and protein predict poor outcome in breast cancer. Mod Pathol 2007;20(6):685–93 [DOI] [PubMed] [Google Scholar]
- 11.Massi D, Tarantini F, Franchi A, Paglierani M, Di Serio C, Pellerito S, et al. Evidence for differential expression of Notch receptors and their ligands in melanocytic nevi and cutaneous malignant melanoma. Mod Pathol 2006;19(2):246–54 [DOI] [PubMed] [Google Scholar]
- 12.Okuyama R, Tagami H, Aiba S. Notch signaling: its role in epidermal homeostasis and in the pathogenesis of skin diseases. J Dermatol Sci 2008;49(3):187–94 [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, De Marco MA, Graziani I, Gazdar AF, Strack PR, Miele L, et al. Oxygen concentration determines the biological effects of NOTCH-1 signaling in adenocarcinoma of the lung. Cancer Res 2007;67(17):7954–9 [DOI] [PubMed] [Google Scholar]
- 14.Aster JC, Pear WS, Blacklow SC. Notch signaling in leukemia. Annu Rev Pathol 2008;3:587–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curry CL, Reed LL, Broude E, Golde TE, Miele L, Foreman KE. Notch inhibition in Kaposi’s sarcoma tumor cells leads to mitotic catastrophe through nuclear factor-kappaB signaling. Mol Cancer Ther 2007;6(7):1983–92 [DOI] [PubMed] [Google Scholar]
- 16.Meng RD, Shelton CC, Li Y, Schwartz GK. Disrupting the notch pathway with gamma-secretase inhibitors suppresses growth and induces apoptosis of sarcoma cell lines with constitutively active ras and notch signaling pathways. Cancer Research 2006;66(8 Supplement):950– [Google Scholar]
- 17.Tolcher AW, Messersmith WA, Mikulski SM, Papadopoulos KP, Kwak EL, Gibbon DG, et al. Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J Clin Oncol 2012;30(19):2348–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LoRusso PM, Rudin CM, Reddy JC, Tibes R, Weiss GJ, Borad MJ, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res 2011;17(8):2502–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham RA, Lum BL, Cheeti S, Jin JY, Jorga K, Von Hoff DD, et al. Pharmacokinetics of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with locally advanced or metastatic solid tumors: the role of alpha-1-acid glycoprotein binding. Clin Cancer Res 2011;17(8):2512–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J, Wiegand R, LoRusso P, Li J. Validation and implementation of a liquid chromatography/tandem mass spectrometry assay for quantitation of the total and unbound RO4929097, a gamma-secretase inhibitor targeting Notch signaling, in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2011;879(19):1537–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu J, Lorusso PM, Matherly LH, Li J. Implications of plasma protein binding for pharmacokinetics and pharmacodynamics of the gamma-secretase inhibitor RO4929097. Clin Cancer Res 2012;18(7):2066–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol 2013;14(7):416–29 [DOI] [PubMed] [Google Scholar]
- 23.Pelullo M, Zema S, Nardozza F, Checquolo S, Screpanti I, Bellavia D. Wnt, Notch, and TGF-beta Pathways Impinge on Hedgehog Signaling Complexity: An Open Window on Cancer. Front Genet 2019;10:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Italiano A, Le Cesne A, Bellera C, Piperno-Neumann S, Duffaud F, Penel N, et al. GDC-0449 in patients with advanced chondrosarcomas: a French Sarcoma Group/US and French National Cancer Institute Single-Arm Phase II Collaborative Study. Ann Oncol 2013;24(11):2922–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gronchi A, Ferrari S, Quagliuolo V, Broto JM, Pousa AL, Grignani G, et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol 2017;18(6):812–22 [DOI] [PubMed] [Google Scholar]
- 26.Van Glabbeke M, Verweij J, Judson I, Nielsen OS. Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer 2002;38(4):543–9 [DOI] [PubMed] [Google Scholar]
- 27.Laurent SA, Hoffmann FS, Kuhn PH, Cheng Q, Chu Y, Schmidt-Supprian M, et al. γ-Secretase directly sheds the survival receptor BCMA from plasma cells. Nat Commun 2015;6:7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pont MJ, Hill T, Cole GO, Abbott JJ, Kelliher J, Salter AI, et al. γ-Secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood 2019;134(19):1585–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data are available from the authors upon reasonable request.


