Abstract
Ixodes ricinus is the most relevant vector for tick-borne diseases in Austria and responsible for the transmission of Borrelia burgdorferi sensu lato (s. l.), which causes Lyme borreliosis in humans; however, also other bacteria and protozoa can be found in ticks and have the potential of infecting people and animals. In this study we collected ticks in popular recreational areas in the city of Vienna in the years 2019 and 2020 and analyzed them for the presence of such putative pathogenic microorganisms. By using reverse line blot (RLB) hybridization we detected DNA of B. burgdorferi s. l., Rickettsia spp., Babesia spp., Candidatus Neoehrlichia mikurensis (CNM) and Anaplasma phagocytophilum. Moreover, we also screened them for the relapsing fever spirochete Borrelia miyamotoi employing real-time PCR. The most frequently detected pathogens were B. burgdorferi s. l. in 28.6% of the ticks in 2019 and 21.3% of the ticks in 2020. The genus Rickettsia was detected in 13.8% of the ticks from 2019 and only in 4.6% from 2020. Babesia spp. were detected in 5.7% in 2019 and 4.2% in 2020. Furthermore, we detected CNM in 4.0% (2019) and 5.6% (2020), A. phagocytophilum in 0.5% (2019) and 1.3% (2020) and finally B. miyamotoi in 3.3% (2019) and 1.7% (2020). Collectively, we show that various microorganisms are prevalent in ticks collected in Vienna and identify hotspots for B. miyamotoi, which we have detected for the first time in the city.
Keywords: Vienna, Borrelia miyamotoi, Tick-borne pathogens, Borrelia, City parks
Introduction
Ixodes ricinus is the most abundant tick species in Austria and is commonly associated with the transmission of a large number of (potentially) pathogenic microorganisms [1–3]. Out of the large number of tick-borne pathogens, spirochetes of the Borrelia burgdorferi sensu lato (s. l.) complex, the causative agent for Lyme borreliosis, are the most frequently found pathogens in Ixodes ticks collected in Austria [4, 5]. While the genospecies B. afzelii, B. burgdorferi sensu stricto (s.s.), B. garinii, B. bavariensis and B. spielmanii are known to cause disease in humans, the genospecies B. bissettii, B. lusitaniae, and B. valaisiana are believed to be of lower importance for human disease [6, 7]. The different possible manifestations of the disease have been linked to different genospecies: B. afzelii is known to cause primarily skin manifestations, B. garinii and B. bavariensis are more frequently linked to neuroborreliosis and B. burgdorferi s.s. seems to affect the joints more than other genospecies [8].
Aside from B. burgdorferi s. l., Anaplasma phagocytophilum, Candidatus Neoehrlichia mikurensis and several Babesia and Rickettsia species have been detected in Ixodes ticks collected in Austria in the past [5, 9, 10].
The intracellular bacterium A. phagocytophilum is known to infest the granulocytes and can lead to flu-like illness, so-called human granulocytic anaplasmosis (HGA), a self-limiting infection in most cases but leading to severe and fatal disease in immunocompromised patients [11–13].
An infection with Candidatus N. mikurensis can cause symptoms, such as fever, malaise, weight loss and septicemia by affecting the vascular endothelium [14–17]; however, recently we showed that this pathogen can persist multiple weeks in human blood without causing any symptoms [18].
The intraerythrocytic parasites of the genus Babesia belong to the phylum of Apicomplexa and can lead to babesiosis, a flu-like to malaria-like illness including symptoms like malaise, chills, myalgia, anemia, fatigue and fever [19]. In immunocompromised, especially in splenectomised, individuals Babesia infections can lead to life-threatening complications like severe hemolysis [19]. B. venatorum infection of an immunocompromised 56-year-old male, most likely caused by a tick bite, was reported in Austria in 2003 [20]. Also, human infections with the species Babesia divergens and Theileria (Babesia) microti have already been reported in Europe [21–23].
Within the genus Rickettsia the spotted-fever group (SFG) species R. helvetica, R. monacensis, R. raoultii and R. slovaca have been detected in ticks collected in Austria [5, 10]. The species R. slovaca and R. raoultii are known to cause scalp eschar, facial edema and cervical lymphadenopathy, the so-called tick-borne lymphadenopathy (TIBOLA) or Dermacentor-borne necrosis erythema and lymphadenopathy (DEBONEL) [24]. The infection with R. helvetica has been occasionally associated with perimyocarditis and eruptive fever in the literature but other studies showed that despite a high prevalence in ticks, R. helvetica poses no risk to humans [18, 25, 26]. R. monacensis infections present with symptoms like fever, headache, general discomfort, joint pain and erythematous rash [27]. Non-SFG Rickettsiae, specifically Candidatus R. mendelii and Candidatus R. thierseensis, have been found in ticks in Austria as well [18, 28].
The relapsing fever spirochete Borrelia miyamotoi has been detected in I. ricinus ticks collected in Austria during the year 2005 [29] but not in Vienna. The disease caused by B. miyamotoi infection is described as a nonspecific febrile illness which can also occur asymptomatically and human cases have sporadically been reported in Austria [18, 30, 31].
Due to this large variety of pathogens known to be present in I. ricinus in Austria and the fact that urban recreational areas in Vienna are regularly visited by the citizens, the infection rate of ticks in these areas is of particular medical interest. To assess this, we collected questing I. ricinus ticks at seven popular green spaces in Vienna and screened them for a large panel of potentially pathogenic microorganisms by using the reverse line blot hybridization assay (RLB) and real-time PCR.
Material and methods
Collecting of ticks
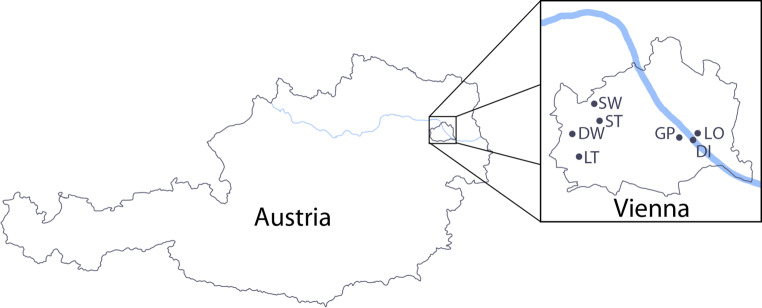
From April to May 2019 questing ticks were collected at Deutschordenswald (48°12′02.6″N 16°13′38.1″E), Donauinsel (48°11′14.3″N 16°28′14.2″E), Grüner Prater (48°11′30.7″N 16°26′11.8″E), Lainzer Tiergarten (48°10′18.5″N 16°14′58.4″E), Nationalpark Lobau (48°11′37.0″N 16°28′19.9″E), Schottenwald (48°14′05.3″N 16°16′04.4″E) and Steinhofgründe (48°12′38.2″N 16°16′32.9″E) (Fig. 1). From May to July 2020 questing ticks were collected again in the same areas with the exception of Schottenwald (due to SARS-CoV‑2 limitations in time and resources). All ticks were collected by dragging a white cotton cloth through the sampling area. The collection sites showed similar features like mixed forests with strong undergrowth, meadows, and wildlife. For tick collection sunny to cloudy, but dry days were chosen and the temperature during sampling varied from 17 °C to 24 °C in 2019 and from 14 °C to 29 °C in 2020.
Fig. 1.
Tick collection sites in Vienna. Schematic map of Austria with magnification of Vienna showing the seven collection sites. DW Deutschordenswald, DI Donauinsel, GP Grüner Prater, LT Lainzer Tiergarten, LO Nationalpark Lobau, SW Schottenwald, ST Steinhofgründe. (The figure is modified from Google Maps, https://maps.google.com/)
Tick identification and DNA extraction
Prior to DNA extraction, the I. ricinus ticks were morphologically identified using a current identification key [32]. No additional molecular identification of the collected ticks was carried out and we considered all screened I. ricinus ticks to belong to the I. ricinus complex which includes I. inopinatus, due to the recently observed inability to distinguish I. ricinus from I. inopinatus molecularly [33]. In 2019, from all locations 30 ticks were tested. In 2020, 40 ticks were tested from every location except from Schottenwald where no sampling took place in 2020. Our study was focused on nymphal ticks as this is the life stage of the highest medical relevance to humans. In case not enough nymphal ticks were flagged we added adult ticks to reach a minimum number of 30 ticks per location. In 2020 exclusively nymphs were investigated. In 2019 we included some adult ticks for the locations Donauinsel (n = 10), Schottenwald (n = 2), Steinhof (n = 2) and Deutschordenswald (n = 10) to reach 30 ticks per collection site.
The DNA was extracted using the DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany). Briefly, individual ticks were washed in 70% ethanol, air-dried and cut into halves using a sterile surgical blade. The lysis step was performed by overnight incubation at 56 °C in 180 µL ATL buffer and 20 µL proteinase K. The following DNA extraction steps were performed according to the manufacturer’s protocol. DNA was eluted with 100 µL pre-warmed (70 °C) AE buffer and stored at −20 °C until further use.
Pathogen detection by RLB
Genus-specific PCR targeting the Anaplasma/Ehrlichia/Neoehrlichia 16S rRNA gene, the Babesia/Theileria 18S rRNA gene, the Borrelia 23S-5S intergenic spacer (IGS), the Rickettsia 16S rRNA gene and the Rickettsia 23S-5S IGS were performed for each tick DNA extract using a 5’biotin-labeled primer per PCR reaction as described elsewhere [5, 34]. The RLB was performed according to a protocol described previously [35]. Briefly, the PCR products were combined for each tick individually in 2x SSPE buffer (Thermo Scientific, Vienna, Austria) consisting of 0.3M NaCl, 0.02 NaH2PO4, 0.002 M EDTA and additionally added 0.1% sodium dodecyl sulphate (SDS, Applichem, Darmstadt, Germany). The combined PCR products were denatured and the single-stranded PCR products were then hybridized to a nylon membrane (Biodyne C, Pall Laboratories, Crailsheim, Germany) with a set of covalently linked genus-specific (catch-all) and species-specific oligonucleotide probes (Eurofins Genomics GmbH, Ebersberg, Germany) allowing the detection of several Borrelia spp., Rickettsia spp., Babesia spp., Anaplasma and Ehrlichia spp. (individual probes used are listed by Schötta et al. 2017 [5]). After incubation with 1:10,000 diluted horseradish peroxidase-labelled streptavidin (Roche Diagnostics GmbH, Mannheim, Germany) visualization of the hybridized PCR products was enabled by the Pierce ECL Western blotting substrate (Thermo Fisher Scientific, Vienna, Austria) and the ChemiDoc touch imaging system (Bio-Rad, Hercules, CA, USA). For quality control of the PCR step, DNA extracts of bacterial cultures (B. burgdorferi s.s., B. afzelii, R. raoultii) or DNA extracts of confirmed-positive ticks (A. marginale, B. canis, C. N. mikurensis) were used. For the quality control of the blotting step, pooled biotinylated PCR amplicons of confirmed-positive ticks (A. phagocytophilum, T. (B.) microti, B. afzelii, R. monacensis, R. raoultii, R. slovaca) were used.
Detection of B. miyamotoi by real-time PCR
To detect B. miyamotoi DNA, we used a real-time PCR targeting the glycerophosphodiester phosphodiesterase gene (glpQ) as published by Reiter et al. 2015 [29]. The PCR reaction (20 µL total volume) contained 10 µl 2 × GoTaq® Probe qPCR Master Mix (Promega), 0.5 µM of each primer, 0.25 µM probe (Sigma-Aldrich, Vienna, Austria) and 4 µl of DNA. The reaction volume was adjusted with PCR-grade water (Sigma-Aldrich, Vienna, Austria). A two-step real-time PCR was run in an Applied Biosystems™ (Thermo Fisher Scientific, Vienna, Austria) QuantStudio 5 Real-Time PCR system with the following conditions: 95 °C for 10 min followed by 50 cycles of 95 °C for 15 s and 60 °C for 1 min. One reaction using a plasmid containing the target sequence and one reaction using PCR-grade water instead of DNA were included for quality control of the PCR run.
Statistical analysis
IBM SPSS Statistics 25.0 was used to analyze the collected dataset by descriptive statistics. Differences within nominal categories (e.g., tick life stage, infection rate) and the significance of co-infections were calculated using Fisher’s exact test or Pearson’s χ2-test. Two-tiered p-values of < 0.05 were considered significant.
Results
Borrelia burgdorferi sensu lato
In 2019, of the 210 ticks 60 (28.6%) and in 2020 of the 240 ticks 51 (21.3%) were positive for at least 1 member of the B. burgdorferi s. l. complex by RLB. The most common genospecies detected in 2019 as well as in 2020 was B. afzelii (16.2% in 2019; 15.0% in 2020), followed by B. burgdorferi s. s. (6.7% in 2019; 3.8% in 2020; Table 1).
Table 1.
Microorganisms detected in ticks in 2019 and 2020
| Year of collection |
Location | No. of ticks |
B. burgdorferi s. l. | Rickettsia spp | Babesia spp | Others | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ba | Bbss | Bg/Bbav | Bsp | Bval | Rh | Rm | Bv | T(B)m | Ap | CNm | Bomy | |||
| 2019 | Deutschordenswald | 20 N, 10 A | 2 N, 4 A | 2 A | 3 A | – | 1 A | 2 N | – | – | 1 N, 1 A | – | 3 N, 2 A | 1 A |
| Donauinsel | 20 N, 10 A | 3 N, 1 A | 1 N | 3 N | 1 N, 2 A | 4 A | 1 N, 4 A | 1 A | – | 1 N | – | – | 1 N | |
| Grüner Prater | 30 N | 2 | 5 | 1 | – | – | 7 | – | – | – | 1 | – | – | |
| Lainzer Tiergarten | 30 N | 10 | – | – | – | – | 2 | – | – | 7 | – | 4 | 4 | |
| Lobau | 30 N | 2 | 3 | 2 | – | – | 7 | – | – | – | – | 1 | 1 | |
| Schottenwald | 28 N, 2 A | 2 N | – | – | – | – | 2 N, 1 A | – | – | 1 N | – | 2 N | – | |
| Steinhof | 28 N, 2 A | 7 N, 1 A | 3 N | – | – | – | 2 N | – | – | 1 N | – | 1 N, 1 A | – | |
| 2020 | Deutschordenswald | 40 N | 6 | – | 1 | – | 1 | 2 | – | – | – | 1 | 3 | – |
| Donauinsel | 40 N | 6 | 3 | 1 | – | 1 | 1 | 1 | 1 | – | – | – | – | |
| Grüner Prater | 40 N | 3 | 2 | – | – | – | 1 | – | 1 | 1 | 1 | 1 | – | |
| Lainzer Tiergarten | 40 N | 9 | – | – | – | 2 | 4 | 1 | 1 | 4 | 1 | 4 | 1 | |
| Lobau | 40 N | 5 | 2 | 1 | – | 1 | 1 | – | – | 1 | – | – | 1 | |
| Steinhof | 40 N | 7 | 2 | – | – | – | – | – | – | 1 | – | – | 2 | |
A adults, N nymphs, Ba B. afzelii, Bbss B. burgdorferi s.s., Bg/Bbav B. garinii/B. bavariensis, Bsp B. spielmanii, Bval B. valaisiana, Rh R. helvetica, Rm R. monacensis, Bv B. venatorum, T(B)m T. (B.) microti, Ap A. phagocytophilum, CNm Candidatus Neoehrlichia mikurensis, Bomy B. miyamotoi
We detected B. garinii/B. bavariensis DNA in 9 of the 210 (4.3%) ticks from 2019 and in 3 of the 240 ticks (1.3%) collected in 2020. B. spielmanii was detected in 3 of the 210 ticks (1.4%) from 2019 and was not detected in any of the ticks collected in 2020, 5 of the 210 ticks (2.4%) from 2019 and 5 of the 240 ticks (2.0%) from 2020 were positive for B. valaisiana. B. spielmanii was only found in three ticks collected at Donauinsel in 2019. The highest number of B. afzelii positive ticks was found in both years at the location Lainzer Tiergarten. In 2019, no other genospecies of the Borrelia burgdorferi s. l. complex was detected at Lainzer Tiergarten; in 2020 we also detected B. valaisiana in 2 of the 40 ticks. In ticks collected at Steinhof we detected only the genospecies B. afzelii and B. burgdorferi s. s. in both years. Ticks from Schottenwald were only collected in 2019 and we detected B. afzelii in 2 out of 30 ticks. Co-infections with multiple Borrelia burgdorferi s. l. species occurred in four ticks, of which two were detected in nymphs (Table 2).
Table 2.
All single microorganisms and co-infections detected within the collected ticks
| Species | No. (%) of infected ticks 2019 (n = 210 n(+) = 97) |
No. (%) of infected ticks 2020 (n = 240 n(+) = 71) |
|---|---|---|
| Single infections | n = 72 | n = 58 |
| B. afzelii | 18 (8.6) | 25 (10.4) |
| B. burgdorferi s.s. | 8 (3.8) | 7 (2.9) |
| B. garinii/B. bavariensis | 7 (3.3) | 2 (0.8) |
| B. spielmanii | 2 (1.0) | 5 (2.1) |
| B. valaisiana | 4 (1.9) | 6 (2.5) |
| R. helvetica | 20 (9.5) | 2 (0.8) |
| R. monacensis | 1 (0.5) | 2 (0.8) |
| T. (B.) microti | 1 (0.5) | 2 (0.8) |
| A. phagocytophilum | 1 (0.5) | 3 (1.3) |
| Cand. N. mikurensis | 7 (3.3) | 3 (1.3) |
| B. miyamotoi | 3 (1.4) | 1 (0.4) |
| Dual infections | n = 20 | n = 10 |
| B. afzelii + T. (B.) microti | 6 (2.9) | 2 (0.8) |
| B. afzelii + B. burgdorferi | 2 (1.0) | 2 (0.8) |
| B. afzelii + Cand. N. mikurensis | – | 2 (0.8) |
| B. afzelii + R. helvetica | 2 (1.0) | 1 (0.4) |
| T. (B.) microti + Cand. N. mikurensis | 2 (1.0) | – |
| B. burgdorferi s.s. + R. helvetica | 1 (0.5) | – |
| B. burgdorferi s.s. + B. garinii/bavariensis | 1 (0.5) | – |
| B. spielmanii + R. helvetica | 1 (0.5) | – |
| B. valaisiana + R. helvetica | 1 (0.5) | – |
| B. afzelii + Cand. N. mikurensis | 1 (0.5) | – |
| R. helvetica + Cand. N. mikurensis | 1 (0.5) | – |
| B. afzelii + B. miyamotoi | – | 1 (0.4) |
| B. burgdorferi s.s. + B. miyamotoi | 1 (0.5) | – |
| B. garinii/bavariensis + B. miyamotoi | – | 1 (0.4) |
| T. (B.) microti + B. miyamotoi | 1 (0.5) | – |
| Cand. N. mikurensis + B. venatorum | – | 1 (0.4) |
| Triple infections | n = 4 | n = 2 |
| B. afzelii + Cand. N. mikurensis + R. helvetica | 1 (0.5) | – |
| B. afzelii + T. (B.) microti + R. helvetica | 1 (0.5) | 1 (0.4) |
| B. afzelii + B. miyamotoi + Cand. N. mikurensis | 1 (0.5) | – |
| B. afzelii + B. burgdorferi s.s. + B. garinii/bavariensis | 1 (0.5) | – |
| B. afzelii + T. (B.) microti + Cand. N. mikurensis | – | 1 (0.4) |
| Quadruple infections | n = 1 | n = 0 |
| B. afzelii + T. (B.) microti + B. miyamotoi + Cand. N. mikurensis | 1 (0.5) | – |
| Quintuple infections | n = 0 | n = 1 |
| B. afzelii + T. (B.) microti + B. miyamotoi + Cand. N. mikurensis + R. helvetica | – | 1 (0.4) |
Of the 210 ticks from 2019, 24 (11.4%) ticks were adult ticks of which 15 (62.5%) were positive for Borrelia burgdorferi s. l., hence, contributing to a significant number of positive ticks. A detailed overview with respect to the tick life stage is shown in Table 1.
Rickettsia species
Of the 210 ticks 29 (13.8%) collected in 2019 and 11 of the 240 ticks (4.6%) from 2020 were positive for Rickettsia species (Table 1); in both years only the species R. helvetica (13.3% in 2019; 3.8% in 2020) and R. monacensis (0.5% in 2019; 0.8% in 2020) were detected. No tick individual was co-infected with two Rickettsia species. In 2019, we detected R. helvetica DNA in ticks from every location and in 2020 we detected R. helvetica at Deutschordenswald, Donauinsel, Grüner Prater, Lainzer Tiergarten and Lobau, but in none of the 40 ticks collected at Steinhof.
Among nymphs the Rickettsia infection rate was 12.4% (23/186) in 2019 and 4.6% (11/240) in 2020; among adults the infection rate in 2019 was 25% (6/24).
Babesia species
Of the 210 ticks 12 (5.7%) collected in 2019 and 10 of 240 ticks (4.2%) collected in 2020 were positive for Babesia spp. (Table 1). All positive ticks from the 2019 collection and 7 positive ticks from the 2020 collection were infected with the species T. (B.) microti; 3 positive ticks from 2020 were infected with B. venatorum. Out of the 12 Babesia positive ticks 7 (58.3%) from 2019 and 5 out of the 10 Babesia positive ticks (50%) from 2020 were collected at the location Lainzer Tiergarten. We did not detect B. venatorum in the 210 ticks collected in 2019. In 2020 we detected B. venatorum in 1 of each of the 40 nymphs collected at Donauinsel, Grüner Prater and Lainzer Tiergarten. Among ticks in the nymphal life stage the Babesia spp. infection rate was 5.9% (11/186) in 2019 and 4.1% (10/240) in 2020; among adult ticks collected in 2019 the infection rate was 4.3% (1/23).
Anaplasma and Ehrlichia species
In 1 of the 210 ticks (0.5%) collected in 2019 and in 3 of the 240 ticks (1.3%) collected in 2020 we detected A. phagocytophilum (Table 1). In both years one positive tick was collected at the location Grüner Prater and in 2020 one additional positive tick was collected each at the location Lainzer Tiergarten and at Deutschordenswald. Among the nymphs the infection rate was 0.5% in 2019 and no adult tick from 2019 tested positive for A. phagocytophilum. For Cand. N. mikurensis 8 of the 210 ticks (4.0%) from 2019 and 14 of the 240 ticks (5.8%) from 2020 were positive. Among the nymphs from 2019 the infection rate was 6.0% and among adult ticks the infection rate was 12.5% in 2019.
Borrelia miyamotoi
We detected B. miyamotoi DNA by real-time PCR in 7 out of the 210 ticks (3.3%) collected at the locations Deutschordenswald, Donauinsel, Lainzer Tiergarten and Lobau in 2019 and in 4 of the 240 ticks (1.7%) collected at the locations Lainzer Tiergarten, Lobau and Steinhof in 2020 (Table 1). We collected the highest number of B. miyamotoi positive ticks in 2019 at Lainzer Tiergarten where 4 out of 30 ticks (13.3%) were positive.
Among nymphs, an infection rate of about 3.2% was calculated in 2019 and 1.6% in 2020. In adult ticks collected in 2019, the infection rate was 4.2%.
Detection of infections and co-infections in Ixodes ricinus ticks
Of the 210 ticks 97 (46.2%) collected in 2019 and 71 of the 240 ticks (29.6%) collected in 2020 tested positive for at least 1 pathogenic species (Table 2). In 2019, 72 ticks (34.8%) were infected with 1 pathogen, 20 ticks (9.5%) showed a dual infection, 4 ticks (1.9%) harboured 3 and one 1 (0.5%) harbored 4 pathogenic species; from the 2020 collection 58 of the 240 ticks (24.2%) were positive for 1 species, 10 (4.2%) for 2 species, 2 (0.8%) for 3 species and in 1 nymph (0.4%) we detected 5 species (Table 2). Co-occurrences of tick-borne pathogens were investigated with the Fisher’s exact test. In both years, detection of the co-occurrence was significant for Babesia spp. and Cand. N. mikurensis (2019: P = 0.036; 2020: P = 0.003) and B. burgdorferi s. l. and Babesia spp. (2019: P = 0.005, 2020: P = 0.038). In 2020, the co-occurrence of B. burgdorferi s. l. and B. miyamotoi was also significant (P = 0.031).
Discussion
In this study, we determined the tick infection rates for numerous tick-borne pathogens in urban city parks and suburban forests of Vienna. A total of 450 ticks (426 nymphs, 24 adults) were collected during the summer months at 7 (sub)urban locations and screened by RLB, a DNA-hybridization method which is feasible for a reliable detection of various tick-borne pathogens on genus and species levels at once. Moreover, we also investigated the ticks of Vienna for the presence of the relapsing fever spirochete B. miyamotoi by real-time PCR. The focus of this study was laid on nymphs, as this life stage of I. ricinus is the medically most relevant stage when it comes to transmitting a pathogen that could cause disease in humans. Next to their small size and thus easily overlooked while feeding, nymphs already underwent one previous bloodmeal during the larval stage and therefore might have taken up microorganisms from the host blood which could now be transmitted during their second blood meal. A recent study conducted in Austria showed that 72.1% of the reported human tick bites are ticks of the nymphal life stage [18].
With 28.6% positive ticks (60/210) in 2019 and 21.3% (51/240) in 2020, B. burgdorferi s. l. was the most common pathogen detected. The percentages calculated lie within the range shown in studies carried out previously in Austria and surrounding countries [5, 36, 37]. Within the B. burgdorferi s. l. complex, the most abundant genospecies was B. afzelii. As mentioned in the introduction, B. afzelii is the main cause of skin manifestations such as erythema migrans; however, in rare cases, B. afzelii can mimic a rickettsial infection by causing a scalp eschar [38]. Furthermore, in a recent case report, it was noted that B. afzelii DNA was detected in ocular tissue [39] making B. afzelii one of the most medically significant borrelial species.
The second most common genus detected over our 2‑year survey was Rickettsia. In 2019, 14.3% out of all ticks tested positive for Rickettsia spp. Strikingly, in 2020 only 4.6% of the ticks tested positive for Rickettsia spp. Next to the Rickettsia species R. helvetica and R. monacensis, which were detected in both years at several of our locations, no other species were detected even though species like R. raoultii, R. slovaca and the novel Candidatus R. thierseensis are known to be present in Austrian I. ricinus ticks [5, 28]. The finding that only R. helvetica and R. monacensis were found in this study might be due to their higher prevalence and distribution compared to the other genospecies.
The tick infection rates for B. miyamotoi, Babesia spp., A. phagocytophilum and Candidatus N. mikurensis were beneath 6.0% in both years and lie within the range previously described [5, 29, 40–42]. An astonishing finding was the significant co-occurrence of Babesia spp. and Candidatus N. mikurensis which was also already seen in a previous investigation of Austrian ticks [5]. It might be of great interest to further study the interaction of different microorganisms within the tick vector and whether this might have an influence on uptake of pathogens or transmission dynamics.
For both years, the location Lainzer Tiergarten was identified as a ‘hotspot’ for B. afzelii. In 2019 at Lainzer Tiergarten, 33.3% of the collected ticks were positive for B. afzelii and no other B. burgdorferi s. l. genospecies were detected. In 2020, 22.5% of the ticks were positive for B. afzelii (81.8% of the Borrelia burgdorferi sl positive ticks). Aside from B. afzelii, Lainzer Tiergarten was also identified as a ‘hotspot’ for the relapsing fever spirochete B. miyamotoi in 2019 with a tick infection rate of 13.3%, compared to the overall infection rate of 3.3% in 2019. In 2020, only 2.5% of the ticks collected at Lainzer Tiergarten were infected with this relapsing fever spirochete, but this is also above the 1.7% calculated among all locations in 2019. Furthermore, 58.3% of the Babesia spp. detected in 2019 and 50.0% of the Babesia spp. detected in 2020 were collected at Lainzer Tiergarten. Identifying hotspots is not just of epidemiological interest but also aids further studies of certain tick-borne pathogens when it comes to cultivation attempts and where to best collect ticks for certain experiments.
The Lainzer Tiergarten, a 2450 hectar Natura 2000 area (the largest coordinated network of protected areas in the world), was originally a fenced off hunting ground used by the Austrian imperial family. In 1919, the region was opened to the public and since 1941 has been classified as a nature reserve. The area supports a large diversity of wildlife, and as currently announced on the website (https://www.lainzer-tiergarten.at/ visited on the 19 January 2022), the Lainzer Tiergarten estimates to host, among other animals, 800–1000 wild boars, 80–100 red deer, 200–250 fallow deer, countless roe deer and around 700 mouflons. We suspect that the large density and diversity of wildlife present plays a large role in creating an unintended hotspot for tick-borne pathogens.
In conclusion, our study emphasizes the need to keep track of ticks and tick-borne pathogens within urban recreational areas to early identify potential health risks.
Funding
Open access funding provided by Medical University of Vienna.
Conflict of interest
A.-M. Schötta, T. Stelzer, G. Stanek, H. Stockinger and M. Wijnveld declare that they have no competing interests.
Footnotes
The authors Anna-Margarita Schötta and Theresa Stelzer contributed equally as first authors.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Radda A, Burger I, Stanek G, Wewalka G. Austrian hard ticks as vectors of Borrelia burgdorferi, overview. Zentralbl Bakteriol Mikrobiol Hyg A. 1986;263(1-2):79–82. doi: 10.1016/s0176-6724(86)80106-7. [DOI] [PubMed] [Google Scholar]
- 2.Rizzoli A, Silaghi C, Obiegala A, Rudolf I, Hubálek Z, Földvári G, et al. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in europe: new hazards and relevance for public health. Front Public Health. 2014;2:251. doi: 10.3389/fpubh.2014.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogelgesang JR, Walter M, Kahl O, Rubel F, Brugger K. Long-term monitoring of the seasonal density of questing ixodid ticks in Vienna (Austria): setup and first results. Exp Appl Acarol. 2020;81:409–420. doi: 10.1007/s10493-020-00511-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaschitz M, Narodoslavsky-Gföller M, Kanzler M, Walochnik J, Stanek G. Borrelia burgdorferi sensu lato genospecies in questing Ixodes ricinus ticks in Austria. Int J Med Microbiol. 2008;298:168–176. doi: 10.1016/j.ijmm.2007.10.001. [DOI] [Google Scholar]
- 5.Schötta A-M, Wijnveld M, Stockinger H, Stanek G. Approaches for reverse line blot-based detection of microbial pathogens in Ixodes ricinus ticks collected in Austria and impact of the chosen method. Appl Environ Microbiol. 2017;83:e00489-17. doi: 10.1128/AEM.00489-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margos G, Vollmer SA, Cornet M, Garnier M, Fingerle V, Wilske B, et al. A new Borrelia species defined by multilocus sequence analysis of housekeeping genes. Appl Environ Microbiol. 2009;75:5410–5416. doi: 10.1128/AEM.00116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379:461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 8.Strle F, Stanek G. Clinical manifestations and diagnosis of lyme borreliosis. Curr Probl Dermatol. 2009;37:51–110. doi: 10.1159/000213070. [DOI] [PubMed] [Google Scholar]
- 9.Blaschitz M, Narodoslavsky-Gföller M, Kanzler M, Stanek G, Walochnik J. Babesia species occurring in Austrian Ixodes ricinus ticks. Appl Environ Microbiol. 2008;74:4841–4846. doi: 10.1128/AEM.00035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaschitz M, Narodoslavsky-Gföller M, Kanzler M, Walochnik J, Stanek G. First detection of Rickettsia helvetica in Ixodes ricinus ticks in Austria. Vector Borne Zoonotic Dis. 2008;8:561–564. doi: 10.1089/vbz.2007.0250. [DOI] [PubMed] [Google Scholar]
- 11.Grankvist A, Andersson PO, Mattsson M, Sender M, Vaht K, Höper L, et al. Infections with the tick-borne bacterium “Candidatus Neoehrlichia mikurensis” mimic noninfectious conditions in patients with B cell malignancies or autoimmune diseases. Clin Infect Dis. 2014;58:1716–1722. doi: 10.1093/cid/ciu189. [DOI] [PubMed] [Google Scholar]
- 12.Dumler JS, Barat NC, Barat CE, Bakken JS. Human granulocytic anaplasmosis and macrophage activation. Clin Infect Dis. 2007;45:199–204. doi: 10.1086/518834. [DOI] [PubMed] [Google Scholar]
- 13.Dumler JS, Madigan JE, Pusterla N, Bakken JS. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45(Suppl 1):S45–S51. doi: 10.1086/518146. [DOI] [PubMed] [Google Scholar]
- 14.Wass L, Grankvist A, Bell-Sakyi L, Bergström M, Ulfhammer E, Lingblom C, et al. Cultivation of the causative agent of human neoehrlichiosis from clinical isolates identifies vascular endothelium as a target of infection. Emerg Microbes Infect. 2019;8:413–425. doi: 10.1080/22221751.2019.1584017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Von Loewenich FD, Geißdörfer W, Disqué C, Matten J, Schett G, Sakka SG, et al. Detection of “Candidatus Neoehrlichia mikurensis” in two patients with severe febrile illnesses: evidence for a European sequence variant. J Clin Microbiol. 2010;48:2630–2635. doi: 10.1128/JCM.00588-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fehr JS, Bloemberg GV, Ritter C, Hombach M, Lüscher TF, Weber R, Keller PM. Septicemia caused by tick-borne bacterial pathogen Candidatus Neoehrlichia mikurensis. Emerg Infect Dis. 2010;16(7):1127–1129. doi: 10.3201/eid1607.091907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glatz M, Müllegger RR, Maurer F, Fingerle V, Achermann Y, Wilske B, et al. Detection of Candidatus Neoehrlichia mikurensis, Borrelia burgdorferi sensu lato genospecies and Anaplasma phagocytophilum in a tick population from Austria. Ticks Tick Borne Dis. 2014;5:139–144. doi: 10.1016/j.ttbdis.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Markowicz M, Schötta AM, Höss D, Kundi M, Schray C, Stockinger H, Stanek G. Infections with tickborne pathogens after tick bite, Austria, 2015-2018. Emerg Infect Dis. 2021;27(4):1048–1056. doi: 10.3201/eid2704.203366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Homer MJ, Aguilar-Delfin I, Telford SR, Krause PJ, Persing DH. Babesiosis. Clin Microbiol Rev. 2000;13:451–469. doi: 10.1128/CMR.13.3.451-469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herwaldt BL, Cacciò S, Gherlinzoni F, Aspöck H, Slemenda SB, Piccaluga PP, et al. Molecular characterization of a non-Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg Infect Dis. 2003;9:942–948. doi: 10.3201/eid0908.020748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mørch K, Holmaas G, Frolander PS, Kristoffersen EK. Severe human Babesia divergens infection in Norway. Int J Infect Dis. 2015;33:37–38. doi: 10.1016/j.ijid.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 22.Hildebrandt A, Gray JS, Hunfeld KP. Human babesiosis in europe: what clinicians need to know. Infection. 2013;41:1057–1072. doi: 10.1007/s15010-013-0526-8. [DOI] [PubMed] [Google Scholar]
- 23.Welc-Falęciak R, Pawełczyk A, Radkowski M, Pancewicz S, Zajkowska J, Siński E. First report of two asymptomatic cases of human infection with Babesia microti (Franca, 1910) in Poland. Ann Agric Environ Med. 2015;22:51–54. doi: 10.5604/12321966.1141394. [DOI] [PubMed] [Google Scholar]
- 24.Parola P, Rovery C, Rolain JM, Brouqui P, Davoust B, Raoult D. Rickettsia slovaca and R. raoultii in tick-borne rickettsioses. Emerg Infect Dis. 2009;15:1105–1108. doi: 10.3201/eid1507.081449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsson K, Lindquist O, Påhlson C. Association of Rickettsia helvetica with chronic perimyocarditis in sudden cardiac death. Lancet. 1999;354:1169–1173. doi: 10.1016/S0140-6736(99)04093-3. [DOI] [PubMed] [Google Scholar]
- 26.Fournier P-E, Allombert C, Supputamongkol Y, Caruso G, Brouqui P, Raoult D. Aneruptive fever associated with antibodies to Rickettsia helvetica in Europe and Thailand. J Clin Microbiol. 2004;42:816–818. doi: 10.1128/JCM.42.2.816-818.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jado I, Oteo JA, Aldámiz M, Gil H, Escudero R, Ibarra V, et al. Rickettsia monacensis and human disease, Spain. Emerg Infect Dis. 2007;13:1405–1407. doi: 10.3201/eid1309.060186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schötta A-M, Wijnveld M, Höss D, Stanek G, Stockinger H, Markowicz M. Identification and characterization of “Candidatus Rickettsia Thierseensis”, a novel spotted fever group Rickettsia species detected in Austria. Microorganisms. 2020;8:1670. doi: 10.3390/microorganisms8111670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reiter M, Schötta A-MM, Müller A, Stockinger H, Stanek G. A newly established real-time PCR for detection of Borrelia miyamotoi in Ixodes ricinus ticks. Ticks Tick Borne Dis. 2015;6(3):303–308. doi: 10.1016/j.ttbdis.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Krause PJ, Fish D, Narasimhan S, Barbour AG. Borrelia miyamotoi infection in nature and in humans. Clin Microbiol Infect. 2015;21(7):631–639. doi: 10.1016/j.cmi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tobudic S, Burgmann H, Stanek G, Winkler S, Schötta A, Obermüller M, et al. Human Borrelia miyamotoi infection, Austria. Emerg Infect Dis. 2020;26(9):2201–2204. doi: 10.3201/eid2609.191501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Estrada-Peña A, Mihalca AD, Petney TN. Ticks of Europe and North Africa. Cham: Springer; 2017. [Google Scholar]
- 33.Wijnveld M, Schötta A, Stelzer T, Duscher G, Leschnik M, Stockinger H, et al. Novel protozoans in Austria revealed through the use of dogs as sentinels for ticks and tick-borne pathogens. Microorganisms. 2021;9:1392. doi: 10.3390/microorganisms9071392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong F, Gilbert GL. Multiplex PCR-based reverse line blot hybridization assay (mPCR/RLB)—a practical epidemiological and diagnostic tool. Nat Protoc. 2006;1(6):2668–2680. doi: 10.1038/nprot.2006.404. [DOI] [PubMed] [Google Scholar]
- 35.Nijhof A, Pillay V, Steyl J. Molecular characterization of Theileria species associated with mortality in four species of African antelopes. J Clin Microbiol. 2005;43(12):5907–5911. doi: 10.1128/JCM.43.12.5907-5911.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Špitalská E, Boldišová E, Štefanidesová K, Kocianová E, Majerčíková Z, Tarageľová VR, et al. Pathogenic microorganisms in ticks removed from Slovakian residents over the years 2008–2018. Ticks Tick Borne Dis. 2021;12(2):101626. doi: 10.1016/j.ttbdis.2020.101626. [DOI] [PubMed] [Google Scholar]
- 37.Răileanu C, Silaghi C, Fingerle V, Margos G, Thiel C, Pfister K, et al. Borrelia burgdorferi sensu lato in questing and engorged ticks from different habitat types in southern Germany. Microorganisms. 2021;9:1266. doi: 10.3390/microorganisms9061266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markowicz M, Schötta A-M, Wijnveld M, Stanek G. Lyme Borreliosis with scalp eschar mimicking rickettsial infection, Austria. Emerg Infect Dis. 2020;26(9):2193–2195. doi: 10.3201/eid2609.191256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindström BE, Skogman BH, Lindström AK, Tallstedt L, Nilsson K. Borrelia ocular infection: a case report and a systematic review of published cases. Ophthalmic Res. 2022;65(2):121–130. doi: 10.1159/000521307. [DOI] [PubMed] [Google Scholar]
- 40.Remesar S, Díaz P, Prieto A, García-Dios D, Panadero R, Fernández G, et al. Molecular detection and identification of piroplasms (Babesia spp. and Theileria spp.) and Anaplasma phagocytophilum in questing ticks from northwest Spain. Med Vet Entomol. 2021;35:51–58. doi: 10.1111/mve.12468. [DOI] [PubMed] [Google Scholar]
- 41.Takumi K, Hofmeester TR, Sprong H. Red and fallow deer determine the density of Ixodes ricinus nymphs containing Anaplasma phagocytophilum. Parasites Vectors. 2021;14:59. doi: 10.1186/s13071-020-04567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Vozmediano A, Krawczyk AI, Sprong H, Rossi L, Ramassa E, Tomassone L. Ticks climb the mountains: Ixodid tick infestation and infection by tick-borne pathogens in the Western Alps. Ticks Tick Borne Dis. 2020;11:101489. doi: 10.1016/j.ttbdis.2020.101489. [DOI] [PubMed] [Google Scholar]