Abstract
As part of environmental management policies in Europe, separate collection of organic household waste and nonorganic household waste has become increasingly common. As waste is often stored indoors, this policy might increase microbial exposure in the home environment. In this study we evaluated the association between indoor storage of organic waste and levels of microbial agents in house dust. The levels of bacterial endotoxins, mold β(1→3)-glucans, and fungal extracullar polysaccharides (EPS) of Aspergillus and Penicillium species were determined in house dust extracts as markers of microbial exposure. House dust samples were collected in 99 homes in The Netherlands selected on the basis of whether separated organic waste was present in the house. In homes in which separated organic waste was stored indoors for 1 week or more the levels of endotoxin, EPS, and glucan were 3.2-, 7.6-, and 4.6-fold higher, respectively (all P < 0.05), on both living room and kitchen floors than the levels in homes in which only nonorganic residual waste was stored indoors. Increased levels of endotoxin and EPS were observed, 2.6- and 2.1-fold (P < 0.1), respectively, when separated organic waste was stored indoors for 1 week or less, whereas storage of nonseparated waste indoors had no effect on microbial agent levels (P > 0.2). The presence of textile floor covering was another major determinant of microbial levels (P < 0.05). Our results indicate that increased microbial contaminant levels in homes are associated with indoor storage of separated organic waste. These increased levels might increase the risk of bioaerosol-related respiratory symptoms in susceptible people.
Separate collection of organic household waste and nonorganic household waste has become increasingly common in many European countries as part of national or local environmental management policies. This often involves indoor storage of separated organic waste, including fruits, vegetables, and food remains in the home. Indoor storage of waste is especially common in apartment buildings in densely populated areas. As microbial decomposition of organic waste starts and possibly proceeds to a substantial extent inside an organic waste bin, the bin might become an important source of bacteria and mold spores inside the house.
Although the specific role of microbial exposure in the development of noninfectious respiratory disease is still not clear, there is strong evidence that microbial contaminants and allergens from house dust mites are related to the prevalence and severity of respiratory symptoms, particularly in damp houses (1, 3, 10, 17, 24, 26). Exposure to bacteria, especially exposure to bacterial endotoxins, is known to be associated with respiratory symptoms (2, 14, 16, 19). It is thought that molds initiate both allergic and nonallergic inflammatory reactions (1, 10, 17, 24). The latter could be related to β(1→3)-glucans, which are cell wall components of most fungi (20–22).
In the present study we investigated the influence of indoor storage of organic waste on microbial levels in the home environment as the first step in a health risk evaluation. The effect of indoor storage of household waste on concentrations of biological contaminants indoors has not been reported previously. We measured the levels of bacterial endotoxins and fungal β(1→3)-glucans, which are known or probable inducers of airway inflammation, and the levels of extracellular polysaccharides (EPS) of Aspergillus and Penicillium species, which were used as markers for fungal exposure. In addition, the levels of house dust mite and cat allergens, which are two well-recognized indoor air allergens associated with housing characteristics, were measured to provide external validation of the results.
MATERIALS AND METHODS
Study population.
In the summer of 1997 samples of settled house dust were collected in 99 households in The Netherlands. Approximately one-half of the houses (53 houses) were selected from a large birth cohort study on the development of asthma and mite allergy in The Netherlands. Most of these houses (>80%) were single-family houses. In addition, we studied 46 households in apartment buildings in a small city of approximately 30,000 inhabitants in The Netherlands. A stratified sampling strategy was devised for both populations such that one-half of the households had a 5- to 30-liter organic waste bin in the kitchen. Data concerning the frequency at which the organic bin was emptied and variables such as the type of floor covering and the presence of pets were collected by questionnaire. Members of all households agreed to participate in the study by signing an informed consent document.
Dust sampling and extraction.
House dust samples were collected by vacuuming 1-m2 portions of living room and kitchen floors by using an internationally standardized protocol, as described previously (7, 18). Samples were taken from smooth and wall-to-wall textile floor coverings. When a rug with a surface area of at least 1 m2 was present, the sample was taken from the rug. Filters were weighed before and after dust samples were collected by using an analytical balance in a preconditioned room at approximately 20°C and 50% relative humidity. Dust samples were stored at −20°C until extraction was performed. Endotoxins, EPS, and allergens were extracted from the dust as described by Douwes et al. (7), and afterwards β(1→3)-glucans were extracted (6). Briefly, the dust samples were suspended in 2.5 to 20 ml (depending on the weight of the dust) of 0.05% (vol/vol) Tween 20 in pyrogen-free water (<0.3 g, 3 ml; 0.3 to 0.5 g, 5 ml; 0.5 to 1.0 g, 10 to 15 ml; >1.0 g, 20 to 50 ml). The suspensions were rocked vigorously for 2 h at room temperature, and after centrifugation each supernatant was stored at −20°C until it was analyzed. After extraction at room temperature, extraction at 120°C was used to dissolve β(1→3)-glucans (6). Each pellet was resuspended in the same volume of 0.05% Tween 20 in pyrogen-free water, vigorously shaken for 15 min at room temperature, autoclaved at 120°C and 105 Pa for 1 h, and then shaken for 15 min. The samples were centrifuged, and each supernatant was collected and stored at −20°C until it was analyzed.
Microbial and allergen analyses.
The concentrations of endotoxins, EPS antigens, house dust mite allergens, and cat allergens were determined by using the dust extracts prepared at room temperature. Bacterial endotoxin levels were measured by a quantitative kinetic chromogenic Limulus amebocyte lysate assay (Kinetic-QCL no. 50-650 U; Bio Whittaker, Walkersville, Md.) as described previously (7). Levels of fungal EPS antigens of Penicillium and Aspergillus spp. were measured with a sandwich enzyme immunoassay (EIA) essentially as described by Douwes et. al. (9). Briefly, microplates were coated overnight at 4°C with isolated anti-Aspergillus/Penicillium-EPS immunoglobulin G antibodies (10 μg/ml) which had been raised in rabbits and isolated from their serum as previously described (11). The specificity of these antibodies for EPS of Aspergillus and Penicillium spp. has been shown by Kamphuis et. al. (11) and Douwes et al. (9), who used EPS of species belonging to other genera and crude fungal allergen extracts, respectively. Then the plates were washed with phosphate-buffered saline containing 0.05% Tween 20 (PBT), and free sites on the well surfaces were blocked by incubation for 0.5 h at 37°C with PBT containing 0.1% milk proteins (Protifar; NV Nutricia, Zoetermeer, The Netherlands) (PBTM). Dust extracts were diluted 1/5, 1/10, and 1/20 with PBTM and incubated for 1 h at 37°C in the microwells. After plates were washed with PBT, the amount of bound EPS was measured by incubating the plates for 1 h at 37°C with peroxidase-labelled rabbit anti-Aspergillus/Penicillium-EPS immunoglobulin G diluted 1/2,000 with PBTM, followed (after another wash with PBT) by incubation for 0.5 h at room temperature with o-phenylenediamine (2 mg/ml in 0.05 M citrate-phosphate buffer; pH 5.5) containing 0.015% H2O2. The reaction was stopped with HCl, and the results were read spectrophotometrically at 492 nm.
The procedure used to calibrate the assay was modified from the previously described procedure as the specific activities of standard preparations containing isolated EPS appeared to differ considerably when the EIA was used and as dose-response curves of many house dust extracts were not parallel to the dose-response curve of the standard. Therefore, we calibrated the EIA in the present study by including in each test plate serial dilutions (1/5 to 1/640) of a sieved bulk house dust extract to which an arbitrary value of 5,000 EPS units per ml was given. When this was done, the obtained results obtained with different dilutions of the same test sample usually varied less than 20%. The limit of detection of the assay was 19 EPS units/ml.
The levels of house dust mite allergen Der p 1 (13) and cat allergen Fel d 1 (4) were measured with monoclonal antibody-based EIA kits (catalog no. 5H8/4C1 and 6F9/3E4; Indoor Biotechnologies, Chester, United Kingdom) by using the protocol of the supplier with some slight modifications. The limits of detection of these tests were 5.36 ng/ml for Der p 1 and 1.47 ng/ml for Fel d 1.
The levels of β(1→3)-glucans in the heated dust extracts were measured with an inhibition EIA (6). The detection limit of the assay was 80 ng/ml. The blank paper filters used for dust sampling contained β(1→3)-glucan (mean, 300 μg per filter). Therefore, a correction for this amount of β(1→3)-glucan was used as described previously.
Statistical analysis.
A statistical analysis was performed by using SAS statistical software (version 6.12; SAS Institute, Cary, N.C.). Indoor microbial and allergen levels were expressed per square meter and per gram of dust, and a sample with undetectable microbial or allergen levels was given a value of two-thirds of the lowest observed amount per gram of dust or per square meter for the specific component determined. Except for Der p 1, the indoor microbial and allergen levels were normally distributed after natural log transformation. Therefore, data were summarized as geometric means (exponent of the average of natural log-transformed concentrations) and geometric standard deviations (exponent of the standard deviation of natural log-transformed concentrations). First, crude unadjusted analyses of the differences in allergen and microbial contaminant levels between houses with organic waste bins and houses without organic waste bins were performed. Endotoxin, glucan, Penicillium/Aspergillus- EPS, and cat allergen levels were evaluated by performing Student's t tests with natural log-transformed concentrations, and Der p 1 levels were evaluated by performing nonparametric Wilcoxon tests because of the nonnormality of Der p 1 levels. Subsequently, natural log-transformed concentrations of microbial contaminants determined per square meter or per gram of dust were used as the response variables in an analysis of covariance. Homes with organic waste bins indoors were compared to homes without organic waste bins indoors. By including potentially confounding variables in the regression model, the corrected effect of organic waste bins indoors compared to no organic waste bins indoors could be determined (12). The possible confounding factors included type of floor covering, population sample, and the presence of pets.
In the final analysis, households with only residual nonorganic waste indoors (n = 26) were compared with households with nonseparated organic and residual waste indoors (n = 25), households with organic waste bins that were emptied at least twice per week (n = 32), and households with organic waste bins that were emptied at most once per week (n = 17), corrected for type of floor covering and population sample, by using the following model: Ln(microbial agent concentration) = 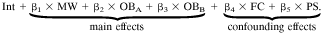
where Ln(microbial agent concentration) is the natural log-transformed concentration of microbial agents; Int is the intercept; MW is the presence of mixed waste indoors (organic and nonorganic waste not separated); OBA is the presence of an indoor organic waste bin that is emptied twice a week or more; OBB is the presence of an indoor organic waste bin that is emptied once a week or less; FC is the type of floor covering (textile or smooth); PS is the population sample (birth cohort study or apartment); and β1 through β5 are the regression coefficients for the respective effects.
RESULTS
The distributions of some relevant variables in the whole study population and both population samples are shown in Table 1. By design, one-half of the households had an organic waste bin indoors. However, people who lived in apartments emptied their organic waste bins less frequently than participants in the birth cohort study emptied theirs. Furthermore, when no organic waste bin was present indoors, more households in apartment buildings than households in the birth cohort study did not separate their waste. Textile floor coverings (rugs or wall-to-wall carpets) were also more common in apartment houses than in the birth cohort study households. The distributions of textile floor coverings were the same for groups of houses defined on the basis of different waste collection characteristics.
TABLE 1.
Distribution of waste characteristics, type of floor covering and presence of pets in the study population
| Group | No. positive/no. tested (%)
|
||
|---|---|---|---|
| Total population | Birth cohort | Apartments | |
| Organic waste bin indoors | 48/99 (48) | 23/53 (43) | 25/46 (54) |
| Emptied once a week or less | 17/99 (17) | 3/53 (6) | 14/46 (30)a |
| Emptied twice a week or more | 31/99 (32) | 20/53 (38) | 11/46 (24) |
| No organic waste bin indoors | 51/99 (51) | 30/53 (57) | 21/46 (46) |
| Only residual waste | 26/99 (26) | 23/53 (43) | 3/46 (7)a |
| Unseparated organic and residual waste | 25/99 (25) | 7/53 (13) | 18/46 (39)b |
| Textile floor covering in living room | 61/99 (62) | 24/53 (45) | 37/46 (80)a |
| Organic waste bin indoors | 29/99 (29) | 9/53 (17) | 20/46 (43)b |
| No organic waste bin indoors | 32/99 (32) | 15/53 (28) | 17/46 (37)c |
| Textile floor covering in kitchen | 4/99 (4) | 0/53 (0) | 4/46 (7) |
| Presence of petsd | 37/96 (39) | 19/51 (37) | 18/45 (40) |
| Organic waste bin indoors | 15/96 (16) | 5/51 (10) | 10/45 (22) |
| No organic waste bin indoors | 22/96 (23) | 14/51 (27) | 8/45 (18) |
P ≤ 0.001 compared with birth cohort data, as determined by a chi-square test.
P ≤ 0.01 compared with birth cohort data, as determined by a chi-square test.
P ≤ 0.05 compared with birth cohort data, as determined by a chi-square test.
Data were not available for three households (two birth cohort households and one apartment household).
Unadjusted analyses of microbial agent and allergen concentrations in homes with and without organic waste bins indoors (Table 2) showed that bacterial endotoxin and fungal Aspergillus/Penicillium-EPS concentrations both in the living room and in the kitchen were significantly higher in homes with organic waste bins (endotoxin, 1.8- to 3.4-fold [P ≤ 0.05]; EPS, 1.8- to 2.4-fold [P ≤ 0.1 and P ≤ 0.05]). We observed a trend toward increased β(1→3)-glucan levels expressed per square meter (1.5- and 1.7-fold [P > 0.1]). In contrast, neither Der p 1 nor Fel d 1 allergen levels were associated with the type of waste container inside the house. Similar results were obtained after we adjusted for possible confounding factors, such as the type of floor covering, the sample population, and the presence of pets (data not shown). The presence of a textile floor covering was strongly associated with both microbial and allergen concentrations in house dust (2- to 100-fold higher levels [P < 0.05]), while the population sample was not significantly associated with measured concentrations in house dust. The presence of pets, especially cats, was a major determinant for cat allergens but not for house dust mite allergens or microbial agents.
TABLE 2.
Endotoxin, β(1→3)glucan, EPS, Der p 1, and Fel d 1 concentrations in house dust from living room and kitchen floors in the presence and in the absence of an organic waste bin indoors
| Group | Endotoxin
|
β(1→3)Glucan
|
EPS
|
Der p 1a
|
Fel d 1
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. positive/ total no.b | Concn
|
No. positive/ total no.b | Concn
|
No. positive/ total no.b | Concn
|
No. positive/ total no.b | Concn
|
No. positive/ total no.b | Concn
|
||||||
| EU/m2 | EU/g of dust | μg/m2 | μg/g of dust | EPS units/m2 | EPS units/ g of dust | ng/m2 | ng/g of dust | ng/m2 | ng/g of dust | ||||||
| Living room | |||||||||||||||
| No organic waste bin | 51/51 | 950 (8.4)c | 4,361 (3.0) | 44/51 | 223 (12.2) | 933 (7.0) | 40/51 | 2,090 (16.1) | 8,426 (5.9) | 9/51 | 38 (4.0) | 69 (3.6) | 40/51 | 240 (28.6) | 899 (16.4) |
| Organic waste bin | 48/48 | 2,443 (6.6)d | 7,765 (3.8)d | 43/48 | 326 (12.1) | 1,010 (5.8) | 42/48 | 5,069 (14.0)e | 15,045 (5.7)e | 10/48 | 43 (4.3) | 72 (3.4) | 40/48 | 234 (24.4) | 671 (13.0) |
| Kitchen | |||||||||||||||
| No organic waste bin | 49/49 | 662 (6.6) | 7,846 (4.5) | 36/49 | 42 (11.6) | 444 (11.7) | 37/49 | 371 (6.3) | 4,012 (4.0) | 1/49 | 24 (1.3) | 44 (1.4) | 31/49 | 24 (13.5) | 221 (12.0) |
| Organic waste bin | 48/48 | 2,276 (8.5)f | 19,169 (5.3)f | 41/48 | 73 (9.7) | 614 (7.4) | 43/48 | 775 (5.5)d | 5,928 (4.0) | 2/48 | 24 (1.3) | 48 (1.9) | 35/48 | 34 (11.0) | 257 (10.8) |
Value were examined nonparametrically with Wilcoxon tests.
Number of samples with detectable concentrations/total number of samples.
Geometric mean (geometric standard deviation).
P ≤ 0.05 compared with data for houses without organic waste bins, as determined by Student's t test.
P ≤ 0.10 compared with data for houses without organic waste bins, as determined by Student's t test.
P ≤ 0.01 compared with data for houses without organic waste bins, as determined by Student's t test.
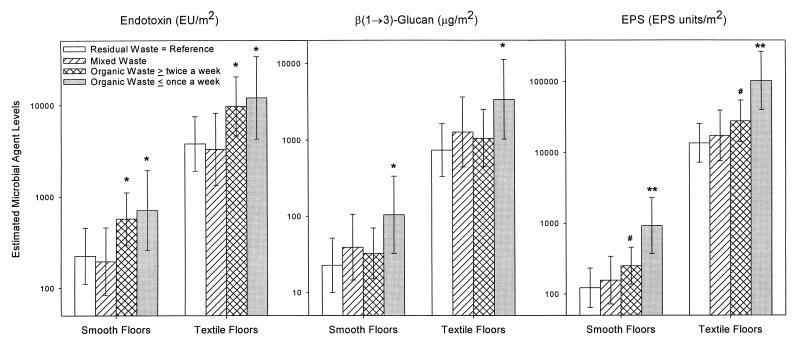
In subsequent regression analyses we made a further distinction between unseparated organic and residual waste (mixed waste) and organic waste stored in a separate bin, and we included the effect of emptying frequency. The data showed that the greatest explained variance occurred with a model in which the effect of organic waste itself, the emptying frequency of the organic waste bin, and two confounding factors, type of floor covering and population sample, were taken into account. The predicted values for microbial contaminant amounts per square meter in the four different groups of homes as derived from this regression model are shown in Fig. 1. The predicted concentrations in living room floor dust in houses with residual waste and smooth floors were 225 endotoxin units/m2 for endotoxin, 23 μg/m2 for β(1→3)-glucans, and 123 EPS units/m2 for EPS. The presence of separated organic waste stored indoors and a low bin-emptying frequency led to significantly increased microbial agent levels (endotoxin, 3.2-fold [P < 0.05]; EPS, 7.6-fold [P < 0.05]; glucan, 4.6-fold [P < 0.05]). The presence of a textile floor covering increased the concentrations 20- to 100-fold (P < 0.05). The combined effect of textile floor covering and the presence of an organic waste bin resulted in 25- to 840-fold increases in microbial agent levels. An organic waste bin indoors that was emptied at least twice a week was associated with moderately enhanced microbial agent levels (endotoxin, 2.6-fold [P < 0.05]; EPS, 2.1-fold [P < 0.1]; glucan, 1.6-fold [P > 0.2]), while storage of unseparated waste had no effect on microbial concentrations (endotoxin, 0.9-fold [P > 0.2]; EPS, 1.3-fold [P > 0.2]; glucan, 1.7-fold [P > 0.2]). Similar results were obtained for kitchen floors; for example, there were 3.6-, 1.5-, and 6.1-fold increases in the endotoxin, glucan, and EPS concentrations, respectively, due the presence of an organic waste bin that was emptied once a week or less frequently (data not shown). Analyses of microbial agent concentrations expressed per gram of dust produced the same results, although the data were less marked.
FIG. 1.
Concentrations of microbial contaminants in living room floor dust (mean ± 95% confidence interval), as predicted by analysis of covariance, stratified by type of floor covering, and adjusted for population sample. ∗∗, P < 0.01; ∗, P < 0.05; #, P < 0.10. P values were obtained by comparing results with results obtained for houses with similar floor covering and only residual waste.
DISCUSSION
Indoor storage of organic waste was associated with significantly increased concentrations of bacterial endotoxins and fungal antigens in dust from both kitchen and living room floors. The type of floor covering was another important and independent determinant of microbial contamination in the home environment. To confirm that increased levels of microbial agents were specifically associated with indoor storage of organic waste and not due to other differences between homes with and without organic waste containers (for instance, the overall hygiene status of the homes), we measured the levels of cat and house dust mite allergens in the dust samples. These two allergens are well-known indoor allergens that would probably be present at higher levels in dirtier homes (for example, homes that are cleaned less frequently) but should not be specifically associated with indoor storage of organic waste. The presence of an organic waste bin indoors was not associated with cat and house dust mite allergen levels in the home environment, indicating that there was a specific association between indoor storage of organic waste and biocontaminant concentrations. The lack of an association between house dust mite allergen levels and waste characteristics might be explained by the fact that most of the house dust samples had undetectable levels of house dust mite allergens. The cat allergen levels were also relatively low compared to the levels in previous studies (23, 25). However, we found that there was a strong association between both of these allergens and textile floor coverings, a well-known determinant of indoor allergen exposure.
Only in relatively few studies conducted to assess the relationships among mold growth, house dust mites, home dampness, and respiratory symptoms have actual levels of exposure to microbial agents been determined. Endotoxin, glucan, and EPS levels were readily detected in house dust samples, as shown in previous studies. The endotoxin levels determined in this study were, however, 2- to 10-fold lower than the levels in previous studies performed by Michel et al. and Douwes et al. (5, 7, 8, 14, 16). This is in contrast to the glucan levels, which were more comparable to the levels found in previous studies performed in Germany and The Netherlands (5, 6, 8). The presence of textile floor coverings was again strongly associated with increased levels of endotoxin, glucan, and EPS. This should be interpreted as indirect validation of our finding that the presence of an organic waste bin results in 1.6- to 7.6-fold increases in microbial agent concentrations. The microbial contaminant levels that were expressed per square meter were more variable than the levels expressed per gram of dust, indicating that there was an association between the amount of dust sampled and the biocontaminant levels expressed per square meter. However, significantly increased microbial exposure levels expressed per gram of dust were found in households with indoor storage of organic waste, which indicated that the association between microbial levels in dust and waste storage does not depend only on the amount of dust in a home.
This study was the first step in a health risk evaluation of source-separated organic waste collection. So far, the health implications of elevated microbial exposure are uncertain, as health effects have not been evaluated directly. In various epidemiological studies, however, bioaerosol-related respiratory symptoms and morbidity in moldy and damp houses have been described (1, 3, 10, 17, 24, 26). More recent studies have demonstrated that endotoxins are quantitatively associated with the severity of airflow limitation and respiratory symptoms in asthmatics (14–16), and epidemiological and toxicological studies have revealed that endotoxins are causative agents of nonspecific, nonallergic airway inflammation (19). Similar associations with respiratory symptoms and nonspecific airway inflammation have also been suggested for β(1→3)-glucans (20–22) and EPS (9). In a recent study, the effects of endotoxins and β(1→3)-glucans on the lung function of children were assessed (5). We found that 25-fold-higher levels of microbial agents in living room floor dust were associated with a 1.6-fold increase in peak flow variability in a subgroup of atopic children with asthmatic symptoms. This implies that there may be similar or even stronger effects due to the presence of an infrequently emptied organic waste bin together with the presence of a textile floor covering. Therefore, health risks associated with indoor storage of organic waste, particularly in asthmatics and other susceptible individuals, must be considered a possibility.
In conclusion, we found that increased microbial contaminant concentrations in the home environment were associated with indoor storage of separated organic waste, which might increase the risk of respiratory diseases related to such contaminants.
ACKNOWLEDGMENTS
We are indebted to the participating households. We thank Bart Flipse, Wobbe van der Meulen, Lützen Portengen, Isabella van Schothorst, Jack Spithoven, and Siegfried de Wind for technical assistance with exposure measurements and laboratory analyses.
This study was supported by The Ministry of Housing, Spatial Planning and the Environment (VROM), by The Netherlands Organization for Scientific Research (NWO), and by an EC research project (Prevention of disease caused by waste handling with special reference to endotoxin and β(1→3)-glucan [BHM4-CT96-0105]).
REFERENCES
- 1.Andriessen J W, Roemer W, Brunekreef B. Home dampness and respiratory health status in European children. Clin Exp Allergy. 1998;28:1129–1200. doi: 10.1046/j.1365-2222.1998.00322.x. [DOI] [PubMed] [Google Scholar]
- 2.Björnsonn E, Norbäck D, Janson C, Windström J, Palmgren U, Ström G, Boman G. Asthmatic symptoms and indoor levels of micro-organisms and house dust mites. Allergy (Copenhagen) 1995;25:423–431. doi: 10.1111/j.1365-2222.1995.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 3.Brunekreef B. Damp housing and adult respiratory symptoms. Allergy (Copenhagen) 1992;47:498–502. doi: 10.1111/j.1398-9995.1992.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 4.Chapman M D, Aalberse R C, Brown M J, Platts-Mills T A E. Monoclonal antibodies to the major feline allergen, Fel D I: II. Single step affinity purification of Fel d I,N-terminal sequence analysis and development of a sensitive two-site immunoassay to assess Fel D I exposure. J Immunol. 1988;140:812–818. [PubMed] [Google Scholar]
- 5.Doekes G, Douwes J, Zuidhof A, van der Zee S, Brunekreef B. Endotoxin and β-(1→3)-d-glucan in house dust in relation to peak flow variability in asthmatic children. Eur Respir J. 1998;12:13S. doi: 10.1164/ajrccm.162.4.9909118. [DOI] [PubMed] [Google Scholar]
- 6.Douwes J, Doekes G, Montijn R, Heederik D, Brunekreef B. Measurement of β(1→3)-glucans in the occupational and home environment with an inhibition enzyme immunoassay. Appl Environ Microbiol. 1996;62:3176–3182. doi: 10.1128/aem.62.9.3176-3182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douwes J, Versloot P, Hollander A, Heederik D, Doekes G. Influence of various dust sampling and extraction methods on the measurement of airborne endotoxin. Appl Environ Microbiol. 1995;61:1763–1769. doi: 10.1128/aem.61.5.1763-1769.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douwes J, Doekes G, Heinrich J, Koch A, Bischof W, Brunekreef B. Endotoxin and β(1→3)-glucan in house dust and the relation with home characteristics: a pilot study in 25 German houses. Indoor Air. 1998;8:255–263. [Google Scholar]
- 9.Douwes J, van der Sluis B, Doekes G, van Leusden F, Wijnands L, van Strien R, Verhoeff A, Brunekreef B. Fungal extracellular polysaccharides in house dust as a marker for exposure to fungi: relation with culturable fungi, reported home dampness, and respiratory symptoms. J Allergy Clin Immunol. 1999;103:494–500. doi: 10.1016/s0091-6749(99)70476-8. [DOI] [PubMed] [Google Scholar]
- 10.Husman T. Health-effects of indoor-air microorganisms. Scand J Work Environ Health. 1996;22:5–13. doi: 10.5271/sjweh.103. [DOI] [PubMed] [Google Scholar]
- 11.Kamphuis H J, De Ruiter G A, Veeneman G H, van Boom J H, Rombouts F M, Notermans S H W. Detection of Aspergillus and Penicillium extracellular polysaccharides (EPS) by ELISA: using antibodies raised against acid hydrolyzed EPS. Antonie Leeuwenhoek. 1992;61:323–332. doi: 10.1007/BF00713940. [DOI] [PubMed] [Google Scholar]
- 12.Kleinbaum D G, Kupper L L, Muller K E, Nizam A. Applied regression analysis and multivariable methods. Pacific Grove, Calif: Brooks/Cole Publishing Company; 1998. Confounding and interaction in regression; pp. 186–211. [Google Scholar]
- 13.Luczynska C M, Arruda L K, Platts-Mills T A E, Miller J D, Lopez M, Chapman M D. A two-site monoclonal antibody ELISA for the quantification of the major Dermatophagoides spp. allergens, Der p I and Der f I. J Immunol Methods. 1989;118:227–235. doi: 10.1016/0022-1759(89)90010-0. [DOI] [PubMed] [Google Scholar]
- 14.Michel O, Ginnani R, Duchateau J, Vertongen F, Le Bon B, Sergysels R. Domestic endotoxin exposure and clinical severity of asthma. Clin Exp Allergy. 1991;21:441–448. doi: 10.1111/j.1365-2222.1991.tb01684.x. [DOI] [PubMed] [Google Scholar]
- 15.Michel O, Ginnani R, Le Bon B, Content J, Duchateau J, Sergysels R. Inflammatory response to acute inhalation of endotoxin in asthmatic patients. Am Rev Respir Dis. 1992;146:352–357. doi: 10.1164/ajrccm/146.2.352. [DOI] [PubMed] [Google Scholar]
- 16.Michel O, Kips J, Duchateau J, Vertongen F, Robert L, Collet H. Severity of asthma is related to endotoxin in house dust. Am J Respir Crit Care Med. 1996;154:1641–1646. doi: 10.1164/ajrccm.154.6.8970348. [DOI] [PubMed] [Google Scholar]
- 17.Peat J K, Dickerson J, Li J. Effects of damp and mould in the home on respiratory health: a review of the literature. Allergy (Copenhagen) 1998;53:120–128. doi: 10.1111/j.1398-9995.1998.tb03859.x. [DOI] [PubMed] [Google Scholar]
- 18.Platts-Mills T A E, De Weck A L. Dust mite allergens and asthma: a world wide problem. International workshop report. J Allergy Clin Immunol. 1989;11:199–206. doi: 10.1016/0091-6749(92)90228-t. [DOI] [PubMed] [Google Scholar]
- 19.Rylander R, Jacobs R R. Endotoxins in the environment: a criteria document. Int J Occup Environ Health. 1997;3:S1–S48. [Google Scholar]
- 20.Rylander R, Persson K, Goto H, Yuasa K, Tanaka S. Airborne β(1-3)-glucan may be related to symptoms in sick buildings. Indoor Environ. 1992;1:263–267. [Google Scholar]
- 21.Rylander R, Norhall M, Engdahl U, Tunsäter A, Holt P G. Airways inflammation, atopy, and (1→3)-β-d-glucan exposures in two schools. Am J Respir Crit Care Med. 1998;158:1685–1687. doi: 10.1164/ajrccm.158.5.9712139. [DOI] [PubMed] [Google Scholar]
- 22.Thorn J, Rylander R. Airways inflammation and glucan in damp rowhouses. Am J Respir Crit Care Med. 1998;157:1798–1803. doi: 10.1164/ajrccm.157.6.9706081. [DOI] [PubMed] [Google Scholar]
- 23.van Strien R T, Verhoeff A P, Brunekreef B, van Wijnen J H. Mite antigen in house dust: relationship with different housing characteristics in The Netherlands. Clin Exp Allergy. 1994;24:843–853. doi: 10.1111/j.1365-2222.1994.tb01807.x. [DOI] [PubMed] [Google Scholar]
- 24.Verhoeff A P, Burge A H. Health risk assessment of fungi in home environments. Ann Allergy Asthma Immunol. 1997;78:544–556. doi: 10.1016/S1081-1206(10)63214-0. [DOI] [PubMed] [Google Scholar]
- 25.Verhoeff A P, van Strien R T, van Wijnen J H, Brunekreef B. House dust mite allergen (Der p 1) and respiratory symptoms in children: a case-control study. Clin Exp Allergy. 1994;24:1061–1069. doi: 10.1111/j.1365-2222.1994.tb02744.x. [DOI] [PubMed] [Google Scholar]
- 26.Verhoeff A P, van Strien R T, van Wijnen J H, Brunekreef B. Damp housing and childhood respiratory symptoms: the role of sensitization to dust mites and molds. Am J Epidemiol. 1995;141:103–110. doi: 10.1093/oxfordjournals.aje.a117398. [DOI] [PubMed] [Google Scholar]