Abstract
Background.
Although the consequences of sleep deficiency for obesity risk are increasingly apparent, experimental evidence is limited and there are no studies on body fat distribution.
Objectives.
To investigate the effects of experimentally-induced sleep curtailment in the setting of free access to food on energy intake, energy expenditure, and regional body composition.
Methods.
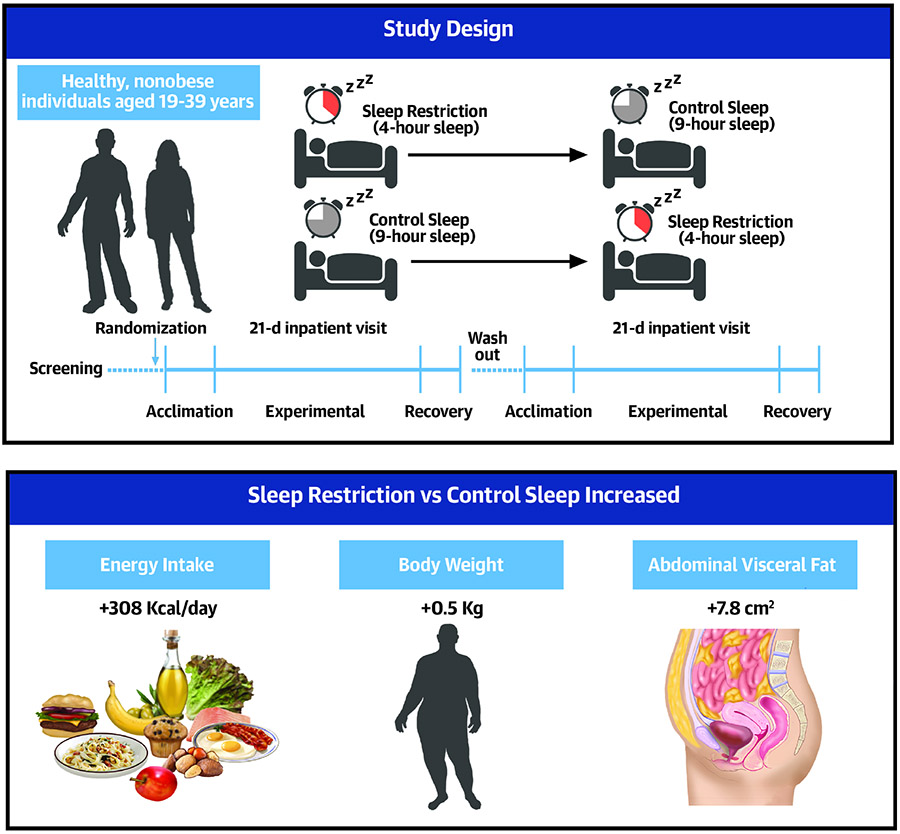
Twelve healthy, nonobese individuals (9 males, age range 19 to 39 years) completed a randomized, controlled, crossover, 21-day inpatient study comprising 4 days of acclimation, 14 days of experimental sleep restriction (4 hour sleep opportunity) or control sleep (9 hour sleep opportunity), and a 3-day recovery segment. Repeated measures of energy intake, energy expenditure, body weight, body composition, fat distribution and circulating biomarkers were acquired.
Results.
With sleep restriction vs control, participants consumed more calories (P=0.015), increasing protein (P=0.050) and fat intake (P=0.046). Energy expenditure was unchanged (all P’s >0.16). Participants gained significantly more weight when exposed to experimental sleep restriction than during control sleep (P=0.008). While changes in total body fat did not differ between conditions (P=0.710), total abdominal fat increased only during sleep restriction (P=0.011), with significant increases evident in both subcutaneous and visceral abdominal fat depots (P=0.047 and P=0.042, respectively).
Conclusions.
Sleep restriction combined with ad libitum food promotes excess energy intake without varying energy expenditure. Weight gain and particularly central accumulation of fat indicate that sleep loss predisposes to abdominal visceral obesity.
Keywords: energy intake, obesity, sleep, sleep restriction, visceral fat, weight gain
Condensed Abstract
In this randomized, controlled, crossover, 21-day inpatient study, healthy adults exposed to experimental sleep restriction vs control sleep in the setting of free access to food increased their energy intake with no changes in energy expenditure. This resulted in weight gain and accumulation of excess fat in the abdominal region, including increases in visceral fat. These data show that sleep restriction in combination with overeating predisposes to obesity and especially central obesity. As abdominal fat accumulation is a potent risk factor for cardiometabolic disease, these data have compelling public health significance.
Tweet
# MayoClinic study shows that shortening sleep causes increased food intake and weight gain, and especially increases in intra-abdominal fat – this highlights the important implications of insufficient sleep for cardiometabolic risk
Introduction
Habitual sleep deficiency affects more than one-third of the US adult population (1), and has been linked to obesity, morbidity and premature mortality (2,3). Although null findings have been reported,(4,5) observational population-based data implicating short sleep duration as a factor promoting obesity are strongly suggestive yet inferential (6-11). Conversely, experimental studies on sleep curtailment and weight regulation are limited and conflicting, and few laboratory-based investigations monitored concurrently both energy intake and energy expenditure (12-17). In addition, whether sleep loss actually induces fat gain is unclear, with major limitations of previous studies including short duration of sleep manipulation and use of surrogate measures of adiposity. Relatedly, an unanswered and more relevant question is where the excess fat is stored, since accumulation of fat in the abdominal cavity (visceral obesity) is more hazardous than other obesity phenotypes (18-21).
In this randomized, controlled, crossover, 21-day inpatient study we sought to investigate the effects of prolonged sleep restriction vs normal (control) sleep on energy intake, energy expenditure, and regional fat storage in healthy, nonobese individuals.
Methods
This was a randomized, controlled, crossover study comparing sleep restriction vs control sleep in a 21-day, inpatient setting. Study periods were separated by a washout interval of at least 3 months. The study was registered at ClinicalTrials.gov (NCT01580761).
Participants
Exclusion criteria were as follows: age <18 or >50 years old, body mass index (BMI) >30 kg/m2, tobacco use, pregnancy or breastfeeding for women, any medical or psychiatric diseases, use of prescription medications with exception of oral contraceptives or intrauterine devices, or second generation antihistamines, and a history of irregular sleep patterns (with regular sleep pattern defined as nocturnal sleep duration of 7-8 hours without daytime naps).
Recruitment was by word of mouth, and advertisements posted on Mayo Clinic websites and clinicaltrials.gov. After a pre-screening phone interview, those who met initial criteria underwent an overnight screening at the Clinical Research and Trial Units at Mayo Clinic Hospital, Saint Marys Campus to confirm eligibility. Medical history and physical examination were conducted to ascertain health status, with absence of sleep disorders demonstrated by polysomnography (PSG). Urine pregnancy test was confirmed as negative in all female participants.
The study was approved by the Mayo Clinic Institutional Review Board and conducted in conformity with the Declaration of Helsinki. All participants provided written informed consent.
Randomization and masking
Randomization to study sequences (sleep restriction or control sleep first), stratified by sex, was generated and allocated by study staff in a 1:1 ratio. Because of the impracticality of blinding sleep opportunities, participants were not masked to group assignment. Investigators were blinded during data processing.
Procedures
One week prior to beginning each inpatient study visit, participants were asked to follow a standardized sleep/wake schedule with ≥8 hours/night of time in bed to reduce the possibility of sleep debt at study entry. Adherence to this schedule was objectively confirmed by wrist actigraphy (MotionWatch 8, CamNtech, Cambridge, UK; mean±SD rest time prior to visits 1 and 2: 515.7±23.3 min and 520.9±22.6 min, respectively). During this time participants were also required to refrain from alcohol, caffeine and vigorous exercise.
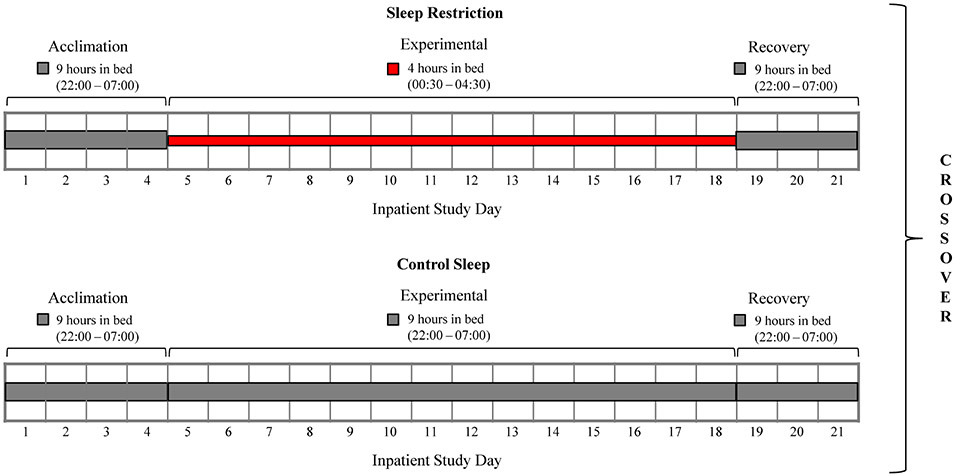
Following admission, participants remained in the research unit for the entire duration of each study period. Each period consisted of an initial 4-day acclimation (baseline) segment, followed by a 14-day experimental phase, and 3 days for recovery, with total in-laboratory study duration of 21 days (Figure 1). A 9-hour time in bed (22:00-7:00) was allowed throughout the entire 21-day control sleep condition, and also during the acclimation and recovery segments of the sleep restriction condition, while nocturnal time in bed was restricted to 4 hours (00:30-4:30) during the 14-day/14-night experimental phase of sleep restriction, similar to previous studies.(14,15,22) Participants were studied in a sedentary setting with ad libitum food access. Participant adherence to sleep prescriptions was ensured by real-time PSG monitoring and direct observation by study staff. See the Supplemental Material for additional information on inpatient study setting.
Figure 1. Study outline.
Each 21-day study period consisted of 4 days/3 nights of acclimation (Days 1-4), then a 14-day/14-night experimental phase (Days 5-18), followed by a 3-day/3-night recovery (Days 19-21). Participants completed both sleep restriction and control sleep conditions in randomized order, after a >3-month wash-out. Gray bar indicates 9 hours of time in bed (from 22:00 to 7:00), while red bar indicates 4 hours of time in bed (from 00:30 to 4:30).
Outcomes
Serial outcome assessments were performed throughout the inpatient protocol (Supplemental Table 1), with repeated measurements taken at the same time of the day to minimize circadian effects. The primary outcome was mean change in daily calorie intake from acclimation to experimental phase, assessed as difference between sleep restriction and control conditions. Secondary outcomes included dietary macronutrients, resting and postprandial energy expenditure via indirect calorimetry, non-exercise activity energy expenditure, physical activity by accelerometry, body weight, whole-body composition from dual energy X-ray absorptiometry (DEXA), abdominal fat distribution from computed tomographic (CT) scans, sleep duration from 24-hour PSG, and appetite-regulatory biomarkers. Detailed description of measurements is provided in the Supplemental Material.
Statistical analysis
With 12 subjects, we had 84% power to detect a clinically meaningful difference of 500 Kcal,(12,16,23,24) given SD for energy consumption of 536 Kcal and two-sided alpha=0.05. Due to skewed distribution of residuals from fitted models, circulating biomarkers were transformed for analysis, and estimates back-transformed for presentation. Mixed models were used, including condition (sleep restriction, control sleep), phase (acclimation, experimental, and recovery) and their interaction as fixed effects, participant as a random effect and baseline value as a covariate. For each outcome, contrasts were applied within the mixed model approach to test between-condition differences in changes from acclimation to experimental phase. Treatment period and sequence order effects were assessed but excluded from final models because nonsignificant (all Ps>0.24). In post-hoc analysis, we investigated time course of changes across study days by applying similar mixed models. Because no correction for multiplicity was applied, findings from this analysis are regarded as exploratory.
Statistical analyses were run using SPSS 25.0 (IBM SPSS Statistics), with the significance level set at P<0.05 (two-sided).
Results
Between May, 2013 and May, 2018, twelve healthy, nonobese individuals (9/3 males/females, 26.5±5.8 years, BMI 24.6±3.7 kg/m2) were enrolled and completed the study (Supplemental Figure 1). One participant withdrew prior to randomization. Characteristics of participants randomized to sleep restriction first (n=6) were comparable to those randomized to control sleep first (n=6; Table 1).
Table 1.
Characteristics of study participants.
| Characteristic | All (N=12) | Sleep Restriction First (n=6) |
Control Sleep First (n=6) |
|---|---|---|---|
| Age, years | 26.5 (5.8) | 26.5 (4.7) | 26.5 (7.1) |
| Male/female, n | 9/3 | 5/1 | 4/2 |
| Body mass index, kg/m2 | 24.6 (3.7) | 23.7 (4.1) | 25.4 (3.5) |
| Race/Ethnicity | |||
| White | 10 | 6 | 4 |
| Othera | 2 | - | 2 |
| Contraceptive use, n | 0 | - | - |
| Caffeine consumption, cups/day | 1.0 (0.0-1.5) | 1.0 (0.0-1.6) | 0.5 (0.0-1.6) |
| Alcohol consumption, drinks/week | 0.0 (0.0-2.8) | 0.0 (0.0-3.6) | 0.0 (0.0-4.0) |
| Habitual sleep duration, hr | 7.4 (1.0) | 7.5 (1.0) | 7.3 (1.0) |
Data are expressed as mean (SD), median (IQR), or count.
One mixed race Native American/White, and one mixed race Hispanic/White.
Outcomes are summarized in Table 2. As per study design, compared to acclimation, PSG-derived 24-hour sleep time was reduced during the experimental segment of the sleep restriction condition (P<0.001), with a net difference relative to control sleep of 3.5 hours (−211.4 min, 95% CI −224.4, −198.5 min; P<0.001). Sleep rebound was seen in the recovery phase (Supplemental Figure 2a). Nocturnal sleep architecture results showed increased sleep efficiency (P<0.001), decreased duration of N1, N2 and R sleep stages (all P’s<0.001), and similar duration of N3 sleep (P=0.413) during experimental sleep restriction compared to control sleep (Supplemental Table 3).
Table 2.
Mixed model comparisons between sleep restriction and control sleep on primary and secondary outcomes.
| Acclimation | Experimental | Recovery | Adjusted Between- condition Differencein Changes from Acclimation to Experimental 95% CI)a |
P- value |
||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Sleep Restriction |
Control Sleep | Sleep Restriction |
Control Sleep | Sleep Restriction |
Control Sleep | ||
| Sleep | ||||||||
| 24-hr TST, min | 485.7 (33.0) | 498.3 (31.2) | 256.1 (26.6) | 480.1 (29.1) | 526.4 (29.5) | 479.8 (22.5) | −211.4 (−224.4, −198.5) | <0.001 |
| Energy Intake | ||||||||
| Calorie, Kcal/day | 2673.7 (681.7) | 2724.6 (793.9) | 3135.5 (917.0) | 2878.4 (860.6) | 2753.9 (807.0) | 2674.4 (940.5) | 308.1 (59.2, 556.8) | 0.015 |
| Protein, gr/day | 100.5 (36.7) | 106.2 (33.6) | 114.2 (43.0) | 109.1 (43) | 95.2 (39.0) | 101.8 (38.1) | 10.9 (0.0, 21.7) | 0.050 |
| Fat, gr/day | 98.9 (32.6) | 98.9 (37.6) | 116.2 (41.0) | 102.7 (36.0) | 103.3 (28.9) | 96.8 (41.4) | 13.5 (0.3, 26.8) | 0.046 |
| Carbohydrate, gr/day | 354.4 (92.2) | 359.5 (113.8) | 418.9 (119.1) | 389.2 (120.2) | 367.3 (112.3) | 354.6 (122.5) | 34.9 (−1.9, 71.6) | 0.063 |
| Sodium, mg/day | 3360.5 (2645.6, 4892.9) | 3707.5 (2554.1, 4841.1) | 4113.0 (3085.3, 5706.9) | 3834.5(2897.0, 5287.0) | 3660.4(2410.3, 4571.8) | 3713.8(2676.6, 4488.5) | 510.0 (57.9, 962.1) | 0.027 |
| Energy Expenditure | ||||||||
| BMR, Kcal/hr | 62.8 (7.9) | 62.0 (9.6) | 63.5 (11.0) | 65.4 (9.6) | 61.2 (9.6) | 63.4 (10.9) | −2.7 (−6.9, 1.5) | 0.208 |
| TEF, Kcal/hr | 58.9 (40.3, 80.7) | 54.9 (39.7, 68.1) | 61.9 (39.2, 96.8) | 42.2 (29.3, 55.9) | 59.2 (54.0, 99.1) | 57.8 (40.2, 79.2) | 16.7 (−6.7, 40.0) | 0.157 |
| Total acceleration, au | 11607.1 (1508.1) | 12304.9 (3506.2) | 11190.3 (2470.4) | 11167.9 (3001.3) | 11114.1 (2329.4) | 11175.8 (2721.8) | 720.2 (−653.7, 2094.1) | 0.297 |
| Sitting, Kcal/hr | 70.4 (55.7, 84.3) | 60.4 (51.9, 68.3) | 71.2 (57.6, 78.2) | 66.9 (53.8, 77.7) | 68.9 (54.0 −77.9) | 70.1 (52.7 −77.3) | −1.1 (−18.1, 15.9) | 0.895 |
| Standing, Kcal/hr | 71.9 (56.3, 92.1) | 67.4 (52.6, 75.6) | 73.3 (60.9, 84.0) | 71.3 (55.6, 81.9) | 74.1 (55.1, 86.0) | 72.8 (55.9, 84.3) | −2.1 (−20.8, 16.5) | 0.819 |
| Walking 1 mph, Kcal/hr | 167.7 (39.8) | 150.2 (33.8) | 168.1 (39.0) | 158.8 (35.8) | 160.9 (43.4) | 164.5 (49.2) | −6.7 (−28.9, 15.5) | 0.546 |
| Walking 2 mph, Kcal/hr | 221.4 (53.4) | 208.5 (47.7) | 237.8 (61.1) | 216.6 (45.8) | 223.0 (58.9) | 223.2 (63.6) | 8.7 (−18.0, 35.3) | 0.516 |
| Walking 3 mph, Kcal/hr | 299.5 (79.0) | 294.6 (64.6) | 319.2 (85.2) | 303.0 (66.0) | 314.4 (84.2) | 309.4 (80.3) | 13.5 (−20.5, 47.5) | 0.428 |
| Body Composition | ||||||||
| Weight, kg | 75.2 (14.8) | 73.8 (13.3) | 76.4 (15.0) | 74.5 (14.0) | 76.7 (15.8) | 74.6 (14.4) | 0.5 (0.1, 0.8) | 0.008 |
| Total body fat, % | 28.6 (6.2) | 27.8 (6.6) | 28.6 (6.0) | 28.1 (6.6) | 29.4 (6.3) | 28.7 (6.8) | −0.3 (−0.9, 0.3) | 0.312 |
| Total fat mass, kg | 21.3 (5.3) | 20.3 (5.7) | 21.7 (5.2) | 20.8 (5.8) | 22.2 (5.7) | 21.1 (6.1) | −0.1 (−0.7, 0.5) | 0.710 |
| Total lean mass, kg | 51.1 (12.0) | 50.7 (11.2) | 52.0 (11.9) | 50.9 (11.1) | 51.0 (11.7) | 50.2 (11.6) | 0.6 (−0.2, 1.5) | 0.138 |
| Android fat mass, lbm | 3.82 (1.34) | 3.46 (1.60) | 3.93 (1.37) | 3.59 (1.65) | 4.00 (1.54) | 3.69 (1.82) | −0.03 (−0.22, 0.17) | 0.782 |
| Subcutaneous fat, cm2 | 162.1 (69.1) | 151.7 (71.1) | 175.4 (70.5) | 157.6 (73.3) | 175.8 (74.3) | 163.3 (76.7) | 7.4 (0.1, 14.6) | 0.047 |
| Visceral fat, cm2 | 69.8 (24.0) | 75.2 (35.4) | 77.5 (30.4) | 75.2 (36.2) | 81.9 (32.7) | 77.3 (37.6) | 7.8 (0.3, 15.3) | 0.042 |
| Total abdominal fat, cm2 | 231.9 (79.9) | 226.9 (96.9) | 252.9 (90.8) | 232.8 (101.3) | 257.7 (96.9) | 240.6 (104.6) | 15.2 (3.6, 26.8) | 0.011 |
| Circulating Biomarkers | ||||||||
| Total ghrelin, pg/mL | 726.4b | 820.0b | 754.0b | 763.8b | 778.8b | 773.2b | 0.05 (−0.00, 0.10) | 0.061 |
| Leptin, ng/mL | 8.2b | 6.6b | 9.9b | 8.1b | 11.4b | 8.5b | −0.00 (−0.10, 0.09) | 0.922 |
| Cortisol, μg/dL | 9.8b | 13.8b | 8.7b | 12.6b | 10.8b | 13.6b | −0.01 (−0.13, 0.11) | 0.870 |
| Anandamide, ng/mL | 0.25b | 0.25b | 0.22b | 0.21b | 0.19b | 0.20b | 0.10 (−0.04, 0.06) | 0.664 |
| 2-AG, ng/mL | 4.6b | 5.7b | 4.3b | 4.2b | 4.0b | 4.3b | 0.14 (−0.02, 0.29) | 0.080 |
Data are means (SD) or medians (IQR).
2-AG, 2-arachidonoylglycerol.
Adjusted between-condition differences (95% CI) in changes from Acclimation to Experimental are obtained from contrasts applied within the mixed model analysis.
Circulating biomarkers were log- (total ghrelin, leptin and cortisol) and cube root-transformed (anandamide and 2-AG) for analysis. Back-transformed means are reported.
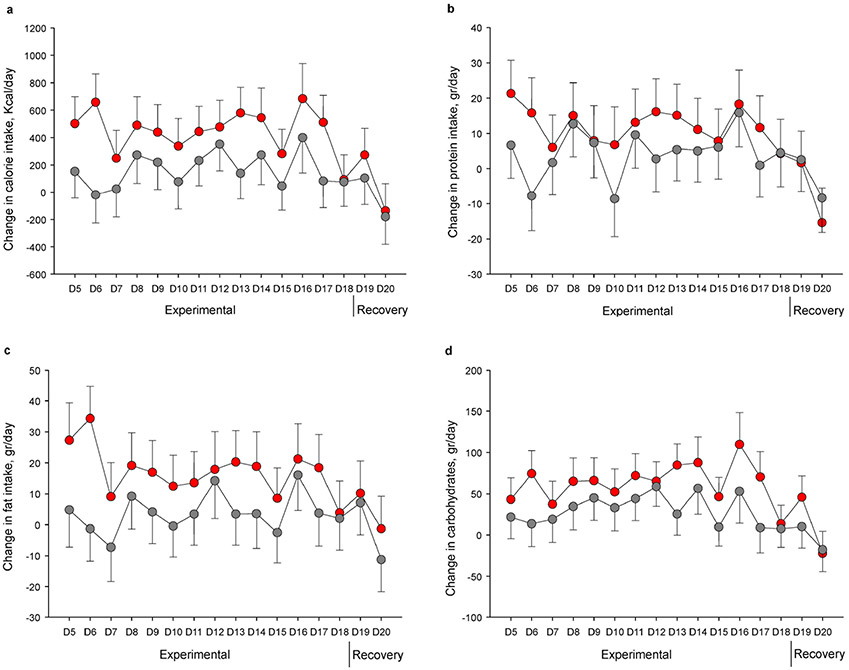
Participants ingested significantly more calories during experimental sleep restriction in comparison with acclimation (~17%; P<0.001), while there was a non-significant increase relative to acclimation during control sleep (~6%; P=0.086). The adjusted between-condition difference in changes in energy intake from acclimation to experimental phases (primary outcome) was 308.1 Kcal/day (95% CI 59.2, 556.8 Kcal/day; P=0.015). Participants increased protein (~13%) and fat intake (~17%) only when undergoing sleep restriction (both P’s <0.001), resulting in a relative increase of 10.9 gr/day (95% CI 0.0, 21.7 gr/day; P=0.050) and 13.5 gr/day (95% CI 0.3, 26.8 gr/day; P=0.046), respectively. Carbohydrate consumption was elevated in both conditions, and the between-condition difference did not achieve significance (34.9 gr/day; 95% CI −1.9, 71.6 gr/day; P=0.063). The temporal profile of changes in food intake is depicted in Figure 2 (a-d), indicating that excess energy consumption was prominent during early exposure to sleep restriction, while it decreased towards acclimation levels during recovery, with similar patterns evident for all nutrients.
Figure 2. Changes in energy intake and diet composition.
Adjusted mean changes from acclimation to experimental and recovery study phases in daily calorie (a), protein (b), fat (c), and carbohydrates (d) consumption across study days. Sleep restriction is presented in red and control sleep in gray. Error bars indicate SE.
Measures of energy expenditure and efficiency did not show evidence of change in response to sleep restriction, as shown by no differences in basal metabolic rate (BMR), thermic effect of food (TEF), energy expenditure of non-exercise activities (sitting, standing, and walking at 1 mph, 2 mph, and 3 mph), and physical activity (expressed as total acceleration) (all P’s >0.16).
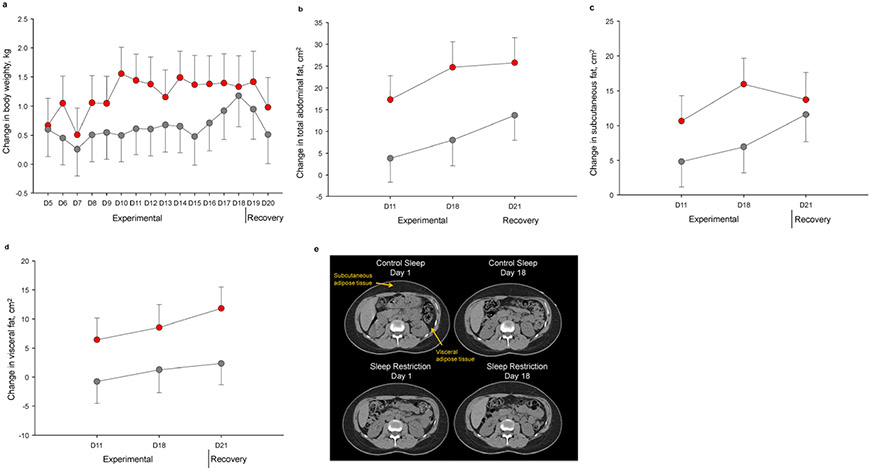
Body weight increased during both experimental sleep restriction and control sleep (both P’s <0.001), but the magnitude of increase was greater following sleep restriction, with a net weight gain of 0.5 kg (95% CI 0.1, 0.8 kg; P=0.008) when participants were exposed to sleep curtailment. As illustrated in Figure 3a, between-condition differences in weight gain were maximal during mid-experimental phase, and dissipated during recovery.
Figure 3. Changes in body weight and regional fat distribution.
Adjusted mean changes from acclimation to experimental and recovery study phases in body weight (a), total abdominal fat (b), subcutaneous fat (c), and visceral fat (d) areas across study days. Sleep restriction is presented in red and control sleep in gray. Error bars indicate SE. e. Representative abdominal computed tomography scans from a study participant during acclimation (Day 1) and experimental (Day 18) phases of control sleep (top left and top right, respectively), and of sleep restriction (bottom left and bottom right, respectively). Arrows indicate subcutaneous and visceral fat.
No significant between-condition differences in changes in body fat percentage, total fat mass, total lean mass, or android fat mass as quantified from DEXA, were noted (all P’s >0.14). By contrast, CT-derived fat measures showed that total abdominal fat area increased significantly during experimental sleep restriction by 9% (P<0.001) but did not during control sleep (P=0.157), yielding a relative increment of 15.2 cm2 (95% CI 3.6, 26.8 cm2; P=0.011). When considering the subcutaneous and visceral fat compartments separately, we found that the subcutaneous fat area expanded significantly during both experimental sleep restriction (~8%, P<0.001) and control sleep phases (~4%, P=0.024). However, the net degree of subcutaneous fat accumulation was higher during sleep restriction (7.4 cm2; 95% CI 0.1, 14.6 cm2; P=0.047). Conversely, ~11% increase in visceral fat area occurred in response to experimental sleep restriction (P=0.005), while no changes were seen in control sleep (P=0.987), thus yielding a between-condition difference of 7.8 cm2 (95% CI 0.3, 15.3 cm2; P=0.042). Evaluation of temporal trajectories of abdominal fat deposition (Figure 3b-e) showed that, in the sleep restriction condition, expansion of both subcutaneous and visceral depots emerged early and persisted through recovery, while increments in subcutaneous fat were noted later during control sleep.
No significant between-condition differences in changes were observed for leptin, total ghrelin, cortisol, 2-arachidonoylglycerol (AG) and anandamide.
Discussion
This study showed that 14 days of experimental sleep restriction in the context of free access to food caused increased energy intake in the absence of changes in energy expenditure, leading to significant weight gain. Furthermore, in this first-ever study assessing the effects of sleep curtailment on body fat distribution, we report the novel observation that expansion in abdominal adipose tissue and especially visceral fat deposition occurred only in response to shortened sleep. During sleep restriction, participants consumed considerably more calories than during control sleep, resulting in an average net difference of 308 Kcal/day. Although the observed difference was lower than anticipated, this increased calorie consumption retains clinical significance given the clear increases noted in clinically relevant outcomes such as weight and abdominal fat, as discussed below. Our findings of increased energy intake are also consistent with prior in-laboratory studies on the effects of sleep truncation on eating behaviors (12-16), although heterogeneity in the magnitude of reported excess intake is apparent and plausibly a function of study design (e.g., degree and duration of sleep loss). In support of this hypothesis, we found that, comparing sleep restriction to control sleep, propensity to overeating peaked during the early experimental sleep restriction phase, then declined as exposure continued, and finally normalized during recovery. A similar trend was reported by Markwald et al,(13) who noted that energy intake was increased during the first 2 days of a 5-day sleep restriction period. This suggests that partial adaptation to the hyperphagic effects of sleep loss may occur over time, though this defense response appears insufficient to entirely dissipate such effects, with consequent detrimental implications for weight gain and obesity, as discussed below.
Short sleep appears also to modulate nutritional choices, with sleep restricted participants consuming significantly more fat and protein, consistent with prior data.(14,15)
Mechanisms underlying increased nutrient intake during sleep restriction remain unclear. Previous research has reported perturbations in the endocrine network modulating appetite, as indicated by increased circulating levels of orexigenic hormones such as ghrelin and endogenous cannabinoids and reduced levels of the satiety hormone leptin (13,25,26). However, other studies did not corroborate these findings (12,16). Consistent with the latter, we did not observe appreciable changes in any of these markers which may implicate central hormonal or neuronal determinants. This is in line with neuroimaging studies reporting that sleep restricted participants exhibit exaggerated activation of brain regions implicated in appetite control and reward in response to visual food cues and especially to unhealthy food (27,28). The lack of compensatory changes seen in the peripheral appetite-regulatory endocrine pathway in the face of a positive energy balance suggests a degree of pathophysiologic dysregulation.
The hyperphagia exhibited during sleep curtailment does not appear to be a function of the longer activity periods, and consequently increased energy demands of protracted wakefulness. In our study, resting energy expenditure in the basal state did not vary, nor did standing or walking energy expenditure. Furthermore, there was no effect of sleep loss on postprandial rise in energy expenditure and participants also maintained similar levels of physical activity in both conditions, thus indicating that overall energy expenditure remained unchanged during sleep restriction relative to control sleep. Data on the impact of sleep restriction on energy expenditure are conflicting, with studies showing increases,(13) decreases,(29) and, for the most part, no changes.(12,16,17,24) While different techniques and measured components of energy expenditure may contribute to mixed results, pooled estimates from a recent meta-analysis (30) support our null findings, corroborating the idea that overeating is not simply a compensatory response to augmented energy output or daily movements with sleep truncation. This also implies that the ensuing net positive balance is essentially driven by a surplus of energy intake, which, in turn, could lead to weight gain. Even if the increased eating behavior during sleep restriction is due to greater access to food during wakefulness, it is notable that we did not see the expected compensatory changes seen in the peripheral appetite-regulatory endocrine pathway, which again suggests a degree of pathologic impairment. Accordingly, greater weight gain occurred during sleep restriction, with a between-condition difference of 0.5 kg, reaffirming that sleep deficiency causes increases in body weight.
Despite being customarily adopted as a proxy for adiposity, scale-measured weight de facto does not convey any information on body fatness as it does not differentiate between fat and fat-free tissues nor does it inform on regional tissue distribution. In our study, imaging modalities enabled us to characterize changes in body composition in response to sleep curtailment. As showed by whole-body DEXA, participants overall gained similar fat mass during both sleep restriction and control sleep conditions, with no incremental effect of sleep loss. Our data are consistent with previous evaluations that used air displacement plethysmography,(24) bioimpedance,(31) as well as DEXA (16) to assess body composition and observed no changes with experimentally-induced insufficient sleep.
However, with respect to fat deposition and its health implications, a pivotal consideration pertains to the location of accumulated fat. By imaging and segregating abdominal fat compartments, CT scans revealed that the subjects’ intraabdominal fat depots increased only during sleep restriction but did not change during their control sleep. Both subcutaneous and visceral abdominal fat increased in sleep restriction, while only a modest increase in the subcutaneous fat but no change in visceral fat occurred during control sleep. Temporal trajectories show increases in intraabdominal adipose tissue largely paralleled those in the subcutaneous compartment during the experimental sleep restriction phase and remarkably continued to increase through recovery, despite normalization of sleep and of energy intake. These data show, for the first time, that experimentally-induced sleep curtailment affects regional fat accumulation, favoring abdominal adiposity, and specifically centralized partitioning of fat into the visceral depot.
It could be argued that expansion of abdominal fat depots in response to sleep restriction simply reflects higher relative energy intake in comparison to controls. However, in healthy individuals, overeating leads initially to fat accumulation in the subcutaneous regions, followed only later, once storage capacity is exceeded, by ectopic fat deposition. Distribution of lipid storage including excess fat is indeed determined by biological properties of subcutaneous fat and specifically its expandability threshold.(32,33) The early and preferential accumulation of ectopic fat in the intraabdominal cavity, as seen in our study, indicates that sleep restriction in conjunction with overeating alters lipid storage mechanisms and potentiates susceptibility to visceral fat deposition. Since the crossover design rules out inter-individual variability in storage of excess energy, extrinsic, presumably sleep-dependent factors are likely implicated in these findings. Dysregulation of the hypothalamic-pituitary-adrenal axis and endocannabinoid system have been implicated as biological mechanisms predisposing to abdominal and visceral fat deposition. (34-36) Although some studies reported that sleep curtailment may impact these systems (26,37), in our exploratory analysis we did not find evidence of changes in cortisol, anandamide or 2-AG in response to sleep loss; hence the mediators of the selective increases in intraabdominal fat are yet to be identified.
Our findings provide the first causal evidence for the epidemiological observations linking short sleep to abdominal obesity and to visceral fat.(7-10) Abdominal (central) obesity is associated with poorer health status and more negative outcomes than generalized excess adiposity and high BMI.(38-40) Within the abdominal fat compartment, mounting evidence suggests a profoundly different pathogenic contribution from subcutaneous vs visceral adipose tissue, two fat depots that are anatomically and functionally distinct. Compared to subcutaneous fat, intraabdominal fat is less insulin-sensitive, metabolically more active and a greater source of pro-inflammatory cytokines, along with exhibiting greater innervation and vascularization (34,41), and excess visceral adipose tissue is associated with cardiometabolic risk factors and overt diseases in several population-based studies, including in nonobese individuals.(18-21) Visceral adiposity is also a much more powerful predictor of unfavorable events and mortality than BMI and subcutaneous fat and retains its prognostic power even after correcting for these other anthropometric measures.(18,19) Thus, visceral fat expansion, which may not be readily apparent by measures of body weight or body fat percent alone, may contribute importantly to the adverse health repercussions of sleep loss.
Short sleep is increasingly pervasive and often a behavioral choice. Recognition of its impact on excess adiposity is pivotal for public health. Strategies to normalize sleep duration to the recommended healthy range of 7-9 hours,(42) and control of food consumption during sleep curtailment, may contribute to alleviate health hazards of insufficient sleep and mitigate obesity and its comorbidities. Relatedly, short bouts of extended (recovery) sleep may not effectively resolve the cumulative adverse metabolic effects of sleep loss. While energy intake and weight normalized during the recovery phase in our study, resumption of adequate sleep over a 3-day period was insufficient to reverse visceral fat deposition, which continued to increase. Thus, the practice of weekend “catch-up sleep” as an attempt to compensate for sleep debt accumulated during weekdays may not offset the increased metabolic risk.
Study strengths and limitations
Our study has numerous strengths, including a robust randomized cross-over study design; prolonged exposure to sleep restriction; ad libitum food intake to mimic real-life free access to food; repeated serial outcome assessments throughout the study; and daily monitoring of energy intake and body weight measures. DEXA and CT imaging, as used here, are the gold standard methods for determination of body composition and regional body fat distribution. The application of a minimally obtrusive, ambulatory PSG recording system yielded precise quantification and qualitative characterization of total 24-hour sleep continuously during the 21-day study. Furthermore, unlike previous studies (13,43), our results were not confounded by treatment period nor order effects. Limitations include a modest sample comprising mostly of normal weight young men, which may hamper generalizability and precluded evaluation of potential sex differences in study outcomes. As our findings were obtained from a highly controlled, in-laboratory study, with transient, two-week duration of exposure to sleep restriction, they may not necessarily apply to states of chronic sleep deficiency. Similarly, findings may not be generalized to patients experiencing sleep loss because of clinical sleep disturbances. Although confounding from circadian fluctuations was minimized by application of a sleep restriction model that preserved sleep midpoint and outcome measurements that were taken at the same time of the day, we cannot exclude occurrence of a degree of circadian disruption influencing our findings. Furthermore, it is conceivable that the impact of sleep truncation on outcomes and specifically on increases in visceral adiposity would be attenuated if participants would have access only to food items of high nutritional quality. The metabolic effects of experimental sleep restriction combined with different diet compositions warrant further investigation.
Conclusions
Our study shows that prolonged experimental sleep restriction in an obesogenic setting promotes excess energy intake without affecting energy expenditure, leading to preferential accumulation of fat in the abdominal compartment, and especially in the visceral depot. Our data provide insights into understanding the linkage between insufficient sleep and heightened cardiometabolic risk, and have important implications for public health policy and initiatives.
Supplementary Material
Central illustration. Effects of experimental sleep restriction on obesity risk.
Twelve healthy individuals completed a randomized, controlled, crossover, 21-day inpatient study of sleep restriction (4-hour sleep opportunity) vs control sleep (9-hour sleep opportunity). During sleep restriction participants increased their daily energy intake by 308 Kcal compared to control sleep. Body weight increased in response to sleep restriction vs control sleep, with significant accumulation of abdominal visceral fat (~11%).
Perspectives.
Competency in Medical Knowledge:
Experimental sleep restriction causes excess energy intake in the absence of changes in energy expenditure, leading to weight gain and increased abdominal visceral fat. Hence, limited sleep combined with overeating predisposes to obesity and especially central obesity.
Translational Outlook:
Future studies should address whether metabolic responses to sleep curtailment are potentiated in individuals with cardiometabolic risk factors, and whether sleeping longer improves health.
Funding
This was supported by NIH HL 114024 and CTSA Grant Number UL1 TR002377. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the NIH. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Abbreviations
- 2-AG
2-arachidonoylglycerol
- BMI
body mass index
- BMR
basal metabolic rate
- CI
confidence of interval
- CT
compute tomography
- DEXA
dual energy X-ray absorptiometry
- IQR
interquartile range
- PSG
polysomnography
- SE
standard error
- TEF
thermic effect of food
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
V.K.S. serves as a consultant for Baker Tilly, Jazz Pharmaceuticals, Bayer, Sleep Number and Respicardia. The other authors have no disclosures.
Clinical Trial: clinicaltrials.gov; https://clinicaltrials.gov/ct2/show/NCT01580761; NCT01580761
References
- 1.Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of healthy sleep duration among adults—United States, 2014. MMWR 2016;65:137–141. [DOI] [PubMed] [Google Scholar]
- 2.St-Onge M-P, Grandner MA, Brown D et al. Sleep duration and quality: Impact on lifestyle behaviors and cardiometabolic health. A scientific statement from the American Heart Association. Circulation 2016;134:e367–e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin J, Jin X, Shan Z et al. Relationship of sleep duration with all- cause mortality and cardiovascular events: A systematic review and dose- response meta- analysis of prospective cohort studies. J Am Heart Assoc 2017;6:e005947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Häusler N, Heinzer R, Haba-Rubio J, Marques-Vidal P. Does sleep affect weight gain? Assessing subjective sleep and polysomnography measures in a population-based cohort study (CoLaus/HypnoLaus). Sleep 2019;42:zsz077. [DOI] [PubMed] [Google Scholar]
- 5.Nagai M, Tomata Y, Watanabe T, Kakizaki M, Tsuji I. Association between sleep duration, weight gain, and obesity for long period. Sleep Med 2013;14:206–210. [DOI] [PubMed] [Google Scholar]
- 6.Chaput J-P, Després J-P, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep 2008;31:517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford ES, Li C, Wheaton AG, Chapman DP, Perry GS, Croft JB. Sleep duration and body mass index and waist circumference among US adults. Obesity 2014;22:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hairston KG, Bryer-Ash M, Norris JM, Haffner S, Bowden DW, Wagenknecht LE. Sleep duration and five-year abdominal fat accumulation in a minority cohort: the IRAS family study. Sleep 2010;33:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogilvie RP, Redline S, Bertoni AG et al. Actigraphy measured sleep indices and adiposity: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2016;39:1701–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stranges S, Cappuccio FP, Kandala N-B et al. Cross-sectional versus prospective associations of sleep duration with changes in relative weight and body fat distribution the Whitehall II study. Am J Epidemiol 2008;167:321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaiswal SJ, Quer G, Galarnyk M, Steinhubl SR, Topol EJ, Owens RL. Association of sleep duration and variability with body mass index: Sleep measurements in a large US population of wearable sensor users. JAMA Intern Med 2020;180:1694–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calvin AD, Carter RE, Adachi T et al. Effects of experimental sleep restriction on caloric intake and activity energy expenditure. Chest 2013;144:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markwald RR, Melanson EL, Smith MR et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A 2013;110:5695–5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep 2013;36:981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St-Onge M-P, Roberts AL, Chen J et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr 2011;94:410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr 2009;89:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cros J, Pianezzi E, Rosset R et al. Impact of sleep restriction on metabolic outcomes induced by overfeeding: a randomized controlled trial in healthy individuals. Am J Clin Nutr 2019;109:17–28. [DOI] [PubMed] [Google Scholar]
- 18.Mongraw-Chaffin M, Allison MA, Burke GL et al. CT-derived body fat distribution and incident cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis. J Clin Endocrinol Metab 2017;102:4173–4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol 2013;62:921–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neeland IJ, Ayers CR, Rohatgi AK et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity 2013;21:E439–E447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Després J-P, Carpentier AC, Tchernof A, Neeland IJ, Poirier P. Management of obesity in cardiovascular practice: JACC focus seminar. J Am Coll Cardiol 2021;78:513–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meier-Ewert HK, Ridker PM, Rifai N et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol 2004;43:678–683. [DOI] [PubMed] [Google Scholar]
- 23.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr 2010;91:1550–1559. [DOI] [PubMed] [Google Scholar]
- 24.Bosy-Westphal A, Hinrichs S, Jauch-Chara K et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts 2008;1:266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiegel K, Leproult R, L’Hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 2004;89:5762–5771. [DOI] [PubMed] [Google Scholar]
- 26.Hanlon EC, Tasali E, Leproult R et al. Sleep restriction enhances the daily rhythm of circulating levels of endocannabinoid 2-arachidonoylglycerol. Sleep 2016;39:653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.St-Onge M-P, McReynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr 2012;95:818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.St-Onge M, Wolfe S, Sy M, Shechter A, Hirsch J. Sleep restriction increases the neuronal response to unhealthy food in normal-weight individuals. Int J Obes 2014;38:411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spaeth AM, Dinges DF, Goel N. Resting metabolic rate varies by race and by sleep duration. Obesity 2015;23:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu B, Shi C, Park CG, Zhao X, Reutrakul S. Effects of sleep restriction on metabolism-related parameters in healthy adults: A comprehensive review and meta-analysis of randomized controlled trials. Sleep Med Rev 2019;45:18–30. [DOI] [PubMed] [Google Scholar]
- 31.Robertson MD, Russell-Jones D, Umpleby AM, Dijk D-J. Effects of three weeks of mild sleep restriction implemented in the home environment on multiple metabolic and endocrine markers in healthy young men. Metabolism 2013;62:204–211. [DOI] [PubMed] [Google Scholar]
- 32.Cuthbertson DJ, Steele T, Wilding JP et al. What have human experimental overfeeding studies taught us about adipose tissue expansion and susceptibility to obesity and metabolic complications? Int J Obes 2017;41:853–865. [DOI] [PubMed] [Google Scholar]
- 33.Gray SL, Vidal-Puig AJ. Adipose tissue expandability in the maintenance of metabolic homeostasis. Nutr Rev 2007;65:S7–S12. [DOI] [PubMed] [Google Scholar]
- 34.Tchemof A, Després J-P. Pathophysiology of human visceral obesity: an update. Physiol Rev 2013;93:359–404. [DOI] [PubMed] [Google Scholar]
- 35.Purnell JQ, Kahn SE, Samuels MH, Brandon D, Loriaux DL, Brunzell JD. Enhanced cortisol production rates, free cortisol, and 11β-HSD-1 expression correlate with visceral fat and insulin resistance in men: effect of weight loss. American Journal of Physiology-Endocrinology and Metabolism 2009;296:E351–E357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blüher M, Engeli S, Klöting N et al. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes 2006;55:3053–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guyon A, Balbo M, Morselli LL et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab 2014;99:2861–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sahakyan KR, Somers VK, Rodriguez-Escudero JP et al. Normal-weight central obesity: implications for total and cardiovascular mortality. Ann Intern Med 2015;163:827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jayedi A, Soltani S, Zargar MS, Khan TA, Shab-Bidar S. Central fatness and risk of all cause mortality: systematic review and dose-response meta-analysis of 72 prospective cohort studies. BMJ 2020;370:m3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coutinho T, Goel K, De Sá DC et al. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: role of “normal weight central obesity”. J Am Coll Cardiol 2013;61:553–560. [DOI] [PubMed] [Google Scholar]
- 41.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 2010;11:11–18. [DOI] [PubMed] [Google Scholar]
- 42.Consensus Conference Panel, Watson NF, Badr MS et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine 2015;11:931–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McNeil J, St- Onge MP. Increased energy intake following sleep restriction in men and women: A one- size- fits- all conclusion? Obesity 2017;25:989–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.