Abstract
Introduction
Less than 1% of all breast cancers are diagnosed in males. In females, postmastectomy breast reconstruction is associated with increased patient satisfaction. However, there is a paucity of literature describing reconstructive options for postmastectomy deformity in the male chest. The purpose of this systematic review was to evaluate postmastectomy reconstruction outcomes in males with breast cancer.
Methods
A systematic review was performed in accordance with PRISMA guidelines. Ovid MEDLINE, Embase, Cochrane, and Web of Science were queried for records pertaining to the study question using medical subject heading (MeSH) terms such as “male breast cancer,” “mastectomy,” and “reconstruction.” No limitations were placed on the year of publication, country of origin, or study size. Study characteristics and patient demographics were collected. Primary outcomes of interest included postoperative complications, recurrence rate, and mortality rate.
Results
A total of 11 articles examining 29 male patients with breast cancer who underwent postmastectomy reconstruction were included for analysis. Literature was most commonly available in the form of case reports. The average age was 59.6 +/−11.4 years. Reconstruction methods included fat grafting (n = 1, 3.4%), silicone implants (n = 1, 3.4%), and autologous chest wall reconstruction with local flaps (n = 26, 89.7%). Postoperative complications occurred in two patients (6.8%), including partial nipple necrosis (n = 1) and hypertrophic scarring (n = 1). Of the studies reporting patient satisfaction, all patients were pleased with the aesthetic appearance of their chest.
Conclusion
This systematic review revealed the limited availability of research regarding postmastectomy chest reconstruction in males with breast cancer. Nevertheless, the evidence available suggests that reconstruction can restore a patient's body image and, thus, should be regularly considered and discussed with male patients. Larger studies are warranted to further shed light on this population.
1. Introduction
Less than 1% of all breast cancers are diagnosed in males; however, the incidence is rising [1–3]. According to the American Cancer Society, about 2,710 new cases of invasive breast cancer will be diagnosed in the United States in 2022 [4]. Less than 0.2% of cancer-related deaths in men can be attributed to male breast cancer [4]. The majority of male breast cancers are invasive ductal carcinoma, accounting for up to 90% of cases [5]. Estimates of in situ carcinoma in men are approximately 10% [4, 6]. Invasive lobular carcinoma is rare in men due to a lack of terminal breast lobules [7]. Men with breast cancer are significantly more likely to have hormone-receptor positive tumors, nodal metastases, and to be diagnosed at a more advanced stage than their female counterparts [1, 8]. Tumors are typically in the central subareolar location of the male breast and often involve the nipple [9].
Several risk factors have been identified for male breast cancer. Underlying genetic alterations differ between male and female breast cancers. Common genetic mutations in male breast cancer occur in the BRCA-2 gene, CHEK2 gene, and PALB2 gene [4, 10, 11]. Klinefelter's syndrome, characterized by an XXY genotype, is associated with testicular dysgenesis, gynecomastia, and an altered balance of androgens and estrogens, which confers a 50-fold increased risk of developing male breast cancer [3, 4]. Exogenous causes of hyperestrogenism in males, such as estrogen treatment in prostate cancer or hormone therapy for male-to-female transgender individuals, can increase the risk of male breast cancer [12, 13]. Endogenous causes of higher estrogen levels including obesity, cirrhosis, mumps orchitis, undescended testes, or testicular injury have also been associated with a higher risk of male breast cancer [3, 14]. Since breast cancer is traditionally regarded as a “female disease,” a diagnosis of breast cancer may induce feelings of demasculinization, altered body image, and embarrassment in males [15, 16]. In a survey of 28 males, 43% reported that a diagnosis of breast cancer may cause them to question their masculinity [9].
Current treatment for male breast cancer has largely been extrapolated from female breast cancer, despite the unique anatomy of the male breast. Due to the paucity of male breast tissue and the typical central subareolar tumor location, the standard treatment in male breast cancer is a modified radical mastectomy, which includes removal of the nipple and axillary node sampling or dissection [6, 8, 17, 18]. This results in an asymmetrical chest, which can create a substantial emotional burden and negative self-image in males [8, 18]. Postmastectomy breast reconstruction has been found to increase patient satisfaction and psychosocial well-being in female patients [19–21]. Although postmastectomy reconstruction options for women have been well described, there is a paucity of literature describing reconstructive options for postmastectomy deformity in the male chest. Oftentimes, postmastectomy reconstruction is not discussed or offered to men during their preoperative consultation, despite federal law requiring health plans that pay for mastectomy to also cover breast reconstruction in both men and women [22]. The purpose of this systematic review was to evaluate postmastectomy reconstruction in males with breast cancer.
2. Methods
This systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [23] and the Cochrane handbook of systematic reviews [24]. A systematic search of Ovid MEDLINE, Embase, Cochrane, and Web of Science was performed using medical subject heading (MeSH) terms and keywords including but not limited to “male breast cancer,” “mastectomy,” and “reconstruction”.
2.1. Study Selection
Using the Rayyan (Qatar Computing Research Institute, Doha, Qatar) systematic review web application, two independent reviewers (P.T. and N.A.) screened each citation. First, studies were screened for relevance based on titles and abstracts. In the event that a screening decision was not unanimous, a third reviewer (R.D.) was consulted to discuss their reasoning until consensus was reached. The remaining studies then underwent full-text review. For inclusion in this study, all papers met the following criteria: (1) addressed males with breast cancer who underwent resection surgery and (2) reconstruction with fat grafting/lipofilling, autologous reconstruction, or implant-based reconstruction. Due to the scarce amount of literature on this topic, no restrictions were set on year of publication, country of origin, or study size. Studies were excluded if they did not report on males with breast cancer, postmastectomy reconstruction, or were not written in the English language. A flowchart outlining the study selection process is shown in Figure 1. Each study was analyzed for patient demographics, breast cancer characteristics, type of mastectomy and reconstruction, postoperative complications, recurrence and mortality rates, and follow-up period.
Figure 1.
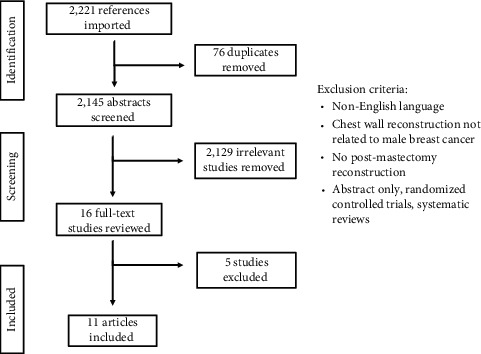
Study selection process flowchart.
3. Results
The majority of articles were case reports (n = 9, 81.8%), followed by retrospective cohort studies (n = 2, 18.2%). Included articles described a total of 29 male patients with breast cancer who underwent postmastectomy reconstruction.
3.1. Patient Demographics and Cancer Characteristics
General study characteristics and breast cancer characteristics of the patients in each study are described in Table 1. Of the 10 studies that explicitly stated patients' age at the time of reconstruction, the average age was 59.6 ± 11.4 years. Only one study reported the body mass index (BMI) in its two patients (35 and 59 kg/m2) [33]. Invasive ductal carcinoma was the most common the breast cancer type (n = 27, 93.1%), followed by papillary ductal carcinoma (n = 1, 3.4%). One study [27] did not specify breast cancer type. All tumors were hormone-receptor positive among the studies that reported receptor status (n = 6). One patient underwent neoadjuvant chemotherapy to decrease tumor size prior to mastectomy [32], and two patients underwent adjuvant chemotherapy with hormone therapy [31, 33].
Table 1.
Patient demographics and cancer characteristics.
| Author, year | Study type | Country | Patients (n)a | Age (yrs) | Breast CA type; stage | Tumor size | Hormone receptor | Medical therapy |
|---|---|---|---|---|---|---|---|---|
| Al-Kalla and Komorowska-Timek, 2014 [25] | Case report | USA | 1 | 68 | IDC; stage 1 | 1 × 2 cm | ER+/PR+ | Not reported |
| Bamba et al., 2017 [26] | Case report | USA | 1 | 78 | IDC | Not reported | Not reported | Not reported |
| Banys-Paluchowski et al., 2016 [1] | Case report | Germany | 1 | 62 | Papillary CA; stage 2 | 16 cm diameter | ER+/PR+ | Not reported |
| Danino et al., 1998 [27] | Case report | France | 1 | 57 | Stage 4 | Not reported | Not reported | Not reported |
| Di Benedetto et al., 1997 [28] | Retrospective cohort | Italy | 11 | 64 + 6.7 | IDC; early: 2 intermediate: 6 advanced: 2 |
Not reported | Not reported | Not reported |
| Elshafiey et al., 2011 [29] | Retrospective cohort | Egypt | 8 (25%) | Not specifiedb | IDC | Not reported | Not specifiedb | Not reported |
| Giunta et al., 2017 [30] | Case report | Italy | 1 | 46 | IDC; stage 1 | Not reported | ER+/PR+ | None |
| Igun, 2000 [31] | Case report | Nigeria | 1 | 35 | IDC; stage 3 | 3 × 3 cm | Not reported | Adjuvant tamoxifen, chemotherapy |
| Nakao et al., 2002 [32] | Case report | Japan | 1 | 59 | IDC; stage 4 | 9 × 6 cm | Not reported | Neoadjuvant chemotherapy |
| Schaverien et al., 2013 [33] | Case report | Scotland | 2 | 35, 59 | IDC; stage 2, stage 1 | 3 × 2 cm 1 × 1 cm | ER+/PR−ER+/PR+ | Adjuvant tamoxifen, chemotherapy, XRT |
| Spear et al., 1997 [34] | Case report | USA | 1 | 49 | IDC, stage 2 | 2.2 cm | ER+/PR+ | None |
Age is presented as n or mean ± standard deviation. aNumber of patients who underwent postmastectomy reconstruction. bNot specified for the 8 patients who underwent postmastectomy reconstruction. CA: carcinoma; IDC: invasive ductal carcinoma; MRM: modified radical mastectomy; XRT: radiation therapy.
3.2. Mastectomy and Chest Reconstruction
Among the 29 patients included in this review, two patients (6.8%) underwent bilateral mastectomy [26, 33]. Ten patients (34.5%) underwent radical mastectomy and five patients (17.2%) underwent modified radical mastectomy. Four studies [1, 25–27] did not identify the type of mastectomy performed, and one study [29] did not specify mastectomy technique among the patients who underwent reconstruction.
Seven studies reported immediate reconstruction following mastectomy. Reconstruction methods included fat grafting [25] (n = 1; 3.4%), silicone implants [26] (n = 1; 3.4%), and autologous chest wall reconstruction with local flaps [1, 27–29, 31–34] (n = 26; 89.7%). One patient (3.4%) underwent primary closure of the mastectomy incision followed by delayed nipple-areolar complex (NAC) reconstruction [30]. Of the 26 local flaps described, myocutaneous latissimus dorsi (LD) flaps (n = 9; 34.6%) were performed most commonly, followed by transverse rectus abdominis muscle (TRAM) flaps (n = 5; 19.2%). Other local flaps included thoracoepigastric flap, deltopectoral flap, internal-external oblique flap, and cutaneous flaps (Table 2).
Table 2.
Mastectomy and reconstruction characteristics.
| Author, year | Mastectomy type | Chest reconstruction |
Type of local flap | Timing of reconstruction | NAC reconstruction |
|---|---|---|---|---|---|
| Al-Kalla and Komorowska-Timek, 2014 [25] | Not specified | Fat grafting | N/A | Immediate | Skate flap with skin graft |
| Bamba et al., 2017 [26] | Bilateral, not specified | Silicone implants | N/A | Delayed | None |
| Banys-Paluchowski et al., 2016 [1] | Not specified | Local flap | Pedicled LD myocutaneous flap | Immediate | None |
| Danino et al., 1998 [27] | Not specified | Local flap | Rotational internal-external oblique flap | Delayed | None |
| Di Benedetto et al., 1997 [28] | MRM: 2 radical mastectomy: 9 | Local flap (n = 11) | Thoracic fasciocutaneous flap: 2 thoracoepigastric flap: 2 LD myocutaneous flap: 5 TRAM flap: 2 |
Immediate | None |
| Elshafiey et al., 2011 [29] | Not specifieda | Local flap (n = 8) | LD myocutaneous flap: 3 TRAM flap: 1 cutaneous local flap: 4 |
Immediate | None |
| Giunta et al., 2017 [30] | MRM | Primary closure with NAC reconstruction | N/A | Delayed NAC reconstruction | FTSG for areola, local flap for nipple |
| Igun, 2000 [31] | MRM | Local flap | TRAM flap | Immediate | None |
| Nakao et al., 2002 [32] | Radical mastectomy | Local flap | Pedicled deltopectoral flap | Immediate | None |
| Schaverien et al., 2013 [33] | Bilateral simple mastectomy | Local flap | Rotational hatchet flaps | Immediate | None |
| Spear and Bowen, 1997 [34] | MRM | Local flap | TRAM flap | Delayed | Skate flap with FTSG to recreate areola |
aNot specified for the 8 patients who underwent postmastectomy reconstruction. FTSG: full-thickness skin graft; LD: latissimus dorsi; MRM: modified radical mastectomy; TRAM: transverse rectus abdominis muscle; NAC: nipple-areola complex.
NAC reconstruction occurred in three patients. Two case reports described using a local skate flap with a skin graft [25, 34], and another utilized a full-thickness skin graft and a subdermal local flap to recreate the areola and nipple, respectively [30]. There were no reports of nipple tattooing.
3.3. Complications, Long-Term Outcomes, and Patient Satisfaction
Of the eight studies that commented on postoperative complications, six articles reported no complications during the recovery process. One study described partial nipple necrosis that did not require revision [25], and another study reported hypertrophic scarring in one of its two patients, which was treated with steroids [33].
Follow-up duration was mentioned in nine of the 11 studies; Bamba et al. [26] did not mention follow-up in their case report, and another study [29] provided a median follow-up of 58 months among their entire cohort of males with breast cancer, not just those who underwent postmastectomy reconstruction. Thus, in the nine studies that mentioned follow-up for our target population, the average follow-up was 27.9 +/−19.6 months. Di Benedetto et al. described a mortality rate of 45.5% (five of 11 patients who underwent postmastectomy reconstruction) [28]. Seven case reports stated that eight of their patients were alive at most recent follow-up. One case report highlighted that its patient experienced three local recurrences, occurring at six, nine, and 18 months [31]. Three studies [26, 27, 29] did not report recurrence, mortality, or follow-up specifically for patients who underwent reconstruction.
Five studies [25, 26, 30, 33, 34] commented on patient satisfaction following reconstruction, in which all patients were reported to be pleased with the appearance of their chest postreconstruction. Table 3 summarizes complications, long-term outcomes, and patient satisfaction in males who underwent postmastectomy reconstruction.
Table 3.
Postoperative complications and long-term outcomes.
| Author, year | Complications | Patient satisfaction | Recurrence | Mortality | Follow-up duration |
|---|---|---|---|---|---|
| Al-Kalla and Komorowska-Timek, 2014 [25] | Partial necrosis of nipple | Satisfied | 0 | 0 | 6 months |
| Bamba et al., 2017 [26] | None | Satisfied | Not reported | Not reported | Not reported |
| Banys-Paluchowski et al., 2016 [1] | None | Not reported | 0 | 0 | 24 months |
| Danino et al., 1998 [27] | Not reported | Not reported | Not reported | Not reported | Not reported |
| Di Benedetto et al., 1997 [28] | Not reported | Not reported | Not reported | 5 (45.5%) | 36 ± 22.1 months |
| Elshafiey et al., 2011 [29] | Not specifieda | Not reported | Not specifieda | Not specifieda | Not specifieda |
| Giunta et al., 2017 [30] | None | Satisfied | 0 | 0 | 18 months |
| Igun, 2000 [31] | None | Not reported | Local × 3 | 0 | 24 months |
| Nakao et al., 2002 [32] | None | Not reported | 0 | 0 | 24 months |
| Schaverien et al., 2013 [33] | Hypertrophic scarring | Satisfied | 0 | 0 | 10 months; 17 months |
| Spear and Bowen, 1997 [34] | None | Satisfied | 0 | 0 | 12 months |
aNot specified for the 8 patients who underwent postmastectomy reconstruction.
4. Discussion
The primary aim of this review was to systematically assess the few available studies on postmastectomy reconstructive methods in males with breast cancer. In general, the literature on this subject is scarce, and there is a lack of high-quality study designs. Only two of the studies included in this review were retrospective cohort studies, while the remainder were case reports.
Local flaps were the most common reconstruction method performed following mastectomy in males. LD flaps from the back were performed most commonly, followed by the TRAM flap. Autologous reconstruction to restore the male chest contour necessitates important aesthetic considerations, such as hair pattern and thickness of subcutaneous fat and skin. The myocutaneous LD flap is an appealing option due to its reliability, proximity to the mastectomy defect, simplicity of use, and similarity in thickness of subcutaneous fat and skin provided [34]. However, in men with hairless backs, an LD flap will create a noticeable unaesthetic patch rather than restoring normal chest cosmesis. This was the case reported by Spear et al. [34], who instead performed a TRAM flap, as the patient's abdomen demonstrated a similar hair pattern and thickness to the patient's chest. The choice of local flap performed should be based on the patient's motivations for pursuing postmastectomy reconstruction.
Only one article reported the use of fat grafting to fill in the residual chest contour defect following mastectomy [25]. Fat grafting is a straightforward technique associated with low complications and minimal donor site morbidity [35]. Reconstruction of the male breast may require small graft volumes and fewer operative sessions to provide a natural feel and satisfactory cosmesis [25]. However, this technique is not without challenges, which include the varying “take” of fat grafting and the potential for fat necrosis and subsequent infection to occur [36]. Additionally, the theoretical risk of local cancer recurrence in fat-grafted breasts secondary to malignant transformation of transferred adipocytes and adipose-derived stem cells exists [37, 38] but has not been observed in multiple studies [37, 39].
Eight of the 11 studies mentioned complication rates, of which only two complications were reported. Al-Kalla et al. reported partial necrosis of the reconstructed nipple in their patient; however, this did not require revision [25]. One of the two patients in the study by Schaverien et al. experienced hypertrophic scarring, which was treated with a local steroid injection [33]. However, the included studies varied greatly in follow-up duration (ranging from six months [25] to three years [28]) and three studies did not mention follow-up at all. This is particularly relevant when considering complications unique to breast reconstruction, such as capsular contracture, which may occur years after implant placement [40, 41].
Despite a federal law mandating that health care plans cover postmastectomy reconstruction in men and women, surgeons do not regularly offer male patients this option [22]. This is likely due to the surgeon's assumption that males are not concerned by the appearance of their chest following breast cancer surgery [33]. However, this generalization can be detrimental and lead to negative psychosocial impacts for men with breast cancer. The patient in the case report by Spear et al. pursued local flap reconstruction 16 months after his mastectomy because he had become depressed about his physical appearance and did not feel comfortable participating in outdoor activities where his chest might be visible [34].
Besides having to cope with a predominantly female disease, men with breast cancer also deal with the physical changes of their chest after surgery. Some men may develop a negative body image as men often associate their chest with masculinity [42]. Many of the articles included in this review reported patient satisfaction following the reconstruction of their chests. Similar to what has been reported in female patients [21], males with breast cancer who undergo postmastectomy chest wall reconstruction may experience a strong psychological benefit.
There were several limitations in this study. Because of the rarity of male breast cancer, the literature lacks high-quality reports that detail postmastectomy reconstruction. Thus, there were only a small number of articles, mostly case reports, available for this review. Secondly, the follow-up duration varied among the studies, which may have affected the occurrence of complications reported. Indications for reconstruction type were not always reported. Finally, we cannot conclude whether the outcomes we observed resulted from patient disease severity or surgical procedure selection. Despite these limitations, the evidence available suggests that postmastectomy chest reconstruction in males can be beneficial from an aesthetic and psychosocial aspect and should be offered regularly in the preoperative setting.
5. Conclusion
This systematic review revealed that there is limited research specific to chest wall reconstruction following breast cancer resection in males. Postmastectomy chest reconstruction should be regularly considered and discussed with men who have breast cancer. Larger studies are warranted to further shed light on this patient population.
Acknowledgments
The authors would like to give special thanks to our librarian C. Scott Dorris for his help throughout this process.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Banys-Paluchowski M., Burandt E., Banys J., et al. Male papillary breast cancer treated by wide resection and latissimus dorsi flap reconstruction: a case report and review of the literature. World Journal of Clinical Oncology . 2016;7(5):420–424. doi: 10.5306/wjco.v7.i5.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White J., Kearins O., Dodwell D., Horgan K., Hanby A. M., Speirs V. Male breast carcinoma: increased awareness needed. Breast Cancer Research . 2011;13(5):p. 219. doi: 10.1186/bcr2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fentiman I. S., Fourquet A., Hortobagyi G. N. Male breast cancer. Lancet . 2006;367(9510):595–604. doi: 10.1016/s0140-6736(06)68226-3. [DOI] [PubMed] [Google Scholar]
- 4.Gucalp A., Traina T. A., Eisner J. R., et al. Male breast cancer: a disease distinct from female breast cancer. Breast Cancer Research and Treatment . 2019;173(1):37–48. doi: 10.1007/s10549-018-4921-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Cancer Society. Breast Cancer in Men . Atlanta, GA, USA: American Cancer Society; 2022. [Google Scholar]
- 6.Korde L. A., Zujewski J. A., Kamin L., et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. Journal of Clinical Oncology . 2010;28(12):2114–2122. doi: 10.1200/jco.2009.25.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ottini L., Palli D., Rizzo S., Federico M., Bazan V., Russo A. Male breast cancer. Critical Reviews in Oncology/Hematology . 2010;73(2):141–155. doi: 10.1016/j.critrevonc.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Giordano S. H., Buzdar A. U., Hortobagyi G. N. Breast cancer in men. Annals of Internal Medicine . 2002;137(8):678–687. doi: 10.7326/0003-4819-137-8-200210150-00013. [DOI] [PubMed] [Google Scholar]
- 9.Thomas E. Original Research: men’s awareness and knowledge of male breast cancer. AJN, American Journal of Nursing . 2010;110(10):32–37. doi: 10.1097/01.NAJ.0000389672.93605.2f. [DOI] [PubMed] [Google Scholar]
- 10.Pritzlaff M., Summerour P., McFarland R., et al. Male breast cancer in a multi-gene panel testing cohort: insights and unexpected results. Breast Cancer Research and Treatment . 2017;161(3):575–586. doi: 10.1007/s10549-016-4085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang M., Zhang Y., Sun C., et al. Association between CHEK2∗1100delC and breast cancer: a systematic review and meta-analysis. Molecular Diagnosis & Therapy . 2018;22(4):397–407. doi: 10.1007/s40291-018-0344-x. [DOI] [PubMed] [Google Scholar]
- 12.Karlsson C. T., Malmer B., Wiklund F., Grönberg H. Breast cancer as a second primary in patients with prostate cancer-estrogen treatment or association with family history of cancer? Journal of Urology . 2006;176(2):538–543. doi: 10.1016/j.juro.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 13.de Blok C. J. M., Wiepjes C. M., Nota N. M., et al. Breast cancer risk in transgender people receiving hormone treatment: nationwide cohort study in The Netherlands. BMJ . 2019;365 doi: 10.1136/bmj.l1652.l1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudlowski C. Male breast cancer. Breast Care . 2008;3(3):183–189. doi: 10.1159/000136825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Co M., Lee A., Kwong A. Delayed presentation, diagnosis, and psychosocial aspects of male breast cancer. Cancer Medicine . 2020;9(10):3305–3309. doi: 10.1002/cam4.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brain K., Williams B., Iredale R., France L., Gray J. Psychological distress in men with breast cancer. Journal of Clinical Oncology . 2006;24(1):95–101. doi: 10.1200/jco.2006.10.064. [DOI] [PubMed] [Google Scholar]
- 17.Fogh S., Kachnic L. A., Goldberg S. I., Taghian A. G., Powell S. N., Hirsch A. E. Localized therapy for male breast cancer: functional advantages with comparable outcomes using breast conservation. Clinical Breast Cancer . 2013;13(5):344–349. doi: 10.1016/j.clbc.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Kinne D. W. Management of male breast cancer. Oncology (Williston Park) . 1991;5(3):45–47. [PubMed] [Google Scholar]
- 19.Qin Q., Tan Q., Lian B., Mo Q., Huang Z., Wei C. Postoperative outcomes of breast reconstruction after mastectomy. Medicine . 2018;97(5) doi: 10.1097/md.0000000000009766.e9766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heneghan H. M., Prichard R. S., Lyons R., et al. Quality of life after immediate breast reconstruction and skin-sparing mastectomy - a comparison with patients undergoing breast conserving surgery. European Journal of Surgical Oncology (EJSO) . 2011;37(11):937–943. doi: 10.1016/j.ejso.2011.08.126. [DOI] [PubMed] [Google Scholar]
- 21.Rubino C., Figus A., Lorettu L., Sechi G. Post-mastectomy reconstruction: a comparative analysis on psychosocial and psychopathological outcomes. Journal of Plastic, Reconstructive & Aesthetic Surgery . 2007;60(5):509–518. doi: 10.1016/j.bjps.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Friedman S., Floyd C., Chrysopoulo M. Men can have breast reconstruction after mastectomy. 2022. https://www.facingourrisk.org/blog/men-can-have-breast-reconstruction-after-mastectomy .
- 23.Moher D., Liberati A., Tetzlaff J., Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ . 2009;339 doi: 10.1136/bmj.b2535.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins J. P T., Green S. Cochrane Handbook for Systematic Reviews of Interventions . Hoboken, NJ, USA: John Wiley & Sons; 2011. [Google Scholar]
- 25.Al-Kalla T., Komorowska-Timek E. Breast total male breast reconstruction with fat grafting. Plastic and Reconstructive Surgery Global Open . 2014;2(11):p. e257. doi: 10.1097/gox.0000000000000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bamba R., Krishnan N. M., Youn R., Economides J. M., Pittman T. A. The use of low-profile silicone breast implants in male breast reconstruction. Plastic and Reconstructive Surgery . 2018;141(2):324e–325e. doi: 10.1097/prs.0000000000004089. [DOI] [PubMed] [Google Scholar]
- 27.Danino A., Ichinose M., Yoshimoto S., Kuroki T., Servant J.-M. External-internal oblique reverse blood supply musculocutaneous flap for chest wall reconstruction. Annals of Plastic Surgery . 1998;41(4):430–433. doi: 10.1097/00000637-199810000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Di Benedetto G., Pierangeli M., Bertani A. Carcinoma of the male breast: an underestimated killer. Plastic and Reconstructive Surgery . 1998;102(3):696–700. doi: 10.1097/00006534-199809010-00012. [DOI] [PubMed] [Google Scholar]
- 29.Elshafiey M. M., Zeeneldin A. A., Elsebai H. I., et al. Epidemiology and management of breast carcinoma in Egyptian males: experience of a single Cancer Institute. Journal of the Egyptian National Cancer Institute . 2011;23(3):115–122. doi: 10.1016/j.jnci.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Giunta G., Rossi M., Toia F., Rinaldi G., Cordova A. Male breast cancer: modified radical mastectomy or breast conservation surgery? a case report and review of the literature. International Journal of Surgery Case Reports . 2017;30:89–92. doi: 10.1016/j.ijscr.2016.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Igun G. O. Rectus abdominis myocutaneous flap in reconstruction for advanced male breast cancer: case report. Central African Journal of Medicine . 2000;46(5):130–132. doi: 10.4314/cajm.v46i5.8537. [DOI] [PubMed] [Google Scholar]
- 32.Nakao A., Saito S., Naomoto Y., Matsuoka J., Tanaka N. Deltopectoral flap for reconstruction of male breast after radical mastectomy for cancer in a patient on hemodialysis. Anticancer Research . 2002;22(4):2477–2479. [PubMed] [Google Scholar]
- 33.Schaverien M. V., Scott J. R., Doughty J. C. Male mastectomy: an oncoplastic solution to improve aesthetic appearance. Journal of Plastic, Reconstructive & Aesthetic Surgery . 2013;66(12):1777–1779. doi: 10.1016/j.bjps.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 34.Spear S. L., Bowen D. G. Breast reconstruction in a male with a transverse rectus abdominis flap. Plastic & Reconstructive Surgery . 1998;102(5):1615–1617. doi: 10.1097/00006534-199810000-00043. [DOI] [PubMed] [Google Scholar]
- 35.de Blacam C., Momoh A. O., Colakoglu S., Tobias A. M., Lee B. T. Evaluation of clinical outcomes and aesthetic results after autologous fat grafting for contour deformities of the reconstructed breast. Plastic and Reconstructive Surgery . 2011;128(5):411e–418e. doi: 10.1097/PRS.0b013e31822b669f. [DOI] [PubMed] [Google Scholar]
- 36.Khouri R. K., Rigotti G., Cardoso E., Khouri R. K., Jr., Biggs T. M. Megavolume Autologous fat transfer. Plastic and Reconstructive Surgery . 2014;133(3):550–557. doi: 10.1097/01.prs.0000438044.06387.2a. [DOI] [PubMed] [Google Scholar]
- 37.Seth A. K., Hirsch E. M., Kim J. Y. S., Fine N. A. Long-term outcomes following fat grafting in prosthetic breast reconstruction. Plastic and Reconstructive Surgery . 2012;130(5):984–990. doi: 10.1097/PRS.0b013e318267d34d. [DOI] [PubMed] [Google Scholar]
- 38.Rowan B. G., Gimble J. M., Sheng M., et al. Human adipose tissue-derived stromal/stem cells promote migration and early metastasis of triple negative breast cancer xenografts. PLoS One . 2014;9(2) doi: 10.1371/journal.pone.0089595.e89595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delay E., Garson S., Tousson G., Sinna R. Fat injection to the breast: technique, results, and indications based on 880 procedures over 10 years. Aesthetic Surgery Journal . 2009;29(5):360–376. doi: 10.1016/j.asj.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Walton L., Ommen K., Audisio R. A. Breast reconstruction in elderly women breast cancer: a review. Cancer Treatment Reviews . 2011;37(5):353–357. doi: 10.1016/j.ctrv.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Lipa J. E., Youssef A. A., Kuerer H. M., Robb G. L., Chang D. W. Breast reconstruction in older women: advantages of autogenous tissue. Plastic and Reconstructive Surgery . 2003;111(3):1110–1121. doi: 10.1097/01.Prs.0000046614.84464.84. [DOI] [PubMed] [Google Scholar]
- 42.Robinson J. D., Metoyer K. P., Jr., Bhayani N. Breast cancer in men: a need for psychological intervention. Journal of Clinical Psychology in Medical Settings . 2008;15(2):134–139. doi: 10.1007/s10880-008-9106-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


