Abstract
Methods
We retrospectively enrolled breast cancer patients who underwent SPECT/CT prior to sentinel lymph node biopsy. Quantification of radiotracer uptake from SPECT/CT data was performed. A radioactivity count threshold (RSPECT) using SPECT/CT was calculated for detecting metastatic sentinel lymph nodes. To localize sentinel lymph nodes exactly, we compared the positions of sentinel lymph nodes localized using SPECT/CT with positions localized surgically using an intraoperative γ-probe.
Results
491 patients were included, with a median of 3 sentinel lymph nodes/patient detected by the γ-probe and 2 sentinel lymph nodes/patient detected by SPECT/CT. As the number of sentinel lymph nodes visualized on SPECT/CT images, the metastasis incidence of lymph nodes in the ≤2 SLNs group was significantly higher than that in the >2 SLNs group (35% vs. 15%, P < 0.001). No metastasis was found in lymph nodes with RSPECT ≤ 30% in the >2 SLNs group, and thus, 30% (157/526) of SPECT/CT-identified nodes would avoid unnecessary removal. The positions of sentinel lymph nodes localized by SPECT/CT and γ-probe were identical in 42% (39/93) of patients.
Conclusions
Quantitative Tc-99 m SC SPECT/CT imaging has the potential to preoperatively locate sentinel lymph nodes and intraoperatively avoid unnecessary sentinel lymph node biopsy.
1. Introduction
According to the data on the global cancer burden updated in 2020, the incidence of female breast cancer has surpassed lung cancer and now ranks first in the world [1]. Axillary lymph node status (AS) is a crucial prognostic factor in breast cancer patients. Accurate lymph node staging can guide treatment that affects the overall survival of patients [2, 3]. Sentinel lymph node (SLN) biopsy (SLNB) has replaced axillary lymph node dissection (ALND) and become an effective method to evaluate AS for patients with clinically node-negative breast cancer [4, 5]. Although SLNB is associated with less postoperative complications [6, 7], 3.5%–10.9% of patients experience lymphoedema and arm numbness caused by excessive SLNs removed [8–10]. Furthermore, more than 70% of excised SLNs have been examined to be healthy [11], prolonging the operating time and increasing the pathology workload and medical expenses of patients.
SLNs in breast cancer are detected using a combination of radiotracer and blue dye [12]. Affected by the small size of radiotracer particles and the long interval time between radiotracer injection and operation, the range of SLN detection is 1–10/patient and the average number of SLNs is approximately 3.24/patient [13]. It is a common practice to continue removing radioactive lymph nodes until the count rates are ≤10% of the hottest nodes by intraoperative γ-probe. However, Schuman et al. found that processing the two hottest lymph nodes and suspicious nodes is sufficient for initial axillary staging of breast cancer [14]. Practically, an intraoperative γ-probe may not detect the hottest SLN successfully for two main reasons: (i) the node is very close to the injection site, obscured by scatter and (ii) the position of the node is too deep to locate. In such circumstances, single-photon emission computed tomography/computed tomography (SPECT/CT) imaging may have an additional value.
SPECT/CT technology has the potential for SLN localization and quantitative analysis of radioactive lymph nodes. Preoperative SLN SPECT/CT imaging provides accurate anatomical information of SLNs with lymphatic drainage into the axilla and extra-axillary regions, distinguishing false-positive results caused by nuclide contamination and lymphatic tortuosity and reducing the false-negative rate caused by patient obesity. It compensates for the limitation of the γ-probe in determining the exact anatomy of SLNs. Moreover, in a recent study, Kwak et al., simulating the common practice of removing SLNs with ex vivo count rate ≥10% of the hottest node detected by γ-probe, proposed the concept of the radioactivity count threshold, which was calculated based on SPECT/CT quantification, and found that no metastatic SLN (m-SLN) was detected when using a threshold of 20% [15]. Nevertheless, the value of quantitative SPECT/CT of SLNs in breast cancer is unknown.
This study aimed to investigate the feasibility of a radioactivity count threshold using 99mTc-SC SPECT/CT quantification in helping surgeons select SLNs that are likely to be metastatic and minimize the number of SLNs removed. In order to help surgeons quickly locate SLNs, we evaluated the accuracy of 99mTc-SC SPECT/CT for preoperatively marking the position of SLNs.
2. Materials and Methods
2.1. Patients Selected
Procedures performed in this study were approved by the institutional review board and ethics committee of Shandong Cancer Hospital. All consecutive breast cancer patients scheduled for SLNB were collected between December 2020 and June 2021. Inclusion criteria were primary breast cancer (T1-3) and no palpable lymph node at diagnosis, ≤2 suspicious nodes on imaging, or ≤2 positive nodes confirmed by needle biopsy ± clip placement. Patients with pregnancy and inflammatory breast cancer were excluded. Several patients were randomly selected for preoperative SLN localization on skin. Informed consent was obtained from each patient.
2.2. Imaging Protocol
A dual-head SPECT/CT scanner with low-energy high-resolution collimators (Discovery NM/CT 670 Pro, GE Healthcare, USA) was used. Each patient was given a peritumoral intradermal injection of 9.25 MBq (0.25 mCi) ±10% of 99mTc-SC (Beijing Shihong Co. Ltd; Beijing, China) for surgery planned on the same day or twice the dose (0.5 mCi) for surgery arranged the next day. Planar scintigraphy (PS) was acquired after 20 min (within 4 h), after the injection of the radiotracer, with 3-minute static images in the anterior and lateral projections (matrix 256 × 256, zoom 1), followed by SPECT/CT (matrix 128 × 128, zoom 1) with the patient lying supine as well as arms positioned above the head. Images were processed by OSEM (ordered-subset expectation maximization) reconstruction and attenuation correction.
2.3. SLN Localization
We have made a simple tool for preoperative SLN localization. Ten metal sticks (diameter: 1.5 × 10−3 m, height: 0.24 m) were arranged at an interval of 0.03 m and fixed with tape orderly. The localization tool was attached to the side with cancer of the patient's chest closely before SPECT/CT. The radiologist diagnosed SLNs on SPECT/CT images and selected the hottest SLN for localization. The position of the hottest SLN was mutually determined by the localization tool, and a laser line of SPECT/CT was marked for surgery reference.
2.4. SLNB and Histopathology
SLNB was operated on the same day as the radiotracer injection or the next day in the morning. A peritumoral intradermal injection of 1% methylene blue (Jichuan Pharmaceutical Group Co. Ltd, Jiangsu, China) was performed 10–15 min before surgery. An intraoperative γ-probe (Neoprobe Corporation, AR-MED Ltd) was used for detecting radioactive nodes. Lymph nodes stained blue and/or ≥10% of the ex vivo counts of the hottest node were removed. Suspicious lymph nodes palpable by hand were also processed. Harvested nodes were sent for pathological examination. SLN status (hot/blue/suspicious), position, radioactivity counts, and pathological results were recorded.
2.5. SLN Quantification
Quantification of radiotracer uptake in the SLNs from SPECT/CT images was processed in the “Volumetrix MI Evolution for Tissue” module of the Xeleris Functional Imaging Workstation (Xeleris Version 4.0). The radiologist identified radioactive lymph nodes and chose semiautomatic segmentation for the region of interest (ROI) on the highest radioactive uptake SPECT layer. Radioactivity counts of SLNs are generated by clicking on the radioactivity concentration of each node. Graphical images describing the location of the ROIs and the generation of radioactivity counts of hot nodes on SPECT/CT are shown in Figure 1. The intraoperative γ-probe detected each extracted SLN for 10–15 s, and the final radioactivity count was obtained by pressing “target button.” The hottest radioactive node detected by either modality (SPECT/CT or γ-probe) was designated as 100%, and the following SLN was calculated as a percentage of the hottest node detected by SPECT/CT quantification or by γ-probe, expressing as RSPECT and Rγ-probe.
Figure 1.

Graphical images describing the semiautomatic segmentation for the region of interest (ROI) and generation of radioactivity counts of hot nodes. (a–f) Coronal, transaxial, and MIP views of SPECT and corresponding hybrid SPECT/CT images.
2.6. Statistical Analysis
Data analysis was processed using SPSS 19.0. Categorical variables of patient characteristics and metastasis incidence of SLNs were analyzed using the chi-square test or univariate Fisher exact test. Numerical variables were tested for normality and applied the Kruskal–Wallis test and two-sample t-test. A P value of <0.05 was considered as statistically significant.
3. Results
3.1. Clinical Characteristics and SLNB
491 consecutive breast cancer patients who underwent PS and 99mTc-SC SPECT/CT prior to SLNB were enrolled. 1504 SLNs were excised using a combination of a radiotracer and blue dye, with a median of 3 SLNs/patient. SLNs were identified by SPECT/CT in 82% (404/491) of the patients, with a median of two SLNs/patient. Surgeons removed every node identified by SPECT/CT in 87% (350/404) of the patients. In the remaining patients, the same nodal level was sampled, but the number of SLNs removed intraoperatively was not equivalent to the number identified by SPECT/CT. The most common reason for this phenomenon was that no radioactivity was detected by the γ-probe at this location.
The clinical characteristics of patients are summarized in Table 1. Patients with early-stage breast cancer had more SLNs identified than those with locally advanced breast cancer, whether detected by SPECT/CT (P < 0.001) or by γ-probe (P=0.049). Additionally, patients younger than 50 y had more SLNs identified by SPECT/CT than those older than 50 y (average: 2.04 vs. 1.55, P=0.001). Patients with a history of tumor excisional biopsy tended to have more intraoperative SLNs (average: 3.50 vs. 3.02, P=0.046). Furthermore, we found that SLN metastasis rates were different in patients with different AJCC stages (P < 0.001) and were statistically higher in male breast cancer than female (75.0% vs. 23.6%, P=0.045).
Table 1.
SPECT/CT and SLNB of 491 breast cancer patients.
| Characteristic | Median (range)/freq (%) | Preoperative SPECT/CT | Intraoperative SLN detection by the combination method∗ | SLN pathology | |||
|---|---|---|---|---|---|---|---|
| SLNs () | Significance | SLNs () | Significance | Patients with metastatic SLNs | Significance | ||
| Total | 491 | 118 (24.0%) | |||||
| Age, y | 50 (25–77) | ||||||
| ≤50 | 255 (51.9%) | 2.04 ± 1.24 | P < 0.001 | 3.15 ± 1.66 | P=0.216 | 66 (25.9%) | P=0.319 |
| >50 | 236 (48.1%) | 1.55 ± 1.36 | 2.97 ± 1.53 | 52 (22.0%) | |||
| BMI, kg/m2 | 24 (16–37) | ||||||
| <24 | 223 (45.4%) | 1.87 ± 1.34 | P=0.285 | 3.17 ± 1.58 | P=0.194 | 60 (26.9%) | P=0.174 |
| ≥24 | 268 (54.6%) | 1.75 ± 1.29 | 2.98 ± 1.62 | 58 (21.6%) | |||
| Gender | |||||||
| Female | 487 (99.2%) | 1.80 ± 1.31 | P=0.289 | 3.06 ± 1.60 | P=0.389 | 115 (23.6%) | P=0.045 |
| Male | 4 (0.8%) | 2.50 ± 1.73 | 3.75 ± 1.71 | 3 (75.0%) | |||
| Primary tumor localization | |||||||
| Upper outer quadrant | 227 (46.2%) | 1.78 ± 1.34 | P=0.577 | 2.94 ± 1.64 | P=0.052 | 57 (25.1%) | P=0.19 |
| Upper inner quadrant | 77 (15.7%) | 1.78 ± 1.25 | 3.47 ± 1.29 | 25 (32.5%) | |||
| Lower outer quadrant | 61 (12.4%) | 1.84 ± 1.43 | 3.03 ± 1.40 | 13 (21.3%) | |||
| Lower inner quadrant | 36 (7.3%) | 2.08 ± 1.16 | 3.44 ± 2.04 | 8 (22.2%) | |||
| Others | 90 (18.3%) | 1.74 ± 1.31 | 2.89 ± 1.61 | 15 (16.7%) | |||
| Tumor size, cm | 2(0.4–6) | ||||||
| ≤2 | 290 (59.1%) | 1.81 ± 1.37 | P=0.904 | 3.06 ± 1.54 | P=0.940 | 61 (21.0%) | P=0.062 |
| >2 | 201 (40.9%) | 1.80 ± 1.24 | 3.07 ± 1.68 | 57 (28.4%) | |||
| AJCC clinical stage (v.8) | |||||||
| 0 | 18 (3.7%) | 1.67 ± 1.33 | P=0.001 | 2.78 ± 2.07 | P=0.049 | 0 (0%) | P < 0.001 |
| I | 241 (49.1%) | 1.92 ± 1.40 | 3.17 ± 1.42 | 32 (13.3%) | |||
| II | 203 (41.3%) | 1.81 ± 1.22 | 3.07 ± 1.67 | 74 (36.5%) | |||
| III | 29 (5.9%) | 0.86 ± 0.88 | 2.31 ± 2.00 | 12 (41.4%) | |||
| Prior excisional biopsy | |||||||
| Yes | 48 (9.8%) | 1.71 ± 1.24 | P=0.595 | 3.50 ± 1.34 | P=0.046 | 8 (16.7%) | P < 0.209 |
| No | 443 (90.2%) | 1.81 ± 1.33 | 3.02 ± 1.62 | 110 (24.8%) | |||
∗ Sentinel lymph nodes detected by the γ-probe and/or blue dye during sentinel lymph node biopsy.
3.2. Advantages of SPECT/CT Imaging over Planar Scintigraphy
PS visualized SLNs in 66% (322/491) patients and SPECT/CT visualized SLNs in 82% (404/491) of the patients, resulting in a 16% improvement in the visualization rate (P < 0.001). The most common region of lymph node drainage was the axilla, accounting for 94% (832/886) of all nodes. In 9% (37/404) of the patients, 33 and 11 lymph nodes drained to internal mammary and supraclavicular regions, respectively. 15 cases were presented as hot nodes on PS but interpreted as false-positive results due to tracer contamination on SPECT/CT images.
3.3. Relationship between the Number of SLNs Shown on SPECT/CT and Lymph Nodes Metastasis
Lymph nodes were visualized on SPECT/CT in 404 patients, and AS was metastatic in 28% (112/404) of them. Of the remaining patients, 34% (30/87) of the patients with no lymph nodes visualized on SPECT/CT had metastatic lymph nodes. Metastasis incidence of lymph nodes had a significant difference between different numbers of SLNs (Table S1). Based on the median number of SLNs visualized on SPECT/CT images, the patients were divided into two groups: ≤2 SLNs and >2 SLNs (Table 2). The metastasis incidence of AS in the ≤2 SLNs group was significantly higher than that in the >2 SLNs group (35% vs. 15%, χ2 = 21.3, P < 0.001).
Table 2.
Comparison of metastasis incidence of axillary lymph nodes with a different number of SLNs by SPECT/CT.
| No. of SLNs | Axillary lymph node status | Total | Metastasis incidence | χ 2 | P value | |
|---|---|---|---|---|---|---|
| Metastasis | Nonmetastasis | |||||
| SPECT ≤ 2 | 119 | 218 | 337 | 35% | 21.3 | <0.001 |
| SPECT > 2 | 23 | 131 | 154 | 15% | ||
3.4. Quantitative SPECT/CT Analysis Identifying Metastatic SLNs
In the >2 SLNs group, 15% (23/154) of patients were metastatic with 36 m-SLNs (6.8%, 36/526). To avoid removing invalid SLNs that were nonmetastatic, we analyzed the RSPECT of m-SLNs in different 10% gradients (Table 3). Based on our data, no m-SLN was detected when RSPECT ≤ 30% was used as a radioactivity count threshold. This would avoid resection of 30% (157/526) of the SPECT/CT-identified nodes, beneficial for 63 (40.9%) patients. A typical case describing the procedure of SLNB combined with SPECT/CT quantification is presented in Figure 2.
Table 3.
R SPECT of m-SLNs in the >2 SLNs group by SPECT/CT.
| R SPECT | No. of visualized SLNs | No. of SLN metastases | Metastasis incidence |
|---|---|---|---|
| R SPECT = 100% | 154 | 17 | 11.0% |
| 90% < RSPECT < 100% | 14 | 2 | 14.3% |
| 80% < RSPECT ≤ 90% | 15 | 1 | 6.7% |
| 70% < RSPECT ≤ 80% | 24 | 1 | 4.2% |
| 60% < RSPECT ≤ 70% | 27 | 1 | 3.7% |
| 50% < RSPECT ≤ 60% | 35 | 5 | 14.3% |
| 40% < RSPECT ≤ 50% | 47 | 7 | 14.9% |
| 30% < RSPECT ≤ 40% | 53 | 2 | 3.8% |
| 20% < RSPECT ≤ 30% | 72 | 0 | 0 |
| 10% < RSPECT ≤ 20% | 55 | 0 | 0 |
| 0 < RSPECT ≤ 10% | 30 | 0 | 0 |
| Total | 526 | 36 | 6.8% |
Figure 2.
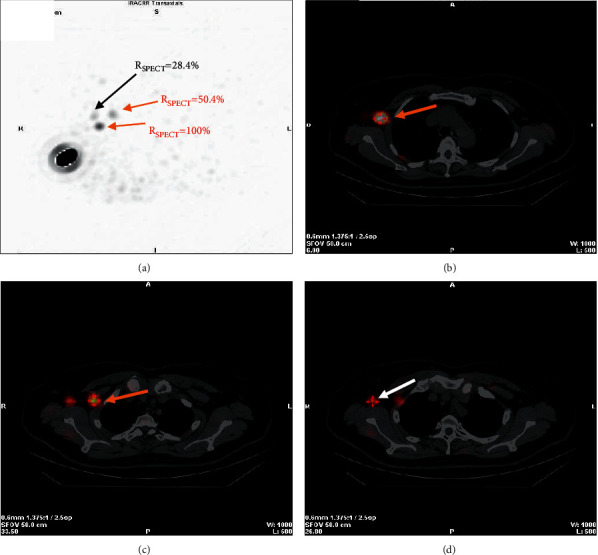
A 60-year-old female patient with invasive ductal carcinoma of the right breast. (a) Maximum intensity projection images. Three hot nodes are visualized with different RSPECT. Two nodes with higher RSPECT (100%, 50.4%) are examined pathologically to be metastatic (red arrows), and another node with lower RSPECT (28.4%) is healthy (black arrow). (b–d), SPECT/CT hybrid images for localizing the two metastatic nodes (red arrows) and the healthy node (white arrow). The surgeon might selectively avoid excising lymph nodes with lower radioactivity (RSPECT ≤ 30%) deriving from preoperative SPECT/CT images.
In 93 patients chosen for localization, radioactivity counts of SLNs were measured both by the γ-probe and SPECT/CT. A total of 29 m-SLNs were pathologically examined in 23 patients. The quantification of radioactive uptake in m-SLNs is listed in Table 4. The majority of m-SLNs occurred in the hottest nodes detected by SPECT/CT (79% (19/24)) or γ-probe (66% (19/29)). Four m-SLNs occurred in the second hottest nodes, and one m-SLN occurred in the third hottest node detected by SPECT/CT. Three m-SLNs mismatched with the SLNs presented on SPECT/CT images, but they were observed to have morphological abnormalities. The γ-probe found metastasis on a fifth hottest node. RSPECT of m-SLNs were all over 30%, but no radioactivity count threshold of Rγ-probe could be found to identify the nature of the lymph node. When using the same radioactivity count threshold in the Rγ-probe, we calculated a test sensitivity of 86% and 44.6% (139/312) of intraoperatively detected nodes that would not need to be removed.
Table 4.
Patients with metastatic sentinel lymph nodes (m-SLNs).
| Patient no. | No. of SLNs by SPECT/CT | m-SLN location | m-SLN radioactivity | Blue dye | ALND | |
|---|---|---|---|---|---|---|
| R SPECT (%) | R γ-probe (%) | |||||
| 1 | 1 | L1 | 100 | 100 | Negative | Yes |
| 2 | 2 | L1 | 100 | 100 | Negative | No |
| 3 | 2 | L1 | 100 | 100 | Negative | Yes |
| 4 | 2 | L1 | 100 | 100 | Positive | Yes |
| 4 | L1 | 50b | 56b | Negative | Yes | |
| 5 | 3 | L1 | 100 | 100 | Positive | Yes |
| 6 | 2 | L1 | 100 | 63b | Negative | No |
| 7 | 1 | L1 | 100 | 100 | Positive | Yes |
| 8 | 0 | L1 | Fail | 100 | Negative | Yes |
| 9 | 1 | L1 | 100 | 100 | Positive | Yes |
| 10 | 3 | L1 | 100 | 100 | Positive | Yes |
| 11 | 2 | L1 | 100 | 100 | Positive | No |
| 12 | 2 | L1 | 100 | 100 | Positive | Yes |
| 12 | L1 | Not | 83b | Positive | Yes | |
| 13 | 3 | L1 | 100 | 100 | Positive | Yes |
| 13 | L1 | 50b | 49b | Positive | Yes | |
| 14 | 3 | L1 | 97b | 100 | Positive | No |
| 15 | 4 | L1 | 51b | 72b | Positive | Yes |
| 15 | L1 | 48c | 41c | Positive | Yes | |
| 15 | L1 | Not | Not | Negative | Yes | |
| 16 | 0 | L1 | Fail | 100 | Negative | Yes |
| 17 | 1 | L1 | 100 | 100 | Positive | Yes |
| 18 | 2 | L1 | 100 | 100 | Positive | Yes |
| 18 | L1 | Not | 10f | Positive | Yes | |
| 19 | 1 | L1 | 100 | 14c | Negative | Yes |
| 20 | 2 | L1 | 100 | 100 | Positive | Yes |
| 21 | 3 | L1 | 100 | 100 | Positive | Yes |
| 22 | 1 | L1 | 100 | 25b | Negative | Yes |
| 23 | 3 | L1 | 100 | 100 | Positive | Yes |
R SPECT, Rγ-probe: the SLN was calculated as a percentage of the hottest node detected by SPECT/CT or γ-probe, and the hottest node detected by either modality was designated as 100%. ALND: axillary lymph node dissection. Fail: no lymph node detected on SPECT/CT. Not: palpable metastatic lymph nodes, which cannot be detected by SPECT/CT or γ-probe. b, c, f: second, third, fifth hottest node.
3.5. SPECT/CT-Guided Intraoperative SLN Localization
93 patients were prospectively selected for SLN localization using SPECT/CT images. Positions of SLNs localized by SPECT/CT and by γ-probe were consistent in 42% (39/93) of the patients, and all were located in the anterior axillary region. In the remaining patients, the localization markers were located inside and/or below the position detected by γ-probe, with a deviation of 1–4 cm in distance. Particularly, a typical overweight patient, with a body mass index of 37 kg/m2, failed SLNB by using a combination of a radiotracer and blue dye. With the help of anatomic images and position markers localized by SPECT/CT, surgeons successfully removed a SLN in level I of the axilla, which was 6.69 cm away from the skin (Figure S1).
4. Discussion
In this study, we preliminarily developed a radioactivity count threshold for identifying m-SLNs using 99mTc-SC SPECT/CT quantification, aiming to reduce invalid SLN removal for breast cancer. Patients with a history of prior excisional biopsy and younger than 50 y tended to have more SLNs detected either by a combination method or by SPECT/CT, implying that they would be the main beneficiaries of our findings. Additionally, it is feasible for advanced SPECT/CT to preoperatively locate SLNs.
It is well known that a combination of radioactive tracers and blue dyes increases the detection rate of SLNs, but it seems reasonable to believe that the removal of more nodes would lead to more adverse effects. Improving the accuracy of SLN detection is our objective. Numerous studies have focused on developing various novel radiotracers or hybrid approaches to improve SLN identification [16–19]; to the best of our knowledge, these methods have not been officially adopted in clinical settings because of their shortcomings. Less attention has been given to the evaluation of the relationship between radioactive uptake in metastatic and healthy SLNs.
Our study tried to investigate the value of quantitative SPECT/CT in distinguishing metastatic and healthy lymph nodes using 99mTc-SC. 99mTc-SC is the commonly used radiocolloid particle with a uniform size of 220 nm after filtration. It can be smoothly transported from the injection site to the SLNs but can cause excessive migration to secondary lymph nodes over time [20]. Based on this limitation, we hypothesized that metastases would more easily occur when fewer lymph nodes were visualized on SPECT/CT images owing to lymphatic duct obstruction by tumor cells, and distant lymph nodes with low tracer uptake affected by time extension would be less likely to metastasize.
As the number of SLNs shown on SPECT/CT images, we found that patients with ≤2 SLNs had significantly higher metastasis incidence than those with >2 SLNs. For radioactive uptake of SLNs, we developed a radioactivity count threshold that could guide selective removal of nodes by analyzing SPECT/CT quantification. When the radioactivity count threshold was set to 30% of the hottest nodes, no m-SLNs were missed, and 30% of the SPECT/CT-identified lymph nodes did not require resection. Our data preliminarily demonstrated that 99 m Tc-SC SPECT/CT has the potential to indicate AS and identify the nature of lymph nodes in breast cancer, assisting surgeons in judging whether lymph nodes could be monitored rather than excised in cases where the anatomic location of the node may be difficult for biopsy.
Clinical factors affecting the number of SLNs detected and metastasis incidence were also analyzed. Patients with younger age (≤50 y) and a history of prior excisional biopsy tended to have more SLNs detected, be exposed to unnecessary SLNB, and have more postoperative risks. These patients would be the target group of our findings. The m-SLNs more frequently occurred in male breast cancer patients than female patients (75% vs. 23.6%), which is consistent with previous studies [21]. Although male breast cancer is a relatively rare malignant cancer, accounting for less than 1.0% of all breast cancers, it has a worse prognosis than female breast cancer [22]. Therefore, the need for a thorough examination and pathological analysis of SLNs should be highlighted.
Preoperative SLN localization would reduce the scope of surgical resection, important for breast-conserving surgery. In a previous study, radioisotope (Co-57) was often appropriately positioned on the side of the body opposite the camera for reference, exposing patients to extra radiation [23]. We innovatively made a simple and safe tool for localizing SLNs. Regrettably, only 42% of the markers were completely consistent with the position determined by γ-probe. The remaining locations were all situated in a fixed range, with a deviation of 1–4 cm from the position using γ-probe. We believe that the posture difference between SPECT/CT imaging and surgery may be the main reason for this discrepancy. Due to the limitation of the small SPECT/CT aperture, patients lay supine with arms positioned above the head, whereas during SLNB, patients most often lay supine with arms extending perpendicular to the body. If possible, patients should maintain the same position during both SPECT/CT imaging and surgery. Our preliminary investigation of preoperatively marking of SLNs on skin according to SPECT/CT images is feasible and reliable, which would aid in probe-directed surgery and reduce operator-dependent variation and time, especially in obese patients.
Our study has several limitations. First, the detection rate of SPECT/CT alone was low (82%), compared with other studies (84.4%–92%) [24, 25]. There is a possibility that composition of the patients is different. Patients in previous research were strictly restrained to clinically node-negative. According to theNational Comprehensive Cancer Network (NCCN) guidelines version 4.2021 of breast cancer, SLNB indications are increasing. We enrolled a group of patients with ≤2 suspicious nodes on imaging or ≤2 positive nodes confirmed by needle biopsy, which is more suitable for the newest requirement clinically. Additionally, our preliminary findings need further validation in more patients from various hospitals and would be more meaningful when combined with recurrence of lymph nodes by follow-up, which we will take into account in the further study. Despite these limitations, we believe that our clinical procedures adhere to the NCCN guidelines and our findings are valuable for future research.
5. Conclusions
99mTc-SC SPECT/CT quantification of SLNs might be helpful to guide selective radioactive node resection during SLNB and minimize unnecessary lymph node removal by establishing radioactivity count thresholds, especially for patients with prior excisional biopsy and younger than 50 y.
Acknowledgments
This work was financially supported by the grants from the National Natural Science Foundation of China (82172866).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
None of the authors have any financial or personal relationships with other people or organizations that could inappropriately influence (bias) this work.
Supplementary Materials
Figure S1: negative PS and positive SPECT/CT identification of hot node in 55-y-old overweight patient (BMI, 37 kg/m2). A, B, Anterior and left lateral PS showed no obvious hot uptake in the left axilla except injection site. C, CT slice showed an enlarged lymph node in the left axilla. D, SPECT/CT hybrid image showed a faint uptake was in concordance with the lymph node and 6.69 cm away from skin. Small metal shadow (white arrow) on hybrid images is our localization tool. Table S1: comparison of metastasis incidence of axillary lymph nodes with different number of SLNs by SPECT/CT.
References
- 1.Sung H., Ferlay J., Siegel R. L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians . 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Verheuvel N. C., Voogd A. C., Tjan-Heijnen V. C. G., Siesling S., Roumen R. M. H. Different outcome in node-positive breast cancer patients found by axillary ultrasound or sentinel node procedure. Breast Cancer Research and Treatment . 2017;165(3):555–563. doi: 10.1007/s10549-017-4342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyman G. H., Giuliano A. E., Somerfield M. R., et al. American society of clinical oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. Journal of Clinical Oncology . 2005;23(30):7703–7720. doi: 10.1200/jco.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Lyman G. H., Somerfield M. R., Giuliano A. E. Sentinel lymph node biopsy for patients with early-stage breast cancer: 2016 American society of clinical oncology clinical practice guideline update summary. Journal of Oncology Practice . 2017;13(3):196–198. doi: 10.1200/jop.2016.019992. [DOI] [PubMed] [Google Scholar]
- 5.Manca G., Rubello D., Tardelli E., et al. Sentinel lymph node biopsy in breast cancer. Clinical Nuclear Medicine . 2016;41(2):126–133. doi: 10.1097/rlu.0000000000000985. [DOI] [PubMed] [Google Scholar]
- 6.Swenson K. K., Nissen M. J., Ceronsky C., Swenson L., Lee M. W., Tuttle T. M. Comparison of side effects between sentinel lymph node and axillary lymph node dissection for breast cancer. Annals of Surgical Oncology . 2002;9(8):745–753. doi: 10.1007/bf02574496. [DOI] [PubMed] [Google Scholar]
- 7.Burak W. E., Hollenbeck S. T., Zervos E. E., Hock K. L., Kemp L. C., Young D. C. Sentinel lymph node biopsy results in less postoperative morbidity compared with axillary lymph node dissection for breast cancer. The American Journal of Surgery . 2002;183(1):23–27. doi: 10.1016/s0002-9610(01)00848-0. [DOI] [PubMed] [Google Scholar]
- 8.Langer I., Guller U., Berclaz G., et al. Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery. Annals of Surgery . 2007;245(3):452–461. doi: 10.1097/01.sla.0000245472.47748.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boughey J. C., Moriarty J. P., Degnim A. C., Gregg M. S., Egginton J. S., Long K. H. Cost modeling of preoperative axillary ultrasound and fine-needle aspiration to guide surgery for invasive breast cancer. Annals of Surgical Oncology . 2010;17(4):953–958. doi: 10.1245/s10434-010-0919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansel R. E., Fallowfield L., Kissin M., et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC trial. Journal of the National Cancer Institute: Journal of the National Cancer Institute . 2006;98(9):599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 11.Johnson L., Pinder S. E., Douek M. Deposition of superparamagnetic iron-oxide nanoparticles in axillary sentinel lymph nodes following subcutaneous injection. Histopathology . 2013;62(3):481–486. doi: 10.1111/his.12019. [DOI] [PubMed] [Google Scholar]
- 12.Yuan L., Qi X., Zhang Y., et al. Comparison of sentinel lymph node detection performances using blue dye in conjunction with indocyanine green or radioisotope in breast cancer patients: a prospective single-center randomized study. Cancer Biology & Medicine . 2018;15(4):452–460. doi: 10.20892/j.issn.2095-3941.2018.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker J. L., Pu M., Tokin C. A., et al. Comparison of [(99mTc)] tilmanocept and filtered [(99mTc)] Sulfur colloid for identification of SLNs in breast cancer patients. Annals of Surgical Oncology . 2015;22(1):40–45. doi: 10.1245/s10434-014-3892-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuman S., Walker G., Avisar E. Processing sentinel nodes in breast cancer: when and how many? Archives of surgery . 2011;146(4):389–393. doi: 10.1001/archsurg.2011.29. [DOI] [PubMed] [Google Scholar]
- 15.Kwak J. J., Kesner A. L., Gleisner A., et al. Utility of quantitative SPECT/CT lymphoscintigraphy in guiding sentinel lymph node biopsy in head and neck melanoma. Annals of Surgical Oncology . 2020;27(5):1432–1438. doi: 10.1245/s10434-019-08078-0. [DOI] [PubMed] [Google Scholar]
- 16.Savolainen H., Volpe A., Phinikaridou A., Douek M., Fruhwirth G., De Rosales R. T. M. (68) Ga-Sienna+ for PET-MRI guided sentinel lymph node biopsy: synthesis and preclinical evaluation in a metastatic breast cancer model. Nanotheranostics . 2019;3(3):255–265. doi: 10.7150/ntno.34727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manca G., Garau L. M., Mazzarri S., et al. Novel experience in hybrid tracers. Clinical Nuclear Medicine . 2021;46(4):e181–e187. doi: 10.1097/rlu.0000000000003478. [DOI] [PubMed] [Google Scholar]
- 18.Bugby S. L., Lees J. E., Perkins A. C. Hybrid intraoperative imaging techniques in radioguided surgery: present clinical applications and future outlook. Clinical and translational imaging . 2017;5(4):323–341. doi: 10.1007/s40336-017-0235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vidal-Sicart S., Vera D. R., Olmos R. A. V. Next generation of radiotracers for sentinel lymph node biopsy: what is still necessary to establish new imaging paradigms? Revista Española de Medicina Nuclear e Imagen Molecular . 2018;37(6):373–379. doi: 10.1016/j.remnie.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Hung J. C., Wiseman G. A., Wahner H. W., Mullan B. P., Taggart T. R., Dunn W. L. Filtered technetium-99 m-sulfur colloid evaluated for lymphoscintigraphy. Journal of Nuclear Medicine : Official Publication, Society of Nuclear Medicine . 1995;36(10):1895–1901. [PubMed] [Google Scholar]
- 21.Flynn L. W., Park J., Patil S. M., Cody H. S., Port E. R. Sentinel lymph node biopsy is successful and accurate in male breast carcinoma. Journal of the American College of Surgeons . 2008;206(4):616–621. doi: 10.1016/j.jamcollsurg.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Wang X., Liu S., Xue Y. Clinicopathological features and prognosis of male breast cancer. Journal of International Medical Research . 2021;49(10) doi: 10.1177/03000605211049977.3000605211049977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gizewska A., Witkowska-Patena E., Osiecki S., Mazurek A., Stembrowicz-Nowakowska Z., Dziuk M. Utility of single-photon emission tomography/computed tomography for sentinel lymph node localization in breast cancer patients. Nuclear Medicine Communications . 2017;38(6):493–499. doi: 10.1097/MNM.0000000000000676. [DOI] [PubMed] [Google Scholar]
- 24.Coffey J. P., Hill J. C. Breast sentinel node imaging with low-dose SPECT/CT. Nuclear Medicine Communications . 2010;31(2):107–111. doi: 10.1097/mnm.0b013e32832ed3a6. [DOI] [PubMed] [Google Scholar]
- 25.Van der Ploeg I. M. C., Nieweg O. E., Kroon B. B. R., et al. The yield of SPECT/CT for anatomical lymphatic mapping in patients with breast cancer. European Journal of Nuclear Medicine and Molecular Imaging . 2009;36(6):903–909. doi: 10.1007/s00259-008-1050-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: negative PS and positive SPECT/CT identification of hot node in 55-y-old overweight patient (BMI, 37 kg/m2). A, B, Anterior and left lateral PS showed no obvious hot uptake in the left axilla except injection site. C, CT slice showed an enlarged lymph node in the left axilla. D, SPECT/CT hybrid image showed a faint uptake was in concordance with the lymph node and 6.69 cm away from skin. Small metal shadow (white arrow) on hybrid images is our localization tool. Table S1: comparison of metastasis incidence of axillary lymph nodes with different number of SLNs by SPECT/CT.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


