Abstract
In clinical scenario surveys, inpatient providers were more likely to report continuing inappropriate (odds ratio, 2.02 [95% confidence interval, 1.35–3.03]; P<.001) or broad-spectrum (1.8 [1.27–2.56]; P=.001) antibiotic therapy when initiated by emergency department providers, than to change to appropriate or narrow-spectrum therapy, respectively. Antibiotic inertia could represent a significant antibiotic stewardship target.
Keywords: antimicrobial stewardship, emergency department, behavioral patterns, cognitive bias, transitions of care
Observational data demonstrates improved survival with appropriate antibiotics given early in sepsis [1]. This has led to widespread adoption of aggressive, early, empiric broad-spectrum antibiotic administration as a mainstay of early management in patients with sepsis [1]. The impact that emergency department (ED) antibiotic selection has on inpatient providers is unknown, and patients started on broad-spectrum therapy may continue it as they move along the care continuum. This therapeutic inertia, as described in chronic condition management in outpatient settings, refers to the failure to advance or deintensify therapy as appropriate [2]. Within the realm of antimicrobial therapy, we postulate that providers may be reluctant to change antimicrobial therapy chosen by a previous team earlier in a patient’s hospital course, a similar concept we call antibiotic inertia [3, 4].
We explored the impact of ED antibiotic decision making on subsequent provider choice using clinical scenario surveys. Our primary outcome was to identify the rate of agreement between ED and inpatient providers on antibiotic selection and continuation after therapy was selected in the ED.
METHODS
This institutional review board–approved study adheres to methodological guidelines for survey studies [5]. We chose 4 infectious syndromes (pneumonia, skin and soft-tissue infection, urinary tract infection, and sepsis) owing to the existence of distinct diagnostic criteria, common ED presentation, and clear indications for broad- or narrow-spectrum empiric coverage in current guidelines. Each syndrome was described in 2 cases; one where broad-spectrum antibiotics would be appropriate per guidelines and another where narrow-spectrum would be appropriate. (see Supplementary Materials for survey and antibiotic list).
Providers were asked to select their empiric antibiotic(s) of choice for each scenario from a list of 26 commonly prescribed antibiotics. Broad-spectrum antibiotics included selection of coverage for methicillin-resistant Staphylococcus aureus or β-lactam antibiotics that cover Pseudomonas aeruginosa. These included vancomycin, daptomycin, linezolid, piperacillin-tazobactam, cefepime, and meropenem. Ertapenem was considered a narrow-spectrum antibiotic, given the lack of Pseudomonas coverage. Narrow-spectrum antibiotics were any other option for each scenario.
The survey was administered over 4 weeks with Qualtrics software May 2020 (Qualtrics, Provo, UT), Qualtrics website: https://www.qualtrics.com/blog/citing-qualtrics/. Providers were contacted by e-mail with an individualized link to the survey. We surveyed only licensed emergency medicine, pulmonary critical care, and internal medicine physicians and advanced practice providers at a quaternary medical center. These providers are the most likely to routinely care for patients with these infections at our institution.
Phase 1 of the survey was administered to ED providers. Based on simple majority for appropriate and inappropriate antibiotics for each scenario, the ED antibiotic choices were incorporated into phase 2. To examine the impact of both appropriate and inappropriate ED antibiotic selection on downstream decision making in phase 2, we created two phase 2 options. Each contained the same 8 clinical scenarios as phase 1 but alternated the ED-selected antibiotic to have 50% appropriate and 50% inappropriate (Supplementary Figure 1 and Supplementary Table 1). The standard for the appropriate antibiotic options were dictated by clinical practice guidelines for each disease state. The primary end point was the rate of continued inappropriate prescribing by inpatient providers. Secondary end points included the rate of appropriate or broad-spectrum prescribing compared between ED and inpatient providers and the rate of inappropriate or broad-spectrum antibiotic continuation based on provider specialty.
We conservatively estimated a 30% response rate from each provider group, and we anticipated that 42 responses in each group being compared would provide 80% power to see a difference in inappropriate prescribing between 18% and 28%. Group comparisons for individual survey items were performed using Pearson χ2 test. Group comparisons using multiple survey items at the same time were performed using logistic regression with generalized estimating equations, with inpatient provider as a cluster. All tests were 2 sided, and differences were considered statistically significant at P < .05.
RESULTS
Phase 1 was distributed to 62 ED providers, with a 32.7% response rate. Phase 2 option A was distributed to 67 internal medicine and 28 intensive care unit (ICU) providers, while option B was distributed to 68 internal medicine and 29 ICU providers with a 24% response rate. ED providers had a median of 9 years in practice (interquartile range, 4.8–13.5 years); phase 2A providers, a median of 5.1 years (3.4–18.6 years); and phase 2B providers, a median of 7.1 years (3.1–14.6 years). Full response rates for all survey phases are displayed in Supplementary Table 2.
Overall, ED providers selected broad-spectrum antibiotics when narrow-spectrum were appropriate in 40% of scenarios, and they selected narrow-spectrum antibiotics when broad-spectrum were appropriate in 35.7% of scenarios. In the narrow-spectrum sepsis scenario, the majority of ED providers selected broad-spectrum antibiotics. The provider responses to phase 1 are in Supplementary Table 3.
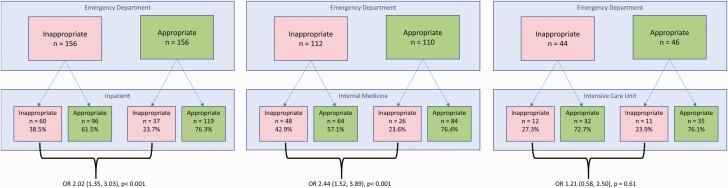
When the inappropriate category was presented, inpatient providers were more likely to continue inappropriate therapy (odds ratio [OR]. 2.02 [95% confidence interval (CI): 1.35–3.03]; P<.001) than if the appropriate category was presented (Figure 1). This difference was statistically significant for internal medicine providers (OR, 2.44 [95% CI: 1.52–3.89]; P<.001), but not for ICU providers (1.21 [.58–2.50]; P=.61). When assessing the impact of the ED selection of narrow-spectrum antibiotic therapy (whether or not it was the appropriate treatment), inpatient providers chose broad-spectrum therapy 43.8% of the time. If the ED selected broad-spectrum antibiotic therapy, the inpatient providers chose broad-spectrum therapy in 58.5% of cases (OR, 1.8 [95% CI: 1.27–2.56]; P=.001). Rates of appropriate antimicrobial selection by inpatient providers based on the scenario and options presented as the ED choice are displayed in Supplementary Table 4.
Figure 1.
Impact of inappropriate emergency department (ED) selection on empiric provider choice. The odds of inpatient providers selecting inappropriate therapy when selected by ED providers are presented for all inpatient providers (left), internal medicine providers only (middle), and intensive care unit (ICU) providers only (right). Odds ratios (ORs) are shown with 95% confidence intervals.
DISCUSSION
We demonstrated that when presented with the inappropriate antibiotic selection by ED providers, inpatient providers will empirically continue an inappropriate selection in 38.5% of cases, >2-fold higher odds than if presented with an appropriate selection. Furthermore, if broad-spectrum antibiotics were started in the ED, whether or not appropriate, inpatient providers continued them in 58.5% of cases, including 20.1% of cases where inpatient providers should have de-escalated. We propose that these findings are due in part to antibiotic inertia, a specific type of cognitive bias with origins in anchoring, diagnostic momentum, and prescribing etiquette. Antibiotic inertia is a hidden challenge to antimicrobial stewardship efforts that presents itself as patients transition between care teams and levels of care during hospital admission. With identification and understanding of this prescribing behavior, it may be possible to develop stewardship interventions targeting inpatient transitions of care.
Cognitive biases in medicine have been well described as a contributing factor to medical errors [6]. They have also been reported in errors in infectious disease diagnosis, though minimal data has assessed their impact on treatment selection [7]. The ED is particularly vulnerable to errors owing to time-pressured decision making, lack of complete information, frequent interruptions, and the need for accurate and timely diagnosis [8, 9]. Overdiagnosis and overtreatment can be harmful, both in placing patients at risk for unnecessary interventions and in attributable cost of unnecessary care [9]. In addition, in an interview-based study of antibiotic decision making on ward rounds, inpatient providers discussed a reluctance to alter the antibiotic plans started first by the ED [4]. Specific reasons for this reluctance were not clarified further [4].
The 2018 recommendation to administer broad-spectrum antibiotic therapy to patients within 1 hour of sepsis recognition led to concern for overuse of antibiotics, with as many as 32.7% of antibiotic prescriptions in the ED being inappropriate [10]. Antibiotic inertia could affect extended durations of these inappropriate initial therapies beyond the ED phase of care.
The impact of ED prescribing on inpatient selection is not well described. We sought to capture inpatient provider behavior as if providers were receiving each patient on transfer from the ED. It is known that 60%–70% of empiric broad-spectrum antibiotics in the ICU are continued even when there is no evidence of resistant organisms [11]. A more recent article published in 2021 found that 55% of antimicrobial use in hospitals was considered inappropriate, though considerations for the underlying driving pressure of prescribing were not part of the investigation [12].
Our data represent hypothesis generation for a significant antimicrobial stewardship target. Based on our estimation that 38.5% of inappropriate ED prescribing is continued, the impact of antibiotic inertia on prescribing trends could be significant and widespread. Further investigation into this phenomenon and potential solutions is needed, including validation of our findings with assessment of antibiotics prescribed in a real-world setting and a provider-facing intervention to interrupt this tendency.
Limitations of this study include the single-center design and low response rate; while we met our conservative calculation for statistical power, this represented only 30% of our inpatient providers. The practice setting is a quaternary academic medical center, and prescribing patterns observed may not reflect those seen in other settings. In addition, this study is unlikely to reflect the practice for patients with an unclear or mixed infection source.
In summary, we found that inpatient providers appeared to exhibit antibiotic inertia (ie, the tendency to continue antibiotics selected in the ED even when inappropriate). This is a preliminary study and further validation of this concept should be conducted based on actual prescribing patterns of similar patient populations.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The study team would like to acknowledge James Steckelberg (1954–2020), MD, the cofounder of the Mayo Clinic Antimicrobial Stewardship Program, for his role in this study. Discussions surrounding the nascent concept of “antibiotic inertia” with Dr Steckelberg in 2017 helped foster interest in this idea. We also thank the Mayo Clinic Survey Research Center for their assistance in planning, testing, and implementing the survey.
Disclaimer. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Center for Advancing Translational Sciences, a component of the National Institutes of Health (Clinical and Translational Science Awards grant UL1 TR00237), and by a research grant from the Mayo Clinic Pharmacy Services Discretionary Fund.
Potential conflicts of interest. F. B. reports receiving funding, unrelated to this work, from the National Institute on Aging, Agency for Healthcare Research and Quality, the Society of Academic Emergency Medicine, and Diagnostic Robotics. A. J. T. reports payment/honoraria for medical writing, unrelated to this (<$5000 from Uptodate.com) and reports serving on the Musculoskeletal Infection Society executive board (unpaid and unrelated). All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Kirstin Kooda, Department of Pharmacy, Mayo Clinic, Rochester, Minnesota, USA.
Fernanda Bellolio, Department of Emergency Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Ross Dierkhising, Division of Clinical Trials and Biostatistics, Mayo Clinic, Rochester, Minnesota, USA.
Aaron J Tande, Department of Infectious Diseases, Mayo Clinic, Rochester, Minnesota, USA.
References
- 1. Levy MM, Evans LE, Rhodes A.. The surviving sepsis campaign bundle: 2018 update. Crit Care Med 2018; 46:997–1000. [DOI] [PubMed] [Google Scholar]
- 2. Brunton S. Therapeutic inertia is a problem for all of us. Clin Diabetes 2019; 37:105–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA.. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med 2015; 175:356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Charani E, Ahmad R, Rawson TM, Castro-Sanchèz E, Tarrant C, Holmes AH.. The differences in antibiotic decision-making between acute surgical and acute medical teams: an ethnographic study of culture and team dynamics. Clin Infect Dis 2019; 69:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eysenbach G. Improving the quality of Web surveys: the Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J Med Internet Res 2004; 6:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Sullivan ED, Schofield SJ.. Cognitive bias in clinical medicine. J R Coll Physicians Edinb 2018; 48:225–32. [DOI] [PubMed] [Google Scholar]
- 7. Vick A, Estrada CA, Rodriguez JM.. Clinical reasoning for the infectious disease specialist: a primer to recognize cognitive biases. Clin Infect Dis 2013; 57:573–8. [DOI] [PubMed] [Google Scholar]
- 8. Singh H, Sittig DF.. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf 2015; 24:103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yealy DM, Mohr NM, Shapiro NI, Venkatesh A, Jones AE, Self WH.. Early care of adults with suspected sepsis in the emergency department and out-of-hospital environment: a consensus-based task force report. Ann Emerg Med 2021; 1–19. [DOI] [PubMed] [Google Scholar]
- 10. Denny KJ, Gartside JG, Alcorn K, Cross JW, Maloney S, Keijzers G.. Appropriateness of antibiotic prescribing in the emergency department. J Antimicrob Chemother 2019; 74:515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Braykov NP, Morgan DJ, Schweizer ML, et al. Assessment of empirical antibiotic therapy optimisation in six hospitals: an observational cohort study. Lancet Infect Dis 2014; 14:1220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Magill SS, Leary EO, Ray SM, Kainer MA, Evans C, Bamberg WM.. Assessment of the appropriateness of antimicrobial use in US hospitals. JAMA Netw Open 2021; 4:e212007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.