Abstract
Background
Organ transplantation from donors with human immunodeficiency virus (HIV) to recipients with HIV (HIV D+/R+) presents risks of donor-derived infections. Understanding clinical, immunologic, and virologic characteristics of HIV-positive donors is critical for safety.
Methods
We performed a prospective study of donors with HIV-positive and HIV false-positive (FP) test results within the HIV Organ Policy Equity (HOPE) Act in Action studies of HIV D+/R+ transplantation (ClinicalTrials.gov NCT02602262, NCT03500315, and NCT03734393). We compared clinical characteristics in HIV-positive versus FP donors. We measured CD4 T cells, HIV viral load (VL), drug resistance mutations (DRMs), coreceptor tropism, and serum antiretroviral therapy (ART) detection, using mass spectrometry in HIV-positive donors.
Results
Between March 2016 and March 2020, 92 donors (58 HIV positive, 34 FP), representing 98.9% of all US HOPE donors during this period, donated 177 organs (131 kidneys and 46 livers). Each year the number of donors increased. The prevalence of hepatitis B (16% vs 0%), syphilis (16% vs 0%), and cytomegalovirus (CMV; 91% vs 58%) was higher in HIV-positive versus FP donors; the prevalences of hepatitis C viremia were similar (2% vs 6%). Most HIV-positive donors (71%) had a known HIV diagnosis, of whom 90% were prescribed ART and 68% had a VL <400 copies/mL. The median CD4 T-cell count (interquartile range) was 194/µL (77–331/µL), and the median CD4 T-cell percentage was 27.0% (16.8%–36.1%). Major HIV DRMs were detected in 42%, including nonnucleoside reverse-transcriptase inhibitors (33%), integrase strand transfer inhibitors (4%), and multiclass (13%). Serum ART was detected in 46% and matched ART by history.
Conclusion
The use of HIV-positive donor organs is increasing. HIV DRMs are common, yet resistance that would compromise integrase strand transfer inhibitor–based regimens is rare, which is reassuring regarding safety.
Keywords: HIV, transplant, organ donation, drug resistance
Under the HOPE Act, 92 donors (58 human immunodeficiency virus [HIV] positive, 34 false-positive) donated 177 organs to recipients with HIV. Many donors (64%) were taking antiretrovirals, and although HIV drug resistance was common (42%), multiclass (12%) and integrase strand transfer inhibitor resistance (4%) was rare.
Transplantation of organs from donors with human immunodeficiency virus (HIV) to recipients with HIV (HIV D+/R+) was pioneered in South Africa (SA) in 2008 [1]. The 2013 HIV Organ Policy Equity (HOPE) Act permitted HIV D+/R+ in the United States, with initial focus on liver and kidney transplantation [2–4]. Theoretical risks associated with this practice include donor-derived opportunistic infection (OI) and superinfection with drug-resistant HIV [5, 6]. These complications have not been observed in SA, albeit in the context of a primarily antiretroviral therapy (ART)–naive donor population (87%), and recipients generally taking protease inhibitor (PI)–based recipient ART which may overcome HIV drug resistance mutations (DRMs) [7].
In the United States, the risk profile of HIV-positive donors may be higher given differing HIV acquisition behaviors associated with transmitted drug resistance (eg, men who have sex with men [MSM]) [8], more prevalent coinfections (eg, hepatitis C [HCV]) [9, 10], and higher rates of ART use and circulating DRMs (<10% in SA vs 20%–40% in the United States) [11–16]. Transient detection of donor HIV strains, including the presence of DRMs, has been described in HIV D+/R+ transplantation in high-income countries [17, 18].
Understanding the risk profile of the US HIV-positive donor population is critical for transplant providers who make time-constrained, point-of-care decisions to accept organs for HIV-positive candidates and may not have access to immunologic data or HIV genotypes for individual donors. The objective of this study was to describe clinical, virologic, and immunologic characteristics of donors under the HOPE Act, with focus on ART use and HIV DRMs, to characterize the safety profile of donors in HIV D+/R+ transplantation.
METHODS
Study Population
The study included deceased donors with reactive HIV tests or HIV history who had organs recovered for transplantation to HIV-positive recipients from 1 March 2016 to 15 March 2020 within the HOPE in Action studies (ClinicalTrials.gov: NCT02602262, NCT03500315, and NCT02602262). HIV screening assays were performed according to Organ Procurement and Transplantation Network (OPTN) protocols [19, 20], including HIV antibody (Ab), antibody/antigen (Ab/Ag), and nucleic acid testing (NAT).
Donors could not have active OI and the transplant team had to anticipate an “effective, safe, and tolerable” recipient ART regimen [21]. There were no restrictions on donor HIV viral load (VL) or CD4. Donors with discordant Ab/NAT results and no known history of HIV infection were suspected to have false-positive (FP) HIV results (“FP donors”) [22]. Confirmatory testing was performed by the Organ Procurement Organization (OPO) or research team, using Western blot or fourth-generation HIV Ag/Ab results (if Ab positive) and/or quantitative HIV VL (if NAT positive).
This study was approved centrally by the Johns Hopkins University Institutional Review Board (IRB0041681) as well as locally at each participating US transplant center. It was exempted from requirements for human subjects research by the Johns Hopkins Institutional Review Board, as it included only data and biospecimens from decedents. Authorization for donation, including collection of biospecimens for research purposes, was confirmed by OPOs in accordance with federal regulations.
National Enumeration of Donors with Reactive HIV testing
To determine the number of donors with reactive HIV testing who completed organ donation during the study period, we used the Scientific Registry of Transplant Recipients (SRTR), which includes data on all US donors, wait-listed candidates, and transplant recipients, submitted by members of OPTN. The Health Resources and Services Administration (US Department of Health and Human Services) provides oversight to OPTN/SRTR contractors. Within limited data sets released by OPTN/SRTR, each donor is assigned an anonymous identifier; the record includes infection serostatus. Donors with reactive HIV tests were identified by “positive” HIV Ab and/or HIV NAT results. SRTR identifiers were cross-referenced with the HOPE database to confirm a match. HIV history and confirmatory assays were not in SRTR. FP donors served as a proxy for contemporaneous HIV-negative US deceased donors; characteristics were contrasted with published OPTN data (kidney donors in 2018; >99.8% HIV negative) [23].
Donor Characteristics
OPOs collected demographic data, comorbid conditions, social history, serologic results, and medications. An additional HIV medical history form obtained the following, if available: HIV diagnosis date, acquisition risk(s), ART experience, OI history, and laboratory data (eg, CD4 T-cell count and percentage, VL, and genotype/phenotype). HIV provider notes were also obtained if available.
Laboratory Testing
All donors underwent serologic testing per OPTN policies [20] for hepatitis B (surface Ag and core Ab), hepatitis C (Ab and NAT), and HIV (Ab, Ab/Ag, and NAT). Donors were predominantly screened using anti-HIV I/II Abs (enzyme-linked immunosorbent or chemiluminescent assay) and multiplex qualitative HIV/HCV/hepatitis B virus (HBV) NAT (Supplementary Table 1). Additional serologic findings included CMV (immunoglobulin [Ig] G), Epstein-Barr virus (IgM/IgG/nuclear antigen), syphilis (rapid plasma reagin), and toxoplasma (IgG). These results were available to providers during donor evaluation.
For HOPE donors, 100 mL of blood was collected to measure CD4 T cells, HIV VL (Abbott RealTime HIV-1 assay; limit of detection, 40 copies/mL), sequencing for DRMs (GenoSure PRIme/Archive assays), and chemokine coreceptor (CC) tropism (Trofile RNA assay; Monogram Biosciences). These results were not entered into SRTR and were not available to providers in real time to inform clinical care.
Major DRMs were defined according to the International Antiviral Society–USA (IAS-USA) [24] and the Stanford University HIV Drug Resistance Database [25]. Multiclass resistance was defined as ≥1 major DRM per the IAS-USA definition, versus >1 drug class.
To detect serum ART, liquid chromatography–tandem mass spectrometry (QExactive; Thermo Fisher Scientific) was performed for 22 drugs [26]: abacavir, amprenavir, atazanavir, darunavir, dolutegravir, efavirenz, elvitegravir, emtricitabine, indinavir, lamivudine, lopinavir, maraviroc, nelfinavir, nevirapine, raltegravir, rilpivirine, ritonavir, saquinavir, stavudine, tenofovir, tipranavir, zidovudine (limit of detection, 10 ng/mL). Bictegravir, cobicistat, and doravirine were not assayed.
Statistical Analysis
Demographic and transplant factors were summarized and compared between HIV-positive and FP groups. Summary statistics were expressed as median (interquartile range [IQR]) for continuous variables and count (percentage) for categorial variables. Continuous variables were compared using Wilcoxon rank-sum testing and categorical variables compared using χ 2 or Fisher exact testing. Analyses were performed using Stata/MP2_v16.1 software (StataCorp). No hypothesis testing was performed between FP and aggregate OPTN donor data.
RESULTS
Counts and Regional Distribution of HOPE Donors
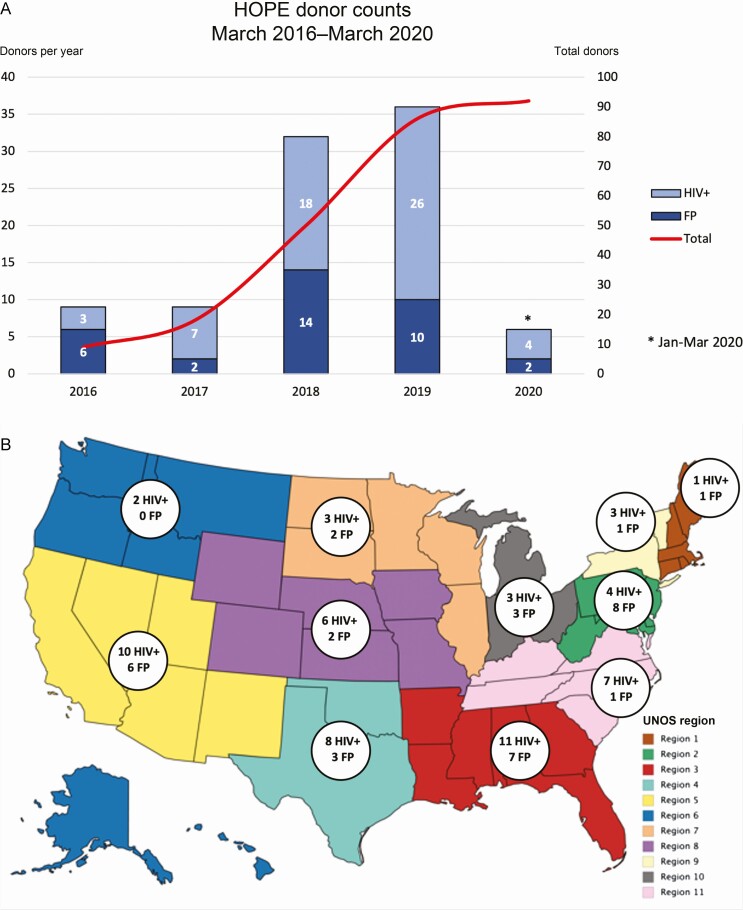
Ninety-two donors (58 HIV positive, 34 FP) donated 177 organs (131 kidneys, 46 livers) to HIV-positive recipients at 24 transplant centers. During the study period, OPTN/SRTR reported 92 donors with any reactive HIV test, of whom 91 were confirmed in our cohort. One FP donor in our cohort was identified by medical history from next of kin yet had a negative HIV testing result, whereas 1 donor in SRTR with reactive HIV testing was involved in a transplant outside the HOPE in Action Consortium. Thus, this series encompassed 98.9% of the US HIV-positive/FP donor total. The number of donors increased each year (Figure 1A). There was ≥1 HIV-positive donor in each of the 11 United Network for Organ Sharing regions, including 26 donors (18 HIV positive, 8 FP) in southeast regions 3 and 11 (Figure 1B).
Figure 1.
A, Number of HOPE organ donors (both human immunodeficiency virus positive [HIV+] and false-positive [FP]) who donated kidneys and/or livers during the study period, by calendar year. Donations increased significantly in 2018 and continued to increase through March 2020. B, National distribution of HOPE donors during the study period. At least 1 HOPE donation occurred in each of the 11 United Network for Organ Sharing (UNOS) regions. Abbreviation: HOPE, HIV Organ Policy Equity.
Donor Characteristics: HIV Positive Versus FP
Donor characteristics are shown in Table 1. Comparing HIV-positive and FP donors, the median age (IQR) was 36 (29–46) versus 31 (23–41) years, respectively (P = .01); 76% versus 65% were male (P = .25); and 37% versus 44% were white (P = .21). The median body mass index (IQR) (calculated as weight in kilograms divided by height in meters squared) was 25.2 (22.7–29) in HIV-positive and 28.4 (22.1–34.8) in FP donors (P = .11).
Table 1.
HOPE Donor Characteristics in Human Immunodeficiency Virus–Positive and False-Positive Donors
| Donors, No. (%)a | P Valueb | |||
|---|---|---|---|---|
| Donor Characteristic | Total (n = 92) | HIV Positive (n = 58) | HIV FP (n = 34) | |
| Age, median (IQR), y | 33.0 (28.0–44.0) | 36.0 (29.0–46.0) | 31.0 (23.0–41.0) | .01 |
| Male sex | 67 (72) | 44 (76) | 22 (65) | .25 |
| Race | ||||
| White | 37 (41) | 22 (38) | 15 (44) | .21 |
| Black | 37 (40) | 27 (47) | 10 (29) | |
| Other | 18 (19) | 9 (16) | 9 (26) | |
| BMI, median (IQR)c kg/m2 | 26.1 (22.7–30.5) | 25.2 (22.7–29.0) | 28.4 (22.1–34.8) | .11 |
| Diabetes | 9 (10) | 5 (9) | 4 (12) | .64 |
| Hypertension | 25 (27) | 16 (28) | 9 (26) | .87 |
| CAD | 3 (3) | 1 (2) | 2 (6) | .29 |
| Cause of death | ||||
| Anoxia | 38 (41) | 27 (47) | 11 (32) | .53 |
| Cerebrovascular | 24 (26) | 14 (24) | 9 (26) | |
| Head trauma | 28 (30) | 15 (26) | 13 (38) | |
| Other | 3 (3) | 2 (3) | 1 (3) | |
| Mechanism of death | ||||
| Drug intoxication | 19 (21) | 15 (26) | 4 (12) | .11 |
| Cardiovascular | 12 (13) | 9 (16) | 3 (9) | .36 |
| Suicide | 9 (10) | 7 (12) | 2 (6) | .33 |
| Results of serologic screening | ||||
| HCV Ab+ | 4 (4) | 1 (2) | 3 (9) | .11 |
| HCV NAT+ d | 3 (3) | 1 (2) | 2 (6) | .56 |
| HBV Ab+ | 9 (10) | 9 (16) | 0 (0) | .02 |
| CMV IgG+ | 72 (79) | 53 (91) | 19 (58) | <.001 |
| Toxoplasma IgG+ | 5 (6) | 3 (6) | 2 (7) | .84 |
| Syphilis (RPR) | 9 (10) | 9 (16) | 0 (0) | .02 |
| Organ donated | ||||
| Kidney (≥1) | 77 (84) | 46 (79) | 31 (91) | .14 |
| Liver | 46 (52) | 34 (59) | 12 (35) | .03 |
| Kidney and liver | 31 (34) | 22 (38) | 9 (26) | .17 |
| KDPI, median (IQR) % | 40 (28– 62) | 41 (30– 63) | 36 (21– 60) | .20 |
| DCD | 11 (12) | 4 (7) | 7 (21) | .05 |
| Steroid given | 60 (66) | 42 (72) | 18 (55) | .08 |
| PHS increased risk | 62 (67) | 47 (81) | 15 (44) | <.001 |
Abbreviations: Ab, antibody; BMI, body mass index; CAD, coronary artery disease; CMV, cytomegalovirus; DCD, donation after circulatory death; FP, false-positive; HBV, hepatitis B virus (hepatitis B core Ab); HCV, hepatitis C virus; HIV, human immunodeficiency virus; HOPE, HIV Organ Policy Equity; IgG, immunoglobulin G; IQR, interquartile range; KDPI, kidney donor profile index; NAT, nucleic acid testing; PHS, Public Health Service; RPR, rapid plasma reagin.
aData represent no. (%) of donors unless otherwise specified.
bComparisons are unadjusted.
cBMI calculated as weight in kilograms divided by height in meters squared.
dTwo other FP donors had FP results of HCV screens with multiplex HIV/HCV/HBV NAT, with negative confirmatory quantitative polymerase chain reaction results (omitted from table).
ug intoxication was the cause of death in 26% of HIV-positive and 12% of FP donors (P = .11). HCV NAT results were positive in 2% of HIV-positive and 6% of FP donors (P = .11). HBV core Ab results were positive in 16% of HIV-positive and 0% of FP donors (P = .02), CMV IgG results in 91% and 58%, respectively (P < .001), and syphilis (rapid plasma reagin) results in 16% and 0% (P = .02). The median kidney donor profile index, a marker of organ quality with lower numbers signifying better quality, was 41 (IQR, 30–63) in HIV-positive and 36 (21–60) in FP (P = .2). There were fewer donations after circulatory death in HIV-positive than in FP donors (7% vs 21%, respectively; P = .05) and more corticosteroid administration (72% vs 55%; P = .08).
FP donors were similar to the overall US donor population in demographic and laboratory features (eg, approximate median age of 35 years, 60% male, 10% with diabetes, 20% donation after circulatory death, 7% HCV Ab positive, and 50% CMV IgG positive). There was a higher proportion of black FP donors (29%) than in OPTN data (15%).
Donor HIV Testing
All HIV-positive donors had reactive anti-HIV Ab; 69% had reactive qualitative NAT (Table 2). FP donors predominantly had isolated reactive anti-HIV Ab (79%), and 15% had isolated reactive multiplex NAT. One FP donor had an FP Ab/Ag test. The FP donor identified by erroneous medical history had negative HIV Ab and NAT results. All FP donors, by definition, had negative confirmatory testing (Supplementary Table 1).
Table 2.
Donor Human Immunodeficiency Virus History, Screening, and Biology
| Donors, No. (%)a | ||
|---|---|---|
| HIV Factor | HIV Positive (n = 58) | HIV FP (n = 34) |
| Reactive HIV screening assayb | ||
| Anti-HIV I/II Ab | 58 (100) | 27 (79) |
| HIV qualitative NAT | 40 (69) | 5 (15) |
| Ab/Ag+ | … | 1 (3) |
| Confirmatory rule-out assayc | ||
| Western blot | … | 25 (74) |
| Ag/Ab (4th generation) | … | 7 (21) |
| Quantitative PCR | … | 4 (12) |
| Time of HIV diagnosis | ||
| Prior knowledge | 41 (71) | … |
| At admission | 14 (24) | … |
| Unknown | 3 (5) | … |
| HIV risk categoryd | ||
| MSM | 25 (43) | … |
| IDU | 13 (22) | … |
| Heterosexual sex | 16 (28) | … |
| Perinatal | 1 (2) | … |
| Other or unknown | 16 (28) | … |
| Reported ART use | ||
| Yes | 37 (64) | … |
| No | 15 (26) | … |
| Unknown | 6 (10) | … |
| CD4 T-cell findings at donatione | ||
| Count, median (IQR), cells/µL | 194 (77–331) | … |
| Proportion, median (IQR), % | 27.0 (16.8–36.1) | … |
| Count <200/µL | 27 (51) | … |
| Proportion <14% | 11 (22) | … |
| HIV VL at donationf | ||
| Median (IQR), copies/mL | 882 (<40 to 20 417) | … |
| Log VL, median (IQR) | 2.9 (1.0–4.3) | … |
| VL <400 copies/mL | 27 (47) | … |
| CC tropismg | ||
| R5 | 19 (68) | … |
| Dual R5-X4 | 9 (32) | … |
| X4 | 0 (0) | … |
Abbreviations: Ab, antibody; Ag, antigen; ART, antiretroviral therapy; CC, chemokine coreceptor; FP, false-positive; HIV, human immunodeficiency virus; HOPE, HIV Organ Policy Equity; IQR, interquartile range; IDU, injection drug use; MSM, men who have sex with men; NAT, nucleic acid testing; PCR, polymerase chain reaction; VL, viral load.
aData represent no. (%) of donors unless otherwise specified.
bNo FP donor had >1 positive screening assay result, and 1 screened positive by medical history only. All NAT-positive FP donors were tested with a multiplex qualitative assay (HIV/hepatitis B virus/hepatitis C virus). One FP donor had reactive HIV Ag (Ab negative).
cSee Supplementary Table 1 for additional information; multiple rule-out assays were used for individual donors.
dRisk categories are not mutually exclusive. “Heterosexual sex” includes sex work and intercourse with sex workers.
eDonation CD4 T-cell counts were available for 53 donors, and CD4 T-cell percentages for 51.
fVL <40 copies/mL was set at 10 copies/mL (1 log) for analysis.
gHIV tropism results in 38 donors with successful assays.
Donor HIV History, Risk Factors, and ART experience
Of HIV-positive donors, 71% had prior known HIV infection, and 24% had HIV discovered at admission (Table 2). HIV acquisition risk factors included MSM status (43%), injection drug use (22%), and heterosexual sex (28%). OI history was unknown in 67%. Three donors had prior OIs: herpes simplex virus infection (acyclovir-resistant genital ulcers) and cryptococcosis; pneumocystosis; and CMV disease and herpes simplex virus esophagitis.
Most HIV-positive donors (64%) were prescribed ART (Table 3), including 90% of those with known HIV diagnosis. ART history was unavailable for 6 donors (10%), 2 of whom had undetectable VLs. The most common regimens included 2 nucleoside reverse-transcriptase inhibitors (NRTIs) plus an integrase strand transfer inhibitor (INSTI) (65%). Overall, 28 (77%) of donor regimens included INSTIs, whereas 8 (22%) included protease inhibitors (PIs), and 3 (8%) included nonnucleoside reverse-transcriptase inhibitors (NNRTIs).
Table 3.
Reported Antiretroviral Therapy Regimens of HOPE Donors at Donation
| ART Regimen | Donors, No. (n = 37) |
|---|---|
| 2 NRTIs + INSTI | 24 (65%) |
| TAF/FTC/EVG/c | 9 |
| ABC/3TC/DTG | 7 |
| TAF/FTC/BIC | 5 |
| TAF/FTC + DTG | 2 |
| TDF/FTC/EVG/c | 1 |
| 2 NRTIs + NNRTI | 4 (11%) |
| TAF/FTC/RPV | 3 |
| TDF/FTC + ETR | 1 |
| 2 NRTIs + PI | 3 (8%) |
| TAF/FTC + ATV/c | 1 |
| TDF/FTC + ATV/c | 1 |
| TDF/FTC + DRV/c | 1 |
| Other | 6 (16%) |
| FTC + DTG | 1 |
| 3TC + DTG + DRV/r | 1 |
| TDF/FTC + DTG + ATV/r | 1 |
| ABC/3TC + TDF + DRV/c | 1 |
| d4t + TAF/FTC + DTG + DRV/c | 1 |
| TDF/FTC/EFV + DRV/r | 1 |
Abbreviations: 3TC, lamivudine; ABC, abacavir; ART, antiretroviral therapy; ATV, atazanavir; BIC, bictegravir; c, cobicistat; d4t, stavudine; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; ETR, etravirine; EVG, elvitegravir; FTC, emtricitabine; HOPE, HIV Organ Policy Equity; INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; r, ritonavir; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
HIV Viral Control
Quantitative HIV VL was performed on all 58 HIV-positive donor samples (Table 2). The median VL (IQR) was 882 (<40 to 20 417) copies/mL, and 47% of donors had a VL <400 copies/mL. Stratifying by ART treatment, the median VL was <40 copies/mL for those taking ART versus 20 417 copies/mL for those who were not. Among those taking ART, 27% had a VL >1000 copies/mL; compared with those with suppressed VL, clinical characteristics were similar (median age, 36 vs 43 years [P = .38]; male sex. 83% vs 72% [P = .45]; and black race, 42% vs 48% [P = .6]), apart from a trend toward more MSM (67% vs 36%; P = .08).
CD4 T-Cell Counts and Percentages
CD4 T-cell counts and percentages were measured in 53 HIV-positive donor samples. Overall, the median CD4 T-cell count (IQR) was 194/µL (77–331/µL); 51% of donors had an absolute CD4 T-cell count <200/µL (Table 2). The median CD4 T-cell percentage (IQR) was 27.0% (16.8%–35.4%), and 22% of donors had a CD4 T-cell percentage <14%. Historical CD4 T-cell percentage was strongly correlated with donation CD4 T-cell percentage (r = 0.72) (Supplementary Figure 1A), and historical absolute CD4 T-cell count was moderately correlated with donation count (r = 0.43) (Supplementary Figure 1B). Stratifying by treatment status, the median CD4 T-cell count was 262/µL for those taking ART and 118/µL for those who were not (P < .01); the median CD4 T-cell percentage was 29.9% for those taking ART and 17.2% (P = .02) for those not taking ART (P = .02).
There were 11 donors with a CD4 T-cell percentage <14%, all of whom were viremic with a median VL (IQR) of 83 770 (2238–380 736) copies/mL. This included 5 donors with newly diagnosed HIV infection and 3 donors prescribed ART (with VLs of 1905, 2111, and 51 827 copies/mL). Geography, demographics, and acquisition risks were indistinguishable between donors with low CD4 T-cell percentages and other donors (data not shown).
Corticosteroid Administration and Discordance Between Absolute CD4 T-Cell Count Percentage
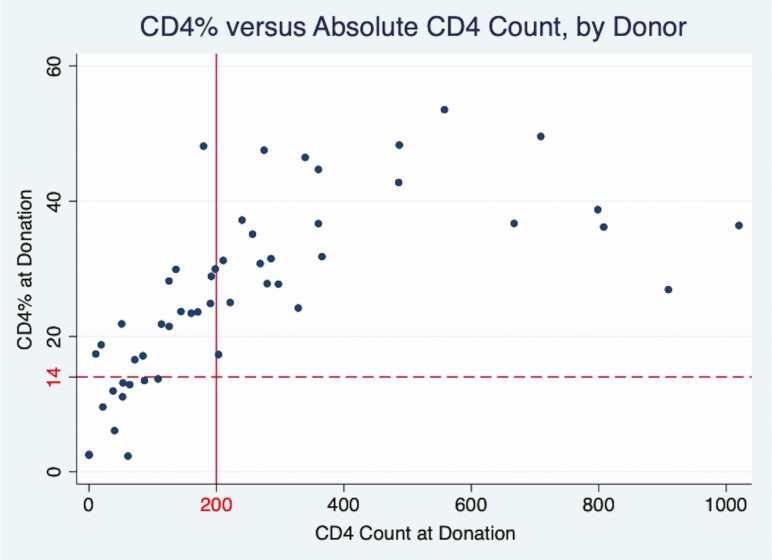
Notably, 59% of donors with CD4 T-cell count <200/µL had discordant CD4 T-cell percentage ≥14% (Figure 2, upper left quadrant); no donor with a CD4 T-cell percentage <14% had a CD4 T-cell count >200/µL (Figure 2, lower right quadrant). In-hospital corticosteroid administration was higher in donors with a CD4 T-cell count <200/µL versus ≥200/µL (81% vs 62%; P = .11).
Figure 2.
Donor CD4 T-cell percentage versus absolute CD4 T-cell count at donation for 51 human immunodeficiency virus–positive donors with both values available. Solid vertical line corresponds to a CD4 T-cell count of 200/µL; dashed horizontal line, to CD4 T-cell percentage of 14% (ie, AIDS-defining thresholds). Of these 51 donors, 27 (53%) had a donation CD4 T-cell count <200/µL, yet 16 (59%) of these 27 had a preserved CD4 T-cell percentage >14% (upper left quadrant). In contrast, no donors with a CD4 T-cell proportion <14% had a CD4 T-cell count >200/µL (bottom right quadrant).
ART Detection by Mass Spectrometry
Mass spectrometry was performed on 54 of 58 HIV-positive donor samples (93%), with detection of ≥1 ART drug in 25 samples (46%) (Table 4). All samples with detectable ART were from donors documented as having ART prescribed (25 of 34 [74%]). Of the 9 donors prescribed ART in whom no ART was detected, 4 had HIV VLs >10 000 copies/mL, and the remainder had a median length of stay of 10 days before donation (IQR, 6–11 days). ART was not detected in any of the 7 donors reported as either not prescribed ART or with unknown ART history. The percentage agreement between historical and laboratory ART detection was 83% (95% confidence interval, 49%–86%; κ = 0.67).
Table 4.
Antiretroviral Therapy Drugs Detected in Serum of HOPE Donors by Mass Spectrometry
| No. With Drug Detected in Serum/Total No. | ||
|---|---|---|
| Individual ART Drug | All Donorsa | Donors With HIV VL <400 Copies/mLb (n = 23) |
| NRTIs | ||
| 3TC | 7/8 | 6/6 |
| ABC | 2/6 | 1/5 |
| d4T | 0/1 | 0/1 |
| FTC | 15/26 | 11/17 |
| TAF | 1/20 | 1/14 |
| TDF | 3/6 | 2/3 |
| NNRTIsc | ||
| RPV | 0/2 | 0/2 |
| PIsc | ||
| ATV | 3/3 | 2/2 |
| DRV | 5/5 | 4/4 |
| RTV | 3/4 | 2/2 |
| INSTIsc | ||
| EVG | 6/10 | 4/5 |
| DTG | 9/11 | 8/9 |
Abbreviations: 3TC, lamivudine; ABC, abacavir; ART, antiretroviral therapy; ATV, atazanavir; d4t, stavudine; DRV, darunavir; DTG, dolutegravir; EVG, elvitegravir; FTC, emtricitabine; HIV, human immunodeficiency virus; HOPE, HIV Organ Policy Equity; INSTIs, integrase strand transfer inhibitors; NNRTIs, nonnucleoside reverse-transcriptase inhibitors; NRTIs, nucleoside reverse-transcriptase inhibitors; PIs, protease inhibitors; RPV, rilpivirine; RTV, ritonavir; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; VL, viral load.
aMass spectrometric data were unavailable for 3 of 37 donors with reported ART use, whose drug regimens are excluded from this table: ABC/3TC/DTG, TAF/FTC/RPV, and TAF/FTC/bictegravir (1 donor each)
bHIV VLs were available for 33 of 34 donors with mass spectrometric data and known ART regimens, including 23 with VLs <400 copies/mL.
cBictegravir, etravirine, and cobicistat were not assayed by mass spectrometry.
HIV DRMs
HIV genotyping was successfully performed in 47 donation samples (81%); 11 assays (19%) failed, 9 among donors with HIV VLs <40 copies/mL. In addition to genotyping of blood at organ donation (“laboratory genotypes”), we collected prior genotype reports from the medical record, available for 14 donors (24%), including 1 for whom the laboratory genotyping failed.
Of donors with any genotype data, 20 of 48 (42%) had ≥1 major DRM (Table 5); 8 had ≥1 historical DRM and 15 had ≥1 laboratory DRM detected. There were no significant differences between donors with and those without DRMs in demographics, HIV acquisition risk factors, HIV VL, CD4 T-cell count or percentage, or ART exposure (data not shown). NNRTI resistance was common (33%), most frequently substitutions at the K103 position of the reverse-transcriptase gene. Three donors had historical NNRTI DRMs, identical to those detected with laboratory genotyping.
Table 5.
Major Drug Resistance Mutations and Multiclass Resistance Profiles in Human Immunodeficiency Virus–Positive HOPE Donors
| Donors | Major DRMs | |||
|---|---|---|---|---|
| NRTIs | NNRTIs | PIs | INSTIs | |
| All detected DRMs (n = 20 [42%])a | (n = 9 [19%]) | (n = 16 [33%]) | (n = 1 [2%]) | (n = 2 [4%]) |
| M184V/I (n = 5), D67N/G/E/H/S/T (n = 2),M41L,A62V, K65R/N/E,L74V/I, T215Y/F/C/D | K103N/S/H/T/R/Q/E (n = 8), V179D/E/F/I/L/T (n = 6), V108I (n = 2), L100I/V,K101E/H/P/Q/R/N, V106A/M/I, Y181C/I/V/S/G | L90M | T66A/I/K, E92Q/G/V, Y143Y/C | |
| Donors with multiclass DRMs (n = 6 [13%]) | ||||
| Donor 1 (ART: ABC/3TC/DTG; VL: <40 copies/mL) | A62V, K65N | L100I, V108I, V179I | … | … |
| Donor 2 (ART: TAF/FTC/EVG/c; VL: 51 827 copies/mL) | M184I | K103N | … | … |
| Donor 3 (ART: TAF/FTC + DRV/c; VL: <40 copies/mL) | M184V | V179I | L90M | … |
| Donor 4 (ART: TDF/FTC + DTG + ATV/r; VL: <40 copies/mL) | M41L, M184V, T215C/Y | K103N | … | Y143C |
| Donor 5 (ART: TDF/FTC/EFV + DRV/r; VL: 1905 copies/mL) | M184V/I | … | … | T66I,E92Q |
| Donor 6 (ART: TAF/FTC/BIC; VL: <40 copies/mL) | D67N | K103N | … | … |
Abbreviations: 3TC, lamivudine; ABC, abacavir; ART, antiretroviral therapy; ATV, atazanavir; BIC, bictegravir; c, cobicistat; DRMs, drug resistance mutations; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; EVG, elvitegravir; FTC, emtricitabine; HOPE, HIV Organ Policy Equity; INSTIs, integrase strand transfer inhibitors; NNRTIs, nonnucleoside reverse-transcriptase inhibitors; NRTIs, nucleoside reverse-transcriptase inhibitors; PIs, protease inhibitors; r, ritonavir; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; VL, viral load (for human immunodeficiency virus).
aA total of 48 human immunodeficiency virus–positive donors had available genotype data, with 47 interpretable laboratory genotypes and 14 historical genotypes, which were combined. Mutations were reported on only 1 donor genotype unless otherwise specified in parentheses.
NRTI mutations were detected in 19%, particularly M184V/I (10%). One donor had historical NRTI DRMs, confirmed with laboratory genotyping. Thymidine analogue mutations were detected in 3 donors (6%). One donor had multi-NRTI resistance (A62V + K65N).
INSTI mutations were seen in 2 donors (4%) on historical genotypes only (T66I + E92Q; Y143C). Multiclass DRMs were detected in 6 donors (13%), all with NRTI resistance plus a second class, commonly NNRTIs. These donors were all reported to have ART prescribed, typically with an NRTI + INSTI or NRTI + PI backbone, and 4 (66%) were virologically suppressed. One donor with perinatal HIV acquisition demonstrated DRMs versus 3 drug classes (including INSTIs), with an HIV VL <40 copies/mL at donation, on an NRTI + INSTI + PI regimen.
Viral Coreceptor Tropism
CC tropism testing was performed in 50 donors; the assay failed in 22, including 16 donors with VLs <40 copies/mL. Of 28 with reportable results, 19 (68%) showed R5 and 9 (32%) mixed R5-X4 tropism. Among those with mixed tropism, the median VL (IQR) was 83 770 (15 488–410 407) copies/mL; the median CD4 T-cell count, 80/µL (42–176/µL), and the median CD4 T-cell percentage, 12.9% (4.6%–20.9%). No donor was reported to be taking maraviroc.
DISCUSSION
In 4 years of the HOPE in Action studies, there were 92 deceased donors with reactive HIV screens who donated 177 organs to recipients with HIV. Among donors, 37% had FP results, and 24% had new HIV diagnoses. Of those with known HIV infection, most (90%) were prescribed ART. Major HIV DRMs were frequent, yet INSTI and multiclass DRMs were rare. Overall, this should reassure providers who aim to minimize the risk of HIV breakthrough in potential recipients due to donor-derived INSTI or multiclass DRMs.
In contrast to SA HIV D+/R+ data—where 8% donors were ART experienced and ≤10% had circulating DRMs [6, 11]—most (64%) US donors were ART experienced, and 42% had ≥1 DRM. NNRTI DRMs were common (33%), including mutations affecting second-generation drugs, such as rilpivirine. This concords with transmitted NNRTI drug resistance patterns in the United States, >10% in some populations [16], as well as rising community prevalence of DRMs against this class (eg, 23% in black MSM) [12, 13, 27]. Thus, relying on NNRTIs as the empirical primary backbone in US HIV D+/R+ transplantation appears to be unfavorable. Doravirine, however, may maintain activity against most detected NNRTI DRMs. Otherwise, multi-NRTI DRMs and TAMs were seen in only 8%, largely maintaining this class.
As in the greater US HIV population [28, 29], INSTI DRMs were uncommon in HOPE donors. Moreover, none were predicted to affect later-generation INSTIs, such as dolutegravir or bictegravir. Multiclass DRMs were detected in only 6 donors (13%), 2 of whom were virologically suppressed on NRTI + INSTI regimens, and all of whom, based on available genotypes, would likely have achieved viral suppression with such regimens. One donor with perinatal HIV acquisition and extensive ART exposure demonstrated DRMs against 3 drug classes (including INSTIs) and had viral suppression on an NRTI + INSTI + PI regimen. This rare case highlights the need for thorough HIV history ascertainment and indicates that broadly active ART (eg, INSTI + PI) may be required in select circumstances. Finally, the frequency of R5-X4 tropic virus (32%) among HIV-positive donors may limit CCR5 inhibitors as posttransplantation ART in HIV D+/R+ transplantation.
Other HIV-positive donor features included frequent HBV, CMV, and syphilis seropositivity, approaching rates seen in North American people living with HIV [30, 31], and higher than in FP donors or the general donor population [19, 23]. In addition, discordance in CD4 T-cell count or percentage was observed in 31% of donors, potentially related to corticosteroid administration (73%), previously associated with CD4 lymphopenia in HIV-uninfected donors without affecting the CD4 T-cell percentage [32].
Detailed HIV history was an important adjunct to OPTN questionnaires and serologic findings. A considerable proportion of donors had historical ART (64%) and genotype (24%) data, key to informing posttransplantation ART selection. Results of mass spectrometry for ART exposure were largely concordant with medical records, showing 83% agreement, particularly in the setting of VL suppression. Of the 9 donors prescribed ART who had no ART detected in serum, 4 had VLs >10 000 copies/mL, consistent with nonadherence. The remainder had a prolonged length of stay, which, if withholding enteral ART during critical illness, may have led to washout of drugs with short elimination half-lives, such as tenofovir alafenamide [33] (rarely detected, even in donors with VL suppression). Overall, medication ascertainment by OPO staff and transplant providers was a very good point-of-care metric to determine ART exposure and emphasizes the critical role of infectious diseases providers in risk stratifying donors in HIV D+/R+ transplantation [34].
The current study had several limitations. Donors were carefully selected, given the novelty of HIV D+/R+ transplantation, and may not reflect the greater potential HIV-positive US donor population. There were only 92 donors over 4 years, far lower than projections (350–600 yearly) [35, 36], possibly reflecting measured adoption of a new practice by OPOs and transplant centers, as well as stigma surrounding donor HIV disclosure and lagging registration for organ donation, despite high willingness [37]. Regardless, annual donations quadrupled over time and occurred across all United Network for Organ Sharing regions, with concentration in the southern United States, overlapping the current HIV epicenter [38] and consistent with estimated HIV-positive deceased donor distribution [39].
Owing to missing historical genotype information, technical assay failure, and imperfect sensitivity of laboratory genotypes, we may have underestimated DRM prevalence in HIV-positive donors. In addition, we were unable to correlate donor DRMs with HIV breakthrough in HOPE recipients as the blinded studies are ongoing. Reassuringly, early studies of the SA [7] and US HIV D+/R+ cohorts [40] have not revealed conclusive donor HIV superinfection, and the HIV D+/R+ pilot study noted only 1 case of HIV viremia, attributed to ART interruption rather than resistant donor virus.
This report highlights the promise of organ donation from deceased donors with HIV. As HIV D+/R+ transplantation expands, further characterization of HIV donors will focus on risk stratification to identify donors with problematic drug resistance (INSTI, multiclass DRMs) and permit posttransplantation ART optimization for recipients.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author Contributions. Conception and design: D. L. S., C. M. D., and A. A. R. T. Obtaining of funding: A. D. R., D. L. S., C. M. D., and A. A. R. T. Collection and assembly of data: D. M. B., O. T. K., B. L. D., S. M. S., Y. E., and R. E. F. Provision and clinical care of study participants: N. M. D., S. S. F., S. H., M. M. R., R. J. F. M., A. K. M., P. G. S., J. C. P., V. S., S. G. M., A. J. G., N. E., M. I. M., S. A. M., C. B. S., G. H., M. M., J. S. H., M. R. P., G. G., J. H., V. A. K., A. A., S. A., E. A. B., C. R. W., N. N., D. L. S., and C. M. D. Conduct and oversight of laboratory operations: A. D. R., Y. E., R. E. F., J. M., G. A. B., C. S. K., H. A. S., W. A. C., M. Seisa, C. J. P., T. C. Q., and A. A. R. T. Administrative, technical, or logistic support: D. M. B., O. T. K., B. L. D., S. M. S., Y. E., R. E. F., K. M., R. P. W., N. N., S. S., M. Shuck, H. W., M. W., and J. O. Interpretation of data: W. A. W., J. D. M., C. M. D., and A. A. R. T. Statistical analysis: W. A. W. and J. D. M. Drafting of manuscript: W. A. W., C. M. D., and A. A. R. T. Critical revision of manuscript for important intellectual content: W. A. W., D. M. B., A. D. R., T. C. Q., S. S. F., P. G. S., S. G. M., M. I. M., G. H., M. M., J. S. H., M. R. P., S. A., E. A. B., C. R. W., C. M. D., and A. A. R. T. Final approval of the article: All authors.
Acknowledgments. The authors appreciate the help and support of all the organ donors and their families. The organ transplants and this research would not be possible without their gift.
The authors also appreciate the assistance and support of the Association of Organ Procurement Organizations and their local members with the HOPE in Action studies, listed here by institution. Columbia University Medical Center: Dominque Piquant. Duke University: Katherine Link, RN, and Marion Hemmersbach-Miller, MD, PhD. Emory University: Thomas Pearson MD, Nicole Turgeon MD, G. Marshall Lyon, MD, MMSc, William Kitchens MD, PhD, Jeryl Huckaby, MSCRA, CCRC, A. Francie Lasseter, RN, Rivka Elbein, RN, BSN, April Roberson, RN, and Elizabeth Ferry, RN. Johns Hopkins University School of Medicine: Ethan Klock, BS, Willa V. Cochran, CRNP, Michelle Morrison, BSN, Sarah Rasmussen, BA, Juli Bollinger, MS, Jeremy Sugarman, MD, the Transplant/Oncology Infectious Diseases Clinical Research Unit, the Division of Transplant Surgery Research Group, and the Epidemiology Research Group in Organ Transplantation. Ochsner School of Medicine: Angela R. Smith, MBA. Massachusetts General Hospital: Margaret Thomas, BS. MedStar Georgetown Transplant Institute: Margaret Coakley, RN, Joseph Timpone, MD, and Alyssa Stucke, BS. Mount Sinai School of Medicine: Brandy Haydel. New York University: Rebecca Dieter, PharmD, Elizabeth J. Klein, BA, and Henry Neumann, MD. Northwestern University Feinberg School of Medicine: Lorenzo Gallon, MD, Leah Goudy, RN, and Michelle Callegari. University of Maryland: Ilise Marrazzo, RN, BSN, MPH, and Towanda Jackson. University of Minnesota: Timothy Pruett, MD, and Mary Farnsworth, CCRC. University of Alabama at Birmingham: Jayme E. Locke, MD, MPH, FACS, FAST, Darnell Mompoint-Williams, CRNP, DNP, and Katherine Basinger, RN, CCRP. University of California San Diego: Kristin Mekeel, MD, and Phirum Nguyen, BS. University of California, San Francisco: Joanne Kwan, Tab Srisengfa, Peter Chin-Hong, MD, and Rodney Rogers. University of Miami Miller School of Medicine: Jacques Simkins, MD, and Carlos Munoz, CRC. University of Pennsylvania: Ty Dunn, MD, and Dierdre Sawinski, MD. University of Pittsburgh: Fernanda Silveira, MD, Kailey Hughes, MPH, and Diana Lynn Pakstis, RN, BSN, MBA. University of Virginia: Jamie Nagy, BA.
Virginia Commonwealth University: Mary Baldecchi. Weill Cornell Medicine: Thangamani Muthukumar, MD, and Melissa D. Eddie, MS, RN. Katharine Robb, RN, Elizabeth Salsgiver, MPH, and Britta Witting, BS. Yale School of Medicine: Marwan M. Azar, Merceditas Villanueva, Richard Formica, and Ricarda Tomlin, BS, CCRP.
Disclaimer. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the Scientific Registry of Transplant Recipients or the US government.
Financial support. This work was supported by the National Institutes of Health (NIH; grants R01 AI20938 to A. A. R. T. and U01 AI138897 and U01 AI134591 to D. L. S. and C. M. D.), the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (A. D. R. and T. C. Q.), the National Institute of Diabetes and Digestive and Kidney Diseases (grant K24 AI144952 to D. L. S.), the Transplantation and Immunology Research Network of the American Society of Transplantation (grant gSAN-201C0WW to W. A. W.), the Johns Hopkins School of Medicine (Discovery Fund for Synergy Award to A. D. R., D. L. S., C. M. D., and A. A. R. T.), the Johns Hopkins University Center for AIDS Research (grant P30 AI094189), and The Living Legacy Foundation.
Data availability. Proposals to access deidentified data from the HOPE in Action studies can be submitted to the Steering Committee (contact: C. M. D.; christinedurand@jhmi.edu), with transfer approved on an individual basis via a formal data use agreement. The HOPE in Action recipient study protocols are available at Clinicaltrials.gov (NCT02602262, NCT03500315, and NCT03734393). The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the Scientific Registry of Transplant Recipients (SRTR). SRTR limited data sets are publicly available and may be requested directly from the SRTR (srtr@srtr.org) under data use agreement.
Potential conflicts of interest. Note that everything listed below is outside the submitted work. B. L. D. reports receiving a Mid-America Transplant Foundation grant (grant 03–2020), consulting fees from Arkansas Regional Organ Recovery Agency and Life Connection of Ohio, and travel support from Southwest Transplant Alliance and the Mid-America Transplant Foundation. S. S. F. served as an unpaid consultant to CareDx and Miromatrix and participated on a data and safety monitoring board (DSMB)/advisory board for Miromatrix. J. C. P. received grant support from Gilead Sciences and Merck, provided consulting to Theratechnologies, and is the spouse of a stock/shareholder in Johnson & Johnson, AbbVie, Merck, and Bristol Myers Squibb (BMS). S. G. M. has received an honorarium for serving on an advisory board for CareDx. M. I. M. reports grant support (including to their institution) from Merck and has served on advisory boards for Viracor Eurofins and Natera. C. B. S. reports grant support to their institution from GlaxoSmithKline, ViiV, Abbott, Merck, Gilead, Chimerix, Shire/Takeda, Schering-Plough, Ablynx, Janssen, Ansun Biopharma, and Karyopharm Therapeutics. G. H. reports research support from Karius and research and salary support from the NIH (grant KL2TR001856). M. M. reports leadership/fiduciary roles with Takeda/Shire. J. S. H. has received grant funding from Merck. M. R. P. has served on an advisory board for Takeda/Shire. S. A. reports research funding from the Cystic Fibrosis Foundation; a Clinical and Translational Science award to their institution from the National Center for Advancing Translational Sciences, NIH; and research and salary support from the NIH (KL2 award to the UC San Diego Altman Clinical and Translational Research Institute); S. A. also reports consulting for BioMx, Merck, Gilead, and Johnson & Johnson. E. A. B. reports research support to their institution from Takeda, Hologic, and Merck; served on an unpaid advisory board for Merck; and served on a DSMB for Amplyx. C. R. W. reports serving on an advisory board for Enzychem Lifesciences and on DSMBs for Biogen, Merck, Atea, and Janssen. D. L. S. reports consulting for CSL Behring, Mallinckrodt, Novartis (honorarium), Sanofi (honorarium), Thermo Fisher Scientific, and Veloxis. C. M. D. reports research support to their institution from AbbVie and GlaxoSmithKline, and payment/honoraria from Gilead (HIV grant review committee). All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
William A Werbel, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Diane M Brown, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Oyinkansola T Kusemiju, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Brianna L Doby, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Shanti M Seaman, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Andrew D Redd, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Yolanda Eby, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Reinaldo E Fernandez, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Niraj M Desai, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Jernelle Miller, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Gilad A Bismut, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Charles S Kirby, Department of Biochemistry, Cellular, and Molecular Biology, Johns Hopkins University, Baltimore, Maryland, USA.
Haley A Schmidt, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
William A Clarke, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Michael Seisa, Laboratory Corporation of America (LabCorp), South San Francisco, California, USA.
Christos J Petropoulos, Laboratory Corporation of America (LabCorp), South San Francisco, California, USA.
Thomas C Quinn, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Sander S Florman, Recanati/Miller Transplantation Institute, The Mount Sinai Hospital, New York City, New York, USA.
Shirish Huprikar, Department of Medicine, Division of Infectious Diseases, The Mount Sinai Hospital, New York City, New York, USA.
Meenakshi M Rana, Department of Medicine, Division of Infectious Diseases, The Mount Sinai Hospital, New York City, New York, USA.
Rachel J Friedman-Moraco, Department of Medicine, Division of Infectious Diseases, Emory University, Atlanta, Georgia, USA.
Aneesh K Mehta, Department of Medicine, Division of Infectious Diseases, Emory University, Atlanta, Georgia, USA.
Peter G Stock, Department of Surgery, University of California San Francisco, San Francisco, California, USA.
Jennifer C Price, Department of Medicine, University of California San Francisco, San Francisco, California, USA.
Valentina Stosor, Division of Infectious Disease and Organ Transplantation, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA.
Shikha G Mehta, Section of Transplant Nephrology, The University of Alabama at Birmingham, Birmingham, Alabama, USA.
Alexander J Gilbert, MedStar Georgetown Transplant Institute, Georgetown University School of Medicine, Washington, DC, USA.
Nahel Elias, Department of Surgery, Division of Transplant Surgery, Massachusetts General Hospital, Boston, Massachusetts, USA.
Michele I Morris, Department of Medicine, Division of Infectious Diseases, University of Miami, Miami, Florida, USA.
Sapna A Mehta, New York University Langone Transplant Institute, New York University Grossman School of Medicine, New York, New York, USA.
Catherine B Small, Department of Medicine, Division of Infectious Diseases, Weill Cornell Medical College, New York, New York, USA.
Ghady Haidar, Department of Medicine, Division of Infectious Diseases, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Maricar Malinis, Department of Medicine, Section of Infectious Diseases, Yale University School of Medicine, New Haven, Connecticut, USA.
Jennifer S Husson, Institute of Human Virology, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Marcus R Pereira, Department of Medicine, Division of Infectious Diseases, Columbia University Medical Center, New York, New York, USA.
Gaurav Gupta, Department of Medicine, Division of Nephrology, Virginia Commonwealth University, Richmond, Virginia, USA.
Jonathan Hand, Department of Infectious Diseases, Ochsner Clinic Foundation, New Orleans, Louisiana, USA.
Varvara A Kirchner, Department of Surgery, University of Minnesota, Minneapolis, Minnesota, USA.
Avinash Agarwal, Department of Surgery, Division of Transplantation, University of Virginia, Charlottesville, Virginia, USA.
Saima Aslam, Department of Medicine, Division of Infectious Diseases and Global Public Health, University of California San Diego, San Diego, California, USA.
Emily A Blumberg, Department of Medicine, Division of Infectious Diseases, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Cameron R Wolfe, Department of Medicine, Division of Infectious Diseases, Duke University School of Medicine, Durham, North Carolina, USA.
Kevin Myer, LifeGift, Houston, Texas, USA.
R Patrick Wood, Department of Surgery, Division of Transplantation, University of Wisconsin, Madison, Wisconsin, USA.
Nikole Neidlinger, Department of Surgery, Division of Transplantation, University of Wisconsin, Madison, Wisconsin, USA; UW Health Organ Procurement Organization, Madison, Wisconsin, USA.
Sara Strell, UW Health Organ Procurement Organization, Madison, Wisconsin, USA.
Marion Shuck, Gift of Hope, Chicago, Illinois, USA.
Harry Wilkins, Gift of Hope, Chicago, Illinois, USA.
Matthew Wadsworth, Life Connection, Dayton, Ohio, USA.
Jennifer D Motter, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Jonah Odim, Division of Allergy, Immunology and Transplantation, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Dorry L Segev, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Christine M Durand, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Aaron A R Tobian, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
HOPE in Action Investigators, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
HOPE in Action Investigators:
Dominque Piquant, Katherine Link, Marion Hemmersbach-Miller, Thomas Pearson, Nicole Turgeon, G Marshall Lyon, William Kitchens, Jeryl Huckaby, A Francie Lasseter, Rivka Elbein, April Roberson, Elizabeth Ferry, Ethan Klock, Willa V Cochran, Michelle Morrison, Sarah Rasmussen, Juli Bollinger, Jeremy Sugarman, Angela R Smith, Margaret Thomas, Margaret Coakley, Joseph Timpone, Alyssa Stucke, Brandy Haydel, Rebecca Dieter, Elizabeth J Klein, Henry Neumann, Lorenzo Gallon, Leah Goudy, Michelle Callegari, Ilise Marrazzo, Towanda Jackson, Timothy Pruett, Mary Farnsworth, Jayme E Locke, Darnell Mompoint-Williams, Katherine Basinger, Kristin Mekeel, Phirum Nguyen, Joanne Kwan, Tab Srisengfa, Peter Chin-Hong, Rodney Rogers, Jacques Simkins, Carlos Munoz, Ty Dunn, Dierdre Sawinski, Fernanda Silveira, Kailey Hughes, Diana Lynn Pakstis, Jamie Nagy, Mary Baldecchi, Thangamani Muthukumar, Melissa D Eddie, Katharine Robb, Elizabeth Salsgiver, Britta Witting, Marwan M Azar, Merceditas Villanueva, Richard Formica, and Ricarda Tomlin
References
- 1. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med 2010; 362:2336–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boyarsky BJ, Segev DL. From bench to bill: how a transplant nuance became 1 of only 57 laws passed in 2013. Ann Surg 2016; 263:430–3. [DOI] [PubMed] [Google Scholar]
- 3. Wilk AR, Hunter RA, McBride MA, Klassen DK. National landscape of HIV+ to HIV+ kidney and liver transplantation in the United States. Am J Transplant 2019; 19:2594–605. [DOI] [PubMed] [Google Scholar]
- 4. Durand CM, Werbel W, Doby B, et al. Clarifying the HOPE act landscape: the challenge of donors with false-positive HIV results. Am J Transplant 2019; 20:617–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Durand CM, Segev D, Sugarman J. Realizing HOPE: the ethics of organ transplantation from HIV-positive donors. Ann Intern Med 2016; 165:138–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muller E, Barday Z. HIV-positive kidney donor selection for HIV-positive transplant recipients. J Am Soc Nephrol 2018; 29:1090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Selhorst P, Combrinck CE, Manning K, et al. Longer-term outcomes of HIV-positive-to-HIV-positive renal transplantation. N Engl J Med 2019; 381:1387–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United Sates, 2010–2016. HIV Surveillance Supplemental Report. Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed 10 November 2020.
- 9. Rao VB, Johari N, du Cros P, Messina J, Ford N, Cooke GS. Hepatitis C seroprevalence and HIV co-infection in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Infect Dis 2015; 15:819–24. [DOI] [PubMed] [Google Scholar]
- 10. Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016; 16:797–808. [DOI] [PubMed] [Google Scholar]
- 11. Chimukangara B, Lessells RJ, Rhee SY, et al. Trends in pretreatment HIV-1 drug resistance in antiretroviral therapy-naive adults in South Africa, 2000-2016: a pooled sequence analysis. EClinicalMedicine 2019; 9:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen I, Connor MB, Clarke W, et al. Antiretroviral drug use and HIV drug resistance among HIV-infected black men who have sex with men: HIV prevention trials network 061. J Acquir Immune Defic Syndr 2015; 69:446–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seal PS, Frontini M, Jhita PK, Deichmann PC, Clark RA. Characteristics and genotype profiles of antiretroviral-naïve patients entering a Southern US HIV outpatient clinic 2009-2012. Int J STD AIDS 2016; 27:554–9. [DOI] [PubMed] [Google Scholar]
- 14. Davy-Mendez T, Eron JJ, Brunet L, Zakharova O, Dennis AM, Napravnik S. New antiretroviral agent use affects prevalence of HIV drug resistance in clinical care populations. AIDS 2018; 32:2593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kassaye SG, Grossman Z, Balamane M, et al. Transmitted HIV drug resistance is high and longstanding in metropolitan Washington, DC. Clin Infect Dis 2016; 63:836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rhee SY, Clutter D, Fessel WJ, et al. Trends in the molecular epidemiology and genetic mechanisms of transmitted human immunodeficiency virus type 1 drug resistance in a large US clinic population. Clin Infect Dis 2019; 68:213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hathorn E, Smit E, Elsharkawy AM, et al. HIV-positive-to-HIV-positive liver transplantation. N Engl J Med 2016; 375:1807–9. [DOI] [PubMed] [Google Scholar]
- 18. Blasi M, Stadtler H, Chang J, et al. Detection of donor’s HIV strain in HIV-positive kidney-transplant recipient. N Engl J Med 2020; 382:195–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seem DL, Lee I, Umscheid CA, Kuehnert MJ; US Public Health Service. . PHS guideline for reducing human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission through organ transplantation. Public Health Rep 2013; 128:247–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Organ Procurement and Transplantation Network. Organ Procurement and Transplantation Network policies. Available at: https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf. Accessed 8 November 2020.
- 21. US Department of Health and Human Service. Human Immunodeficiency Virus (HIV) Organ Policy Equity (HOPE) act safeguards and research criteria for transplantation of organs infected with HIV. Federal Register 2015; 80:34912–21. [Google Scholar]
- 22. Durand CM, Halpern SE, Bowring MG, et al. Organs from deceased donors with false-positive HIV screening tests: an unexpected benefit of the HOPE act. Am J Transplant 2018; 18:2579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2018 annual data report: kidney. Am J Transplant 2020; 20(suppl s1):20–130. [DOI] [PubMed] [Google Scholar]
- 24. Wensing AM, Calvez V, Ceccherini-Silberstein F, et al. 2019 update of the drug resistance mutations in HIV-1. Top Antivir Med 2019; 27:111–21. [PMC free article] [PubMed] [Google Scholar]
- 25. Stanford University HIV drug resistance database. 5 October 2020. Available at: https://hivdb.stanford.edu/. Accessed 10 November 2020.
- 26. Grabowski MK, Reynolds SJ, Kagaayi J, et al. The validity of self-reported antiretroviral use in persons living with HIV: a population-based study. AIDS 2018; 32:363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levintow SN, Okeke NL, Hué S, et al. Prevalence and transmission dynamics of HIV-1 transmitted drug resistance in a southeastern cohort. Open Forum Infect Dis 2018; 5:ofy178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Menza TW, Billock R, Samoff E, Eron JJ, Dennis AM. Pretreatment integrase strand transfer inhibitor resistance in North Carolina from 2010-2016. AIDS 2017; 31:2235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen I, Zhang Y, Cummings V, et al. Analysis of HIV integrase resistance in black men who have sex with men in the United States. AIDS Res Hum Retroviruses 2017; 33:745–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chun HM, Fieberg AM, Hullsiek KH, et al. ; Infectious Disease Clinical Research Program HIV Working Group. . Epidemiology of hepatitis B virus infection in a US cohort of HIV-infected individuals during the past 20 years. Clin Infect Dis 2010; 50:426–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Remis RS, Liu J, Loutfy MR, et al. Prevalence of sexually transmitted viral and bacterial infections in HIV-positive and HIV-negative men who have sex with men in Toronto. PLoS One 2016; 11:e0158090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Serrano OK, Kerwin S, Payne WD, Pruett TL. CD4 count in HIV- brain-dead donors: insight into donor risk assessment for HIV+ donors. Transplantation 2017; 101:831–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. HIVinfo. Drug database. Available at: https://clinicalinfo.hiv.gov/en/drugs/. Accessed 20 November 2020.
- 34. Hemmersbach-Miller M, Wood RP, Wolfe CR. Donor evaluation in the era of HIV-positive organ transplantation: the importance of the infectious diseases specialist. Am J Transplant 2020; 20:2589–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boyarsky BJ, Hall EC, Singer AL, Montgomery RA, Gebo KA, Segev DL. Estimating the potential pool of HIV-infected deceased organ donors in the United States. Am J Transplant 2011; 11:1209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Richterman A, Sawinski D, Reese PP, et al. An assessment of HIV-infected patients dying in care for deceased organ donation in a United States urban center. Am J Transplant 2015; 15:2105–16. [DOI] [PubMed] [Google Scholar]
- 37. Doby BL, Tobian AAR, Segev DL, Durand CM. Moving from the HIV organ policy equity act to HIV organ policy equity in action: changing practice and challenging stigma. Curr Opin Organ Transplant 2018; 23:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Centers for Disease Control and Prevention. HIV in the United States by region. 26 October 2020. Available at: https://www.cdc.gov/hiv/statistics/overview/geographicdistribution.html. Accessed 28 December 2020.
- 39. Cash A, Luo X, Chow EKH, et al. HIV+ deceased donor referrals: a national survey of organ procurement organizations. Clin Transplant 2018; 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bonny TS, Kirby C, Martens C, et al. Outcomes of donor-derived superinfection screening in HIV-positive to HIV-positive kidney and liver transplantation: a multicentre, prospective, observational study. Lancet HIV 2020; 7:e611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.