Abstract
Background
Low vitamin D levels may increase the risk of tuberculosis disease; however, previous observational cohort studies showed variable results. We investigated the relationship between vitamin D levels in infancy and subsequent development of tuberculosis disease throughout childhood.
Methods
We enrolled pregnant women at 20–28 weeks’ gestation attending antenatal care in a periurban South African setting in the Drakenstein Child Health Study. Serum 25(OH)D concentrations were measured in newborn infants aged 6–10 weeks. Children were followed prospectively for tuberculosis infection and disease using annual tuberculin skin testing, radiographic examinations, and microbiological diagnosis with GeneXpert, culture, and smear testing. Univariable and multivariable Cox regression was performed and HRs with 95% CIs were calculated.
Results
Children were followed for tuberculosis disease for a median of 7.2 years (IQR, 6.2–7.9). Among 744 children (<1% with human immunodeficiency virus (HIV), 21% HIV-exposed without HIV), those who were vitamin D deficient in early infancy were not at increased risk of developing tuberculosis disease (adjusted HR, .8; 95% CI, .4–1.6). Infants in the lowest vitamin D concentration tertile were at similar risk of tuberculosis as the highest tertile (adjusted HR, .7; 95% CI, .4–1.4). Vitamin D deficiency was associated with tuberculin conversion ≤2 years of age at a <30-nmol/L (adjusted OR, 1.9; 95% CI, 1.2–3.2), but not <50-nmol/L (adjusted OR, 1.5; 95% CI, .8–2.9), cutoff.
Conclusions
In a setting with hyperendemic rates of tuberculosis, vitamin D concentrations in infancy did not predict tuberculosis disease at any point in childhood. However, very low vitamin D levels were associated with tuberculin conversion in young children.
Keywords: tuberculosis, pediatrics, micronutrients, vitamin D
Low vitamin D may increase the risk of tuberculosis; however, previous cohort studies have had variable results. In a South African birth cohort, vitamin D levels in infancy were not associated with tuberculosis over a median of 7.2 years’ follow-up.
Over 1 million children develop tuberculosis disease every year, representing substantial morbidity and mortality [1, 2]. The risk of tuberculosis disease is greatest in early childhood, so understanding and identifying which children are at high risk is essential to target and prioritize finite health resources [3–5]. However, other than children recently exposed to a tuberculosis case [4, 6, 7] or with a positive skin test [4], there is uncertainty about which children health services should target and how nutritional deficiencies play a role in the development of pediatric tuberculosis.
Globally, undernutrition is a major contributor to tuberculosis disease [8, 9] and several studies have investigated whether there is an association between micronutrient deficiencies and tuberculosis disease, with varied results [10–13]. Of all micronutrient deficiencies, vitamin D is commonly linked to tuberculosis disease in observational studies. Patients with tuberculosis often present with lower vitamin D concentrations; however, whether low vitamin D levels predict subsequent incident tuberculosis infection or disease is unclear [11]. Adult studies have shown conflicting data, with some studies reporting an association between low vitamin D levels and prevalent (at time of tuberculosis disease diagnosis) or incident (diagnosed during follow-up) tuberculosis disease. However, others have shown no association between low vitamin D levels and tuberculosis disease [11]. No population-based studies have been performed in infants or in settings with a high burden of both tuberculosis and vitamin D deficiency.
In a birth cohort study from South Africa, we studied the relationship between serum vitamin D levels in infancy and subsequent development of tuberculosis disease throughout childhood. We also assessed whether vitamin D concentrations were associated with tuberculin skin test conversion.
METHODS
Study Design and Participants
The study cohort as well as the subcohort tested for vitamin D has been described previously [3, 14, 15]. Briefly, we enrolled pregnant women between 20 and 28 weeks’ gestation attending antenatal care in Paarl, a periurban setting outside of Cape Town, South Africa. Participants were recruited from 2 neighboring community clinics, TC Newman and Mbekweni, serving impoverished communities. Infants received the bacillus Calmette–Guérin (BCG) vaccination at birth. All mothers accessed care in the public sector, which has a strong primary healthcare program, including an effective mother-to-child human immunodeficiency virus (HIV) prevention and antiretroviral therapy program. Women were followed through pregnancy and childbirth, and newborn infants were followed into childhood. Vitamin D supplementation was not routinely provided to infants in the national health services, unless born premature. Exclusion criteria for pregnant women were being younger than 18 years and intending to leave the area within 1 year.
We obtained ethics approval from the University of Cape Town Faculty of Health Sciences Human Research Ethics Committee (reference numbers 401/2009 and 651/2013) and the Provincial Child Health Research Committee. Mothers provided written informed consent at enrollment, which was renewed annually.
Procedures
Surveys focusing on maternal health were administered at enrollment and antenatal data were concurrently collected. Detailed birth information was obtained at delivery. Obstetric care and all births took place at the regional hospital in Paarl. Follow-up visits, including clinical examinations, were done at 6, 10, and 14 weeks; 6 and 12 months; and then annually until the end of follow-up. Data for environmental exposures, household characteristics, respiratory risk factors, anthropometry, and child symptoms were obtained at scheduled visits. Missed visits were rebooked with a study mobile phone network system or by study community-based fieldworkers. Mothers were counselled about respiratory symptoms at every visit and advised to attend the study site or contact study staff between scheduled study visits whenever the child developed cough or difficulty breathing.
All mothers were tested for HIV during pregnancy with Abbott Determine HIV 1/2 rapid HIV antibody test (Abbott Laboratories, North Chicago, IL, USA). If positive, a confirmatory enzyme-linked immunosorbent assay was done. All mothers living with HIV received ART as per national guidelines. Infants of mothers living with HIV were tested with DNA polymerase chain reaction (PCR; Cobas Ampliprep System; Roche Molecular Systems, Branchburg, NJ, USA) at age 6 weeks and 6 weeks after the end of breastfeeding as per national guidelines. Children were re-tested at 18 months with the rapid antibody test [16, 17].
Serum samples were taken from infants between 6 and 10 weeks of age. Vitamin D status was assessed through serum 25-hydroxyvitamin D [25(OH)D] concentration (nmol/L) and measured at Vitas AS (Oslo, Norway; a reference laboratory in Europe with a Vitamin D External Quality Assessment Scheme certificate) from specimens stored at –80°C using liquid chromatography–tandem mass spectrometry. Clinicians did not have access to vitamin D results when making diagnoses, as measurements were done on biobanked samples several years after collection.
Data were collected on infant factors relevant to vitamin D and tuberculosis disease risk based on prior studies with this cohort [3, 15] as well as the medical literature [18]. Infant variables included sex, height-for-age z score (HAZ), weight-for-age z score (WAZ), maternal HIV, gestational age, breastfeeding practices in the first year of life, and season of birth. Season of birth was categorized into summer (December–February), autumn (March–May), winter (June–August), and spring (September–November). We also collected maternal factors including age, smoking, educational level achieved, and various markers for household socioeconomic status. Socioeconomic status was assessed using a validated composite score comprising 4 variables including asset ownership, household income, employment, and education [14].
Tuberculin skin tests were done at the 6-month visit and then at 12, 24, 36, 48, and 60 months of age, and at the time of a lower respiratory tract infection (LRTI) [3]. Tuberculin skin test conversion was defined as an induration reaction greater than or equal to 10 mm, to minimize the risk of misclassification due to BCG vaccination or exposure to environmental mycobacteria [19, 20]. As tuberculin skin test boosting may occur after recurrent tests, children with reactive but negative skin test results (1–9 mm) were not given another test and were censored from conversion analysis at that time point. Because most children were censored by 2 years of age in our cohort, we only used tuberculin skin test results before this age in this analysis. Children with positive tuberculin skin tests were screened for tuberculosis disease and, if none, were referred to local tuberculosis clinics for isoniazid preventive therapy.
Children were followed up for tuberculosis disease from birth at regular study visits as previously described [3]. Tuberculosis disease was diagnosed by experienced physicians and nurses in local tuberculosis community clinics or by study staff, and chest radiographs were read and reported by an experienced clinician. Trained staff collected induced sputum for microbiological confirmation using liquid culture and nucleic acid amplification (Xpert MTB/RIF; Cepheid, Sunnyvale, CA, USA) from children with a tuberculin skin test induration of 10 mm or greater, those presenting with an LRTI, and in participants in whom tuberculosis disease was considered presumptive. A chest radiograph was taken in all children with presumptive (or possible) tuberculosis disease.
Statistical Analysis
Children were included in this analysis if they had a vitamin D measurement at 6–10 weeks of age. We summarized continuous variables as medians with interquartile ranges (IQRs) and categorical variables using proportions.
Our primary outcome was tuberculosis disease incidence after 10 weeks of age to the end of follow-up (30 January 2021). For tuberculosis disease incidence, time-to-event was constructed between birth and the date of tuberculosis. Follow-up was censored at death, development of tuberculosis disease, end of follow-up, or until 30 January 2021. We compared tuberculosis disease incidence in infants with and without vitamin D deficiency using hazard ratios (HRs) and 95% confidence intervals (CIs) obtained from Cox proportional hazards models. We completed this analysis independently for the entire follow-up and then conducted a landmark analysis (ie, analyzing only subjects at that time point still eligible for the analysis) prior to 1 year of age. Two-sample likelihood ratio tests were used.
To assess whether there was a dose–response relationship between vitamin D concentration, we categorized vitamin D concentrations into tertiles, as used elsewhere and to enable comparison with such studies [11, 12, 15]. We compared incident tuberculosis disease among participants at each tertile level. We also compared our results using vitamin D cutoffs. Children were categorized into distinct categories based on their serum 25(OH)D concentration, including deficient (<50 nmol/L), insufficient (50–74 nmol/L), and sufficient (≥75 nmol/L). There is no consensus on the definition of vitamin D deficiency [18]. Due to this, we conducted separate analyses with <50-nmol/L and <30-nmol/L cutoffs for vitamin D deficiency in our cohort [18].
Last, we assessed the relationship between vitamin D levels and tuberculin conversion at 1 year of age or younger and 2 years of age using logistic regression models. We assessed the odds of a tuberculin conversion at 1 year of age or younger or 2 years of age independently for each vitamin D deficiency cutoff and in each vitamin D tertile. Multivariable models were built including all relevant variables related to tuberculin conversion in this cohort [3, 15]. All analyses were performed using Stata (version 14.1; StataCorp, College Station, TX, USA).
RESULTS
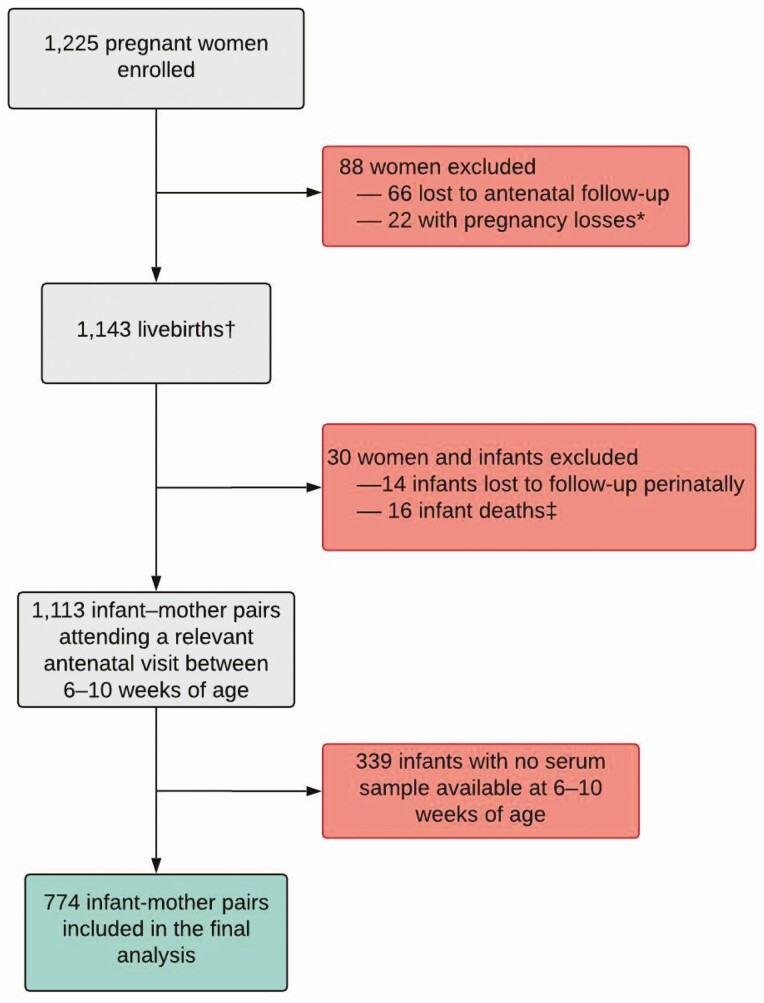
Pregnant women were recruited and enrolled between 5 March 2012 and 31 March 2015, with 1225 women included in the birth cohort (Figure 1). Of 1143 live births, 30 (3%) were excluded because of perinatal death or study termination and 339 infants were enrolled but did not have a valid test for vitamin D. In total, 774 (2 [<1%] living with HIV, 166 [21%] HIV-exposed living without HIV) children were tested for vitamin D and included in our analysis (Table 1). The median WAZ and HAZ scores at the time of the vitamin D measurement were −0.61 (IQR, −1.32, 0.04) and 0.01 (IQR, −0.86, 0.87).
Figure 1.
Study flow diagram of eligibility and enrollment of mothers and infants in the Drakenstein Child Health Study: Cape Town, South Africa. *Loss of pregnancy due to miscarriage, stillbirth, or intrauterine death (23 infants [including 1 set of twins]). †Including 4 pairs of twins and 1 set of triplets. ‡No postnatal data collected.
Table 1.
Sociodemographic and Clinical Characteristics of 774 Mother–Infant Pairs
| Variables | Total n (%) | Median (IQR) |
|---|---|---|
| Maternal characteristics | ||
| Median (IQR) age, years | … | 26 (22–31) |
| Age group | ||
| <20 years | 89 (11.5) | |
| 20–24 years | 253 (32.7) | |
| 25–29 years | 209 (27.0) | |
| ≥30 years | 223 (28.8) | |
| Education | ||
| Primary | 58 (7.5) | |
| Some secondary | 413 (53.4) | |
| Completed secondary | 256 (33.1) | |
| Some tertiary | 47 (6.1) | |
| HIV-positive status | 166 (21.4) | |
| Maternal smoking in pregnancy | 182 (23.5) | |
| Infant characteristics | ||
| Birth weight, g | 3100 (2750, 3420) | |
| Weight-for-age z scorea | −0.39 (−1.15, 0.33) | |
| Height-for-age z scorea | −0.83 (−1.75, −0.01) | |
| Female | 366 (47.3) | |
| Gestation delivery, weeks | 39 (38, 40) | |
| Prematurity (<37 weeks) | 98 (12.7) | |
| Breastfeeding initiated | 718 (92.8) | |
| HIV-positive status | 2 (0.3) | |
| Season of birth | ||
| Summer (December–February) | 220 (28.4) | |
| Autumn (March–May) | 195 (25.2) | |
| Winter (June–August) | 189 (24.4) | |
| Spring (September– November) | 170 (22.0) | |
| Household characteristics | ||
| Socioeconomic statusb | ||
| Lowest | 190 (23.8) | |
| Moderate low | 190 (25.2) | |
| Moderate high | 195 (25.6) | |
| High | 193 (25.4) |
Percentages refer to within-characteristic column totals among participants within each clinic and in entire study. Percentages may not total 100% because within-column percentages were rounded to the nearest integer. Column totals vary across different characteristics due to missing values for some participants.
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range.
aWe derived z scores from World Health Organization child growth standards at birth and at every follow-up visit; we used the median of all the weight-for-age z scores for each child to summarize nutrition status over the duration of follow-up.
bSocioeconomic status comprised a comprehensive composite of asset ownership, household income, employment, and education.
The median vitamin D concentration was 37.4 nmol/L (IQR, 25.8–47.3). The number of infants classified as vitamin D deficient at cutoffs of less than 50 nmol/L and less than 30 nmol/L were 624 (81%; 95% CI, 78–83%) and 242 (31%; 95% CI, 28–35%), respectively. Almost half of the cohort (n = 382; 49%) had vitamin D levels between 30 and 50 nmol/L. Only 1% of infants were vitamin D sufficient at a cutoff of 75 nmol/L or greater.
Children were followed for tuberculosis disease for a median of 7.2 years (IQR, 6.2–7.9 years). Over 5167 child-years of follow-up, 62 children were diagnosed with tuberculosis disease (1123 cases per 100 000 person-years; 95% CI, 868–1452). One (1.4%) case manifested as disseminated tuberculosis disease; the remaining 61 cases were diagnosed with pulmonary tuberculosis. Among all cases, 12 (19.7%) were microbiologically confirmed.
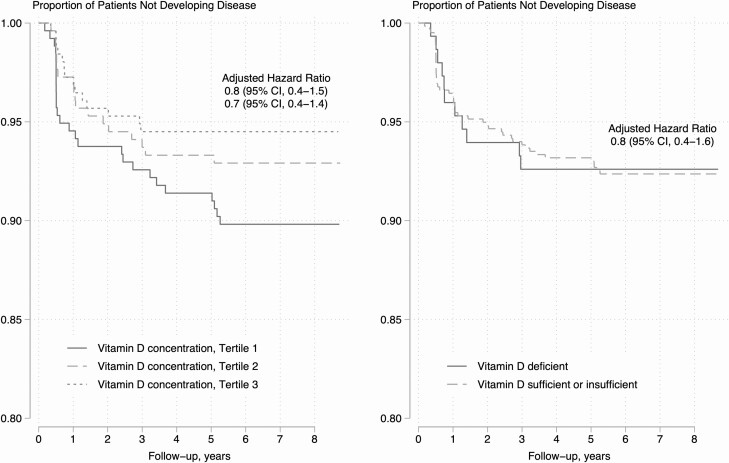
There was no observed statistical relationship between vitamin D levels in early infancy and tuberculosis disease incidence during childhood regardless of the cutoff used (Table 2 and Supplementary Table 1; Figure 2). The incidence of tuberculosis disease per 100 000 child-years was not statistically higher among infants who were and were not vitamin D deficient at a cutoff of less than 50 nmol/L (1137 [95% CI, 854–1513] vs 1066 [95% CI, 591–1926]). The incidence of tuberculosis disease was similar when using an alternative cutoff of less than 30 nmol/L (1202 [95% CI, 891–1620]).
Table 2.
Association Between Vitamin D Concentrations and Risk of Incident Tuberculosis Disease
| Median (IQR) | Person-years | Incident Tuberculosis Diseasea | Univariable Model, HR (95% CI) | Multivariable Model,b AHR (95% CI) | |
|---|---|---|---|---|---|
| All follow-upc | |||||
| Vitamin D deficient, <50 nmol/L | 33.9 (22.4, 41.4) | 4146.7 | 51 | 1.1 (.6–2.1) | .8 (.4-1.6) |
| Vitamin D deficient, <30 nmol/L | 17.3 (9.0, 24.3) | 1595.6 | 26 | 1.3 (.8–2.3) | 1.5 (.7-3.1) |
| Vitamin D concentration | |||||
| Tertile 1 (n = 258) | 18.6 (9.8, 25.8) | 1706.8 | 27 | 1 (referent) | 1 (referent) |
| Tertile 2 (n = 258) | 37.4 (34.3, 41.0) | 1698.1 | 19 | .7 (.4–1.3) | .8 (.5–1.5) |
| Tertile 3 (n = 258) | 51.4 (47.3, 58.8) | 1773.3 | 16 | .6 (.3–1.1) | .7 (.4–1.4) |
| Ptrend | .083 | .4229 | |||
| Less than 1 year of age | |||||
| Vitamin D deficient, <50 nmol/L | 33.9 (22.4, 41.5) | 624.0 | 22 | .9 (.4–2.1) | .7 (.3–1.7) |
| Vitamin D deficient, <30 nmol/L | 17.3 (9.0, 24.3) | 242.0 | 13 | 1.3 (.6–2.5) | 1.5 (.7–3.1) |
| Vitamin D concentration | |||||
| Tertile 1 (n = 258) | 18.6 (9.8, 25.8) | 250.4 | 16 | 1 (referent) | 1 (referent) |
| Tertile 2 (n = 258) | 37.4 (34.2, 41.0) | 253.8 | 10 | .6 (.3–1.4) | .8 (.4–1.8) |
| Tertile 3 (n = 258) | 51.4 (47.3, 58.8) | 254.6 | 8 | .5 (.2–1.1) | .8 (.3–1.9) |
| Ptrend | .101 | .5607 |
Abbreviations: AHR, adjusted hazard ratio; CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; IQR, interquartile range.
aThis is the number of incident tuberculosis cases in the specified row but may not include all cases in the comparison group. For example, in the first row describing the “Vitamin D deficient, <50 nmol/L” group, the number of incident tuberculosis disease cases is 51 but the number of cases in the vitamin D insufficient/sufficient comparator group is not listed.
bAll models were adjusted for sex of the child, study site, season of birth, and maternal HIV using Cox regression models.
cAll follow-up time was restricted to certain ages based on distance from birth. The specified time indicates the starting point time. For example, the primary outcome is follow-up for tuberculosis disease starting at <1 year of age until the end of follow-up.
Figure 2.
Low vitamin D concentrations or vitamin D deficiency and the risk of subsequently developing tuberculosis disease throughout childhood. Models in both panels are adjusted for child sex, maternal HIV status, and study site. Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
In a multivariable model adjusting for maternal HIV, breastfeeding, sex, socioeconomic status, and study site, the hazard of tuberculosis disease was statistically similar among children with and without vitamin deficiency when defining vitamin D deficiency at either less than 50 nmol/L (adjusted HR [AHR], .8; 95% CI, .4–1.6) or less than 30 nmol/L (AHR, 1.5; 95% CI, .7–3.1). When adding tuberculin skin test conversion at 2 years of age or younger to this model, the hazard of tuberculosis disease did not alter substantially at either less than 50 nmol/L (AHR, 1.0; 95% CI, .5–2.2) or less than 30 nmol/L (AHR, 1.0; 95% CI, .5–1.8), respectively. This null relationship did not change when we restricted follow-up time or controlled for additional potential confounders (Supplementary Table 1). There was a consistent null relationship between vitamin D deficiency and incidence of tuberculosis disease when we restricted follow-up to before 12 months of life (<50 nmol/L: AHR, .7; 95% CI, .3–1.7; <30 nmol/: AHR, 1.5; 95% CI, .7–3.1). Similarly, there was no statistical relationship between vitamin D tertiles and incident tuberculosis disease (Ptrend = .5607). Compared with the highest vitamin D tertile, children in the lowest tertile had a similar risk of developing incident tuberculosis disease (adjusted hazard ratio [AHR], .8; 95% CI, .3–1.9) (Figure 2). In multivariable linear regression models, we found no relationship between vitamin D concentrations in infancy and subsequent risk of tuberculosis disease over follow-up (0.4 nmol/L; 95% CI, −0.2, 1.0) or during the first year of life (−1.0 nmol/L; 95% CI, −6.7, 4.6).
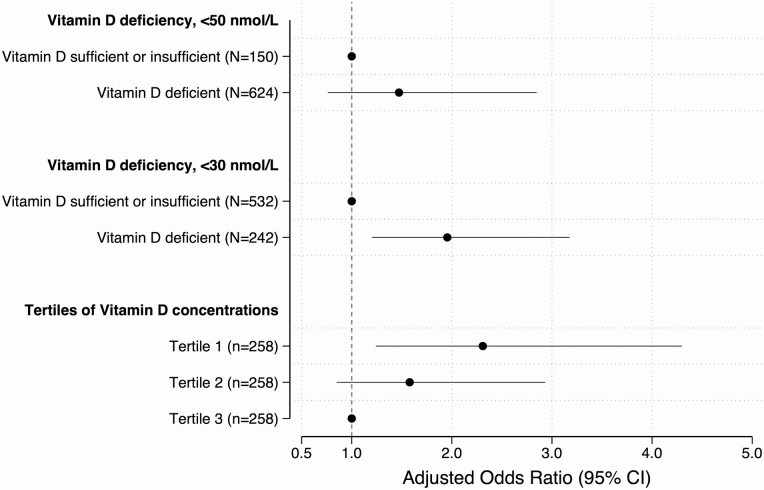
In a multivariable logistic regression model adjusting for study site, season of birth, household tuberculosis exposure, breastfeeding, sex, and maternal HIV, vitamin D deficiency in infancy was associated with tuberculin conversion at 2 years of age or younger at a cutoff of less than 30 nmol/L (AOR, 1.9; 95% CI, 1.2–3.2) but not at less than 50 nmol/L (AOR, 1.5; 95% CI, .8–2.8). Lower vitamin D tertiles were associated with tuberculin conversion at 1 year of age or younger (Ptrend = .0048) and 2 years of age or younger (Ptrend = .0083). Compared with the highest tertile, children in the lowest vitamin D tertile had over 2 times greater odds of tuberculin conversion at and before age 1 year (AOR, 2.7; 95% CI, 1.4–5.3) and at and before age 2 years (AOR, 2.3; 95% CI, 1.2–4.3). Children in the middle tertile were at nonstatistically greater odds of tuberculin conversion at age 1 year or younger (AOR, 1.9; 95% CI, 1.0–3.8) and age 2 years (AOR, 1.6; 95% CI, .8–2.9) (Figure 3).
Figure 3.
Vitamin D concentrations and the odds of tuberculin skin test conversion in the first 2 years of life. All models were adjusted for sex of the child, household tuberculosis exposure, breastfeeding, study site, and maternal HIV. Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
DISCUSSION
In this prospective, population-based birth cohort study in South Africa, we found no relationship between vitamin D levels in early infancy and incident tuberculosis disease throughout childhood. This persisted after controlling for a range of potential confounders, including socioeconomic status and tuberculosis exposure, as well as when restricting follow-up time and varying cutoffs for vitamin D deficiency. However, children with the lowest vitamin D levels in early infancy were more likely to convert their tuberculin skin test in the first 2 years of life. In settings with hyperendemic tuberculosis disease and where vitamin D deficiency is ubiquitous, such as South Africa, vitamin D concentrations in infancy may not predict subsequent tuberculosis disease in childhood.
Our results suggesting no relationship between vitamin D and incident tuberculosis disease conflict with some, but not all, prior observational studies [11, 20, 21]. Several observations may explain these differences. First, almost all previous studies were among groups at high risk of tuberculosis disease, such as household contacts of tuberculosis cases or persons living with HIV. A recent individual-participant meta-analysis found a dose–response relationship between vitamin D levels and incident tuberculosis disease [11], predominantly driven by persons living with HIV [20]. In a household contact study from Peru, however, no relationship between vitamin D and incident tuberculosis disease was found [11]. Our study is among the first population-based studies to investigate this question. Second, we only measured vitamin D levels in infants; previous studies included predominantly adults. Our study extends prior results to young children. Vitamin D levels in young children, especially in studies from sub-Saharan Africa [18], indicate that vitamin D levels may be lowest at this young age and this may modify our results [18]. Last, most previous studies have been limited by short follow-up, typically in the range of 1–2 years [11].
Our null finding may be partially explained by the extraordinarily high prevalence of vitamin D deficiency at the most used cutoff, less than 50 nmol/L [11, 18]. Over 80% of infants in our study were vitamin D deficient at this cutoff and only 1% of infants were vitamin D sufficient. Our findings may be distinct from results from other settings where vitamin D deficiency is less common [18, 22]. A systematic review of vitamin D prevalence levels in Africa found wide heterogeneity by age, location, and deficiency cutoffs [18]. Among a few studies of newborn infants in Africa, the prevalence of vitamin D deficiency ranged from 9% in Tanzania to 98% in Tunisia (at a <50-nmol/L cutoff). It is unclear if a relationship between vitamin D and incident tuberculosis disease is modified by the background burden of vitamin D deficiency or tuberculosis disease.
Our results of an association between vitamin D levels and tuberculin skin test conversion at 1 and 2 years of age were largely dependent on the vitamin D cutoff used. Using a more inclusive definition of less than 50 nmol/L, we found no statistical association between vitamin D status and tuberculin conversion. With a more stringent <30-nmol/L cutoff, we found a positive association. The distinct results using different cutoffs in our study is not surprising given that approximately 50% of our cohort had vitamin D concentrations between 30 and 50 nmol/L. Our results suggest that children may be at particularly high risk to convert their tuberculin skin test if their vitamin D levels are especially low. The distribution of vitamin levels should be considered rather than standardized cutoffs when investigating the relationship between tuberculosis and micronutrients. A recent clinical trial of vitamin D supplementation among 6- to 13-year-old children found no impact on QuantiFERON, QIAGEN conversion after 3 years of follow-up [23]. It is unclear if our study provides elucidation on these trial results as there are substantial setting and study design differences in participant ages, baseline vitamin D levels, the background force of tuberculosis infection, and the use of diagnostic test for conversion.
There are several strengths of our study. We prospectively followed up participants through childhood. In addition, the community-based sample allowed us to understand the degree of exposure to vitamin D deficiency in this population. These results may not be generalizable to settings with a low burden of tuberculosis disease. However, the prevalence of tuberculosis disease and vitamin D deficiency is high in many African and low-income countries; furthermore, the inclusion of 2 distinct communities, with risk factors such as poor nutrition, HIV exposure, or poverty, is likely to make these results generalizable to similar communities. Last, surveillance for tuberculosis disease included a wide range of tests such as tuberculin skin tests, chest radiographs, smear and culture, and Xpert MTB/RIF. Children in our cohort were also assessed for other diseases, which may increase tuberculosis case detection. Our study also has limitations. An important limitation was that we only measured vitamin D levels at 6–10 weeks of age; micronutrient levels are not constant and fluctuate throughout early childhood. For example, in the United States, a high-income, low-tuberculosis-burden setting, there were wide fluctuations in vitamin D concentration from infancy to early childhood and transient vitamin D deficiency was common [22]. How vitamin D concentrations fluctuate over time in young children from South Africa is unknown but is likely to be distinct from children from the United States and other high-income countries. It is also unclear whether vitamin D trajectories, rather than a vitamin D measurement at 1 point in time, provide additional prognostic value for incident tuberculosis disease. Maternal vitamin D status and vitamin supplementation during pregnancy, potential modifiers of infant vitamin D levels, were not assessed. In addition, boosting through BCG vaccination or repeated tuberculin skin tests may lead to false-positive conversion results. To attempt to address this, any child with a positive skin test reaction of any size did not have a repeat skin test, potentially impacting our conversion results. The use of interferon-γ assays may have limited bias through BCG boosting, but the need for a blood sample, laboratory testing, and cost are not likely feasible in low- and middle-income settings [24]. We attempted to minimize this bias by using a conservative skin test conversion cutoff (10-mm induration) for this population [25].
In conclusion, in a setting with hyperendemic tuberculosis disease and where vitamin D deficiency is ubiquitous, vitamin D levels in infancy did not predict subsequent tuberculosis disease at any point in childhood. Whether children with vitamin D deficiency had greater risk of converting their tuberculin skin test depended on the cutoff definition used for vitamin D deficiency.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. L. M. analyzed the data and wrote the first draft of the manuscript. H. J. Z. is the principal investigator, obtained funding, conceived and designed the study, and assisted with drafting of the manuscript. M. P. N. is the lead microbiologist. All authors contributed to the interpretation of results and reviewed, contributed to, and approved drafts of the manuscript as well as the final manuscript.
Acknowledgments. The authors thank the study staff, the clinical and administrative staff of the Western Cape Government Health Department at Paarl Hospital and at the clinics for support of the study, members of the study International Advisory Board for their advice, our collaborators, and the families and children who participated in the study.
Financial support. This work was supported by the Bill & Melinda Gates Foundation (grant number OPP 1017641), Medical Research Council South Africa, and the National Research Foundation South Africa. M. P. N. is supported by an Australian National Health and Medical Research Council Investigator Grant (APP1174455).
Potential conflicts of interest. H. J. Z. and M. P. N. report grants from the Bill & Melinda Gates Foundation. H. J. Z. also reports grants from Medical Research Council South Africa, the National Research Foundation South Africa, and the National Institutes of Health during completion of the study. All other authors report no potential interests. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Leonardo Martinez, Department of Epidemiology, School of Public Health, Boston University, Boston, Massachusetts, USA.
Jabulani R Ncayiyana, Division of Epidemiology and Biostatistics, School of Public Health and Family Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa; Division of Public Health Medicine, School of Nursing and Public Health, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa.
Elizabeth Goddard, Department of Pediatrics and Child Health, Red Cross War Memorial Children’s Hospital, and SA-MRC Uniton Child and Adolescent Health, University of Cape Town, Cape Town, South Africa.
Maresa Botha, Department of Pediatrics and Child Health, Red Cross War Memorial Children’s Hospital, and SA-MRC Uniton Child and Adolescent Health, University of Cape Town, Cape Town, South Africa.
Lesley Workman, Department of Pediatrics and Child Health, Red Cross War Memorial Children’s Hospital, and SA-MRC Uniton Child and Adolescent Health, University of Cape Town, Cape Town, South Africa.
Tiffany Burd, Department of Pediatrics and Child Health, Red Cross War Memorial Children’s Hospital, and SA-MRC Uniton Child and Adolescent Health, University of Cape Town, Cape Town, South Africa.
Landon Myer, Division of Epidemiology and Biostatistics, School of Public Health and Family Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
Mark P Nicol, Division of Infection and Immunity, School of Biomedical Sciences, University of Western Australia, Perth, Australia; Division of Medical Microbiology, University of Cape Town, Cape Town, South Africa.
Heather J Zar, Department of Pediatrics and Child Health, Red Cross War Memorial Children’s Hospital, and SA-MRC Uniton Child and Adolescent Health, University of Cape Town, Cape Town, South Africa.
References
- 1. Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Glob Health 2014; 2:e453–9. [DOI] [PubMed] [Google Scholar]
- 2. Jenkins HE, Tolman AW, Yuen CM, et al. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. Lancet 2014; 383:1572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martinez L, le Roux DM, Barnett W, Stadler A, Nicol MP, Zar HJ. Tuberculin skin test conversion and primary progressive tuberculosis disease in the first 5 years of life: a birth cohort study from Cape Town, South Africa. Lancet Child Adolesc Health 2018; 2:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martinez L, Cords O, Horsburgh CR, Andrews JR; Pediatric TB Contact Studies Consortium. . The risk of tuberculosis in children after close exposure: a systematic review and individual-participant meta-analysis. Lancet 2020; 395:973–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era [State of the Art]. Int J Tuberc Lung Dis 2004; 8:392–402. [PubMed] [Google Scholar]
- 6. Dodd PJ, Yuen CM, Becerra MC, Revill P, Jenkins HE, Seddon JA. Potential effect of household contact management on childhood tuberculosis: a mathematical modelling study. Lancet Glob Health 2018; 6:e1329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martinez L, Shen Y, Handel A, et al. Effectiveness of WHO’s pragmatic screening algorithm for child contacts of tuberculosis cases in resource-constrained settings: a prospective cohort study in Uganda. Lancet Respir Med 2018; 6:276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sinha P, Lönnroth K, Bhargava A, et al. Food for thought: addressing undernutrition to end tuberculosis. Lancet Infect Dis 2021. Available at: https://doi.org/10.1016/S1473-3099(20)30792-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lönnroth K, Williams BG, Cegielski P, Dye C. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol 2010; 39:149–55. [DOI] [PubMed] [Google Scholar]
- 10. Bhargava A, Pai M, Bhargava M, Marais BJ, Menzies D. Can social interventions prevent tuberculosis? The Papworth experiment (1918–1943) revisited. Am J Respir Crit Care Med 2012; 186:442–9. [DOI] [PubMed] [Google Scholar]
- 11. Aibana O, Huang CC, Aboud S, et al. Vitamin D status and risk of incident tuberculosis disease: a nested case-control study, systematic review, and individual-participant data meta-analysis. PLoS Med 2019; 16:e1002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aibana O, Franke MF, Huang CC, et al. Impact of vitamin A and carotenoids on the risk of tuberculosis progression. Clin Infect Dis 2017; 65:900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aibana O, Acharya X, Huang CC, et al. Nutritional status and tuberculosis risk in adult and pediatric household contacts. PLoS One 2016; 11:e0166333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zar HJ, Barnett W, Myer L, Stein DJ, Nicol MP. Investigating the early-life determinants of illness in Africa: the Drakenstein Child Health Study. Thorax 2015; 70:592–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ncayiyana JR, Martinez L, Goddard E, Myer L, Zar HJ. Prevalence and correlates of vitamin D deficiency among young South African infants: a birth cohort study. Nutrients 2021; 13:1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zar HJ, Pellowski JA, Cohen S, et al. Maternal health and birth outcomes in a South African birth cohort study. PLoS One 2019; 14:e0222399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pellowski J, Wedderburn C, Stadler JAM, et al. Implementation of prevention of mother-to-child transmission (PMTCT) in South Africa: outcomes from a population-based birth cohort study in Paarl, Western Cape. BMJ Open 2019; 9:e033259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mogire RM, Mutua A, Kimita W, et al. Prevalence of vitamin D deficiency in Africa: a systematic review and meta-analysis. Lancet Glob Health 2020; 8:e134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children, 2nd ed. Geneva, Switzerland: World Health Organization, 2014. [PubMed] [Google Scholar]
- 20. Department of Health, South Africa. Guidelines for the management of tuberculosis in children. Pretoria, South Africa: Department of Health, 2013. [Google Scholar]
- 21. Tenforde MW, Yadav A, Dowdy DW, et al. ; NWCS319 and ACTG 5175 Study Team. . Vitamin A and D deficiencies associated with incident tuberculosis in HIV-infected patients initiating antiretroviral therapy in multinational case-cohort study. J Acquir Immune Defic Syndr 2017; 75:e71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang G, Liu X, Bartell TR, Pearson C, Cheng TL, Wang X. Vitamin D trajectories from birth to early childhood and elevated systolic blood pressure during childhood and adolescence. Hypertension 2019; 74:421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ganmaa D, Uyanga B, Zhou X, et al. Vitamin D supplements for prevention of tuberculosis infection and disease. N Engl J Med 2020; 383:359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization. Guidelines on the management of latent tuberculosis infection. Geneva, Switzerland: World Health Organization, 2015. [PubMed] [Google Scholar]
- 25. Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis 2006; 10:1192–204. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.