Abstract
Background
Current treatment of vulvovaginal candidiasis (VVC) is largely limited to azole therapy. Ibrexafungerp is a first-in-class triterpenoid antifungal with broad-spectrum anti-Candida fungicidal activity. The objective of this study was to evaluate the efficacy and safety of ibrexafungerp compared with placebo in patients with acute VVC.
Methods
Patients were randomly assigned 2:1 to receive ibrexafungerp (300 mg twice for 1 day) or placebo. The primary endpoint was the percentage of patients with a clinical cure (complete resolution of vulvovaginal signs and symptoms [VSS] = 0) at test-of-cure (day 11 ± 3). Secondary endpoints included the percentage of patients with mycological eradication, overall success (clinical cure and mycological eradication), clinical improvement (VSS ≤ 1) at test-of-cure, and symptom resolution at follow-up (day 25 ± 4).
Results
Patients receiving ibrexafungerp had significantly higher rates of clinical cure (50.5% [95/188] vs 28.6% [28/98]; P = .001), mycological eradication (49.5% [93/188] vs 19.4% [19/98]; P < .001), and overall success (36.0% [64/178] vs 12.6% [12/95]; P < .001) compared with placebo. Symptom resolution was sustained and further increased with ibrexafungerp compared with placebo (59.6% [112/188] vs 44.9% [44/98]; P = .009) at follow-up. Post hoc analysis showed similar rates of clinical cure and clinical improvement at test-of-cure for Black patients (54.8% [40/73] and 63.4% [47/73], respectively) and patients with a body mass index >35 (54.5% [24/44] and 68.2% [30/44], respectively) compared with overall rates. Ibrexafungerp was well tolerated. Adverse events were primarily gastrointestinal and mild in severity.
Conclusions
Ibrexafungerp provides a promising safe and efficacious oral treatment that mechanistically differs from current azole treatment options for acute VVC.
Keywords: Candida albicans, ibrexafungerp, SCY-078, vulvovaginal candidiasis, placebo
Ibrexafungerp was statistically superior to placebo in reduction of signs and symptoms of acute vulvovaginal candidiasis regardless of the endpoint assessed. This advantage was maintained across subpopulations including African American patients or patients with a body mass index >35.
Vulvovaginal candidiasis (VVC) is the second most common cause of vaginitis and is attributed to infection caused by Candida albicans in 85%–95% of women [1–3]. Fifty percent of all women will experience ≥1 episode of VVC by 25 years of age, and approximately 75% of women will experience a VVC episode in their lifetime (with ≥2 episodes occurring in 40%–45% of women) [1]. Comorbid conditions including uncontrolled diabetes, genetic predisposition, immunocompromising conditions, and medications (eg, antibiotics) have been associated with VVC [4, 5]. Given the wide availability of over-the-counter therapies, which often results in patient self-treatment in lieu of professional care, the exact incidence of VVC is grossly under reported [4, 5].
VVC treatments have historically been predominantly limited to the azole class of fungistatic agents. Routine treatment includes topical azole formulations, up to week-long courses, or oral fluconazole given as 150-mg single doses [6, 7]. Limitations of these treatments include possible intolerance to topical formulations, idiosyncratic hepatotoxicity with fluconazole, and fluconazole-associated risk of fetal harm if taken during pregnancy [6, 8–10]. Given the previous lack of approved non-azole therapies, treatment options for VVC have been limited in cases of azole-resistant organisms or for patients with azole intolerance or for whom its use is contraindicated. New oral treatments are warranted, particularly those with broad fungal coverage, a good safety profile (especially no risk of fetal harm), and limited drug-to-drug interactions.
Ibrexafungerp (formerly SCY-078) is the first triterpenoid class antifungal [11]. Its mechanism of action, similar to the echinocandins, targets the glucan synthase enzyme, resulting in decreased (1,3)-β-D-glucan polymers. This polymer reduction weakens the fungal cell wall and leads to fungal cell death. Ibrexafungerp exerts fungicidal activity against numerous Candida isolates—including many that are azole or echinocandin resistant [12, 13]. The possibility for off-target effects (eg, cytochrome P450 interactions), as observed with azole treatments, is notably lessened with the use of glucan synthase inhibitors, since the target exists only in fungal cell walls and is not found in human cells [12].
In preclinical studies, ibrexafungerp levels in vaginal tissue measured 2- to 9-fold higher than in plasma [14, 15], indicating favorable vaginal tissue penetration. Additionally, ibrexafungerp activity is not negatively affected by low vaginal pH (4.5) environment, a characteristic that is common in patients with VVC [6, 14, 16]. In 2019, the Food and Drug Administration (FDA) released industry guidance for drug development in the treatment of VVC. Based on those recommendations, which included trial design and efficacy endpoints, we designed a phase 3, multicenter, randomized, double-blind, placebo-controlled superiority study to evaluate the efficacy and safety of oral ibrexafungerp in the treatment of acute VVC [17]. Given the non–life-threatening nature of VVC, the placebo-controlled design was well aligned with regulatory guidance: VVC complications were not expected to occur from delayed treatment, and patients were allowed to receive rescue therapy, as needed, after establishing failure of the assigned study drug.
Efficacy endpoints in the present study including clinical cure—defined as absence of all signs and symptoms of VVC (vulvovaginal signs and symptoms [VSS] = 0) and responder (successful) outcome (absence of signs and symptoms plus vaginal swab culture negative for growth of Candida species)—differed from previous studies that had established specific criteria (eg, clinical cure, VSS ≤2) [18–20]. Also different from some previous studies [18, 21, 22], test-of-cure (TOC) and follow-up (FU) visits occurred on days 7–14 and 21–30, respectively, in accordance with FDA guidance recommendations [17]. The dose of ibrexafungerp used in this study—300 mg twice a day (BID) for 1 day—was based on results from the phase 2 DOVE study that showed as total milligram dosing increased, gastrointestinal-related adverse events also increased without a corresponding improvement in efficacy [23].
METHODS
VANISH 303 (NCT03734991) evaluated the efficacy and safety of oral ibrexafungerp compared with placebo in female patients with acute VVC. Enrolled patients were ≥12 years of age with acute VVC, which was defined as a minimum composite VSS of ≥4 with at least 2 signs or symptoms having a score ≥2. Other inclusion criteria included a positive microscopic examination with 10% potassium hydroxide (KOH) revealing yeast forms and a normal vaginal pH (≤4.5) and use of contraception in patients of reproductive potential. Exclusion criteria included any condition that may have interfered with the diagnosis or evaluation of response to therapy including mixed infections; the use of systemic and/or topical (vaginal) antifungal treatment products within 28 days of baseline; patients pregnant or lactating; patients with a known human immunodeficiency virus (HIV) infection and/or receiving treatment that could compromise their immune response; and patients with active cervical/vaginal cancer.
Patients were randomly assigned 2:1 to receive ibrexafungerp (300-mg BID for 1 day) or matching placebo administered BID for 1 day using an interactive response system. Randomization was stratified according to diabetes mellitus diagnosis (yes or no). All site and sponsor personnel were blinded to treatment assignments except for a team member who was responsible for drug distribution logistics. Study drugs were provided by SCYNEXIS, Inc. Patients could withdraw or be discontinued from the study if they withdrew consent, experienced an adverse event (AE) that warranted study discontinuation, or were lost to FU or if the investigator believed that it was in their best interest to withdraw.
This study was conducted in accordance with the general principles of the Declaration of Helsinki. Each study site obtained institutional review board approval before study initiation and each patient provided written consent for study participation.
Study Assessments
Vulvovaginal samples were assessed locally using 10% KOH at screening, and fungal cultures were assessed by a central laboratory at baseline, TOC visit (day 11 ± 3), and if symptoms were present at FU visit (day 25 ± 4). Susceptibility testing was performed per Clinical and Laboratory Standards Institute M27-A3 guidelines. Samples were also tested at screening to determine vaginal pH and to identify other possible pathogens. Vulvovaginal signs (edema, erythema, and excoriation/fissures) and symptoms (burning, itching, and irritation) were rated using the VSS scale at screening, day 1, and TOC and FU visits. The VSS scale is a standardized, predefined scale for which each sign and symptom was given a numerical rating based on severity (absent = 0; mild = 1; moderate = 2; severe = 3) to calculate a total composite score (range, 0–18). Patients recorded vulvovaginal symptoms in a diary on days 1 to TOC visit. If the patient experienced persistence, worsening, or recurrence of symptoms (eg, symptoms ≥3) then rescue antifungal therapy was allowed following vaginal examination with samples for KOH and culture. VSS ratings leading to rescue therapy were recorded, and vulvovaginal samples were obtained for testing. If rescue medication was administered before or at the TOC visit, the patient was considered an early termination due to lack of efficacy. If rescue medication was administered after the TOC visit but before the FU visit, then the patient was considered an early termination due to lack of efficacy after the TOC visit. Safety assessments included physical exams, vital signs, laboratory tests, and vitals at screening and TOC visit, and continuous AEs monitoring throughout the study.
Outcomes
The primary objective of this study was to evaluate the efficacy of ibrexafungerp versus placebo in patients with acute VVC. The primary endpoint was the percentage of patients with a clinical cure at the TOC visit (Table 1). Secondary endpoints included the percentage of patients with mycological eradication, overall success (clinical cure and mycological eradication), clinical improvement at the TOC visit, complete resolution of symptoms at the FU visit, and safety and tolerability. All AEs were coded using MedDRA (version 21.1). Outcomes are defined in Table 1.
Table 1.
Efficacy Endpoint Definitions
| Endpoint | Definition |
|---|---|
| Clinical cure | Complete resolution of signs and symptoms of vulvovaginal infection without need for further antifungal treatment and topical vaginal drug therapy for the treatment of vulvovaginal irritation/pruritus before or at the TOC visit. VSS = 0 at TOC visit |
| Complete resolution of symptoms at FU visit | Complete resolution of symptoms in patients at FU visit regardless of clinical cure at TOC visit. Symptom score = 0 at FU visit |
| Clinical improvement | Partial or complete resolution of signs and symptoms with total composite score ≤1 at TOC visit without need for further antifungal treatment and topical drug therapy for the treatment of vulvovaginal irritation/pruritus before or at the TOC visit. VSS ≤ 1 at TOC visit |
| Mycological eradication | Negative culture for Candida species without the need for further antifungal treatment at TOC visit |
| Overall success | Clinical cure and mycological eradication at TOC visit |
Abbreviations: FU, follow-up; TOC, test of cure; VSS, vulvovaginal signs and symptoms.
Post hoc analyses were conducted in Black patients and patients with a body mass index (BMI) >35 to evaluate clinical cure and clinical improvements rates at TOC and the percentage of patients with VSS ≤2 at TOC.
Statistical Analysis
All analyses were conducted using SAS software version 9.4 (SAS Institute, Inc, Cary, North Carolina). A sample size of 366 patients (ibrexafungerp n = 244; placebo, n = 122) was calculated to provide 90% power to detect a superior difference between ibrexafungerp and placebo based on a Pearson’s Chi-squared test with a Type 1 error rate of 5%. This was based on an assumed 50% and 30% clinical cure rate for ibrexafungerp and placebo, respectively [23, 24], a 10% patient dropout, and an estimated 20% of patients without mycological culture-confirmed infection at baseline. Data from all study sites were pooled for analysis and the data set analyzed. Efficacy analyses used the modified intention-to-treat (mITT) population, which consisted of randomly assigned patients who had a positive culture for Candida species at baseline and received ≥1 dose of study drug. The safety set included all randomly assigned patients who received ≥1 dose of study drug and had ≥1 postbaseline evaluation. A patient was considered a nonresponder if they did not meet the clinical response criteria for categorical responses or were missing categorical response data at specific visits. Treatment differences between ibrexafungerp and placebo were compared using a Cochran-Mantel-Haenszel test adjusted for diabetes mellitus diagnosis (yes or no). Descriptive statistical methods were used to summarize data including safety data, by treatment group. All statistical tests were 2-sided and interpreted at a 5% significance level. For change from baseline in VSS scores, an ANCOVA model was used rather than a 2-way ANOVA model.
Role of the Sponsor
The role of the sponsor in the design, execution, analysis, reporting, and funding is fully disclosed. The authors’ personal interests, financial or nonfinancial, relating to this research and its publication have been disclosed.
RESULTS
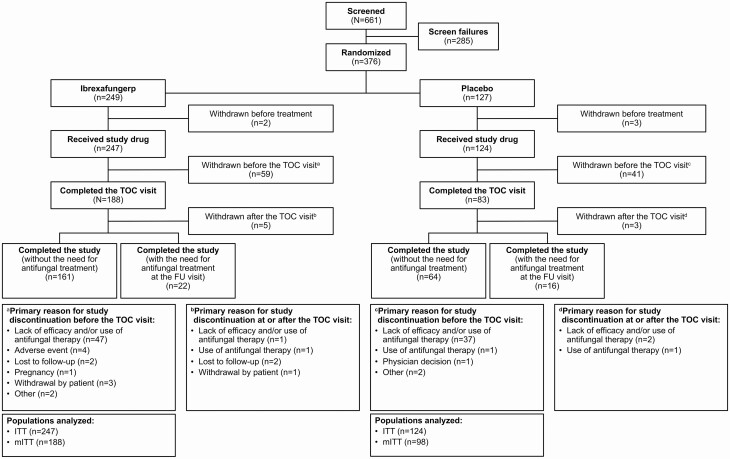
Patients were enrolled between January 2019 and September 2019 at 27 study sites in the United States. A total of 376 patients were randomly assigned to receive ibrexafungerp (n = 249) or placebo (n = 127), with 247 and 124 patients, respectively, receiving the study drug (Figure 1), which composed the intention-to-treat (ITT) population that was used for safety analyses. Approximately 20% of patients in the ITT population did not have a positive culture for Candida species at baseline and were not included in the mITT population (ibrexafungerp [n = 188]; placebo [n = 98]); efficacy results are reported for the mITT population.
Figure 1.
Patient disposition. Abbreviations: FU, follow-up; ITT, intention-to-treat; mITT, modified intention-to-treat; TOC, test of cure.
Overall demographics were similar between treatment groups, including the severity of acute VVC at baseline (Table 2). The majority of patients were not diabetic (ibrexafungerp, 90.4%; placebo 91.8%). All patients in the mITT population had a positive culture for ≥1 Candida species with the majority (>90%) testing positive for C. albicans (Table 2). Baseline susceptibility testing between treatment groups was comparable. No fluconazole-resistant isolates of C. albicans were identified at baseline. At TOC, there was a <2-fold change in minimum inhibitory concentration (MIC) susceptibility following ibrexafungerp exposure.
Table 2.
Baseline Characteristics (mITT Population)
| Ibrexafungerp (n = 188) | Placebo (n = 98) | |
|---|---|---|
| Age, y | ||
| Mean ± SD | 33.5 ± 10.36 | 36.0 ± 12.46 |
| Median (min, max) | 32.5 (18, 67) | 34.0 (17, 66) |
| Race, n (%) | ||
| White | 103 (54.8) | 53 (54.1) |
| Black | 73 (38.8) | 43 (43.9) |
| Asian | 4 (2.1) | 0 |
| American Indian or Alaska Native | 2 (1.1) | 0 |
| Other | 6 (3.2) | 2 (2.0) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 54 (28.7) | 18 (18.4) |
| Non-Hispanic or Latino | 134 (71.3) | 80 (81.6) |
| BMI (kg/m2)a, n (%) | ||
| ≤35 | 144 (76.6) | 76 (77.6) |
| >35 | 44 (23.4) | 22 (22.4) |
| Diabetes mellitus | ||
| Yes | 18 (9.6) | 8 (8.2) |
| No | 170 (90.4) | 90 (91.8) |
| Composite VSS score | ||
| Median (min, max) | 9.0 (5, 18) | 9.0 (4, 17) |
| Candida species | ||
| Candida albicans | 173 (92.0) | 90 (91.8) |
| Candida glabrata | 11 (5.9) | 11 (11.2) |
| Candida tropicalis | 4 (2.1) | 1 (1.0) |
| Candida dubliniensis | 2 (1.1) | 0 |
| Candida lusitaniae | 1 (0.5) | 1 (1.0) |
| Candida parapsilosis | 1 (0.5) | 0 |
| Candida krusei | 0 | 1 (1.0) |
| Saccharomyces species | 1 (0.5) | 0 |
Abbreviations: BMI, body mass index; max, maximum; min, minimum; mITT, modified intention-to-treat; SD, standard deviation; VSS, vulvovaginal signs and symptoms.
aBaseline BMI is calculated as baseline weight/baseline height2.
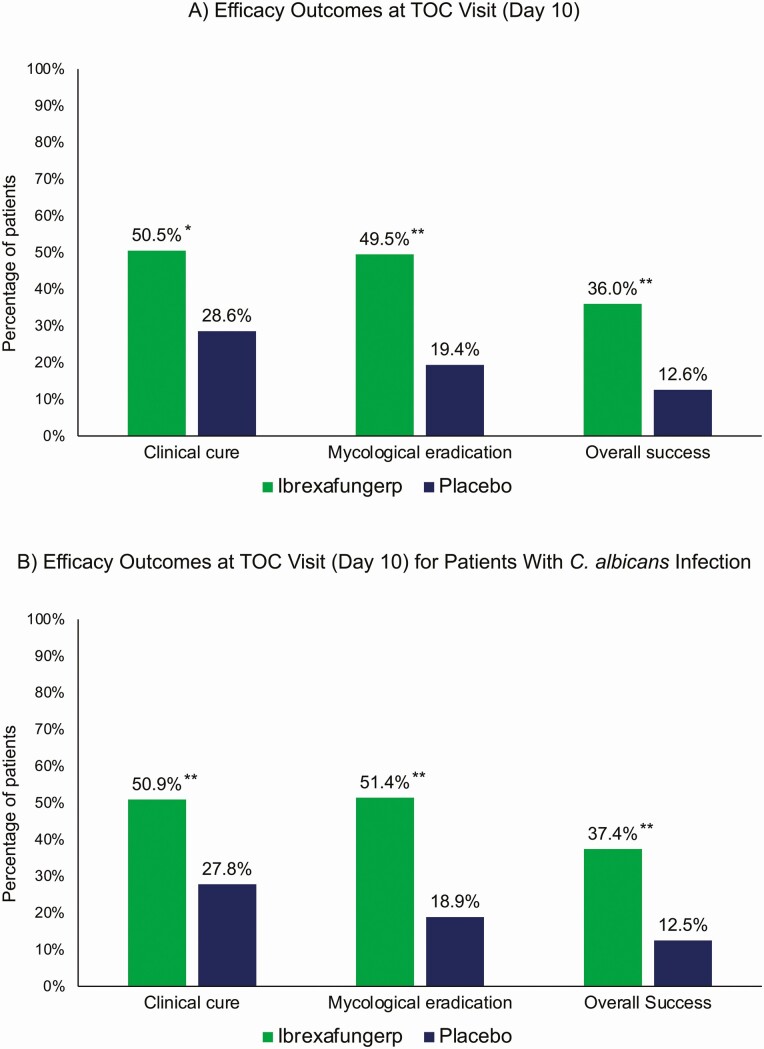
Ibrexafungerp demonstrated statistical superiority over placebo in the primary endpoint and all key secondary endpoints. The clinical cure rate (VSS = 0) at TOC visit was significantly higher in patients receiving ibrexafungerp (50.5% [95/188 patients]) compared with placebo (28.6% [28/98 patients]) (relative risk [RR] = 1.71 [95% confidence interval [CI], 1.205–2.431]; P = .001) (Figure 2A) and in patients with C. albicans (50.9% [88/173 patients] vs 27.8% [25/90 patients]), respectively; RR = 1.81 [95% CI, 1.247–2.623]; (P < .001) (Figure 2B). The percentage of patients with complete symptom resolution at day 25, regardless of clinical cure at TOC, was significantly improved with ibrexafungerp (59.6% [112/188 patients]) compared with placebo (44.9% [44/98 patients]) (P = .009). Although clinical cure was defined as VSS = 0, the percentage of patients with clinical improvement (VSS ≤ 1) at the TOC visit was significantly higher with ibrexafungerp than placebo (64.4% [121/188 patients] vs 36.7% [36/98 patients]; P < .001).
Figure 2.
Select efficacy endpoints. A, Efficacy outcomes at TOC visit (day 10): clinical cure (ibrexafungerp, 95/188 patients; placebo, 28/98 patients), mycological eradication (ibrexafungerp, 93/188 patients; placebo, 19/98 patients), and overall success (ibrexafungerp, 64/178 patients; placebo, 12/95 patients). B, Efficacy outcomes at TOC visit (day 10) for patients with C. albicans infections, clinical cure (ibrexafungerp, 88/173 patients; placebo, 25/90 patients), mycological eradication (ibrexafungerp, 89/173 patients; placebo, 17/90 patients), and overall success (ibrexafungerp, 61/163 patients; placebo, 11/88 patients). *P = .001, **P < .001 for comparisons between ibrexafungerp and placebo. Abbreviation: TOC, test of cure.
Mycological eradication rates at TOC were significantly higher with ibrexafungerp (49.5% [93/188 patients]) than placebo (19.4% [19/98 patients]) (RR = 2.87 [95% CI, 1.799–4.574]; P < .001) and in patients with C. albicans (51.4% [89/173 patients]) vs 18.9% [17/90 patients]), respectively; RR = 3.06 [95% CI, 1.860–5.030]; P < .001) (Figure 2A, 2B). Mycological outcomes at FU were limited, as samples were collected in only 20% of patients (samples not required in asymptomatic patients and those who received rescue antifungals). Overall success (both clinical cure and mycological eradication) rates at TOC visit was significantly higher with ibrexafungerp (36.0% [64/178 patients]) than placebo (12.6% [12/95 patients]) (RR = 3.19 [95% CI, 1.772–5.756]; P < .001) and in patients with C. albicans (37.4% [61/163 patients] vs 12.5% [11/88 patients]); respectively; RR = 3.52 [95% CI, 1.852–6.678]; P < .001) (Figure 2A, 2B).
Before the updated FDA guidance, a common definition of clinical cure was VSS ≤ 2. In a post hoc analysis, the ibrexafungerp clinical cure rate at TOC using VSS ≤ 2 was 70% (131/188 patients). In other post hoc analyses, clinical cure and clinical improvement rates with ibrexafungerp at TOC were similar for Black patients (54.8% [40/73 patients] and 63.4% [47/73 patients], respectively) compared with the overall clinical cure and clinical improvement rates (50.5% and 64.4%, respectively). In patients with BMI >35, clinical cure and clinical improvement rates were similar (54.5% [24/44 patients] and 68.2% [30/44 patients], respectively) to the overall clinical cure and clinical improvement rates with ibrexafungerp.
Ibrexafungerp was well tolerated, with 39.7% (98/247 patients) reporting a treatment-related treatment-emergent AE (TEAE) compared with placebo (16.9% [21/124 patients]). The majority of TEAEs were gastrointestinal and mild in severity (Table 3) with increased rates of treatment-related diarrhea (22.3% [55/247] vs 4.0% [5/124] patients, respectively) and nausea (10.9% [27/247] vs 5.0% [4/124] patients, respectively) reported with ibrexafungerp versus placebo. TEAEs led to discontinuation from study and treatment in 2 patients following the first dose of ibrexafungerp (vomiting and dizziness related to ibrexafungerp) and to study discontinuation in 2 patients after completing ibrexafungerp treatment (bacterial vaginosis; not related to ibrexafungerp). No patients receiving placebo discontinued treatment or the study, and no patients experienced a TEAE that led to dose interruption. Serious adverse events (SAEs) were reported in 1 patient receiving ibrexafungerp (pneumonia and bronchial hyperreactivity) and in 2 patients receiving placebo (diabetes mellitus and hypokalemia) and were not considered to be related to study treatment. No deaths were reported in the study. Two pregnancies were reported in the ibrexafungerp group, both resulting in a live birth with no delivery or newborn complications reported.
Table 3.
Summary of Treatment-Related Treatment-Emergent Adverse Events (TEAEs) Reported in >2% of Patients
| Ibrexafungerp (n = 247) | Placebo (n = 124) | |
|---|---|---|
| Patients with ≥1 TEAE | 98 (39.7) | 21 (16.9) |
| Mild | 78 (31.6) | 17 (13.7) |
| Moderate | 24 (9.7) | 4 (3.2) |
| Severe | 1 (0.4) | 0 |
| Diarrhea | 55 (22.3) | 5 (4.0) |
| Mild | 38 (15.4) | 4 (3.2) |
| Moderate | 17 (6.9) | 1 (0.8) |
| Nausea | 27 (10.9) | 5 (4.0) |
| Mild | 24 (9.7) | 5 (4.0) |
| Moderate | 2 (0.8) | 0 |
| Severe | 1 (0.4) | 0 |
| Abdominal pain | 13 (5.3) | 0 |
| Mild | 12 (4.9) | 0 |
| Moderate | 1 (0.4) | 0 |
| Abdominal discomfort | 11 (4.5) | 2 (1.6) |
| Mild | 6 (2.4) | 2 (1.6) |
| Moderate | 5 (2.0) | 0 |
| Dizziness | 9 (3.6) | 2 (1.6) |
| Mild | 7 (2.8) | 2 (1.6) |
| Moderate | 2 (0.8) | 0 |
| Abdominal pain upper | 7 (2.8) | 1 (0.8) |
| Mild | 6 (2.4) | 1 (0.8) |
| Moderate | 1 (0.4) | 0 |
| Flatulence | 6 (2.4) | 1 (0.8) |
| Mild | 5 (2.0) | 1 (0.8) |
| Moderate | 1 (0.4) | 0 |
| Headache | 6 (2.4) | 3 (2.4) |
| Mild | 5 (2.0) | 3 (2.4) |
| Moderate | 1 (0.4) | 0 |
Mild was defined as awareness of sign or symptom, but easily tolerated and not requiring medical attention. Moderate was defined as discomfort enough to cause some interference with daily activity and may require medical attention. Severe was defined as intense enough to disrupt daily activities and likely required medical attention.
DISCUSSION
To our knowledge, VANISH 303 is the largest placebo-controlled study in the treatment of VVC completed to date. Our study results showed that ibrexafungerp is well tolerated and demonstrated statistical superiority in improved overall clinical cure rates at the TOC visit compared with placebo (50.5% vs 28.6%, respectively; P = .001) and in patients with C. albicans, the Candida species associated with >85% of VVC infections [2, 3]. Symptom resolution was sustained at FU visit and further increased to 59.6% with ibrexafungerp and 44.9% with placebo (P = .009). Additional benefit with ibrexafungerp was evident with increased clinical improvement (VSS ≤ 1) rates at the TOC visit compared with placebo (64.4% vs 36.7%, respectively; P < .001). We also evaluated the efficacy of ibrexafungerp in Black patients and patients with a BMI >35. These patients tended to have similar clinical cure and clinical improvement rates at TOC compared with the overall study population. These data indicate that race and BMI ≥35 do not affect ibrexafungerp efficacy, and thus these patients would not require dose adjustments. Furthermore, ibrexafungerp was well tolerated with the majority of TEAEs being gastrointestinal related and mild in nature, similar to the phase 2 DOVE study [23].
This is the second clinical study to evaluate the efficacy of ibrexafungerp in acute VVC. Our clinical cure rate was similar to that reported with ibrexafungerp 300 mg BID for 1 day (51.9% [14/27 patients]) in the phase 2 DOVE study [23]. Historical comparisons with current therapies for VVC are difficult because of differences in study methodology and dosing regimens. Before the new FDA guidance, study endpoints, including definition of clinical cure, varied widely. Clinical cure was defined as VSS = 0 in our study using the new FDA guidance [17] versus VSS ≤ 2 in many previous azole studies. Clinical cure rates (VSS = 0) of 47.4% to 57.9% on days 7 and 14 have been reported in patients receiving single-dose fluconazole [25] versus 50.5% with single-day ibrexafungerp in our study; clinical cure rate (VSS ≤ 2) of 80.9% on day 14 was reported with single-dose fluconazole versus 70% with ibrexafungerp in our study [19]. However, in our study, an improved and sustained response was observed at the FU visit, whereas some previous studies evaluating various regimens of fluconazole reported an 11%–20% decrease in sustained response from days 7–14 to days 28–35 [18, 19, 26].
In addition to demonstrating the efficacy and safety of ibrexafungerp in the treatment of acute VVC, our study also provides a large placebo cohort, demonstrating a placebo effect. A placebo clinical cure rate of 45% (10/22 patients) has previously been reported compared with 73% (35/48 patients) with oral itraconazole [24]. Similarly, the placebo clinical cure rate in our study was more than half that of ibrexafungerp (28.6% vs 50.5%, respectively). These data suggest a clinically significant placebo effect in the treatment of acute VVC—one that should be accounted for in future study designs and not dismissed in the clinical setting.
The results of our study are limited to women ≥18 years of age. Although our inclusion criteria permitted females ≥12 years of age, no one younger than 18 years of age was enrolled in the ibrexafungerp group. Our study is also limited by the use of a placebo arm. Finally, a lower number of patients with non-albicans Candida species were enrolled in the study and clinical efficacy was not determined. Therefore, our results are limited to patients with acute VVC attributed to C. albicans.
In conclusion, ibrexafungerp is a novel, oral antifungal with a good safety profile and superior efficacy to placebo. In June 2021, ibrexafungerp (BREXAFEMME®) was approved as the first and only non-azole treatment for VVC. A second phase 3 study, VANISH-306 (NCT03987620), evaluating the efficacy and safety of ibrexafungerp 300 mg BID for 1 day in the treatment of acute VVC in an international patient population, has been completed.
Notes
Acknowledgments. The VANISH 303 study was funded by SCYNEXIS, Inc. The authors thank the patients, the investigators, and the investigational staff of the VANISH 303 study. The authors acknowledge the medical writing assistance of Laura Jung, PharmD, and Joe Scobey, PhD of PRECISIONscientia in Yardley, Pennsylvania, USA, which was supported financially by SCYNEXIS, Inc, in compliance with international Good Publication Practice guidelines.
Financial support. This work was supported by SCYNEXIS, INC. SCYNEXIS, INC provided funding to Case Western Reserve University to act as a central Mycology laboratory for the trial.
Data availability. Qualified scientific and medical researchers may make requests for individual participant data that underlie the results (text, tables, figures, and supplement) reported in this article, after de-identification, at medicalaffairs@SCYNEXIS.com. Methodologically sound proposals for such data will be evaluated and approved by SCYNEXIS, Inc, in its sole discretion. All approved researchers must sign a data access agreement prior to accessing the data. Data will be available as soon as possible but no later than within 1 year of the acceptance of the article for publication, and for 3 years following article publication. SCYNEXIS, Inc, will not share identified participant data or a data dictionary.
Potential conflicts of interest. J. R. S. has received support or grants (to institution) from Hologic, Inc, SCYNEXIS, Inc, and Mycovia Pharmaceuticals, consulting fees from SCYNEXIS, Inc, Hologic, Inc, and Talis Biomedical, educational fees from Hologic, Inc, and stock options in Talis Biomedical. R. S. has received support or grants (to institution) from SCYNEXIS, Inc and Mycovia Pharmaceuticals for clinical research and consulting fees from SCYNEXIS, Inc, and Mycovia Pharmaceuticals for advisory panels. S. A. S. reports being contracted to participate in the sponsors’ clinical trial. J. K. G. received compensation as a principal investigator for studies sponsored by SCYNEXIS, Inc. S. N. L. reports payment for clinical trial services from Altus Research during the conduct of the study and outside of the submitted work. B. T. C. received compensation as a principal investigator for studies sponsored by SCYNEXIS, Inc. D. A. A., N. E. A., and I. A. H are employed by and hold stocks in SCYNEXIS, Inc. K. B. E. received consultant fees as the Preclinical Pharmacology Subject Matter Expert from SCYNEXIS, Inc. M. A. G. received support, grants, or contracts (to institution) for SCYNEXIS, Inc, and Mycovia Pharmaceuticals and received consulting fees from SCYNEXIS, Inc. for manuscript writing. P. N. has served as a consultant for Mycovia Pharmaceuticals, Hologic, Inc, and SCYNEXIS, Inc. J. D. S. has received research funds from SCYNEXIS, Inc and Mycovia Pharmaceuticals.
All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Jane R Schwebke, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Ryan Sobel, Jefferson Vulvovaginal Health Center, Department of Obstetrics and Gynecology Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, Pennsylvania, USA.
Janet K Gersten, New Age Medical Research Corp, Miami, Florida, USA.
Steven A Sussman, Lawrence OB/GYN Clinical Research, Lawrenceville, New Jersey, USA.
Samuel N Lederman, Altus Research Inc, Lake Worth, Florida, USA.
Mark A Jacobs, Life Research, Inc, Houston, Texas, USA.
B Todd Chappell, WR-Medical Research Center of Memphis, LLC, Memphis, Tennessee, USA.
David L Weinstein, Consultants in Women’s Healthcare, Inc, St. Louis, Missouri, USA.
Alfred H Moffett, Jr, OB-GYN Associates of Mid-Florida, PA, Leesburg, Florida, USA.
Nkechi E Azie, SCYNEXIS, Inc, Jersey City, New Jersey, USA.
David A Angulo, SCYNEXIS, Inc, Jersey City, New Jersey, USA.
Itzel A Harriott, SCYNEXIS, Inc, Jersey City, New Jersey, USA.
Katyna Borroto-Esoda, KBE Consulting, Raleigh, North Carolina, USA.
Mahmoud A Ghannoum, Case Western Reserve University and University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA.
Paul Nyirjesy, Jefferson Vulvovaginal Health Center, Department of Obstetrics and Gynecology Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, Pennsylvania, USA.
Jack D Sobel, Wayne State University, Detroit, Michigan, USA.
References
- 1. Makanjuola O, Bongomin F, Fayemiwo SA. An update on the doles of non-albicans Candida species in vulvovaginitis. J Fungi 2018; 4:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martin Lopez JE. Candidiasis (vulvovaginal). BMJ Clin Evid 2015; 2015:0815. [PMC free article] [PubMed] [Google Scholar]
- 3. Mendling W, Brasch J, Cornely OA, et al. . Guideline: vulvovaginal candidosis (AWMF 015/072), S2k (excluding chronic mucocutaneous candidosis). Mycoses 2015; 58:1–15. [DOI] [PubMed] [Google Scholar]
- 4. Jeanmonod R, Jeanmonod D. Vaginal candidiasis. Available at: https://www.ncbi.nlm.nih.gov/books/. Accessed 9 March 2021.
- 5. Sobel JD. Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol 2016; 214:15–21. [DOI] [PubMed] [Google Scholar]
- 6. Paavonen JA, Brunham RC. Vaginitis in nonpregnant patients: ACOG practice bulletin number 215. Obstet Gynecol 2020; 135:1229–30. [DOI] [PubMed] [Google Scholar]
- 7. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. . Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 8. Bérard A, Sheehy O, Zhao JP, et al. . Associations between low- and high-dose oral fluconazole and pregnancy outcomes: 3 nested case-control studies. CMAJ 2019; 191:E179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mølgaard-Nielsen D, Pasternak B, Hviid A. Use of oral fluconazole during pregnancy and the risk of birth defects. N Engl J Med 2013; 369:830–9. [DOI] [PubMed] [Google Scholar]
- 10. Diflucan [package insert]. New York, NY: Pfizer, Inc; 2020. [Google Scholar]
- 11. Hasim S, Coleman JJ. Targeting the fungal cell wall: current therapies and implications for development of alternative antifungal agents. Future Med Chem 2019; 11:869–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Azie N, Angulo D, Dehn B, Sobel JD. Oral ibrexafungerp: an investigational agent for the treatment of vulvovaginal candidiasis. Expert Opin Investig Drugs 2020; 29:893–900. [DOI] [PubMed] [Google Scholar]
- 13. Scorneaux B, Angulo D, Borroto-Esoda K, et al. . SCY-078 is fungicidal against Candida species in time-kill studies. Antimicrob Agents Chemother 2017; 63:e01961-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Larkin EL, Long L, Isham N, et al. . A novel 1,3-Beta-d-glucan inhibitor, ibrexafungerp (formerly SCY-078), shows potent activity in the lower pH environment of vulvovaginitis. Antimicrob Agents Chemother 2019; 63:e02611-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wring S, Borroto-Esoda K, Solon E, Angulo D. SCY-078, a novel fungicidal agent, demonstrates distribution to tissues associated with fungal infections during mass balance studies with intravenous and oral [14C]SCY-078 in albino and pigmented rats. Antimicrob Agents Chemother 2019; 63:e02119-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sobel JD, Borroto-Esoda K, Azie N, Angulo D. In vitro pH activity of ibrexafungerp against fluconazole-susceptible and -resistant Candida isolates from women with vulvovaginal candidiasis. Antimicrob Agents Chemother 2021; May 17:AAC.00562-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Food and Drug Administration. Vulvovaginal candidiasis: developing drugs for treatment. guidance for industry. Available at: https://www.fda.gov/. Accessed 9 March 2021.
- 18. Zhou X, Li T, Fan S, et al. . The efficacy and safety of clotrimazole vaginal tablet vs. oral fluconazole in treating severe vulvovaginal candidiasis. Mycoses 2016; 59:419–28. [DOI] [PubMed] [Google Scholar]
- 19. Sobel JD, Kapernick PS, Zervos M, et al. . Treatment of complicated Candida vaginitis: comparison of single and sequential doses of fluconazole. Am J Obstet Gynecol 2001; 185:363–9. [DOI] [PubMed] [Google Scholar]
- 20. Fan S, Liu X, Liang Y. Miconazole nitrate vaginal suppository 1200 mg versus oral fluconazole 150 mg in treating severe vulvovaginal candidiasis. Gynecol Obstet Invest 2015; 80:113–8. [DOI] [PubMed] [Google Scholar]
- 21. Kutzer E, Oittner R, Leodolter S, Brammer KW. A comparison of fluconazole and ketoconazole in the oral treatment of vaginal candidiasis; report of a double-blind multicentre trial. Eur J Obstet Gynecol Reprod Biol 1988; 29:305–13. [DOI] [PubMed] [Google Scholar]
- 22. Sekhavat L, Tabatabaii A, Tezerjani FZ. Oral fluconazole 150 mg single dose versus intra-vaginal clotrimazole treatment of acute vulvovaginal candidiasis. J Infect Public Health 2011; 4:195–9. [DOI] [PubMed] [Google Scholar]
- 23. Angulo D, Tufa M, Azie N. A phase 2b, dose-selection study evaluating the efficacy and safety of oral ibrexafungerp vs fluconazole in vulvovaginal candidiasis (DOVE). Am J Obstet Gynecol 2019; 221:673. [Google Scholar]
- 24. Stein GE, Mummaw N. Placebo-controlled trial of itraconazole for treatment of acute vaginal candidiasis. Antimicrob Agents Chemother 1993; 37:89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nyirjesy P, Alessio C, Jandourek A, Lee JD, Sandison T, Sobel JD. CD101 topical compared with oral fluconazole for acute vulvovaginal candidiasis: a randomized controlled trial. J Low Genit Tract Dis 2019; 23:226–9. [DOI] [PubMed] [Google Scholar]
- 26. Li T, Zhu Y, Fan S, Liu X, Xu H, Liang Y. A randomized clinical trial of the efficacy and safety of terconazole vaginal suppository versus oral fluconazole for treating severe vulvovaginal candidiasis. Med Mycol 2015; 53:455–61. [DOI] [PubMed] [Google Scholar]