Abstract
The widespread lockdowns put in place to limit the spread of the new coronavirus disease (COVID-19) offers a rare opportunity in understanding how human presence influence ecosystems. Using data from long-term seabird monitoring, we reveal a previously concealed guarding effect by tourist groups on an iconic seabird colony in the Baltic Sea. The absence of tourists in 2020 lead to a sevenfold increase in presence of white-tailed eagles Haliaeetus albicilla, a sevenfold increase in their disturbance of breeding common murres Uria aalge and causing 26% lower murre productivity than the long-term average. Eagles did not prey on murres, but their frequent disturbances delayed egg laying and facilitated egg predation from herring gulls Larus argentatus and hooded crows Corvus cornix. Based on our findings, we suggest that human presence could be used as a strategic measure in guarding seabird colonies, and that a social-ecological systems perspective is vital for long-term success in protected area management.
Keywords: COVID-19, Seabirds, Sea eagles, Social-ecological system, Disturbance
1. Introduction
An emerging lesson from over a hundred years of biodiversity conservation is that humans are intrinsic parts of most ecosystems (Liu et al., 2007). While early conservation efforts tried to exclude humans with the goal of maintaining undisturbed or pristine ecosystems, a social-ecological systems perspective is increasingly applied in conservation (Mace, 2014). Social-ecological systems are complex and they often exhibit nonlinearity (Sugihara et al., 2012). Disentangling their dynamics is complicated as humans are ever-present in almost all ecosystems, and scientific experiments are difficult for practical, logistical or ethical reasons. The “anthropaus” or “global human confinement experiment” created in 2020 by the lockdowns during the coronavirus disease 2019 (COVID-19) has consequently created a unique experimental setting for quantifying the role of humans in ecosystems (Bates et al., 2020; Rutz et al., 2020). Evidence on effects of the lockdowns on the world's ecosystems will be important and useful in informing future biodiversity conservation policies (Corlett et al., 2020).
Several apex predators, including the temperate northern hemisphere's sea eagles (genus: Haliaeetus), were severely affected by environmental pollutants, especially the organochlorine contaminants DDT and PCB, in the 1950s–1970s. Those pollutants, combined with mortality due to persecution, lead poisoning from ammunition when feeding on shot game, and low winter survival, led to drastic declines in most populations (Elliott et al., 2011; Helander et al., 2009, Helander et al., 2008; Wayland et al., 2003). The phasing out of the organochlorine contaminants and lead in shotgun ammunition, protection, and winter feeding have resulted in a recovery of sea eagle populations that can be regarded as one of the most remarkable conservation achievements in human history (Stier et al., 2016). This recovery has in some areas surpassed previous population sizes, and the sea eagles have become a threat to other species, including several seabird populations (Henson et al., 2019; Hipfner et al., 2012), adding to the ongoing discussion on new conservation challenges with predator recovery (Cruz et al., 2019; Marshall et al., 2016; Stier et al., 2016).
Oftentimes, it is not the predation itself from sea eagles that causes the greatest harm to breeding seabirds, but the disturbance by their mere appearance in seabird colonies (Hipfner et al., 2012). It appears as if seabird species breeding in sheltered places, such as burrow-breeding puffins (Fratercula sp.), are less affected than openly breeding species like the cliff-breeding murres and kittiwakes (Hipfner et al., 2012). Predator-prey modeling studies have indicated that sea eagles actually can eradicate local seabird populations (Henson et al., 2019). The conflict between sea eagle recovery and seabird conservation have been clearly recognized in North America (e.g. Hipfner et al., 2011; Parrish et al., 2001). Also in Europe, particularly in Scandinavia, the recovery of sea eagles has been significant (Gjershaug et al., 2008; Helander et al., 2008) and seabirds have declined in many areas, particularly along the coast of mainland Norway (Anker-Nilssen et al., 2015). However, in Scandinavia disentangling the possible influence of sea eagles on seabird population declines have been obstructed by concurrent changes in climate and marine ecosystems, including changes in prey fish stocks, and many reports of impact from eagles remain anecdotal (Hipfner et al., 2012).
Common murres Uria aalge on the island of Stora Karlsö in the Baltic Sea have been placed under protection since 1880, resulting in a substantial population recovery after centuries of hunting and egg collection (Hentati-Sundberg and Olsson, 2016; Olsson and Hentati-Sundberg, 2017). Parallel to seabird protection, tourism on this island expanded in the 1920s, and Stora Karlsö is today the largest and most visited seabird colony in the Baltic Sea. Up until 2020, the number of sea eagles visiting the island during the seabird breeding season has been low and mainly concentrated around a large colony of great cormorants Phalacrocorax carbo sinensis. Here we took advantage of the COVID-19 lockdown to investigate and quantify how the absence of tourists in 2020 affected eagle numbers and behavior; and how those changes were reflected in the behavior and breeding success of cliff-breeding common murres. In documenting this interaction, our goal is to inform future management and conservation of seabirds and eagles.
2. Materials and methods
We studied common murres and sea eagles (white-tailed eagle, Haliaeetus albicilla) on the island of Stora Karlsö, Baltic Sea, Sweden (57°17′1 N, 17°58′2E).
COVID-19 first appeared in Wuhan, China, in late 2019, and started to spread in Europe in the beginning of 2020. Sweden got it first confirmed case on Jan 31st 2020, and its first event of death on March 11th. On March 11th, COVID-19 was declared a global pandemic by the WHO (Orlowski and Goldsmith, 2020). Sweden did not impose a complete lockdown in response to the COVID-19 pandemic. However, starting March 29th, 2020, the government restricted public gatherings to a maximum of 50 people (Orlowski and Goldsmith, 2020). Following this regulation, the company that owns the island of Stora Karlsö decided to cancel the tourist traffic, which usually runs from early May until the end of August, for the whole 2020 season. Data for human presence on the island was supplied by the ticket office for Karlsö Jagt- och Djurskyddsförenings AB, the company running the tourist traffic to the island.
We used a CCTV camera system to study how eagles affected common murres on a section of the colony with approximately 40 breeding pairs of common murres. We analyzed video footage from 2019 (normal tourist season) and 2020 (no tourists due to Covid-19 lock-down). The CCTV cameras filmed continuously for the whole breeding season, in this paper we analyze the period from the onset of egg laying until the end of the incubation period (May 1st–June 4th) in 2019 and 2020.
Disturbances were defined as occasions when birds synchronously left the breeding ledges, for which we noted time, number of birds before the disturbance, and the return of birds at two-minute intervals after the disturbance until 85% of the birds were again present. In many cases, the videos could not reveal the exact cause of the disturbance, but daily observations in the colony revealed that disturbances were usually caused by sea eagles, although at one point a migrating common crane Grus grus caused a moderate disturbance. The general pattern of disturbances corresponds to observations of common murres being disturbed by bald eagles Haliaeetus leucocephalus in North America (Parrish, 1995).
From 2010 to 2020, we also performed standardized monitoring of 89 to 178 common murre pairs and recorded timing of breeding (phenology), breeding success and feeding frequency from daily observations. Breeding performance was calculated as the number of breeding attempts within a number of study plots multiplicated with the hatching success, i.e., the reproductive output per breeding pair. Although common murres occasionally relay after losing eggs early in the season, we here considered only the first breeding attempt (egg laid) per year. Breeding performance monitoring was performed approx. 50 m north of the area where the video recordings were performed. The monitoring data and video recording were collected in Västerberget, the largest out of three major sub-colonies on Stora Karlsö. (Olsson and Hentati-Sundberg, 2017). Complementary data on breeding performance were collected from one exposed breeding ledge with between 25 and 30 breeding pairs of common murres in the second largest sub-colony, Stornasar, in 2009–2012 and in 2020. In this sub-colony, daily observations were made in 2009–2012. In 2020, the breeding status was recorded for 22 days between June 10 and July 18.
Observations of white-tailed eagles on Stora Karlsö were retrieved from the Swedish “Species Observation System”, an open national biodiversity database where any amateur or professional naturalists can submit their observations. We calculated the maximum number of eagles observed per month for April–July each year, from which we calculated an annual average of number of eagles present on the island.
3. Results
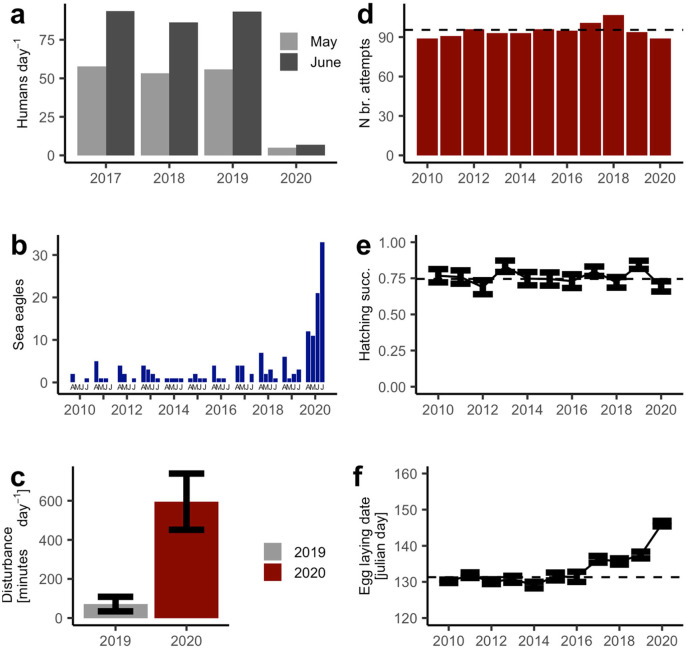
The closing of the tourist traffic to the island lead to a decline of the human presence by 92% compared to the previous three years (Fig. 1a). Concomitantly, sea eagles, which are sensitive to human presence (Grubb and King, 1991; Radović and Mikuska, 2009), increased dramatically, and their behavior changed notably. Before the lockdown, between 2010 and 2019, the maximum monthly number of eagles reported from Stora Karlsö varied between 0 and 7 (mean = 2.25), whereas, during the lockdown in 2020, the maximum number of eagles reported varied between 11 and 33 (mean 19.25) – an increase by 760% (Fig. 1b). The eagles disturbed the murres by flying past the breeding cliffs, causing massive flushes of birds (Video 1). In 2020, for the first time, we also observed eagles attacking murres around the breeding cliffs and on the water, often leading to even stronger reactions with murres evacuating the ledges in panic (Video 2). From 2019 to 2020, the total disturbance time increased from 72 to 602 min day−1 (Fig. 1c). The increase in disturbance time was a combined effect of an increase in number of disturbance events (5.7 and 15.3 events per day for 2019 and 2020, respectively) and an increased duration of disturbance events (12.5 and 39.0 min per event for 2019 and 2020, respectively). No murres were observed being caught by eagles, but disturbances by eagles often resulted in unattended eggs being predated by herring gulls Larus argentatus and hooded crows Corvus cornix while the murres where absent (Video 3), and eggs were also observed falling out off from the ledges during the disturbances (Video 4).
Fig. 1.
Effects of the COVID-19 lockdown in 2020 at Stora Karlsö compared with previous year(s) on (a) human presence, (b) eagle presence (maximum number observed per month, April–July), (c) common murre disturbance, (d) common murre breeding attempts, (e) common murre hatching success, and (f) common murre egg-laying date. Dashed horizontal lines in (d)–(f) denote the average value for 2010–2019.
The disturbances of murres by eagles in 2020 lead to a 26% lower breeding performance than the average for the reference period 2010–2019 for the annually monitored ledges in Västerberget. This was an effect of both a low number of breeding attempts (6.8% lower than average) and a low hatching success (21% lower than average) (Fig. 1d–e). Moreover, the phenology was ten days later than the average for the reference period (Fig. 1f). In the Stornasar subcolony, there were zero successful breeding attempts in 2020. In 2009–2018, the number of breeding attempts varied between 26 and 28 and the breeding success varied between 46 and 69%. At most visits in 2020, there were the normal number of adult birds present, but the extended and frequent disturbances in this sub-colony obviously caused a widespread breeding failure in this sub-colony. Among the murre pairs that managed to hatch a chick in the Västerberget sub-colony, the feeding frequencies and adult birds' time spent on the ledges did not differ between 2019 and 2020 which indicates that food shortage was probably not contributing to the change in murre productivity (t-tests, P > 0.7).
Because of the lockdown, the remaining human activities on the island were concentrated around the largest sub-colony where we make the regular seabird monitoring, and our own presence supposedly explains why this was the sub-colony least affected by eagle disturbance.
4. Discussion
Sea eagles are sensitive to human presence (Grubb and King, 1991; Radović and Mikuska, 2009). The absence of tourists due to the COVID-19 lockdown allowed us to isolate and quantify the effect of increased eagle activities and hence disturbances on breeding seabirds. Although seabird monitoring programs, including ours, are usually maintained to detect bottom-up marine ecosystem changes (Aebischer et al., 1990; Piatt et al., 2007), we here reveal a sudden top-down effect, emerging as a side-effect of a human pandemic. The fact that adult murres' feeding frequencies and time spent on the ledges were not affected, support the conclusion of a solely top-down effect during the 2020 field season.
The comeback of sea eagles following successful conservation measures and their negative effect on seabird colonies is a well-known conservation dilemma (e.g. Cruz et al., 2019; Henson et al., 2019). However, hard evidence for the sea eagle effects on seabirds have been difficult to obtain and differentiate from other gradual ecosystem changes (Hipfner et al., 2012). The common murre population studied here has been growing rapidly since the 1970s and this is the first season of widespread breeding failures we have observed since we started field work in 1997. Recent demographic modeling using murre survival rates obtained in this colony indicates that at least 40% of the breeding pairs needs to produce a chick annually to maintain population numbers (Hentati-Sundberg et al., 2020). As the eagle disturbance affected different sub-colonies differentially, we cannot provide a colony-wide productivity figure for 2020, but our judgement is that the murre productivity observed in 2020 is too low to be long-term sustainable for this population.
Future field studies will reveal whether the return of tourists to the island post-COVID-19 lockdown will reverse the state of the murre colony to “normal”, or if the anthropaus has permanently shifted the behavior of eagles into a long-term threat to the breeding seabirds (c.f. Corlett et al., 2020). If the return of tourists improves the conditions for seabirds, we suggest that human presence can be applied also in other areas to mediate eagle disturbances. Such a strategy, “tourists as seabird guardians” is in line with a social-ecological systems approach to protected area management, but needs to be balanced with the risk of tourists also disturbing the seabirds, as has been seen in other areas (Anderson and Keith, 1980; Carney and Sydeman, 1999). The exact measures with regards to tourist presence will need to be context specific, based on a solid knowledge base and also consider possibly conflicting goals such as between conserving seabirds versus eagles.
The COVID-19 lockdowns have provided multiple examples of the invisible human hand in mediating ecological dynamics (Primack et al., this volume), which reinforces the need for a social-ecological systems perspective for conservation (Mace, 2014). Disentangling such complex and possibly nonlinear social-ecological dynamics should be a key priority in future conservation research and will require continued funding for field-based monitoring programs (Bates et al., 2020; Birkhead, 2014; Lindenmayer et al., 2010).
The following are the supplementary data related to this article.
Common murres disturbed from their ledges by an approaching white-tailed eagle.
Common murres disturbed from their ledges by an actively hunting white-tailed eagle.
Subadult Herring gull Larus argentatus preying on murre egg following eagle disturbance (lower ledge).
Common murre egg rolling down during fly out caused by eagle disturbance (lower ledge).
CRediT authorship contribution statement
JHS: Conceptualization, Methodology, Investigation, Formal Analysis, Visualization, Writing. PAB: Methodology, Formal Analysis, Writing, AH: Conceptualization, Methodology OO: Conceptualization, Methodology, Investigation, Formal Analysis, Writing, Funding acquisition.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgments
We thank Lovisa Dück, Juho Könönen, Emma Engwall and Ludvig Palmheden and numerous other field workers for data collection support, WWF Sweden and Karlsö Jagt- och Djurskyddsförenings AB for economic and logistic support, and Joakim Hjelm and Carl Folke for valuable comments on the manuscript.
References
- Aebischer N.J., Coulson J.C., Colebrookl J.M. Parallel long-term trends across four marine trophic levels and weather. Nature. 1990;347:753–755. doi: 10.1038/347753a0. [DOI] [Google Scholar]
- Anderson D.W., Keith J.O. The human influence on seabird nesting success: conservation implications. Biol. Conserv. 1980;18:65–80. doi: 10.1016/0006-3207(80)90067-1. [DOI] [Google Scholar]
- Anker-Nilssen T., Barrett R.T., Lorentsen S.-H., Strøm H., Bustnes J., Christensen-Dalsgaard S., Descamps S., Erikstad K.E., Fauchald P., Hanssen S.A., Lorentzen E., Moe B., Reiertsen T.K., Systad G.H. SEAPOP. De ti første årene. Nøkkeldokument. 2015:2005–2014. [Google Scholar]
- Bates A.E., Primack R.B., Moraga P., Duarte C.M. COVID-19 pandemic and associated lockdowns as a “global human confinement experiment” to investigate biodiversity conservation. Biol. Conserv. 2020;248:108665. doi: 10.1016/j.biocon.2020.108665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhead T. Stormy outlook for long.term ecology studies. Nature. 2014;514:405. doi: 10.1038/514405a. [DOI] [PubMed] [Google Scholar]
- Carney K.M., Sydeman W.J. A review of human disturbance effects on nesting colonial waterbirds. Waterbirds Int. J. Waterbird Biol. 1999;22:68–79. [Google Scholar]
- Corlett R.T., Primack R.B., Devictor V., Maas B., Goswami V.R., Bates A.E., Koh L.P., Regan T.J., Loyola R., Pakeman R.J., Cumming G.S., Pidgeon A., Johns D., Roth R. Impacts of the coronavirus pandemic on biodiversity conservation. Biol. Conserv. 2020;246:8–11. doi: 10.1016/j.biocon.2020.108571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz J., Windels S.K., Thogmartin W.E., Crimmins S.M., Grim L.H., Larson J.H., Zuckerberg B. Top-down effects of repatriating bald eagles hinder jointly recovering competitors. J. Anim. Ecol. 2019;88:1054–1065. doi: 10.1111/1365-2656.12990. [DOI] [PubMed] [Google Scholar]
- Elliott K.H., Elliott J.E., Wilson L.K., Jones I., Stenerson K. Density-dependence in the survival and reproduction of bald eagles: linkages to chum salmon. J. Wildl. Manag. 2011;75:1688–1699. doi: 10.1002/jwmg.233. [DOI] [Google Scholar]
- Gjershaug J.O., Kålås J.A., Nygård T., Herzke D., Folkestad A.O. Monitoring of raptors and their contamination levels in Norway. Ambio. 2008;37:420–424. doi: 10.1579/0044-7447(2008)37[423:MORATC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Grubb T., King R. Assessing human disturbance of breeding bald eagles with classification tree models. J. Wildl. Manag. 1991;55:500–511. [Google Scholar]
- Helander B., Bignert A., Asplund L. Using raptors as environmental sentinels: monitoring the white-tailed sea eagle Haliaeetus albicilla in Sweden. Ambio. 2008;37:425–431. doi: 10.1579/0044-7447(2008)37[425:URAESM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Helander B., Axelsson J., Borg H., Holm K., Bignert A. Ingestion of lead from ammunition and lead concentrations in white-tailed sea eagles (Haliaeetus albicilla) in Sweden. Sci. Total Environ. 2009;407:5555–5563. doi: 10.1016/j.scitotenv.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Henson S.M., Desharnais R.A., Funasaki E.T., Galusha J.G., Watson J.W., Hayward J.L. Predator–prey dynamics of bald eagles and glaucous-winged gulls at Protection Island, Washington, USA. Ecol. Evol. 2019;9:3850–3867. doi: 10.1002/ece3.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentati-Sundberg J., Olsson O. Amateur photographs reveal population history of a colonial seabird. Curr. Biol. 2016;26:226–228. doi: 10.1016/j.cub.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Hentati-Sundberg J., Olin A., Evans T., Isaksson N., Berglund P.A., Olsson O. A mechanistic framework to inform the spatial management of conflicting fisheries and top predators. J. Appl. Ecol. 2020;58(1):125–134. [Google Scholar]
- Hipfner M.J., Morrison K., Darvill R. Peregrine falcons enable two species of colonial seabirds to breed successfully by excluding other aerial predators. Waterbirds Int. J. Waterbird Biol. 2011;34:82–88. [Google Scholar]
- Hipfner M.J., Blight L.K., Lowe R.W., Wilhelm S.I., Robertson G.J., Barrett R.T., Anker-Nilssen T., Good T.P. Unintended consequences: how the recovery of sea eagle Haliaeetus spp. populations in the northern hemisphere is affecting seabirds. Mar. Ornithol. 2012;40:39–52. [Google Scholar]
- Lindenmayer D.B., Likens G.E., Krebs C.J., Hobbs R.J. Improved probability of detection of ecological “surprises”. Proc. Natl. Acad. Sci. 2010;107:21957–21962. doi: 10.1073/pnas.1015696107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Dietz T., Carpenter S.R., Alberti M., Folke C., Moran E., Pell A.N., Deadman P., Kratz T., Lubchenco J., Ostrom E., Ouyang Z., Provencher W., Redman C.L., Schneider S.H., Taylor W.W. Complexity of coupled human and natural systems. Science (80-.) 2007;317:1513–1516. doi: 10.1126/science.1144004. [DOI] [PubMed] [Google Scholar]
- Mace G.M. Whose conservation? Science (80-.) 2014;345:1558–1560. doi: 10.1126/science.1254704. [DOI] [PubMed] [Google Scholar]
- Marshall K.N., Stier A.C., Samhouri J.F., Kelly R.P., Ward E.J. Conservation challenges of predator recovery. Conserv. Lett. 2016;9:70–78. doi: 10.1111/conl.12186. [DOI] [Google Scholar]
- Olsson O., Hentati-Sundberg J. Population trends and status of four seabird species (Uria aalge, Alca torda, Larus fuscus, Larus argentatus) at Stora Karlsö in the Baltic Sea. Ornis Svecica. 2017;27:64–93. [Google Scholar]
- Orlowski E.J.W., Goldsmith D.J.A. Four months into the COVID-19 pandemic, Sweden’s prized herd immunity is nowhere in sight. J. R. Soc. Med. 2020;113:292–298. doi: 10.1177/0141076820945282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish J.K. Influence of group size and habitat type on reproductive success in common murres (Uria aalge) Auk. 1995;112:390–401. [Google Scholar]
- Parrish J.K., Marvier M., Paine R.T. Direct and indirect effects: interactions between bald eagles and common murres. Ecol. Appl. 2001;11:1858–1869. [Google Scholar]
- Piatt J.F., Sydeman W.J., Browman H. Seabirds as indicators of marine ecosystems. Mar. Ecol. Prog. Ser. 2007;352:199. doi: 10.3354/meps07070. [DOI] [Google Scholar]
- Radović A., Mikuska T. Population size, distribution and habitat selection of the white-tailed eagle Haliaeetus albicilla in the alluvial wetlands of Croatia. Biologia (Bratisl). 2009;64:156–164. doi: 10.2478/s11756-009-0011-0. [DOI] [Google Scholar]
- Rutz C., Loretto M., Bates A.E., Davidson S.C., Duarte C.M., Jetz W., Johnson M., Kato A., Kays R., Mueller T., Primack R.B., Ropert-coudert Y., Tucker M.A., Wikelski M., Cagnacci F. Quantify the effects of human activity on wildlife. Nat. Ecol. Evol. 2020 doi: 10.1038/s41559-020-1237-z. [DOI] [PubMed] [Google Scholar]
- Stier A.C., Samhouri J.F., Novak M., Marshall K.N., Ward E.J., Holt R.D., Levin P.S. Ecosystem context and historical contingency in apex predator recoveries. Sci. Adv. 2016;2 doi: 10.1126/sciadv.1501769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara G., May R.M., Ye H., Hsieh C., Deyle E.R., Fogarty M.J., Munch S.B. Detecting causality in complex ecosystems. Science (80-.) 2012;338:496–500. doi: 10.1126/science.1227079. [DOI] [PubMed] [Google Scholar]
- Wayland K., Wilson L.K., Elliott J.E., Miller J.R., Froese M.W. Mortality, morbidity, and lead poisoning Western Canada, 1986–98. J. Raptor Res. 2003;37:8–18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Common murres disturbed from their ledges by an approaching white-tailed eagle.
Common murres disturbed from their ledges by an actively hunting white-tailed eagle.
Subadult Herring gull Larus argentatus preying on murre egg following eagle disturbance (lower ledge).
Common murre egg rolling down during fly out caused by eagle disturbance (lower ledge).