Abstract
Background and study aims Optimal peri-colonoscopic management of clopidogrel remains unclear. Cold snare polypectomy is safe and effective for removing polyps ≤ 10 mm and clips can control intraprocedural bleeding. We conducted a randomized controlled trial to compare continuation of clopidogrel versus temporary replacement of clopidogrel with aspirin for routine colonoscopy using cold snare polypectomy for polyps ≤ 10 mm.
Patients and methods Between August 2016 and August 2019, consenting participants at 12 centers were randomized to continuation of clopidogrel as a single or dual antiplatelet agent, or to temporarily take aspirin alone from 7 days prior to 2 days after routine colonoscopy. Endoscopists were blinded to group allocation. Cold snare polypectomy was used to remove polyps ≤ 10 mm, with endoscopic clips applied if intraprocedural bleeding continued for > 2 minutes. Follow-up was performed on Day 30. The trial was stopped early due to delayed patient enrollment.
Results Two hundred seventy-six consecutive polyps ≤ 10 mm were removed from 107 patients. Of the patients, 61.7 % were male with a median age of 69 years (interquartile range [IQR] 63 to 76.75). Fifty-nine patients continued on clopidogrel and 48 temporarily took aspirin instead. One hundred thirty-four polyps were removed from 49 patients who continued on clopidogrel vs 142 from 43 patients temporarily took aspirin instead ( P = 0.33). Intraprocedural bleeding requiring clips occurred in 11 of 49 patients who continued on clopidogrel and in two of 43 patients who temporarily took replacing with aspirin instead ( P = 0.02). More post-procedural minor bleeding was seen in the aspirin arm (six of 43 vs one of 49; P = 0.03). One patient in each arm had acute coronary syndrome, which was medically managed. None of the patients had clinically significant post-procedural bleeding.
Conclusions Continuation of clopidogrel in patients undergoing cold snare polypectomy for colorectal polyps ≤ 10 mm does not appear to increase the rate of clinically significant postpolypectomy bleeding. It is associated with an increase in intraprocedural bleeding, which can be successfully treated with clips.
Introduction
With the aging population, the presence of concurrent cardiovascular disease means that increasing proportions of patients who require colonoscopy are also taking antiplatelet agents, such as the P2Y12 inhibitor clopidogrel, as single or dual therapy with aspirin. Peri-endoscopic management creates a conundrum; temporary interruption could place patients at risk of thromboembolic complications whereas peri-endoscopic continuation creates concern about post-polypectomy bleeding.
Current international guidelines recommend continuing clopidogrel for low-risk procedures such as diagnostic colonoscopy, but discontinuing it for high-risk procedures 1 2 . However, these guidelines consider colonoscopic polypectomy a high-risk procedure without differentiating between removal of small polyps (< 10-mm diameter), which are considered low risk 3 , and larger polyps. In contrast, aspirin monotherapy has been shown to be safe in colonoscopic polypectomy.
Modest data favor cold snare over hot snare polypectomy 4 for removal of small polyps in patients on anticoagulants 5 and mechanical endoscopic clips are available if peri-endoscopic bleeding occurs. The Clopidogrel Uninterrupted Postpolypectomy Bleeding Trial 6 demonstrated a statistically non-significant increased number of cases of delayed post-polypectomy bleeding in the patients on uninterrupted clopidogrel. However, the majority of these polyps were removed via hot snare. Another randomized controlled trial (RCT) using cold snare polypectomy techniques compared discontinuation of thienopyridines with continuation had one patient with a clinically significant bleeding while continuing their antiplatelet agent but, given the small sample size, this was not statistically significant 7 .
The primary aim of this study was to compare temporary replacement of clopidogrel with aspirin with continuation of clopidogrel during routine colonoscopy, with regard to intraprocedural and post-procedural bleeding after cold snare polypectomy of polyps ≤ 10 mm in diameter and endoscopic placement of hemoclips for intraprocedural hemostasis.
Patients and method
Study design and study participants
A parallel group, endoscopist-blinded RCT was performed to compare temporary interruption of clopidogrel with a switch to aspirin 7 days prior to colonoscopy and recommencement of clopidogrel 2 days after colonoscopy with continuation of clopidogrel. The protocol has been outlined in detail elsewhere 8 . Any patient aged > 18 years scheduled for routine colonoscopy who was on single-agent clopidogrel or dual antiplatelet therapy with aspirin and clopidogrel was invited to participate. Exclusion criteria included liver cirrhosis, chronic renal impairment (estimated glomerular filtration rate ≤ 30 mL/min/1.73 m 2 ), history of bleeding diathesis, thrombocytopenia of any cause (platelet count ≤ 90 x10 9 /L), use of other concurrent anticoagulation/antiplatelet agents, percutaneous coronary intervention (bare metal stent within the last 30 days or drug-eluting stent within the last 12 months), acute coronary syndrome within the last 90 days, any other concern by treating physician(s), and inability to provide informed consent. The study was conducted at 12 centers in Australia and New Zealand. Written informed consent from individual patients or their representatives was obtained to participate in the trial.
Randomization
Sites were provided with uniquely identified sealed envelopes containing randomization instructions according to a computer-generated randomization schedule, in a ratio of 1:1. The endoscopist performing the procedure was blinded to the randomization arm.
Intervention
For those randomized to the interrupted clopidogrel arm, clopidogrel was stopped 7 days prior to colonoscopy and restarted 2 days after colonoscopy. Patients on single-agent clopidogrel were started on low-dose aspirin daily for the period off clopidogrel. If patients were on dual antiplatelet therapy, clopidogrel was stopped and aspirin continued.
Procedures
Colonoscopy was performed by a consultant endoscopist or endoscopy fellow under their direct supervision. If polyps were found, management was at the discretion of the endoscopist to ensure optimal patient care. However, to be included in the primary endpoint analysis, cold snare polypectomy of polyps ≤ 10 mm was required. The polypectomy site was observed for persistent bleeding over 2 minutes. If hemostasis had not occurred, adjunctive therapy in the form of mechanical through-the-scope clips was used. In addition, ≤ 10 polyps were removed during a single colonoscopy to mitigate risk of bleeding due to multiple polypectomies.
Management of polyps > 10 mm was at the discretion of the endoscopist, with options including piecemeal cold snare, endoscopic mucosal resection or rescheduling the procedure. If > 10 polyps or any polyp > 10 mm was removed, those patients were not included in the safety analysis or primary endpoint.
Follow-up occurred on day 30 via clinic or telephone to assess for study outcomes and adverse events (AEs).
Study outcomes
A composite primary end point was used, which captured both intraprocedural and post-procedural risk of bleeding. This comprised: 1) use of endoscopic clips post-polypectomy to control persistent intraprocedural bleeding (defined as bleeding persisting for > 2 minutes); 2) major delayed bleeding, which was symptomatically or clinically overt and associated with an unplanned admission or readmission to hospital for rectal bleeding; or 3) bleeding that directly contributed to death.
Secondary endpoints were other bleeding and thromboembolic complications including: 1) minor bleeding defined as any sign or symptom of per-rectal bleeding that did not fit the above criteria; 2) need for further intervention for bleeding such as endoscopic, surgical or radiological intervention; 3) requirement for red blood cell transfusion; 4) transient ischemic attack; 5) stroke; 6) myocardial infarction 9 ; and 7) any other serious AE.
Sample size calculation
The proportion of patients in a treatment arm who experienced a post-polypectomy intraprocedural bleed requiring an endoscopic clip or major delayed bleeding was calculated using the number of Full Analysis Set patients in that treatment arm as the denominator. Our conjecture was that the proportion in the control arm (π c ) was 0.10 and we tested the null hypothesis that the two treatment arms had the same proportions (H 0 : π t = π c ) with a two-sided binomial test conducted at the 5 % significance level (α = 0.05). Under the specific alternative that the treatment arms differed by 0.06 or more (|π t – π c | > 0.06), we required at least 980 evaluable patients (490 in each treatment arm) in order for the two-sided test to have 80 % power (East 6, Cytel Inc., Cambridge, Massachusetts, United States). Provision was made for two interim analyses of the primary end point, equally spaced after n = 331 and n = 661 patients were accrued 8 .
Statistical methods
Statistical analyses were performed using SPSS version 25, Chicago, Illinois, United States. Data are presented as median (interquartile range [IQR]) and frequency (%). A Mann-Whitney U test was used to compare continuous data. For categorical data, Chi-squared and Fisher’s exact tests were used. A multivariable logistic regression was used to evaluate the effect on the comparison of the treatment arms of variables that might be associated with the composite primary end point. P ≤ 0.05 was considered significant.
Ethics and data safety monitoring board
The trial received ethical approval from the following ethics committees: Alfred Hospital Human Research and Ethics Committee HREC number HREC/16/Alfred/22 to conduct the study at public hospitals in Victoria, New South Wales and Queensland; Epworth Health Care reference number EF2016–128; Calvary Health Care Adelaide HREC number 17-CHREC-F001; St John of God Health Care local reference number 1020 and by the Canterbury District Health Board, New Zealand, study reference 16/STH/112.The trial was registered on the Australian New Zealand Clinical Trials Registry (ANZCTR), registration number ACTRN12616000895482.
An independent data safety monitoring board was appointed to review all serious AEs, to interpret interim analysis of the primary endpoint and to review any protocol amendments. It was composed of three gastroenterologists, one of whom has a Masters in Public Health with specialization in biostatistics.
Results
Patient characteristics
Between August 2016 and August 2019, 109 participants were randomized and completed follow-up. Due to slow accrual over multiple sites, the data safety monitoring board deemed that achieving the accrual target would not be feasible and the trial was ceased. Two patients were excluded due to having > 10 polyps (n = 1) and a polyp > 10 mm being removed (n = 1) ( Fig. 1 ). Of the 107 patients included in the primary endpoint analysis, the median age was 69 years (IQR 63 to 77) and 66 % of participants were male ( Table 1 ). Sixty-six patients were on clopidogrel only and 41 were on dual antiplatelet therapy with clopidogrel and aspirin. Indications for clopidogrel use and for colonoscopy are detailed in Table 1 . The total number of patients invited to participate was incomplete at some sites so a CONSORT diagram could not be completed. Follow-up was complete for all patients included.
Fig. 1.
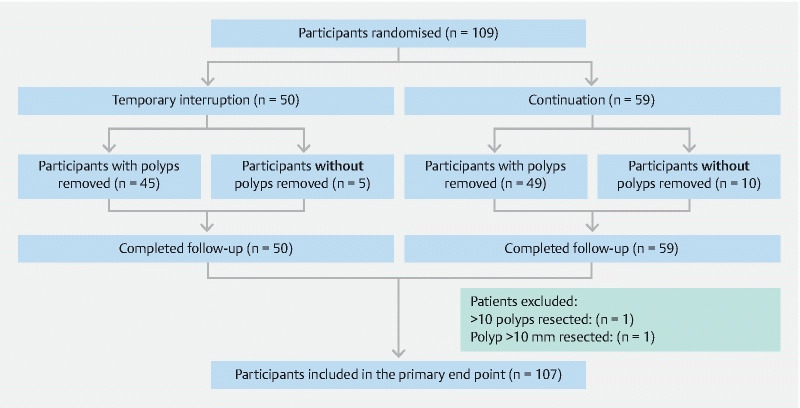
Flow diagram of study cohort.
Table 1. Patient characteristics and indication for clopidogrel and colonoscopy. * .
| Total n = 107 | Continuation of clopidogrel n = 59 | Temporary interruption of clopidogrel n = 48 | |
| Age | 69 [63 to 77] | 72 [61 to 79] | 68.5 [64 to 73] |
| Male | 66 (61.7 %) | 38 (64.4 %) | 28 (58.3 %) |
| Clopidogrel only | 66 (61.7 %) | 34 (58 %) | 32 (67 %) |
| Clopidogrel and aspirin | 41(38 %) | 25 (42 %) | 16 (33 %) |
| Indication for clopidogrel | |||
| Transient ischemic attack | 22 | 9 | 13 |
| Stroke | 20 | 7 | 13 |
| Peripheral vascular disease | 13 | 5 | 8 |
| Ischemic heart disease | 60 | 40 | 20 |
| Cardiac stents | 41 | 26 | 15 |
| Cardiac bypass surgery | 12 | 8 | 4 |
| Other | 6 | 4 | 2 |
| Indication for colonoscopy | |||
| Fecal occult blood test | 25 | 11 | 14 |
| Per-rectal bleeding | 18 | 9 | 9 |
| Iron deficiency anemia | 25 | 15 | 10 |
| Altered bowel habit | 25 | 13 | 12 |
| Polyp surveillance | 29 | 17 | 12 |
| Surveillance for history of CRC | 3 | 0 | 3 |
| Family history of CRC | 7 | 3 | 4 |
| Known polyp for therapy | 4 | 3 | 1 |
| Abdominal pain | 5 | 2 | 3 |
| Inflammatory bowel disease | 1 | 0 | 1 |
| Other | 12 | 5 | 7 |
CRC, colorectal cancer.
All results shown as median [interquartile ratio], n or n (%).
Polyp characteristics
Of the patients who continued clopidogrel, 49 of 59 (83.1 %) had polyps removed compared with 43 of 48 (89.6 %) of those who took aspirin temporarily as a replacement for clopidogrel ( P = 0.33). A median of two polyps (IQR 1 to 4) were removed per patient with a median size of 4 mm (IQR 3 to 5). The distribution of the size of polyps removed is outlined in Fig. 2 . The polyps removed predominantly had a Paris 0-IIa endoscopic appearance (208 of 276), and were in the left colon (187 of 276), with no significant difference between the two arms ( P = 0.06 and 0.39, respectively) ( Table 2 ).
Fig. 2.
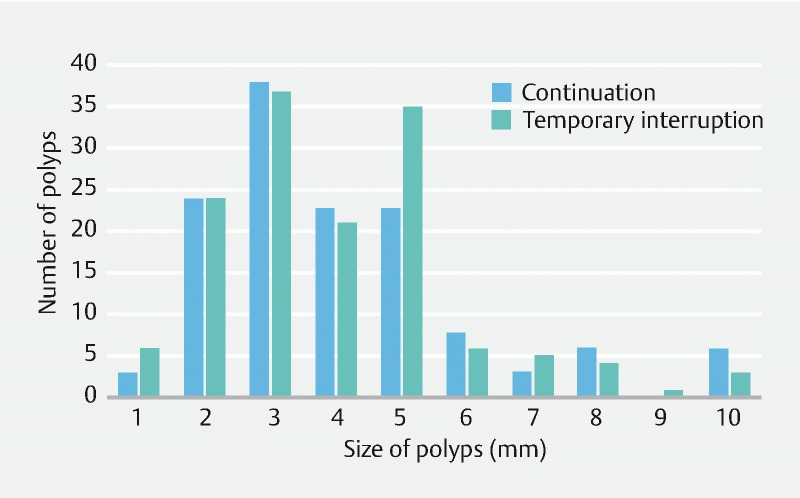
Size of polyps removed from the continuation of clopidogrel and temporary interruption arms.
Table 2. Polyp number and characteristics. 1 .
| Total n = 107 |
Continuation of clopidogrel n = 59 |
Temporary interruption of clopidogrel n = 48 | P value | |
| Number of patients with one or more polyps removed | 92 (86.0 %) | 49 (83.0 %) | 43 (89.6 %) | 0.33 |
| Median number of polyps per patient | 2 [1 to 4] | 2 [1 to 4] | 2 [1 to 4] | 0.29 |
| Median size | 4 [3 to 5] | 4 [3 to 5] | 4 [3 to 4] | 0.64 |
| Endoscopic appearance 1 | 0.06 | |||
|
8 | 7 | 1 | |
|
3 | 1 | 2 | |
|
57 | 32 | 25 | |
|
208 | 94 | 114 | |
| Location | 0.39 | |||
|
187 | 90 | 96 | |
|
89 | 44 | 46 |
Paris Classification.
Including and distal to the splenic flexure.
Primary endpoint
The composite primary endpoint (intraprocedural endoscopic clip use, major post-procedural bleeding or bleeding contributing to death) was seen in 13 of 107 patients (12 %) with 11 of 59 patients (19 %; 95 % CI: 9.7 % to 30.9 %) in the continuation arm and two of 48 (4 %; 95 % CI: 0.5 % to 14.3 %) in the temporary replacement arm ( P = 0.02). All of the events related to intraprocedural bleeding that persisted beyond 2 minutes require endoscopic clip application. No cases of clinically significant post-procedural bleeding were seen in either arm in this study ( Table 3 ).
Table 3. Number of patients with postpolypectomy bleeding and thromboembolic complications.
| Total n = 107 |
Continuation of clopidogrel n = 59 |
Temporary interruption of clopidogrel n = 48 | P value | |
| Intraprocedural bleeding requiring endoscopic clips | 13 | 11 | 2 | 0.02 |
| Post-procedural bleeding | 0.03 | |||
|
7 | 1 | 6 | |
|
0 | 0 | 0 | |
| Death | 0 | 0 | 0 | – |
| Thromboembolic complications | 2 | 1 | 1 | 0.88 |
| Composite primary endpoint | 13 | 11 (18.6 %) [95 % CI; 9.7 % to 30.9 %] |
2 (4.17 %) [95 % CI; 0.5 % to 14.3 %] |
0.02 |
CI, confidence interval.
Adjustment for factors potentially associated with the composite endpoint in multivariate logistic regression did not alter the statistically significant difference between the treatment arms ( Table 4 ).
Table 4. Multivariable logistic regression including factors with possible associations with the composite endpoint.
| Variable | Odds ratio | 95 % confidence interval | P value |
| Arm (temporary interruption vs continuation of clopidogrel) | 5.91 | 1.19 to 29.35 | 0.03 |
| Gender (male vs female) | 0.77 | 0.21 to 2.87 | 0.70 |
| Age (per 1 year) | 1.00 | 0.95 to 1.06 | 0.93 |
| Dual antiplatelet use prior to colonoscopy | 1.39 | 0.40 to 4.84 | 0.60 |
| Number of polyps removed | 1.02 | 0.76 to 1.38 | 0.88 |
Secondary endpoints
Two patients had cardiovascular complications, one in each arm ( P = 0.88). The first, a 63-year-old man on dual antiplatelet therapy for ischemic heart disease with a history of cardiac stents and coronary artery bypass grafts, was randomized to standard of care after discussion with the treating cardiology team. He developed symptoms of angina during recovery after a colonoscopy during which six polyps were removed, the largest polyp measuring 6 mm. Subsequent serial cardiac troponin I levels were negative. The next day, the patient presented with an elevated troponin I level (62 ng/L, normal range < 26 ng/L). A day later, he was discharged the on medical management. The second patient was a 67-year-old man with a history of cardiac stents for ischemic heart disease who was on dual antiplatelet therapy and randomized to the continuation arm. He had no polyps removed at colonoscopy. However, 6 days after the procedure, he presented with unstable angina with a troponin I rise to 60 ng/L, which required an inpatient coronary angiogram. The patient had no intervention and was medically managed.
Intraprocedural bleeding requiring clips occurred in 11 of 49 patients continuing on clopidogrel and in two of 43 patients who were temporarily placed on with aspirin ( P = 0.02). More post-procedural minor bleeding was seen in the temporary replacement arm (six of 43 vs one of 49; P = 0.03).
Discussion
Small polyps are commonly found during routine colonoscopy and rescheduling the procedure with temporary interruption of anticoagulants for subsequent removal of small polyps is not always practical, and exposes patients to the risks of additional bowel preparation, sedation, and colonoscopy. The current trial, therefore, compared the safety of temporary substitution of clopidogrel with aspirin to continuation of clopidogrel for routine colonoscopy. The protocol specified removal of all polyps ≤ 10 mm using cold snare and application of clips if there was ongoing bleeding after 2 minutes. The primary composite end point—use of rescue endoscopic clips post-polypectomy or major delayed bleeding—was chosen to encompass the bleeding issues that are of serious concern to a colonoscopist when removing polyps. Despite the predetermined sample size not being achieved due to poor accrual, a statistically significantly greater frequency of events occurred in the continuation-of-clopidogrel arm compared with the arm in which use of the drug was temporarily interrupted. Importantly, all of these events involved intraprocedural clip use for persistent intraprocedural post-polypectomy bleeding. There was a significantly increased risk of minor post-procedural bleeding in patients in whom clopidogrel was withheld, but clinically important post-procedural bleeding events were not observed.
Continuation of clopidogrel for routine colonoscopy is currently recommended due to the low-risk nature of these procedures. However, colonoscopic polypectomy has been considered a high-risk procedure, with temporary interruption of clopidogrel recommended. The majority of patients who undergo routine colonoscopy will not have polyps, and thus, temporary interruption for elective colonoscopy places the majority of them patients at unnecessary risk of thromboembolic events, which although infrequent, can have potentially devastating consequences. The PARIS (patterns of non-adherence to antiplatelet regimens in stented patients) registry 10 found the adjusted hazard ratios for major AEs for disruption and interruption of antiplatelet therapy were 1.50 (95 % CI 1.14–1.97, P = 0.004) and 1.41 (95 % CI 0.94–2.12; P = 0.10), respectively. It should be noted these were clinically significant AEs including cardiac death, definite or probable stent thrombosis, and myocardial infarction. Thus, it is important to state from the outset that individualized peri-endoscopic management of clopidogrel based on risk-benefit assessment is required. Patients who have had recent coronary percutaneous intervention, acute coronary syndrome or any concern from treating physicians should have their colonoscopy procedure deferred, if possible, until P2Y12 receptor antagonists can be safely temporarily interrupted.
Data for eligible patients were incomplete and deemed not appropriate for inclusion. Recruitment was much more difficult than expected. This related to three main factors. First, there were fewer patients than anticipated who remained on long-term antiplatelet therapy and required colonoscopy, principally due to changes in antiplatelet management guidelines during the study, whereby clopidogrel was stopped 12 months following drug-eluting stent insertion and not continued as long-term prophylaxis. Second, many of these patients had comorbidities such as generalized vasculopathy, and treating physicians (cardiologists, neurologist and vascular surgeons) were reluctant to withhold clopidogrel when the option existed to perform the procedure while these individuals were on antiplatelet therapy. Last, eligible patients were often elderly and informed consent was often declined.
There are increasing data favoring the safety of cold over hot snare polypectomy 4 . In the CUP (Clopidogrel Uninterrupted Postpolypectomy Bleeding) trial, the majority of patients who experienced delayed post-polypectomy bleeding had polyps resected by hot snare polypectomy 6 . Conversely, there are modest data supporting cold snare polypectomy when anticoagulation is continued 5 and our trial adds data to support the use of cold snare polypectomy to resect polyps ≤ 10 mm in patients on clopidogrel.
Although the accrual target was not achieved, 134 polyps were safely removed from patients who continued on clopidogrel. What constitutes intraprocedural bleeding remains undefined, yet it is common with cold snare polypectomy. In this study, if bleeding persisted, we used 2 minutes as a practical time limit before deeming intervention appropriate, so as not to prolong the length of the procedure unnecessarily, particularly if multiple polyps were removed.
While there is minimal evidence supporting the use of prophylactic clip closure to prevent delayed post-polypectomy bleeding following uncomplicated polypectomy 11 , in this study, clip use for control of persistent intraprocedural bleeding was appropriate, particularly as the endoscopist was blinded to clopidogrel cessation. Of interest, despite blinding, more clips were used for intraprocedural control of bleeding in the continued clopidogrel arm compared with the temporary-replacement-with-aspirin arm. Clips may stop the bleeding successfully, but they do carry a high cost.
The most salient limitation of this study was poor accrual. The study was powered to detect a small difference (6 %) in the rates, either 10 % vs 4 % or 10 % vs 16 %. The trial was stopped early, prior to the first scheduled interim analysis, for poor accrual. It transpired that the difference in the rates that was observed in the curtailed study, 14.5 % (95 %CI 3.0 % to 25.9 %) was larger than the conjectured difference.
While some readers may surmise that curtailment may have produced a false-positive outcome, we can only restate that no interim analysis was conducted before curtailment, and so, the false-positive rate was controlled at the 0.05 level. The “lesson learned” for the conduct of other randomized studies in this domain is that when there is unreliable information about event rates, it may be prudent to specify in the protocol blinded sample size re-estimation at a time point before the first scheduled interim analysis and to foreshadow the possibility of a protocol amendment at this time.
While no significant post-procedural bleeding events were seen during the trial, curtailment of the trial would have reduced the chance of detecting rare events and differences between the treatment arms in the rates of these rare events. The alternate option is that these questions might better be answered by observational studies, which are methodologically inferior reflect clinical practice.
We suggest that clopidogrel should be continued for diagnostic colonoscopy as currently recommended 1 12 and, when small subcentimeter polyps are found at colonoscopy, that a pragmatic approach be used. This includes cold snare polypectomy and application of endoscopic clips for any continued bleeding post-resection, which is quick and easy to do and also provides effective mechanical hemostasis. The associated additional cost is easily offset by avoiding the additional cost of repeat colonoscopy with temporary interruption of clopidogrel, notwithstanding the significant inconvenience to the patient and burden on limited endoscopic services. This study found that significantly more endoscopic clips were required in the continuation arm compared with the temporary replacement arm and their use in this context appears justified, as no clinically significant bleeding sequelae were seen following polypectomy of sub-centimeter polyps in patients on continued clopidogrel. Thus, despite the need to address intraprocedural bleeding, it was not associated with subsequent adverse outcomes. A larger study, possibly registry based, may be required to reassure endoscopists that serious AEs truly are rare in patients who continue on clopidogrel.
Conclusions
In conclusion, this RCT that compared the safety of continuation with temporary replacement of clopidogrel for diagnostic colonoscopy demonstrated that removal of sub-centimeter polyps with cold snare polypectomy in patients who continue on clopidogrel was associated with a greater risk of intraprocedural bleeding than if the drug was stopped. However mechanical hemostasis was achieved with endoscopic clips with obviation of further bleeding issues. These results underscore that, while there is a risk associated with performing polypectomy on patients receiving therapeutic clopidogrel, awareness of this risk and appropriate intraprocedural actions may sufficiently mitigate the risk of clinically significant post-polypectomy bleeding. Such practice will avert the need for unnecessary repeat colonoscopy and also allow patients to remain on their usual cardioprotective medication.
Acknowledgments
Dr. Ket is the recipient of a Monash University PhD scholarship.
Funding Statement
PhD scholarship for Dr Shara Ket
Footnotes
Competing interests The authors declare that they have no conflict of interest.
Correction.
Cold snare polypectomy of colorectal polyps ≤ 10 mm on clopidogrel: Australian and New Zealand randomized controlled trial Shara Ket, Douglas Tjandra, David G. Hewett et al. Endoscopy International Open 2022; 10: E745–E752. DOI: 10.1055/a-1813-1019 For this article the authorship list was corrected. Authors of this article are: Shara Ket 1, 2 , Douglas Tjandra 3 , David G. Hewett 4 , Ammar O. Kheir 4, 5, 6 , Andrew J. Metz 3 , AlanMoss 7, 8 , Ravinder Ogra 9 , William Tam 10, 11, 12 , Spiro Raftopoulos 13, 14 , John Reynolds 15 , Robyn Secomb 1 , Lauren Cavalieri 16 , Paul Urquhart 16 , Peter R. Gibson 1, 2 , Gregor Brown 1, 2, 17 . 1 Department of Gastroenterology, Alfred Health, Melbourne, Victoria, Australia 2 Monash University, Central Clinical School, Melbourne, Victoria, Australia 3 Department of Gastroenterology, Melbourne Health, Victoria, Australia 4 Department of Gastroenterology, Queen Elizabeth II Jubilee Hospital, Queensland 5 Digestive Disease Institute, Cleveland Clinic Abu Dhabi, Abu Dhabi, UAE 6 Faculty of Medicine, University of Queensland, Brisbane, Queensland, Australia 7 Department of Endoscopic Services, Western Health, Melbourne, Victoria, Australia 8 Department of Medicine – Western Health, Melbourne Medical School, The University of Melbourne, Victoria, Australia 9 Middlemore Hospital, Auckland, New Zealand 10 Royal Adelaide Hospital, South Australia, Australia 11 University of Adelaide, South Australia, Australia 12 Calvary North Adelaide Hospital, South Australia, Australia 13 Sir Charles Gairdner Hospital, Western Australia 14 Peel Health Campus, Western Australia 15 Biostatistics Consulting Platform, Faculty of Medicine, Nursing & Health Sciences, Monash University, Melbourne, Australia 16 Eastern Health, Victoria, Australia 17 Epworth Hospital, Richmond, Victoria, Australia This was corrected in the online version on 4 November 2022.
References
- 1.Veitch A M, Vanbiervliet G, Gershlick A H et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Endoscopy. 2016;48:385–402. doi: 10.1055/s-0042-102652. [DOI] [PubMed] [Google Scholar]
- 2.Acosta R D, Abraham N S, Chandrasekhara V et al. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc. 2016;83:3–16. doi: 10.1016/j.gie.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 3.Heldwein W, Dollhopf M, Rösch T et al. The Munich Polypectomy Study (MUPS): prospective analysis of complications and risk factors in 4000 colonic snare polypectomies. Endoscopy. 2005;37:1116–1122. doi: 10.1055/s-2005-870512. [DOI] [PubMed] [Google Scholar]
- 4.Ket S N, Mangira D, Ng A et al. Complications of cold versus hot snare polypectomy of 10-20mm polyps: a retrospective cohort study. JGH Open. 2019;4:172–177. doi: 10.1002/jgh3.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horiuchi A, Nakayama Y, Kajiyama M et al. Removal of small colorectal polyps in anticoagulated patients: a prospective randomized comparison of cold snare and conventional polypectomy. Gastrointest Endosc. 2014;79:417–423. doi: 10.1016/j.gie.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 6.Chan F KL, Kyaw M H, Hsiang J C et al. Risk of Postpolypectomy bleeding with uninterrupted clopidogrel therapy in an industry-independent, double-blind, randomized trial. Gastroenterology. 2019;156:918–9250. doi: 10.1053/j.gastro.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 7.Won D, Kim J S, Ji J S et al. Cold snare polypectomy in patients taking dual antiplatelet therapy: a randomized trial of discontinuation of thienopyridines. Clin Transl Gastroenterol. 2019;10:e00091. doi: 10.14309/ctg.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ket S, Metz A, Moss A et al. Study design of endoscopic polypectomy on clopidogrel (EPOC): A randomised controlled trial. Contemp Clin Trial Comm. 2019;16:100479. doi: 10.1016/j.conctc.2019.100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert J S, Jaffe A S et al. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 10.Mehran R, Baber U, Steg P G et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet. 2013;382:1714–1722. doi: 10.1016/S0140-6736(13)61720-1. [DOI] [PubMed] [Google Scholar]
- 11.Mangira D, Ket S, Majeed A et al. Postpolypectomy prophylactic clip closure for the prevention of delayed postpolypectomy bleeding: A systematic review. JGH Open. 2018;2:105–110. doi: 10.1002/jgh3.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gralnek I M, Dumonceau J M, Kuipers E J et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1–46. doi: 10.1055/s-0034-1393172. [DOI] [PubMed] [Google Scholar]


