Abstract
BACKGROUND
Venoarterial extracorporeal membrane oxygenation (VA-ECMO) increases left ventricular (LV) afterload, potentially provoking LV distention and impairing recovery. LV mechanical unloading (MU) with intra-aortic balloon pump (IABP) or percutaneous ventricular assist device (pVAD) can prevent LV distension, potentially at the risk of more complications, and net clinical benefit remains uncertain.
OBJECTIVES
This study aims to determine the association between MU and outcomes for patients undergoing VA-ECMO.
METHODS
The authors queried the Extracorporeal Life Support Organization registry for adults receiving peripheral VA-ECMO from 2010 to 2019 and stratified them by MU with IABP or pVAD. The primary outcome was in-hospital mortality; secondary outcomes included on-support mortality and complications during VA-ECMO.
RESULTS
Among 12,734 VA-ECMO patients, 3,399 (26.7%) received MU: 2,782 (82.9%) IABP and 580 (17.1%) pVAD. MU patients were older (age 56.3 vs 52.7 years) and, before extracorporeal membrane oxygenation, more often required >2 vasopressors (41.7% vs 27.2%) and had respiratory (21.1% vs 15.9%), renal (24.6% vs 15.8%), and liver failure (4.4% vs 3.1%) (all P < 0.001). MU patients had lower in-hospital mortality (56.6% vs 59.3%, P = 0.006), which persisted in multivariable modeling (adjusted OR [aOR]: 0.84; 95% CI: 0.77–0.92; P < 0.001). MU was associated with more cannula site bleeding (aOR: 1.25; 95% CI: 1.11–1.40; P < 0.001) and hemolysis (aOR: 1.27; 95% CI: 1.03–1.57; P = 0.02). Compared to pVAD, MU patients with IABP had similar mortality (aOR: 0.80; 95% CI: 0.64–1.01; P = 0.06) and less medical bleeding (aOR: 0.45; 95% CI: 0.31–0.64; P < 0.001), cannula site bleeding (aOR: 0.72; 95% CI: 0.54–0.96; P = 0.03), and renal injury (aOR: 0.78; 95% CI: 0.62–0.98; P = 0.03).
CONCLUSIONS
Among adults receiving VA-ECMO, MU was associated with lower in-hospital mortality despite increased complications including hemolysis and cannulation site bleeding. Compared to pVAD, MU with IABP was associated with similar mortality and lower complication rates.
Keywords: intra-aortic balloon pump, percutaneous ventricular assist device, survival, unloading, venoarterial extracorporeal membrane oxygenation
Venoarterial extracorporeal membrane oxygenation (VA-ECMO) provides temporary circulatory support for patients with cardiogenic shock or cardiac arrest refractory to standard therapies. Peripheral cannulation for VA-ECMO results in retrograde flow to the proximal aorta and substantial increase in left ventricular (LV) afterload, often leading to increased LV end-diastolic pressure and decreased stroke volume.1 This phenomenon of LV distention can result in pulmonary edema, thrombus formation in the left heart due to stasis, and myocardial ischemia from decreased transcoronary perfusion gradient, potentially impairing myocardial recovery and contributing to poor outcomes with VA-ECMO.2,3
Available strategies to prevent LV distension include medical therapy to enhance LV ejection, such as inotropes or arterial vasodilators, or mechanical unloading (MU) most commonly using intra-aortic balloon pump (IABP) counter-pulsation or percutaneous ventricular assist device (pVAD).4 Although a clear physiologic rationale exists for MU during VA-ECMO, there are no randomized controlled trials comparing LV unloading strategies. Recent observational studies have suggested lower mortality among VA-ECMO patients receiving MU, but a survival benefit has not been consistent and MU has been associated with increased complications, including bleeding, limb ischemia, hemolysis and renal injury.5–11
Uncertainty remains about a net benefit of MU during VA-ECMO. Furthermore, there are no large studies comparing MU modality (IABP vs pVAD), timing (upfront vs delayed), and outcomes across different clinical indications for VA-ECMO such as acute myocardial infarction (AMI) shock and chronic heart failure (CHF). We leveraged the Extracorporeal Life Support Organization (ELSO) registry to compare outcomes, including in-hospital mortality and complication rates, among adult VA-ECMO patients managed with and without a MU strategy.
METHODS
DATA SOURCE.
The ELSO registry is a voluntary international registry of extracorporeal membrane oxygenation (ECMO) that by 2019 included 463 centers, with the majority located in North America (59.8%) and Europe (17.3%).12 Patient characteristics, pre-ECMO interventions, ECMO circuit details, adverse events, and outcomes are recorded using a standardized data collection form. Clinical diagnoses and comorbidities are reported with International Classification of Diseases-9th/10th Revision-Clinical Modification (ICD-9/10-CM) codes. Procedures occurring after ECMO initiation including insertion or removal of MU devices are reported using Current Procedural Terminology (CPT) codes (Supplemental Table 1). Studies using the ELSO database are exempt from Institutional Review Board approval due to the retrospective analysis of de-identified data.
STUDY POPULATION.
We included adults (age ≥18 years) receiving VA-ECMO from 2010 to 2019 with peripheral femoral-femoral cannulation. We excluded patients with multiple VA-ECMO runs, central cannulation, and other nonfemoral arterial cannulation approaches. We excluded patients with pulmonary embolism as the primary indication for VA-ECMO, given that LV function is typically preserved and thus LV distension is rare, as well as patients with heart transplant, congenital heart disease, and valvular heart or aortic disease where cannulation is often central or there could be anatomic considerations precluding a MU strategy with IABP or pVAD.
EXPOSURE AND OUTCOMES.
We stratified patients based on the use of MU with either IABP or pVAD, and we identified these devices from the pre-ECMO support form and/or CPT codes entered for device insertion and removal. MU was defined as either: 1) presence of IABP or pVAD in the 24 hours before ECMO initiation without coding for device removal at the time of ECMO cannulation; or 2) CPT code for IABP or pVAD insertion at or after ECMO initiation. In cases where multiple devices were used, the device inserted closest to ECMO initiation was considered the prevailing MU device. MU devices were categorized based on the timing of insertion relative to ECMO initiation: 1) “upfront” was defined as MU device insertion before or at the time of ECMO cannulation; and 2) “delayed” was defined as device placement any time after ECMO initiation.
The primary outcome was in-hospital mortality. Secondary outcomes included on-support mortality and rates of important complications including bleeding events, hemolysis, ischemic stroke, limb ischemia, and renal injury (Supplemental Table 2). Medical bleeding included hemorrhagic stroke and pulmonary or gastrointestinal bleeding. Nonmedical bleeding included tamponade as well as mediastinal, surgical site, or cannulation site bleeding. The primary diagnoses for VA-ECMO were identified using ICD-9/10-CM codes (Supplemental Table 3) and organized into the following groups: AMI, CHF, myocarditis, and ventricular tachycardia/ventricular fibrillation (VT/VF). Concomitant organ failures at the time of ECMO support, including renal, liver, and respiratory failure, were also categorized according to ICD-9/10-CM codes (Supplemental Table 4).
STATISTICAL ANALYSIS.
Categorical variables are presented as count (%) and continuous variables with mean ± SD or median (IQR), as appropriate. Differences between groups were tested by 1-way analysis of variance or the Wilcoxon rank sum for continuous variables and the chi-square test for categorical variables. Time-to-event data are not provided; therefore, in-hospital mortality, on-support mortality, and complication rates were analyzed as dichotomous outcomes and compared with chi-square testing and multivariable logistic regression modeling. Covariates for multivariable modeling included age, sex, race, weight, AMI as primary indication for ECMO, pre-ECMO cardiac arrest, VA-ECMO duration, year of support, concomitant organ failures, pH before ECMO initiation, and number of vasopressors at the time of ECMO. Because of the moderate rate of missing observations for pH before ECMO (30.2%), we performed multiple imputation on pH, using sequential regression using IVEware (University of Michigan Survey Research Center, Institute for Social Research, Ann Arbor, Michigan, USA) creating a single imputation dataset to perform the analysis. As a sensitivity analysis, we generated all logistic regression models without imputed pH. We examined the association of MU with in-hospital mortality across important subgroups of age, sex, pre-ECMO cardiac arrest, extracorporeal cardiopulmonary resuscitation (ECPR), and primary diagnosis.
Given the potential for confounding in this observational dataset, we conducted additional confirmatory analyses using propensity-matching for the exposure groups: 1) MU vs no MU; 2) upfront vs delayed MU; and 3) IABP vs pVAD. To calculate the propensity score, we used the same variables as the multivariable logistic regression models. Given that pVADs only appeared in the most recent years of the registry, the analytic cohort for IABP vs pVAD was restricted to the years from 2017 to 2019. Cases were matched with controls using a caliper width of 0.2 × SD of the logit of the propensity score, using nearest-neighbor matching without replacement.13 Subjects were matched 1:1 for all analyses except for the MU timing, where we matched upfront and delayed MU patients 2:1 given the small number of patients with delayed MU. Between-group balance in matched covariates was assessed by calculating the standardized difference, with a threshold of 10% used to define matching success (Supplemental Table 5).
We defined MU rate for each center as the number of VA-ECMO patients managed with a MU device divided by the total number of VA-ECMO patients at that center. For this analysis, because MU rate could be disproportionately skewed by centers with very low ECMO volumes, we excluded centers with <3 years of data entered into the registry and <5 total cases of VA-ECMO per year. We compared in-hospital mortality across tertiles of MU rate with multivariable logistic regression modeling as above and tested for a statistical interaction between the use of MU and MU rate. A value of P < 0.05 was used to define statistical significance. All analyses were performed using SAS version 9.4.
RESULTS
PATIENT CHARACTERISTICS.
Among 17,390 adult patients receiving VA-ECMO from 2010 to 2019, 12,734 patients met the study’s inclusion criteria. MU was used in 3,399 patients (26.7%), of which 2,819 (82.9%) were managed with IABP and 580 (17.1%) with pVAD (Figure 1). MU patients were older (56.3 years vs 52.7 years), more often White (58.5% vs 54.3%) and male (76.3% vs 68.5%), were heavier (87.0 kg vs 83.4 kg), and were more likely to have AMI as the primary reason for ECMO (43.0% vs 21.6%) (P < 0.001 for all). At VA-ECMO initiation, MU patients were more likely to have concomitant respiratory (21.1% vs 15.9%), renal (24.6% vs 15.8%), and liver failure (4.4% vs 3.1%), and were more likely to be on >2 vasopressors (41.7% vs 27.2%) (P < 0.001 for all). MU patients had slightly higher pH at cannulation (7.24 vs 7.21, P < 0.001), were less likely to have pre-ECMO arrest (52.0% vs 55.0%, P = 0.009), and were less likely to receive ECPR (19.1% vs 36.2%, P < 0.001) (Table 1). After propensity matching, baseline characteristics were well-balanced (all SD <10%) between 3,079 patients with MU and 3,079 controls without MU (Supplemental Table 5).
FIGURE 1. Flow Diagram of Patient Selection.
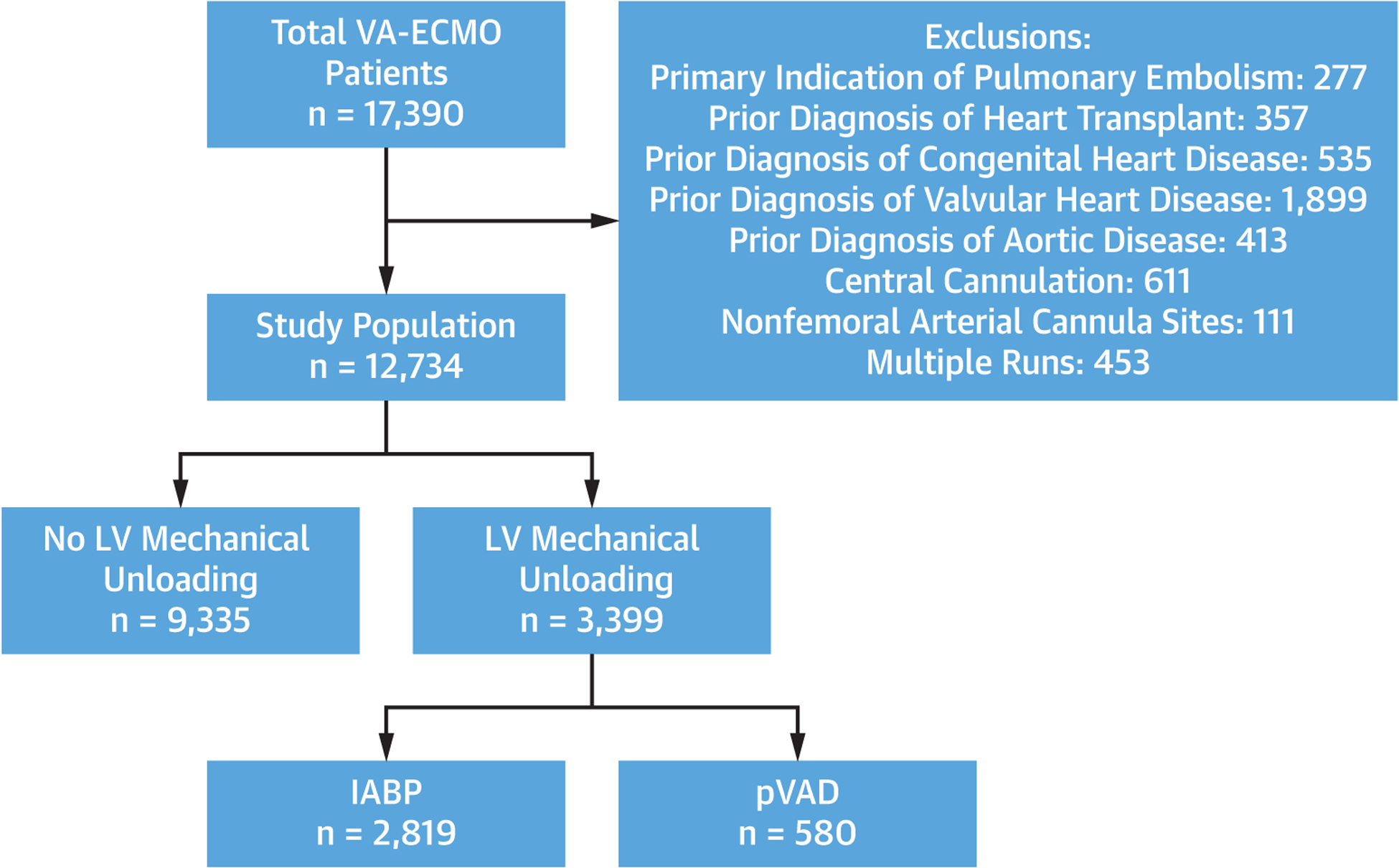
Flow chart of patient selection for the analytic cohort. IABP = intra-aortic balloon pump; LV = left ventricle; pVAD = percutaneous ventricular assist device; VA-ECMO = venoarterial extracorporeal membrane oxygenation.
TABLE 1.
Baseline Characteristics of Adults Supported With VA-ECMO Stratified by Left Ventricular Mechanical Unloading Use
| Total (N = 12,734) | No Mechanical Unloading (n = 9,335) | Mechanical Unloading (n = 3,399) | P Value | |
|---|---|---|---|---|
| Age, y | 53.7 ± 14.5 | 52.7 ± 15.1 | 56.3 ± 12.5 | <0.001 |
| Male | 8,925 (70.6) | 6,341 (68.5) | 2,584 (76.3) | <0.001 |
| Weight kg | 84.4 ± 22.4 | 83.4 ± 22.6 | 87.0 ± 21.4 | <0.001 |
| Caucasian | 7,057 (55.4) | 5,070 (54.3) | 1,987 (58.5) | <0.001 |
| Primary diagnosis | ||||
| Acute myocardial infarction | 3,220 (27.6) | 1,804 (21.6) | 1,416 (43.0) | <0.001 |
| Chronic heart failure | 2,987 (25.6) | 2,047 (24.5) | 940 (28.5) | <0.001 |
| VT/VF | 944 (8.1) | 595 (7.1) | 349 (10.6) | <0.001 |
| Myocarditis | 202 (1.7) | 157 (1.9) | 45 (1.4) | 0.06 |
| Concomitant organ failure | ||||
| Respiratory | 2,198 (17.3) | 1,481 (15.9) | 717 (21.1) | <0.001 |
| Renal | 2,313 (18.2) | 1,477 (15.8) | 836 (24.6) | <0.001 |
| Liver | 440 (3.5) | 289 (3.1) | 151 (4.4) | <0.001 |
| ECPR modality | 4,030 (31.6) | 3,380 (36.2) | 650 (19.1) | <0.001 |
| Pre-ECMO arrest | 6,755 (54.2) | 5,000 (55.0) | 1,755 (52.0) | 0.009 |
| Pre-ECMO MAP, mm Hg | 62.17 ± 21.74 | 61.48 ± 21.71 | 63.81 ± 21.72 | <0.001 |
| Pre-ECMO pH | 7.22 ± 0.18 | 7.21 ± 0.18 | 7.24 ± 0.17 | <0.001 |
| Vasopressors/inotropes | <0.001 | |||
| 0–2 | 8,778 (68.9) | 6,795 (72.8) | 1,983 (58.3) | |
| >2 | 3,956 (31.1) | 2,540 (27.2) | 1,416 (41.7) | |
| Time on ECMO, d | 4.00 (2.00–7.00) | 4.00 (2.00–7.00) | 5.00 (3.00–8.00) | <0.001 |
Values are mean ± SD, n (%), or median (IQR).
ECMO = extracorporeal membrane oxygenation; ECPR = extracorporeal cardiopulmonary resuscitation; MAP = mean arterial pressure; VA-ECMO = venoarterial extracorporeal membrane oxygenation; VT/VF = ventricular tachycardia/ventricular fibrillation.
VA-ECMO AND LV MU USE OVER TIME.
The proportion of patients managed with MU remained stable at ~25% until 2018 when there was a sharp increase in pVAD use and concomitantly in overall MU rate, reaching 35% of VA-ECMO cases in 2019 (P-trend = 0.004) (Central Illustration). IABP was the predominant MU device used, but the proportion of pVAD cases rapidly increased over the final 3 years of the study, reaching 46.4% of MU devices in 2019.
CENTRAL ILLUSTRATION. Left Ventricular Mechanical Unloading during Venoarterial Extracorporeal Membrane Oxygenation: Temporal Trends and Association With Outcomes.
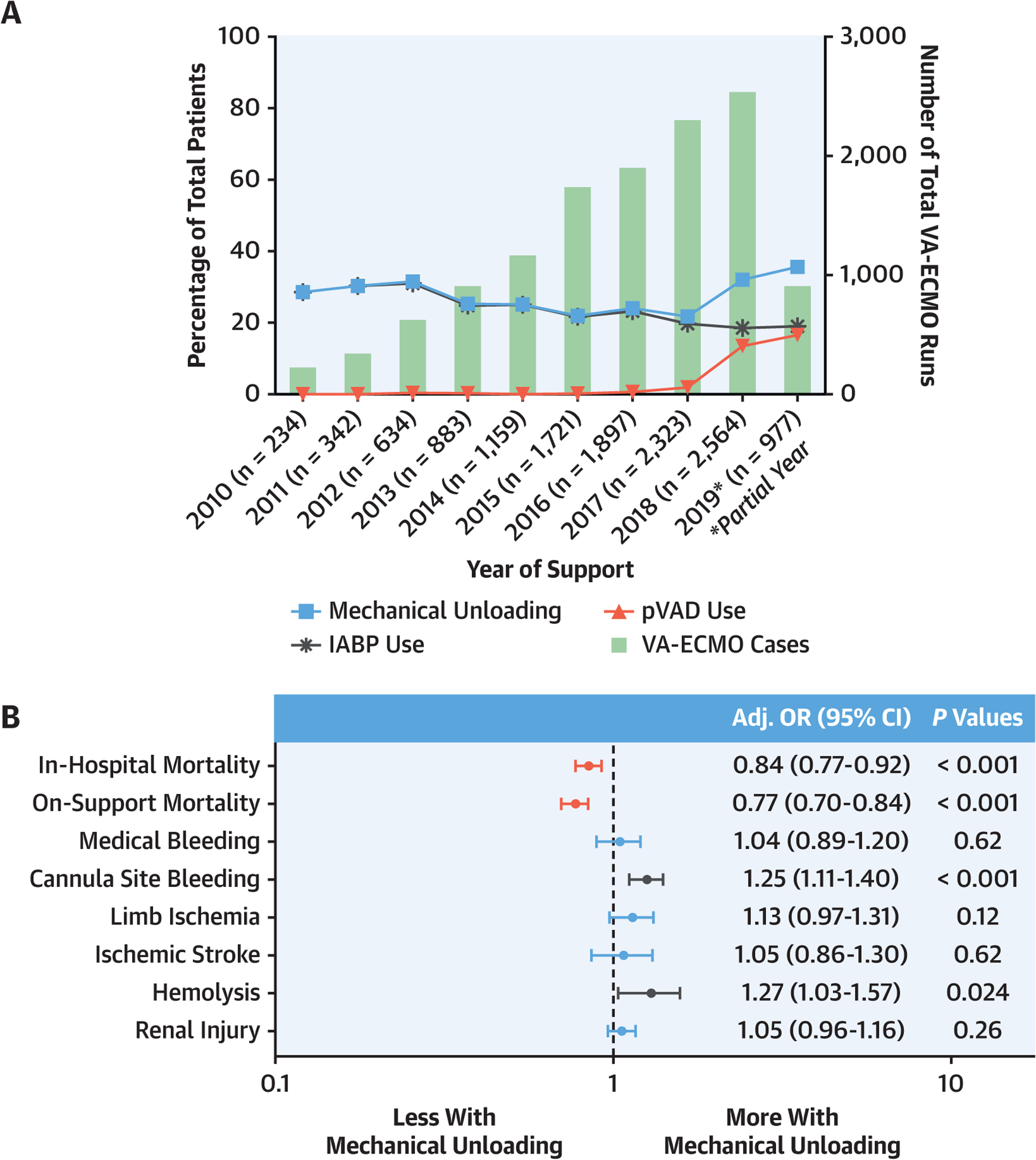
(A) Rates of left ventricular mechanical unloading with intra-aortic balloon pump (IABP) or percutaneous ventricular assist device (pVAD) in patients undergoing venoarterial extracorporeal membrane oxygenation (VA-ECMO) from 2010 to 2019. (B) Forest plot of the OR (95% CI) from multivariable logistic regression modeling examining the association of left ventricular mechanical unloading and outcomes in VA-ECMO patients.
MU AND SURVIVAL.
MU patients had lower unadjusted on-support (41.5 % vs 47.9%) and in-hospital (56.6% vs 59.3%) mortality (Table 2). After multivariable logistic regression modeling, MU retained an independent association with lower on-support mortality (adjusted OR [aOR]: 0.77; 95% CI: 0.70–0.84; P < 0.0001) and in-hospital mortality (aOR: 0.84; 95% CI: 0.77–0.92; P < 0.001) (Central Illustration). These findings remained similar when the multivariable models were constructed without imputing pH (Supplemental Table 6) and in the propensity-matched cohort (Supplemental Table 7). The association of MU with lower in-hospital mortality remained fairly consistent across clinical sub-groups, but a significant interaction effect was observed in patients aged <50 years and those experiencing pre-ECMO cardiac arrest (Figure 2).
TABLE 2.
Outcomes in Adults Supported With VA-ECMO Stratified by Left Ventricular Mechanical Unloading Use
| Total (N = 12,734) | No Mechanica Unloading (n = 9,335) | Mechanical Unloading (n = 3,399) | P Value | |
|---|---|---|---|---|
| In-hospital mortality | 7,456 (58.6) | 5,533 (59.3) | 1,923 (56.6) | 0.006 |
| On-support mortality | 5,878 (46.2) | 4,468 (47.9) | 1,410 (41.5) | <0.001 |
| Medical bleeding | 1,091 (8.6) | 761 (8.2) | 330 (9.7) | 0.005 |
| Hemorrhagic stroke | 296 (2.3) | 211 (2.2) | 85 (2.5) | 0.42 |
| Pulmonary | 274 (2.2) | 209 (2.2) | 65 (1.9) | 0.26 |
| Gastrointestinal | 593 (4.7) | 393 (4.2) | 200 (5.9) | <0.001 |
| Nonmedical bleeding | 2,662 (20.9) | 1,818 (19.5) | 844 (24.8) | <0.001 |
| Cannula site | 1,849 (14.5) | 1,242 (13.3) | 607 (17.9) | <0.001 |
| Surgical site | 909 (7.1) | 643 (6.9) | 266 (7.8) | 0.07 |
| Mediastinal site | 14 (0.1) | 10 (0.1) | 4 (0.1) | 0.87 |
| Tamponade | 247 (1.9) | 165 (1.8) | 82 (2.4) | 0.019 |
| Hemolysis | 467 (3.7) | 308 (3.3) | 159 (4.7) | <0.001 |
| Ischemic stroke | 514 (4.0) | 355 (3.8) | 159 (4.7) | 0.026 |
| Limb ischemia | 1,028 (8.1) | 720 (7.7) | 308 (9.1) | 0.013 |
| Renal injury | 4,229 (33.2) | 2,979 (31.9) | 1,250 (36.8) | <0.001 |
| Cr elevation 1.5–3 | 523 (4.1) | 434 (4.6) | 89 (2.6) | <0.001 |
| Cr elevation >3 | 1,251 (9.8) | 818 (8.8) | 433 (12.7) | <0.001 |
| Need for RRT | 3,436 (27.0) | 2,375 (25.4) | 1,061 (31.2) | <0.001 |
Values are n (%).
Cr = creatinine; RRT = renal replacement therapy; other abbreviation as in Table 1.
FIGURE 2. Left Ventricular Mechanical Unloading and In-Hospital Mortality Across Subgroups.
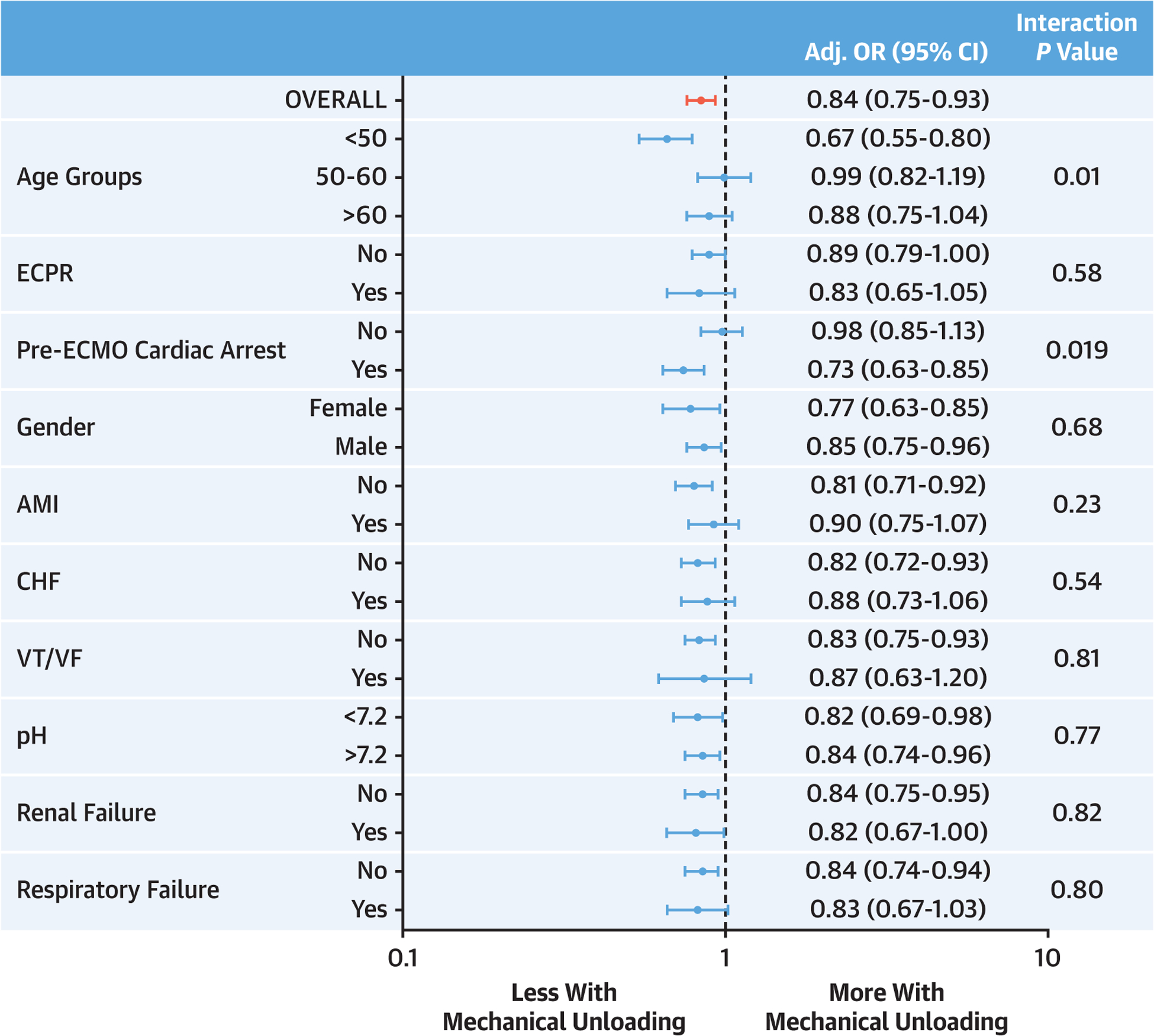
Forest plot of the OR (95% CI) from multivariable logistic regression modeling examining the interaction of key clinical subgroups on the association of left ventricular mechanical unloading and in-hospital mortality in VA-ECMO patients. CHF = congestive heart failure; ECMO = extracorporeal membrane oxygenation; ECPR = extracorporeal cardiopulmonary resuscitation; VA-ECMO = venoarterial extracorporeal membrane oxygenation; VT/VF = ventricular tachycardia/ventricular fibrillation.
MU AND COMPLICATIONS.
Complications were more common in the MU group, including medical bleeding, cannula site bleeding, tamponade, hemolysis, ischemic stroke, limb ischemia, and renal injury (Table 2). In multivariable modeling, MU was independently associated with increased odds of cannula site bleeding and hemolysis (Central Illustration). These relationships were unchanged in sensitivity analyses without multiple imputation for pre-ECMO pH (Supplemental Table 6). The findings were similar in the propensity-matched cohort except that the association between MU and hemolysis was no longer statistically significant (Supplemental Table 7).
TIMING OF MU.
The vast majority of the MU patients in our cohort were treated with an upfront MU approach, with 2,937 (86.4%) patients receiving a MU device before VA-ECMO and 114 (3.3%) placed concomitantly with ECMO initiation. In a propensity-matched cohort of 666 patients with upfront MU compared to 333 patients with delayed MU (Supplemental Table 5), there were no differences in on-support or in-hospital mortality (42.2% vs 44.4%, P = 0.50; 56.0% vs 59.8%; P = 0.26, respectively). The rates of ECMO complications were similar between the groups except for a higher incidence of renal injury with delayed MU (35.9% vs 45.0%; P = 0.005) (Supplemental Table 8).
IABP VS pVAD FOR LV MU.
From 2017 to 2019, there were 1,123 patients managed with IABP and 555 with pVAD. Compared to pVAD patients, those managed with IABP were less likely to be supported for ventricular arrhythmia, less likely to have pre-ECMO arrest, and had lower rates of concomitant renal, liver, and respiratory failure (P < 0.001 for all) (Table 3). After propensity matching, baseline characteristics were well-balanced (all SD <10%) between 560 patients with IABP and 560 patients with pVAD (Supplemental Table 5). On-support and in-hospital mortality rates were lower in patients supported with IABP (Table 4), but after multivariable modeling, no significant differences in mortality were observed between IABP and pVAD groups (Figure 3). These findings were unchanged without imputing pH (Supplemental Table 9) and with propensity matching (Supplemental Table 10).
TABLE 3.
Baseline Characteristics of Adults Supported With VA-ECMO Stratified by Type of Mechanical Unloading Device
| Total (N = 1,678) | IABP (n = 1,123) | pVAD (n = 555) | P Value | |
|---|---|---|---|---|
| Age, y | 56.84 ± 12.07 | 57.01 ± 12.42 | 56.51 ± 11.33 | 0.43 |
| Male | 1,303 (77.7) | 872 (77.7) | 431 (77.8) | 0.60 |
| Weight, kg | 87.98 ± 21.17 | 85.19 ± 20.24 | 93.56 ± 21.90 | <0.001 |
| Caucasian | 990 (59.0) | 633 (56.4) | 357 (64.3) | <0.001 |
| Primary diagnosis | ||||
| Acute myocardial infarction | 700 (42.5) | 449 (40.8) | 251 (45.9) | 0.05 |
| Chronic heart failure | 401 (24.3) | 243 (22.1) | 158 (28.9) | 0.002 |
| VT/VF | 173 (10.5) | 96 (8.7) | 77 (14.1) | <0.001 |
| Myocarditis | 29 (1.8) | 23 (2.1) | 6 (1.1) | 0.14 |
| Concomitant organ failure | ||||
| Respiratory | 437 (26.0) | 230 (20.5) | 207 (37.3) | <0.001 |
| Renal | 514 (30.6) | 292 (26.0) | 222 (40.0) | <0.001 |
| Liver | 107 (6.3) | 52 (4.6) | 53 (9.5) | <0.001 |
| ECPR modality | 323 (19.2) | 231 (20.6) | 92 (16.6) | 0.05 |
| Pre-ECMO arrest | 945 (56.7) | 580 (52.2) | 365 (65.9) | <0.001 |
| Pre-ECMO MAP, mm Hg | 63.03 ± 20.31 | 64.83 ± 20.15 | 68.12 ± 20.45 | 0.009 |
| Pre-ECMO pH | 7.24 ± 0.17 | 7.24 ± 0.17 | 7.24 ± 0.16 | 0.86 |
| Vasopressors/inotropes | 0.001 | |||
| 0–2 | 958 (57.1) | 651 (58.0) | 307 (55.4) | |
| >2 | 720 (42.9) | 472 (42.0) | 248 (44.6) | |
| Time on ECMO, d | 5.00 (3.00–8.00) | 5.00 (3.00–8.00) | 5.00 (3.00–9.00) | 0.51 |
Values are mean ± SD, n (%), or median (IQR).
IABP = intra-aortic balloon pump; pVAD = percutaneous ventricular assist device; other abbreviations as in Table 1.
TABLE 4.
Outcomes Among Adults Undergoing VA-ECMO Stratified by Device Used for Left Ventricular Mechanical Unloading
| Total (N = 1,678) | IABP (n = 1,123) | pVAD (n = 555) | P Value | |
|---|---|---|---|---|
| In-hospital mortality | 949 (56.6) | 611 (54.4) | 338 (60.9) | 0.011 |
| On-support mortality | 740 (44.1) | 486 (43.3) | 254 (45.8) | 0.33 |
| Medical bleeding | 154 (9.2) | 76 (6.8) | 78 (14.1) | <0.001 |
| Hemorrhagic stroke | 44 (2.6) | 21 (1.9) | 23 (4.1) | 0.006 |
| Pulmonary | 29 (1.7) | 14 (1.2) | 15 (2.7) | 0.031 |
| Gastrointestinal | 89 (5.3) | 44 (3.9) | 45 (8.1) | <0.001 |
| Nonmedical bleeding | 362 (21.6) | 221 (19.7) | 141 (25.4) | 0.007 |
| Cannula site | 252 (15.0) | 148 (13.2) | 104 (18.7) | 0.002 |
| Surgical site | 115 (6.9) | 75 (6.7) | 40 (7.2) | 0.68 |
| Mediastinal site | 4 (0.2) | 1 (0.1) | 3 (0.5) | 0.07 |
| Tamponade | 36 (2.1) | 26 (2.3) | 10 (1.8) | 0.49 |
| Hemolysis | 80 (4.8) | 49 (4.4) | 31 (5.6) | 0.26 |
| Ischemic stroke | 72 (4.3) | 42 (3.7) | 31 (5.6) | 0.26 |
| Limb ischemia | 167 (10.0) | 109 (9.7) | 58 (10.5) | 0.63 |
| Renal injury | 573 (34.1) | 348 (31.0) | 225 (40.5) | <0.001 |
| Cr 1.5–3 | 29 (1.7) | 21 (1.9) | 8 (1.4) | 0.52 |
| Cr >3 | 183 (10.9) | 104 (9.3) | 79 (14.2) | 0.002 |
| Need for RRT | 498 (29.7) | 296 (26.4) | 202 (36.4) | <0.001 |
FIGURE 3. VA-ECMO Outcomes With IABP vs pVAD for Left Ventricular Mechanical Unloading.
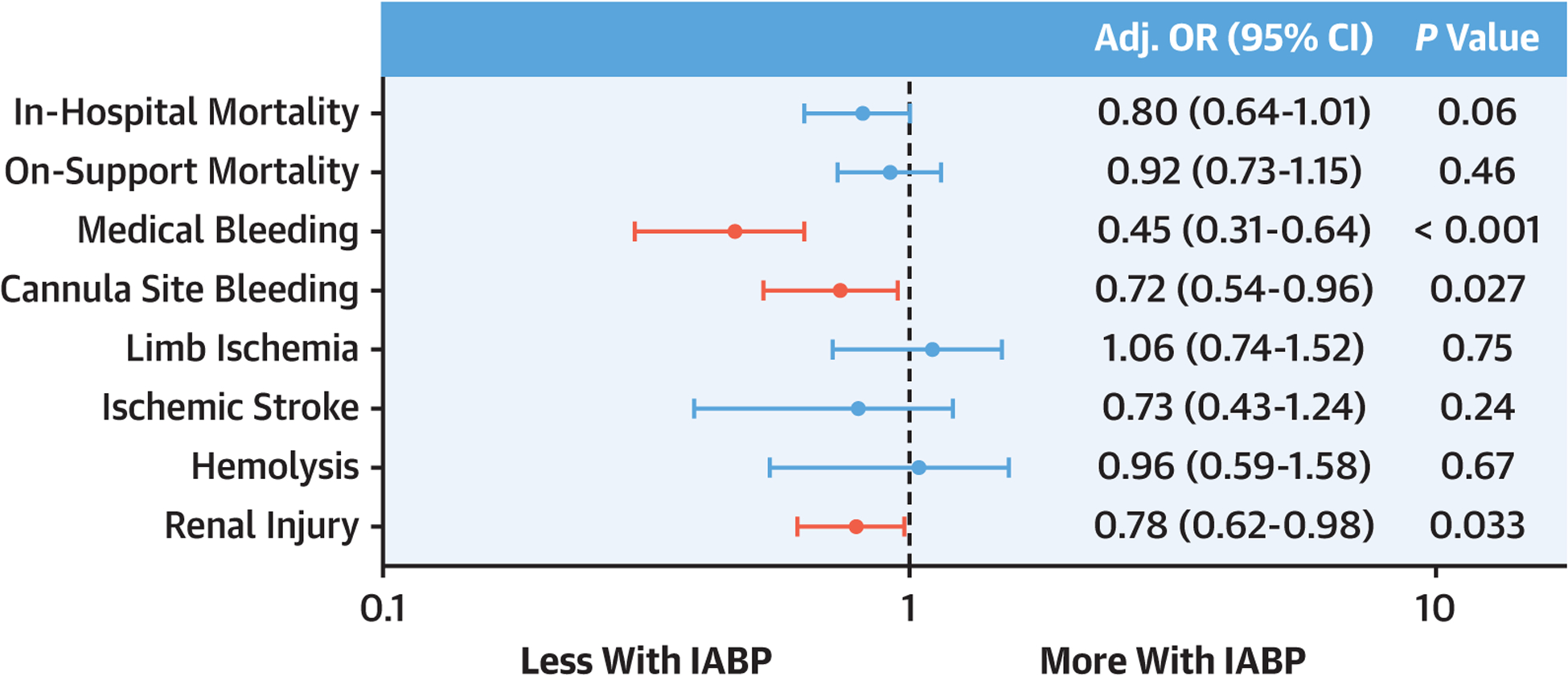
Forest plot of the OR (95% CI) from multivariable logistic regression modeling examining the association of left ventricular mechanical unloading with IABP versus pVAD and outcomes in VA-ECMO patients. IABP = intra-aortic balloon pump; pVAD = percutaneous ventricular assist device; other abbreviation as in Figure 2.
Patients receiving MU with IABP vs pVAD had lower rates of bleeding complications, including cannula site bleeding (13.2% vs 18.7%) and medical bleeding (6.8% vs 14.1%), with significant proportional differences in hemorrhagic stroke (1.9% vs 4.1%) and gastrointestinal bleeding (3.9% vs 8.1%) (Table 4). Renal injury was also less frequent with IABP (31.0% vs 40.5%). The rates of hemolysis, ischemic stroke, and limb ischemia were similar between the groups. After multivariable adjustment, MU with IABP remained independently associated with lower odds of medical bleeding, cannula site bleeding, and renal injury (Figure 3). These findings were unchanged in the propensity-matched cohort (Supplemental Table 10). Without imputing pH, all findings were similar except that the association with cannula site bleeding was no longer significant (Supplemental Table 9).
CENTER MU RATE.
After excluding low-volume centers, 118 sites (32%) and 10,547 patients (83%) remained in the analytic cohort. MU rate ranged from 0%–19% in the lowest tertile to 32%–59% in the highest tertile. Crude inpatient mortality was similar across tertiles of MU rate: 1st tertile 57.2%, 2nd tertile 60.0%, 3rd tertile 58.2% (P = 0.06). In multivariable modeling, the association of MU with lower inpatient mortality was only observed in the 2nd and 3rd tertiles of MU rate (Supplemental Table 11), and there was a significant interaction between MU and the center MU rate (interaction P = 0.006). Center annual VA-ECMO volume did not have a significant association with inpatient mortality or an interaction with MU (data not shown).
DISCUSSION
We report the largest multicenter study examining the impact of MU on both mortality and major complications in adults supported with VA-ECMO. The main findings of our study are the following: 1) The use of MU during VA-ECMO has increased considerably over the past decade, driven predominantly by a sharp increase in use of the pVAD modality since 2017. 2) MU is associated with lower in-hospital and on-support mortality compared to VA-ECMO alone. Younger patients (age <50 years) and those experiencing cardiac arrest before ECMO cannulation may benefit most from MU strategies. 3) MU during VA-ECMO is associated with increased rates of important complications including cannula site bleeding and hemolysis. 4) Among VA-ECMO patients managed with MU, the use of IABP compared to pVAD was associated with similar survival and lower odds of medical bleeding, cannula site bleeding, and renal injury.
LV MU USE OVER TIME.
Approximately one-quarter of VA-ECMO patients in our study received MU, consistent with reports from other observational studies, but we found a substantial increase in MU use over the last few years, coinciding with a dramatic increase in the use of pVAD starting in 2017.6,10,14–16 This shift in device use parallels a rapid increase in the use of pVAD devices for temporary mechanical circulatory support.17
LV MU AND MORTALITY IN VA-ECMO.
In the absence of a randomized clinical trial comparing the effects of LV MU on outcomes in VA-ECMO, the most important finding of our study is the strong association of MU, regardless of the specific device used, with lower inpatient mortality in adult VA-ECMO patients. Our work extends the findings from recent observational studies into a substantially larger multinational registry, with a consistent HR/OR for mortality with MU around 0.8 across these studies.5,7 Importantly, our study includes large numbers of patients managed with both IABP and pVAD, whereas the Russo et al7 analysis was predominantly IABP (only 5% pVAD) and Schrage et al5 only examined pVAD use.
The lower mortality observed with MU is particularly compelling given that MU was associated with significantly higher rates of complications, suggesting a potent physiologic advantage with MU. The benefit of MU could be explained by a variety of physiologic effects including increased coronary blood flow; reduced LV pressures, volumes and wall stress; and improved right ventricular performance.4,18–20 Unfortunately, we lack data on hemodynamic or echocardiographic parameters during VA-ECMO to elucidate the mechanisms leading to improved survival with MU.
Similar to prior studies, the lower mortality associated with MU was consistent across different subgroups and clinical phenotypes of cardiogenic shock including AMI, CHF, and ECPR.5,7 We observed a significant interaction between MU and both age and cardiac arrest, where patients age <50 years and those with arrest before ECMO initiation had substantially lower mortality with MU. Younger patients may have a greater capacity for myocardial recovery and may be less likely to experience complications from MU devices. Cardiac arrest can result in substantial low-flow time and ischemic insult to the myocardium, perhaps leaving the LV particularly vulnerable to distension in the face of increased afterload from the ECMO circuit. This finding is consistent with a recent ELSO analysis showing 30% lower mortality with MU in patients receiving ECPR.9 Our findings suggest that clinicians should consider age and preceding cardiac arrest when weighing the risks and benefits of MU during VA-ECMO.
LV MU AND ECMO COMPLICATIONS.
Several complications including cannula site bleeding, hemolysis, renal injury, and medical bleeding were more common in the MU group. However, after accounting for baseline characteristics, MU was only independently associated with increased odds of cannula site bleeding and hemolysis. Although earlier studies did not detect increased complications with MU, these analyses had methodologic limitations and were significantly underpowered to detect differences in adverse events.7,21 Our results from a large multicenter registry with standard adverse event definitions extends the findings of recent studies and highlights significant risks associated with MU devices in VA-ECMO.5,8 Higher rates of hemolysis with MU have now been consistently observed and is likely related to increased shear stress on red blood cells imposed by these devices.5,7,8 Increased cannula site bleeding is not surprising and likely secondary to vascular injury from the additional arterial access required for MU devices.22 Importantly, the rates of devastating brain injury, such as intracranial hemorrhage and ischemic stroke, were similar with MU. Cannula site bleeding and hemolysis are not strongly associated with mortality in VA-ECMO, potentially allowing for a net benefit of MU even with a higher burden of complications.23
TIMING OF MU.
The optimal timing of MU in VA-ECMO patients has not been established.14 While mortality was slightly lower with upfront MU in our study, we could not identify a clear difference in survival with upfront vs delayed MU. Schrage et al5 found that delayed MU with pVAD was not associated with the same survival benefit observed with upfront pVAD in VA-ECMO, but the sample size for delayed pVAD was small, absolute outcomes were similar to upfront placement, and there was no direct comparison between upfront and delayed strategies. We view our analysis of MU timing as exploratory. The vast majority of MU patients in our study received an upfront device, and delayed MU could be under-represented if centers failed to enter CPT codes for devices inserted after VA-ECMO initiation. We observed higher rates of renal injury with delayed MU, and there are theoretical risks associated with a bailout MU strategy, potentially exposing patients to complications from LV distension as well as increased procedural risk with device placement after a period of ECMO exposure. Optimal metrics to prompt MU have not been established, and prospective studies comparing a pre-emptive versus bailout MU strategy in VA-ECMO are needed.
CENTER MU RATE.
There was considerable variability in the MU rate across VA-ECMO centers, ranging from 0%−59%, and the association of MU with lower mortality was confined to centers in the upper 2 tertiles of MU rate (MU rate >19%). Notably, center VA-ECMO volume was not associated with mortality and did not have an interaction with MU on outcomes, suggesting the relevance of MU rate is not mediated simply through high-volume ECMO centers. Although we view this analysis as hypothesis-generating, it suggests that centers that more frequently deploy MU devices during VA-EMO may have better outcomes with this strategy. This finding is not altogether surprising given that the decision to insert a MU device and optimal management of those devices during VA-ECMO support is nuanced and requires a multidisciplinary team.
IABP VS pVAD AS MU STRATEGIES ON VA-ECMO.
After accounting for indicators of illness severity at the time of ECMO initiation, mortality rates were similar for patients receiving MU with IABP vs pVAD. Importantly, MU with pVAD was associated with a higher burden of complications, including medical bleeding, cannula site bleeding, and renal injury. Although increased complication rates could be related to residual confounding from higher acuity of illness in the pVAD population, these are complications frequently observed with pVAD in clinical practice. Higher rates of renal injury could be related to pigment nephropathy from hemolysis, a major adverse event commonly complicating pVAD support.24 Although we did not observe differences in hemolysis between the devices, the rate of hemolysis in our study was low compared to other recent reports, likely driven by ELSO’s strict definition of hemolysis and the absence of routine laboratory measures, such as lactate dehydrogenase or plasma free hemoglobin, to allow a more granular analysis of hemolysis burden.5 Bleeding is a common complication of pVAD support, and small studies of patients with cardiogenic shock have shown trends to more bleeding complications with pVAD versus IABP.17,25–29 Schrage et al5 reported more severe bleeding complications in VA-ECMO patients managed with pVAD versus VA-ECMO alone. The ELSO registry lacks details on anticoagulation protocols and laboratory parameters of coagulation to better understand this bleeding risk, but the larger bore arterial access required for pVAD placement and a tendency toward higher intensity of anticoagulation with these devices are important factors to consider. Medical bleeding events have been associated with increased mortality in VA-ECMO, and further studies are needed to understand the mechanisms leading to higher bleeding rates with pVAD and mitigate the complications associated with these effective unloading devices.23
The decision to insert a MU device is complex, and key parameters to trigger MU are not well defined and may extend beyond traditional hemodynamic metrics of left heart congestion.30,31 The need for LV unloading depends on the complex interplay between native right and left heart function, systemic arterial properties, and ECMO flows. Furthermore, the degree of LV unloading achieved can vary considerably across medical and device therapies, with modern pVAD devices typically offering the most potent unloading.32 Given the lower mortality associated with MU in VA-ECMO and the signal for increased complications with pVAD, a randomized study of MU devices in VA-ECMO is urgently needed to guide the optimal LV unloading strategy.
STUDY LIMITATIONS.
ELSO is a self-reported registry, and there could be differential reporting of concomitant devices and adverse events across sites. Procedure codes for insertion/removal of MU devices and the exact timing of placement are not mandatory fields in the registry, so underreporting of MU devices is likely and a more granular time-to-unloading analysis was implausible. The majority of MU devices in our study were in place before VA-ECMO; thus, the majority of MU patients were escalated to VA-ECMO from IABP or pVAD rather than having these devices placed at or after ECMO initiation specifically for managing LV distension. Echocardiographic data are not captured and invasive hemodynamic data were highly missing. Only in-hospital outcomes were available, precluding an analysis of longer-term survival or functional outcomes.
We were limited to analyzing complications collected in the ELSO registry, precluding an assessment of venous thromboembolism or arterial thromboembolic complications. Anticoagulation strategy and laboratory values such as coagulation parameters and platelet count are not collected. The ELSO registry uses ICD-9/10-CM billing codes for primary and nonmandatory secondary diagnoses, including concomitant organ failures. As such, assessment of the primary condition for VA-ECMO support is restricted to broad categories, there is minimal information on chronic comorbidities, and there are no standardized criteria for pre-ECMO organ failures. Finally, given the observational nature of this study, causality between MU and the reported outcomes cannot be inferred.
CONCLUSIONS
In this large, multicenter, international registry of adults supported with VA-ECMO, we found that the use of LV MU is rapidly increasing and associated with decreased in-hospital mortality at the expense of more complications, including hemolysis and cannulation site bleeding. These associations were consistent across different clinical phenotypes of cardiogenic shock. Both IABP and pVAD devices were associated with lower mortality, but pVAD was associated with an increased risk of important complications including bleeding events and renal injury. Randomized clinical trials are urgently needed to evaluate the impact of MU and compare different devices for LV unloading in VA-ECMO patients.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS:
Among adults with cardiogenic shock requiring venoarterial extracorporeal hemodynamic and oxygenation support, mechanical LV unloading with either IABP or pVAD is associated with improved survival to discharge.
TRANSLATIONAL OUTLOOK:
Translational research and randomized trials are needed to understand the mechanisms by which mechanical unloading promotes ventricular recovery and survival and guide selection of the optimum modality for individual patients.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
Dr Tonna has received a Career Development Award from the National Institutes of Health/National Heart, Lung, And Blood Institute (K23 HL141596); has received speaker fees and travel compensation from LivaNova, unrelated to this work; and is the Chair of the ELSO Registry Scientific Oversight Committee. Dr Kapur has received institutional research support and speaker/consulting honoraria from Abbott, Abiomed, Boston Scientific, Getinge, LivaNova, Medtronic, MDStart, Precardia, and Zoll. Dr Shaefi has received grants from the National Institutes of Health (K08 GM134220-01 and R01 DK125786-01). Dr Garan has received research support from Abbott Vascular and Verantos; and has received consultant fees from Abiomed and NupulseCV. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- AMI
acute myocardial infarction
- CHF
congestive heart failure
- ECPR
extracorporeal cardiopulmonary resuscitation
- IABP
intra-aortic balloon pump
- LV
left ventricle
- MU
mechanical unloading
- pVAD
percutaneous ventricular assist device
- VA-ECMO
venoarterial extracorporeal membrane oxygenation
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Footnotes
APPENDIX For supplemental tables, please see the online version of this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
REFERENCES
- 1.Cevasco M, Takayama H, Ando M, Garan AR, Naka Y, Takeda K. Left ventricular distension and venting strategies for patients on venoarterial extracorporeal membrane oxygenation. J Thorac Dis. 2019;11:1676–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao P, Khalpey Z, Smith R, Burkhoff D, Kociol RD. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest. Circ Heart Fail. 2018;11:e004905. [DOI] [PubMed] [Google Scholar]
- 3.Truby LK, Takeda K, Mauro C, et al. Incidence and implications of left ventricular distention during venoarterial extracorporeal membrane oxygenation support. ASAIO J. 2017;63:257–265. [DOI] [PubMed] [Google Scholar]
- 4.Donker DW, Brodie D, Henriques JPS, Broome M. Left ventricular unloading during veno-arterial ECMO: a review of percutaneous and surgical unloading interventions. Perfusion. 2019;34:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrage B, Becher PM, Bernhardt A, et al. Left ventricular unloading is associated with lower mortality in cardiogenic shock patients treated with veno-arterial extracorporeal membrane oxygenation: results from an international, multicenter cohort study. Circulation. 2020;142(22): 2095–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aso S, Matsui H, Fushimi K, Yasunaga H. The effect of intraaortic balloon pumping under venoarterial extracorporeal membrane oxygenation on mortality of cardiogenic patients: an analysis using a nationwide inpatient database. Crit Care Med. 2016;44:1974–1979. [DOI] [PubMed] [Google Scholar]
- 7.Russo JJ, Aleksova N, Pitcher I, et al. Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J Am Coll Cardiol. 2019;73:654–662. [DOI] [PubMed] [Google Scholar]
- 8.Pappalardo F, Schulte C, Pieri M, et al. Concomitant implantation of Impella® on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail. 2017;19: 404–412. [DOI] [PubMed] [Google Scholar]
- 9.Tonna J, Selzman C, Bartos J, et al. Abstract 117: critical care management, hospital case volume, and survival after extracorporeal cardiopulmonary resuscitation. Circulation. 2020;142. A117–A117. [Google Scholar]
- 10.Cheng R, Hachamovitch R, Makkar R, et al. Lack of survival benefit found with use of intra-aortic balloon pump in extracorporeal membrane oxygenation: a pooled experience of 1,517 patients. J Invasive Cardiol. 2015;27:453–458. [PubMed] [Google Scholar]
- 11.Chen K, Hou J, Tang H, Hu S. Concurrent initiation of intra-aortic balloon pumping with extracorporeal membrane oxygenation reduced in-hospital mortality in postcardiotomy cardiogenic shock. Ann Intensive Care. 2019;9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ELSO. ECLS Registry Report: International Summary. Extracorporeal Life Support Organization; 2020. Accessed March 1, 2021. https://www.elso.org/Portals/0/Files/Reports/2020_January/International%20Summary%20January%202020_page1.pdf [Google Scholar]
- 13.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10: 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Na SJ, Yang JH, Yang JH, et al. Left heart decompression at venoarterial extracorporeal membrane oxygenation initiation in cardiogenic shock: prophylactic versus therapeutic strategy. J Thorac Dis. 2019;11:3746–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park TK, Yang JH, Choi SH, et al. Clinical impact of intra-aortic balloon pump during extracorporeal life support in patients with acute myocardial infarction complicated by cardiogenic shock. BMC Anesthesiol. 2014;14:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garan AR, Takeda K, Salna M, et al. Prospective comparison of a percutaneous ventricular assist device and venoarterial extracorporeal membrane oxygenation for patients with cardiogenic shock following acute myocardial infarction. J Am Heart Assoc. 2019;8:e012171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amin AP, Spertus JA, Curtis JP, et al. The evolving landscape of Impella use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation. 2020;141:273–284. [DOI] [PubMed] [Google Scholar]
- 18.Cheng A, Swartz MF, Massey HT. Impella to unload the left ventricle during peripheral extra-corporeal membrane oxygenation. ASAIO J. 2013;59:533–536. [DOI] [PubMed] [Google Scholar]
- 19.Meuwese CL,de Haan M, Zwetsloot PP, et al. The hemodynamic effect of different left ventricular unloading techniques during veno-arterial extra-corporeal life support: a systematic review and meta-analysis. Perfusion. 2020:267659119897478.. [DOI] [PubMed] [Google Scholar]
- 20.Lim HS. The effect of Impella CP on cardio-pulmonary physiology during venoarterial extra-corporeal membrane oxygenation support. Artif Organs. 2017;41:1109–1112. [DOI] [PubMed] [Google Scholar]
- 21.Lin LY, Liao CW, Wang CH, et al. Effects of additional intra-aortic balloon counter-pulsation therapy to cardiogenic shock patients supported by extra-corporeal membranous oxygenation. Sci Rep. 2016;6:23838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Jong MM, Lorusso R, Al Awami F, et al. Vascular complications following intra-aortic balloon pump implantation: an updated review. Perfusion. 2018;33:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung M, Cabezas FR, Nunez JI, et al. Hemo-compatibility-related adverse events and survival on venoarterial extracorporeal life support. J Am Coll Cardiol HF. 2020;8:892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pieri M, Sorrentino T, Oppizzi M, et al. The role of different mechanical circulatory support devices and their timing of implantation on myocardial damage and mid-term recovery in acute myocardial infarction related cardiogenic shock. J Interv Cardiol. 2018;31:717–724. [DOI] [PubMed] [Google Scholar]
- 25.Unverzagt S, Buerke M, de Waha A, et al. Intra-aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock. Cochrane Database Syst Rev. 2015: Cd007398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tepper S, Masood MF, Baltazar Garcia M, et al. Left ventricular unloading by Impella device versus surgical vent during extracorporeal life support. Ann Thorac Surg. 2017;104: 861–867. [DOI] [PubMed] [Google Scholar]
- 27.Lauten A, Engstrom AE, Jung C, et al. Percutaneous left-ventricular support with the Impella-2.5–assist device in acute cardiogenic shock: results of the Impella-EUROSHOCK-registry. Circ Heart Fail. 2013;6:23–30. [DOI] [PubMed] [Google Scholar]
- 28.Ouweneel DM, Eriksen E, Sjauw KD, et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017;69:278–287. [DOI] [PubMed] [Google Scholar]
- 29.Alushi B, Douedari A, Froehlig G, et al. Impella versus IABP in acute myocardial infarction complicated by cardiogenic shock. Open Heart. 2019;6:e000987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donker DW, Sallisalmi M, Broomé M. Right–left ventricular interaction in left-sided heart failure with and without venoarterial extracorporeal membrane oxygenation support—a simulation study. ASAIO J. 2021;67:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain P, Salama M, Everett K, Reyelt L, Kapur NK. To vent or not to vent: a loaded question during venoarterial extracorporeal membrane oxygenation support for cardiogenic shock. Circ Cardiovasc Interv. 2021;14:e010537. [DOI] [PubMed] [Google Scholar]
- 32.Donker DW, Brodie D, Henriques JPS, Broomé M. Left ventricular unloading during veno-arterial ECMO: a simulation study. ASAIO J. 2019;65:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


