Abstract
Background.
Pericoronary adipose tissue (PCAT) attenuation and low-attenuation non-calcified plaque (LAP) burden can both predict outcomes.
Objectives.
To assess the relative and additive values of PCAT attenuation and LAP to predict future risk of myocardial infarction.
Methods.
In a post-hoc analysis of the multicenter SCOT-HEART trial, we investigated the relationships between the future risk of fatal or non-fatal myocardial infarction and PCAT attenuation measured from CT coronary angiography using multivariable Cox regression models including plaque burden, obstructive coronary disease and cardiac risk score (incorporating age, sex, diabetes, smoking, hypertension, hyperlipidaemia and family history).
Results.
In 1697 evaluable participants (58±10 years), there were 37 myocardial infarctions after a median follow-up of 4.7 years. Mean PCAT was −76±8 Hounsfield units (HU) and median LAP burden was 4.20 [0–6.86] %. PCAT attenuation of the right coronary artery (RCA) was predictive of myocardial infarction (hazard ratio (HR) 1.55, p=0.017, per 1 standard deviation increment) with an optimum threshold of −70.5 HU (HR 2.45, p=0.01). In multivariable analysis, adding PCAT-RCA ≥−70.5 HU to LAP burden >4% (optimum threshold for future myocardial infarction; HR 4.87, p<0.0001) led to improved prediction of future myocardial infarction (HR 11.7, p<0.0001). LAP burden showed higher area-under-the-curve (AUC) compared to PCAT attenuation for the prediction for myocardial infarction [0.71 (0.62–0.80) versus 0.64 (0.54–0.74); p<0.001], with increased AUC when the two metrics are combined [0.75 (0.65–0.85); p=0.037].
Conclusion.
CT coronary angiography-defined LAP burden and PCAT attenuation have marked and complementary predictive value for the risk of fatal or non-fatal myocardial infarction.
Keywords: Pericoronary adipose tissue, non-calcified plaque burden, low-attenuation non-calcified plaque burden, computed tomography angiography, coronary artery disease, risk stratification
Introduction
Coronary computed tomography angiography (CCTA) plays an important role in the risk stratification of patients with established coronary artery disease. However, most myocardial infarctions occur in coronary artery segments without prior obstructive disease.(1,2) Therefore, interest has turned to assessments of coronary plaque morphology and burden to identify patients at risk of future coronary events. An important advantage of CCTA is that it can assess the composition of atherosclerotic plaque and adjacent structures, not just the arterial lumen. In a recent analysis of the SCOT-HEART population, quantitative plaque analysis showed that both plaque type and burden on CCTA are important predictors of adverse outcomes, with low-attenuation plaque burden being the strongest predictor of future events,(3) outperforming cardiovascular risk scores, coronary artery calcium scoring or the presence and severity of obstructive coronary artery disease.
Recent studies have shown that perivascular inflammation may play a crucial role in the formation of coronary atherosclerotic plaques, with inflammation being a potential marker of plaque instability and vulnerability(4) which can lead to plaque rupture and subsequent myocardial infarction.(5) Pericoronary adipose tissue (PCAT) attenuation measured from CCTA has been able to detect coronary inflammation in patients undergoing cardiac surgery.(6) Increased PCAT attenuation from CCTA can predict cardiovascular mortality in stable coronary disease(7,8) and shows a strong association with culprit coronary plaques in patients with acute coronary syndrome.(9,10)
To date, however, no studies have compared the prognostic value of both PCAT attenuation and quantitative plaque burden to predict myocardial infarction. We hypothesised that the combination of PCAT attenuation and quantitative plaque burden measures could provide additive and improved prediction of myocardial infarction in patients with stable chest pain. We aimed to investigate the prognostic value of PCAT attenuation and quantitative plaque burden to predict future myocardial infarction in the CCTA arm of the randomized, controlled SCOT-HEART trial.
Methods
Study Design
The Scottish Computed Tomography of the HEART (SCOT-HEART) multicentre randomized controlled trial evaluated the use of CCTA in addition to standard care in patients with suspected angina pectoris (ClinicalTrials.gov ., Unique identifier: NCT01149590).(11) The study was approved by the local ethics committee and written informed consent was provided by all participants. The study complied with the Declaration of Helsinki. A detailed description of the study can be found in the Supplemental Appendix.
Computed tomography
All participants underwent coronary artery calcium scoring and CCTA using 64-multidetector (Brilliance 64, Philips Healthcare, North Andover, Massachusetts; or Biograph mCT, Siemens, Erlangen, Germany) or 320-multidetector (Aquilion One, Toshiba Medical Systems, Tochigi, Japan) row scanners at one of three imaging sites in Scotland. CCTA was performed following intravenous injection of 50 to 70 mL of iodine-based contrast medium at a flow rate of 5.5 to 6.5 mL/sec. The Toshiba Aquilion One scanner used wide volume collimation, 0.75 sec gantry rotation and tube current based on body-mass index. The Philips 64 Detector Row Brilliance CT utilised 0.625 mm collimation, gantry rotation varied with heart rate − 0.42 sec to 0.5 sec and tube current based on body-mass index. The Biograph mCT scanner used wide volume collimation, 0.30 sec rotation time and tube current based on body-mass index. Based on heart rate and rhythm, prospective or retrospective gating were utilised.
Analysis of atherosclerotic plaque and pericoronary adipose tissue
CCTA data sets were exported in Digital Imaging and Communications in Medicine (DICOM) format, for measurement of coronary plaque and PCAT using semi-automated software (Autoplaque, Version 2.5, Cedars Sinai Medical Centre, Los Angeles, USA).(12) For each patient, total plaque and PCAT analysis was performed, as described previously.(11,13–15) A detailed description of the analysis of atherosclerotic plaque and pericoronary adipose tissue can be found in the Supplemental Appendix.
Biomarkers
In a subset of 781 patients, we had plasma stored at −80°C which was obtained from blood samples collected into ethylene diamine tetra-acetic acid. Biomarkers’ analysis is described in the Supplemental Appendix.
Clinical outcomes
The primary outcome for this sub-study was the incidence of the combination of fatal or non-fatal myocardial infarction. Cardiovascular risk factor information was obtained from the SCOT-HEART database. Follow up information on cardiovascular events and mortality were obtained from the electronic Data Research and Innovation Service of the National Health Services Scotland and confirmed by reviewing electronic health records where required. Events which occur in Scotland are captured as part of the national coding data which is available through eDRIS. Previous analysis has demonstrated an excellent correlation (>95%) between clinical events identified through Scottish health record linkage and clinical events recorded and adjudicated as part of clinical trials(16). Follow-up was administratively censored for patients without an event on 31 January 2018.
Statistical methods
We assessed the distribution of data with the Shapiro-Wilk test. Categorial variables were reported as frequencies (percentages). Quantitative data are presented as mean ± standard deviation or, if not normally distributed, as median [interquartile interval]. Statistical significance was assessed using Pearson chi-square test, Student t-test, one-way analysis of variance (ANOVA), Kruskal–Wallis test, or Mann–Whitney U test as appropriate. Correlations for variables that were normally distributed were assessed using Pearson’s correlation coefficient, while those not normally distributed variables were assessed using Spearman rank order correlation. Correlation coefficients of <0.2 were regarded as very weak, 0.20 to <0.40 as weak, 0.40 to <0.60 as moderate, 0.6 to <0.80 as strong, and 0.8 to 1 as very strong. Blood biomarkers values were log transformed for this analysis. Outcome data were analysed using Cox proportional hazards regression and presented graphically using cumulative incidence plots using the Kaplan–Meier method. Hazard ratios (HR) and 95% confidence interval (CI) were calculated from the Cox model. Deaths not classified as coronary heart disease deaths were censored for both the Cox regression analysis and the cumulative incidence plots. Univariable analysis was performed for all quantified plaque measures and PCAT attenuation, PCAT volume, cardiovascular risk score (including individual clinical characteristics), Agatston coronary artery calcium score, presence of obstructive disease and presence of high-risk plaque features. Multivariable models were constructed including the individual plaque parameters, cardiovascular risk score and Agatston coronary artery calcium score. Coronary artery calcium score and plaque burdens were log transformed for analysis. Receiver operating characteristic (ROC) analysis was performed to identify the optimum cut-off for PCAT attenuation to identify patients at increased risk of fatal or non-fatal myocardial infarction using Youden J statistic. We used ROC analysis and pairwise comparisons according to DeLong et al. to compare areas under the curves(17). We have refitted the survival analyses using the dichotomous measures for LAP burden and PCAT-RCA. The ROC curves are based on Cox-derived models, fixed at 5 years of follow-up and deaths not classified as coronary heart disease deaths were censored. A statistically significant difference was defined as a 2-sided P value <0.05. Statistical analysis was performed using R (Version 4.0.2, R Foundation for Statistical Computing, Vienna, Austria).
Results
Study Population
Evaluable CCTA scans were present for 1697 patients. Participants were 58±10 years, 56% were male, and the mean 10-year cardiovascular risk score was 18±11% (Table 1). The median total plaque burden for all patients was 39 [0 to 49] %, with non-calcified plaque burden 36 [0 to 46] %, low-attenuation plaque burden 4 [0 to 7] %, and calcified plaque burden 0.4 [0 to 3] %.
Table 1.
Study population
| All participants | ||
|---|---|---|
| Number | 1697 | |
| Male | 959 (57%) | |
| Age (years) | 58±10 | |
| Body mass index (kg/m 2 ) | 30±5 | |
| Atrial fibrillation | 33 (2%) | |
| Previous coronary heart disease | 171 (10%) | |
| Previous cerebrovascular disease | 75 (4%) | |
| Previous peripheral vascular disease | 30 (2%) | |
| Smoking status | Current smoker | 320 (19%) |
| Ex-smoker | 569 (34%) | |
| Non-smoker | 807 (48%) | |
| Hypertension | 58 (35%) | |
| Diabetes mellitus | 191 (11%) | |
| Family history | 736 (44%) | |
| Total cholesterol (mg/dL) | 191±71 | |
| Symptoms | Typical angina | 630 (37%) |
| Atypical angina | 413 (24%) | |
| Nonanginal | 654 (39%) | |
| Cardiovascular risk score | 18±11 | |
| CACS (Agatston units) | 21 [0 to 230] | |
| CCTA findings | Normal | 611 (36%) |
| Non-obstructive | 651 (38%) | |
| Obstructive | 435 (26%) | |
Number (%), mean ± SD or median [interquartile interval].
CACS, Agatston coronary artery calcium score; CCTA, coronary computed tomography angiography.
Peri-coronary adipose tissue and its attenuation
Figure 1 shows representative examples of plaque burden and pericoronary adipose tissue quantification from the SCOT-HEART trial patients. The volume of adipose tissue around the proximal right coronary artery (639±189 mm3) was higher than the proximal left anterior descending artery (591±162 mm3) and proximal left circumflex artery (369±130 mm3; p<0.0001 for both; Supplemental Figure i). Mean PCAT attenuation across all vessels was normally distributed around a mean of −76.0±8.0 HU. It was higher in men (p<0.0001) and those with obstructive coronary artery disease (p=0.0023), lower body-mass index (p<0.001) or typical angina (p<0.001; Table 2) but was similar between different age groups and other baseline characteristics (Table 2). There was also no difference across different Agatston score categories (0, 1–99, 100–400 and >400 Agatston units, p=0.06).
Figure 1:
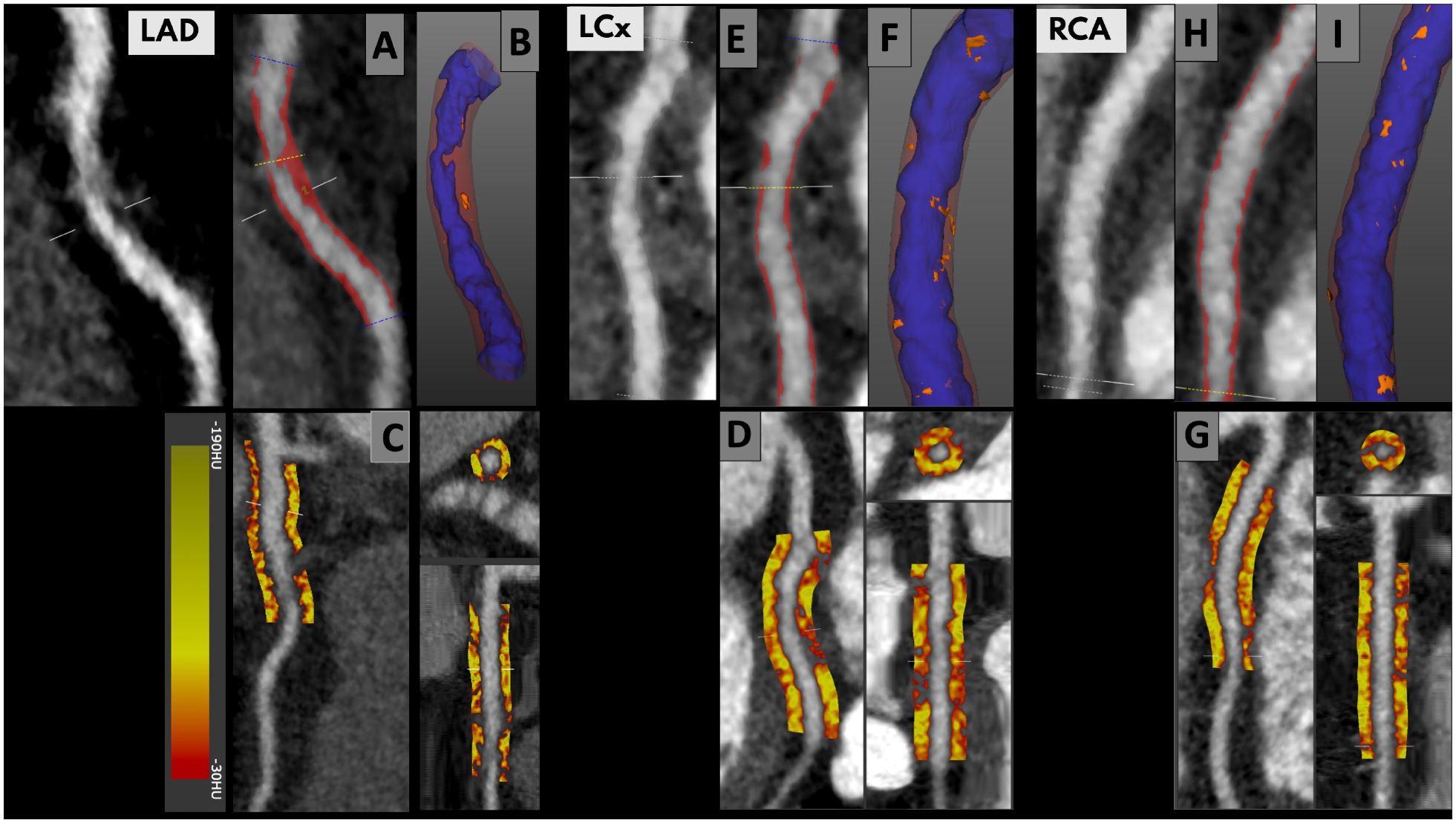
Plaque burden (left upper panels; red is non-calcified plaque) with 3-dimensional plaque composition (right upper panels; orange is low attenuation non-calcified plaque) and peri-coronary adipose tissue quantification (lower panels) and in the left anterior descending artery (A-C), the left circumflex artery (D-F) and the right coronary artery (G-I). PCAT analysis focused on the proximal right coronary artery (10–50 mm from ostium), left anterior descending coronary artery (0–40 mm from left main stem bifurcation) and left circumflex (0–40 mm from left main stem bifurcation). We considered PCAT attenuation (reported in Hounsfield units [HU]) as the average attenuation of all adipose tissue containing voxels (range −190 HU to −30 HU) within an outer radial distance from the vessel wall of 3 mm.
Table 2.
Mean peri-coronary adipose tissue attenuation across all vessels according to baseline characteristic variables.
| PCAT (HU) | PCAT(HU) | P | |||
|---|---|---|---|---|---|
| Age (years) | <60 | −75.6 ± 8.1 | ≥ 60 | −76.0 ± 8.5 | 0.511 |
| Sex | Male | −73.5 ± 6.8 | Female | −76.7 ± 6.9 | <0.001 |
| Body mass index (kg/m 2 ) | <30 | −74.3 ± 7.2 | ≥30 | −75.7 ± 6.7 | <0.01 |
| Atrial fibrillation | Yes | −76.0 ± 9.1 | No | −75.9 ± 8.3 | 0.912 |
| Previous coronary heart disease | Yes | −75.8 ± 8.4 | No | −75.9 ± 8.3 | 0.791 |
| Previous cerebrovascular disease | Yes | −75.2 ± 7.8 | No | −75.9 ± 8.3 | 0.343 |
| Previous peripheral vascular disease | Yes | −76.2 ± 6.4 | No | −74.9 ± 7.1 | 0.514 |
| Smoking status | Current/ex smoker | −76.1 ± 8.1 | Non-smoker | −75.6 ± 8.3 | 0.617 |
| Hypertension | Yes | −76.0 ± 7.8 | No | −75.9 ± 8.5 | 0.143 |
| Diabetes mellitus | Yes | −76.5 ± 7.8 | No | − 75.8 ± 8.4 | 0.113 |
| Family history of CHD | Yes | −76.3 ± 7.9 | No | −75.6 ± 8.7 | 0.311 |
| Hypercholesterolaemia | Yes | −75.9 ± 8.3 | No | −75.7 ± 8.3 | 0.751 |
| Typical angina | Yes | −75.0 ± 8.2 | No | −76.5 ± 8.3 | <0.001 |
| Cardiovascular risk score | <10 % | −76.09 ± 7.9 | >10% | −75.85 ± 8.4 | 0.742 |
| CCTA coronary stenosis | Obstructive (>70%) | −74.8 ± 8.3 | Non-obstructive | −75.8 ± 8.1 | 0.002 |
PCAT: Pericoronary adipose tissue attenuation expressed in mean ± SD
CCTA, coronary computed tomography angiography, CHD, coronary heart disease
Peri-coronary adipose tissue attenuation and coronary artery disease
There was a moderate to strong correlation between the attenuation of peri-coronary adipose tissue measured in the right and left anterior descending coronary arteries (r=0.54, p<0.0001), the right and left circumflex coronary arteries (r=0.58, p<0.0001) and the left anterior descending artery and left circumflex artery (r=0.66, p<0.0001; Supplemental Figure ii A). There were regional variations in PCAT attenuation between the three coronary arteries; PCAT-RCA was normally distributed around a mean of −75.88±8.2 HU, with PCAT-LAD showing lower mean values at −77.0±7.8 HU and PCAT-LCx showing the highest values at −73.4±7.7 HU; p<0.0001 (Supplemental Figure ii B). Mean PCAT attenuation increased with the presence of non-obstructive or obstructive disease as compared to normal coronary arteries (Table 2). For patients with coronary artery disease (n=1086), there was a weak correlation between PCAT-RCA and total (r=0.2, p<0.01), non-calcified (r=0.21, p<0.01) and low-attenuation plaque burden (r=0.077, p=0.02) but no correlation with calcified plaque burden (r=0.026, p=0.43) (Supplemental Figure iii). Weaker correlations were observed between total and non-calcified plaque burden, and PCAT attenuation in the left anterior descending (r=0.14, p<0.01 and r=0.17, p<0.01 respectively) and left circumflex (r=0.14, p<0.01 and r=0.11, p<0.01 respectively) coronary arteries. No association was observed between low attenuation plaque burden and calcified plaque burden and PCAT attenuation in the left anterior descending (r=−0.027, p=0.39 and r=−0.032, p=0.30 respectively) and left circumflex (r=−0.055, p=0.11 and r=0.049, p=0.15 respectively) coronary arteries (Supplemental Figure iii). PCAT-RCA was higher in patients with high-risk plaque features (≥ 1), compared to those without high-risk plaque features (−74.0±6.8 versus −77.0±7.1 HU, p<0.0001) independent of the location of plaque (right versus left coronary artery, proximal versus distal segments). Similarly, PCAT-LAD and PCAT-LCx was higher for patients with high-risk plaque features (−76.4 ± 7.5 versus −77.3± 7.9 HU, p=0.017 and −71.9± 7.2 versus −73.9± 7.9 HU, p<0.001 respectively).”
PCAT attenuation and systemic cardiac and inflammatory biomarkers
There were no associations between mean PCAT attenuation values (global) and high-sensitivity cardiac troponin I (r=0.019, p=0.62) or brain natriuretic peptide (r=0.019, p=0.68); all biomarkers values are log-transformed. Similarly, there were no associations between logtransformed high-sensitivity c-reactive protein (r=0.005, p=0.9), interleukin-6 (r=0.06, p=0.12), tumour necrosis factor-a (r=0.013, p=0.63), endothelin-1 (r=−0.032, p=0.4), vascular endothelial growth factor (r=0.0016, p=0.97) or metalloproteinase-9 (r=0.043, p=0.27; Supplemental Figure iv).
Clinical outcomes
Over a median follow-up of 4.7 [4.0 to 5.7] years, fatal or non-fatal myocardial infarctions occurred in 37 (2.2%) patients (35 non-fatal and 2 fatal). PCAT-RCA attenuation was higher in patients who suffered a myocardial infarction (−72.5±8.3 HU versus −76.5± 7.8 HU, p=0.014), but there was no difference in PCAT-LAD (−76.3±8.6 HU vs −77.0±7.8 HU, p=0.45) or PCAT-LCx (−71.6±7.3 HU vs −73.3±7.7 HU, p=0.26; Supplemental Figure v). Patients sustaining a myocardial infarction also had higher total, non-calcified, low-attenuation and calcified plaque burden, higher Agatston calcium score, higher cardiovascular risk score, increased presence of obstructive disease on CCTA and increased qualitative presence of high-risk plaque features (at least one) (Table 3).
Table 3.
Peri-coronary adipose tissue attenuation and quantitative plaque burden in patients with and without subsequent myocardial infarction.
| No event | Event | p value | |
|---|---|---|---|
| Plaque burden (%) | 38.9 [0 to 49.2] | 50.1 [43.0 to 53.8] | <0.001 |
| Noncalcified plaque burden (%) | 35.4 [0 to 45.3] | 42 [37.39 to 48.95] | <0.001 |
| Low-attenuation plaque burden (%) | 4.1 [0 to 6.7] | 7.4 [4.8 to 9.1] | <0.001 |
| Calcified plaque burden (%) | 0.39 [0 to 2.69] | 3.18 [0.9 to 7.97] | <0.001 |
| PCAT-RCA (HU) | −76.0 ± 7.8 | −72.5 ± 8.3 | 0.009 |
| PCAT-LAD (HU) | −77.0 ± 7.8 | −76.2 ± 8.6 | 0.543 |
| PCAT-LCx (HU) | −73.3 ± 7.7 | −71.6 ± 7.3 | 0.334 |
| CACS (Agatston Units) | 19 [0 to 218] | 283 [59 to 1041] | <0.001 |
| Obstructive disease | 416 (25%) | 19 (51%) | <0.001 |
| Cardiovascular risk score (%) | 18 ± 11 | 22 ± 12 | 0.039 |
| High-risk plaque features (≥1) | 565 (34%) | 23 (62%) | <0.001 |
| PCAT-RCA Volume (mm3) | 639± 189 | 656± 197 | 0.480 |
Median [interquartile interval], mean ± standard deviation or number (%).
CACS, coronary artery calcium score; HU, Hounsfield units; PCAT, pericoronary adipose tissue attenuation; RCA, right coronary artery; LAD, left anterior descending coronary artery; LCx, left circumflex coronary artery.
On univariable analysis, PCAT-RCA attenuation was a predictor of myocardial infarction (HR 1.55, 95% CI 1.08 to 2.22, p=0.017), but neither PCAT-LAD nor PCAT-LCx were associated with myocardial infarction (Figure 2 and Supplemental Table i). Univariable analysis also identified the burden of non-calcified, low-attenuation and calcified plaque as well as Agatston coronary calcium score, presence of obstructive coronary artery disease, cardiovascular risk score and qualitative presence of high-risk plaque features as predictors of myocardial infarction (Table 4). Male sex, age, body-mass index and volume of PCAT-RCA were not predictors of future myocardial infarction (p=0.061, p=0.860, p=0.260 and p=0.654 respectively). In multivariable analysis, only the low-attenuation plaque burden (HR 1.80, 95% CI 1.16 to 2.81, p=0.011, per doubling) and PCAT-RCA (HR 1.54 95%1.02 to 2.13, p=0.040, per standard deviation increment) remained predictors of myocardial infarction (Table 4 and Supplemental Table ii).
Figure 2:
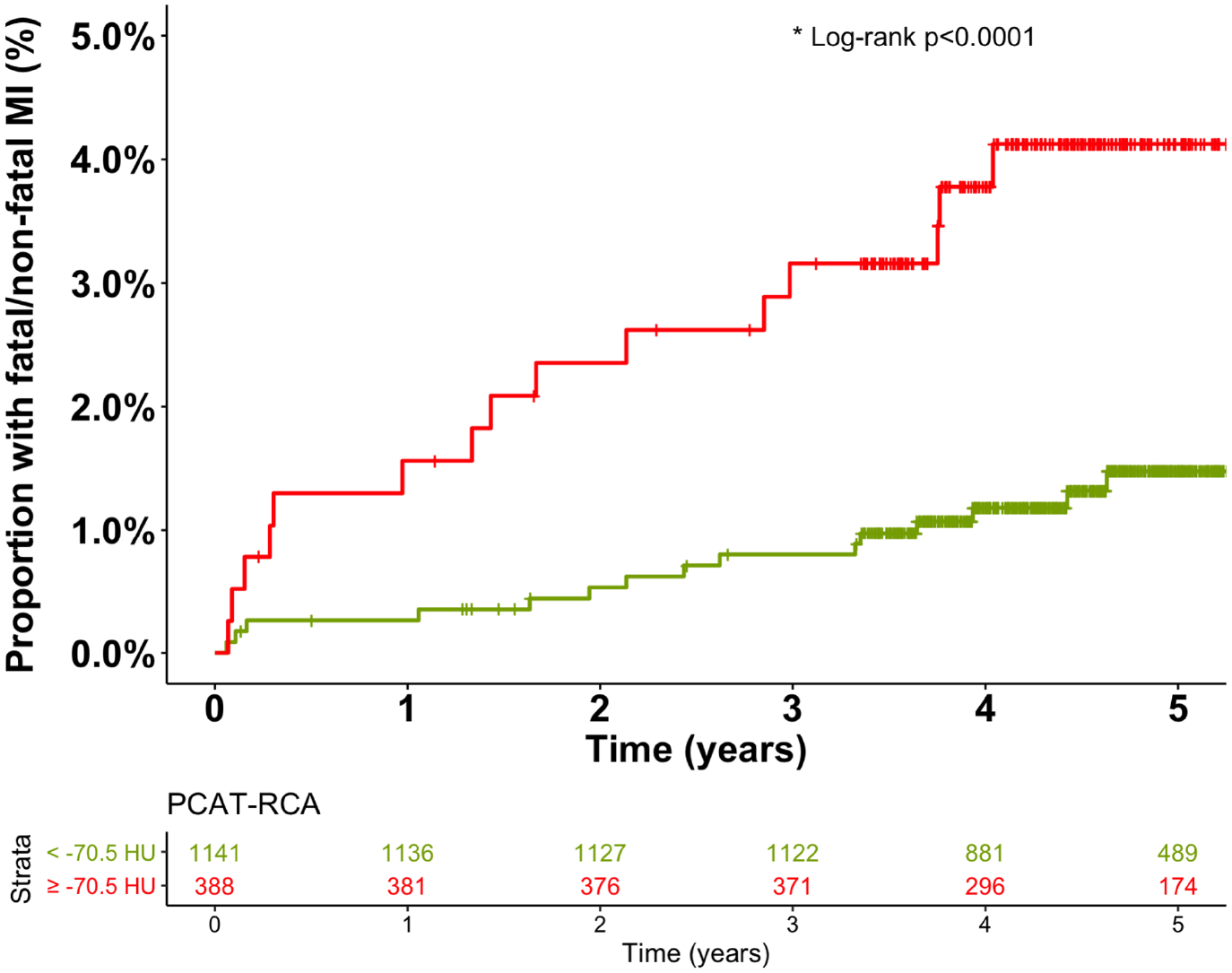
Peri-coronary adipose tissue of the right coronary artery (PCAT-RCA) and fatal or nonfatal myocardial infarction. Cumulative incidence of fatal or nonfatal myocardial infarction in patients with and without PCAT-RCA ≥ −70.5 HU.
Table 4.
Univariable and multivariable analysis for the prediction of myocardial infarction
| Univariable | Multivariable# | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Total plaque burden* | 1.44 (1.15–1.18) | <0.001 | 1.33 (0.97–1.82) | 0.072 |
| NCP burden* | 1.41 (1.14–1.75) | <0.001 | 1.30 (0.96–1.75) | 0.088 |
| LAP burden* | 1.87 (1.36–2.57) | <0.001 | 1.80 (1.16–2.80) | 0.009 |
| CP burden* | 1.70 (1.26–2.12) | <0.001 | 1.55 (0.92–2.63) | 0.102 |
| PCAT-RCA§ | 1.55 (1.08–2.22) | 0.017 | 1.54 (1.02–2.12) | 0.038 |
| Cardiovascular risk score | 1.03 (1.00–1.05) | 0.046 | 1.01 (0.98 – 1.05) | 0.374 |
| CACS* | 1.2 (1.10–1.3) | <0.001 | 0.93 (0.74 – 1.16) | 0.493 |
| Obstructive disease | 3.02 (1.60–5.8) | <0.001 | 1.09 (0.47 – 2.52) | 0.835 |
| High-risk plaque features (≥1) | 3.12 (1.60–6.06) | <0.001 | 0.95 (0.41–2.19) | 0.901 |
| PCAT-RCA volume§ | 1.082 (0.77–1.52) | 0.654 | 1.00 (0.99–1.00) | 0.615 |
Multivariable analysis includes the individual quantitative plaque measure, Agatston calcium score, obstructive disease and cardiovascular risk score. Full model results are presented in Table i in the Supplemental Appendix. Multivariable analysis of cardiovascular risk score, CACS, obstructive disease, presence of high-risk plaque features and adipose tissue volume (PCAT-RCA volume) includes all different types of plaque burden.
Per doubling.
Per 1 standard deviation increment in PCAT attenuation.
HR, hazard ratio; CI, confidence interval; NCP, non-calcified plaque; LAP, low-attenuation plaque; CP, calcified plaque; PCAT-RCA, pericoronary adipose tissue attenuation of the right coronary artery; CACS, coronary artery calcium score.
Based on the Youden’s index of the ROC curves, the optimal cut-off of the right coronary artery PCAT attenuation was −70.5 HU for the primary endpoint of fatal or non-fatal myocardial infarction. Patients with PCAT-RCA above ≥−70.5 HU were nearly 2.5 times more likely to suffer a myocardial infarction (HR 2.45, 95% CI 1.23 to 4.80; p=0.001, Figure 2). Patients with low-attenuation plaque burden (greater than 4%) were nearly 5 times more likely to suffer a myocardial infarction (HR 4.87, 95% CI 2.03 to 11.78, p<0.0001, Supplemental Figure vii). When the two metrics were combined, patients with both low-attenuation plaque burden >4% and PCAT-RCA ≥−70.5 HU were at the greatest risk of myocardial infarction (HR 11.7, 95% CI 3.3 to 40.9, p<0.0001), followed by those with low-attenuation plaque burden >4% and PCAT-RCA <−70.5 (HR 5.1, 95% CI 1.5 to 17.7, p<0.0001; Figure 3 and Supplemental Table iii). In ROC analysis, low-attenuation plaque burden showed significantly higher area-under-the-curve (AUC) compared to PCAT-RCA attenuation for the prediction for myocardial infarction [0.71 (0.62–0.80) versus 0.64 (0.54–0.74); p<0.001], with the combination of both parameters yielding significantly improved predictive value [AUC 0.75 (0.65–0.85) vs 0.71 (0.62–0.80); p=0.037] compared to LAP alone (p=0.037, Figure 4).
Figure 3:
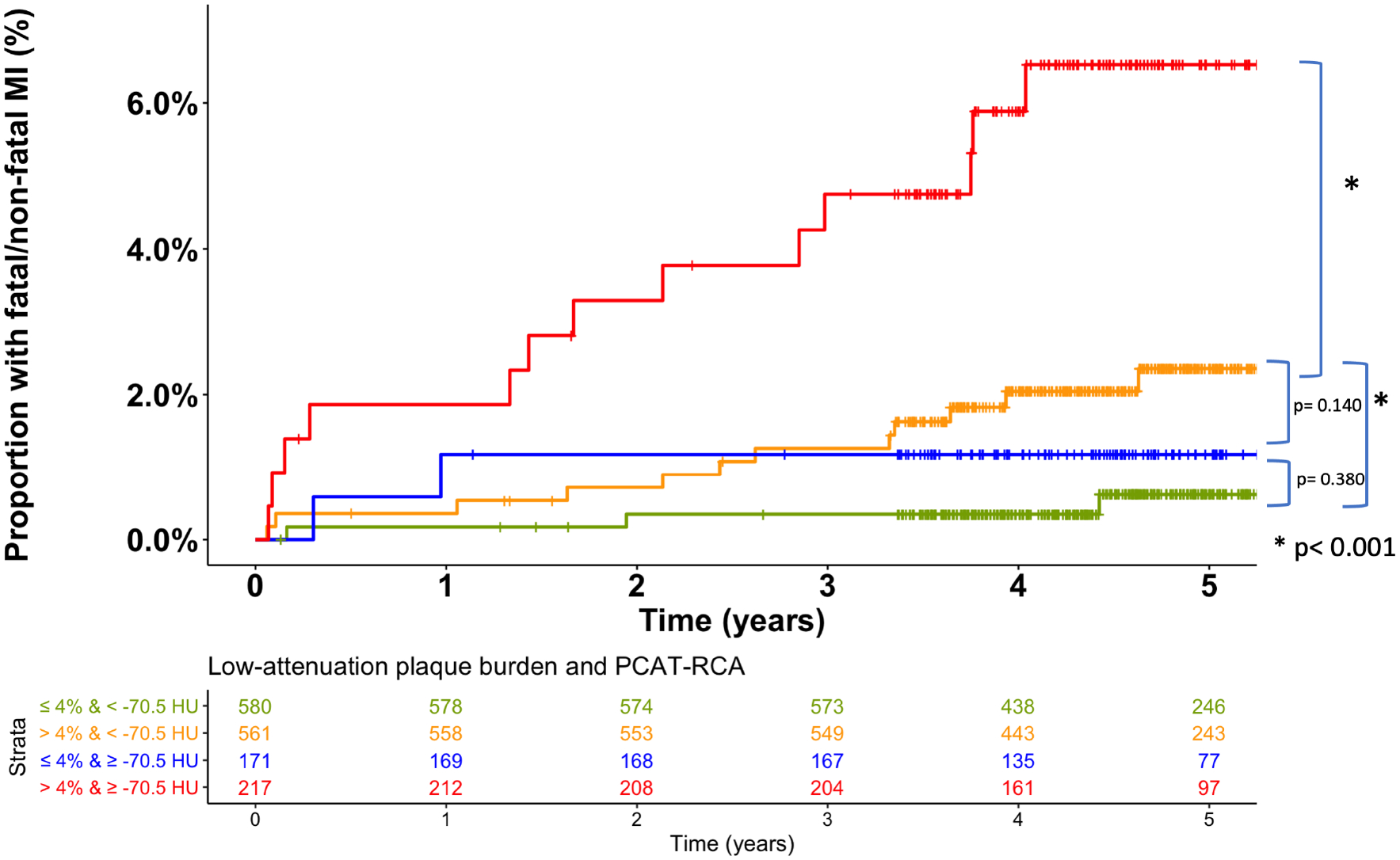
Cumulative incidence of fatal or nonfatal myocardial infarction in patients with and without peri-coronary adipose tissue of the right coronary artery (PCAT-RCA) ≥ −70.5 HU and with and without low-attenuation plaque burden (LAP) above 4%. Patients with LAP burden>4 % and PCAT-RCA ≥−70.5 HU (purple line) were at the greatest risk of myocardial infarction (HR 11.7, 95% CI 3.3 to 40.9, p<0.0001), followed by people with LAP burden >4 % and PCAT-RCA <−70.5 HU (green line); HR= 5.1 (1.5 −17.7), p<0.0001.
Figure 4: Comparison of receiver operator characteristics (at 5 years) and respective area under the curve (AUC).
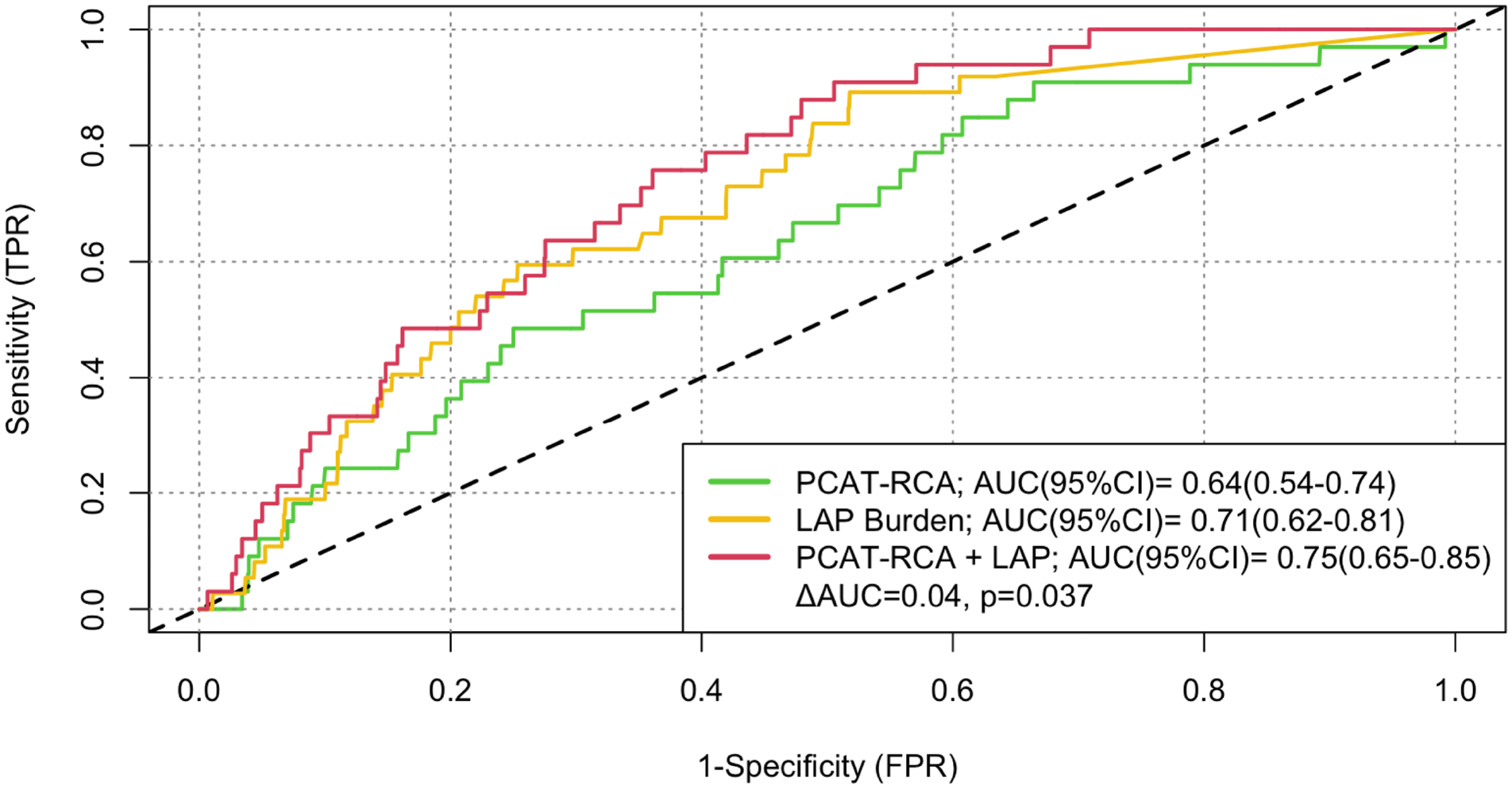
Low-attenuation plaque (LAP) burden is a stronger predictor comparted to peri-coronary adipose tissue of the right coronary artery (PCAT-RCA); p<0.001. The combination of the two metrics increase the AUC from 0.71 for LAP alone to 0.75(ΔAUC= 0.04, p=0.037).
In patients with non-obstructive coronary artery disease, high PCAT attenuation (≥ −70.5 HU) was associated with higher risk of myocardial infarction (Supplemental Figure vi and Table iv).
Discussion
In this post-hoc analysis of a prospective multicentre study, we have confirmed that pericoronary adipose tissue attenuation measured around the proximal right coronary artery can identify patients who are at an increased risk of myocardial infarction. This association persisted following adjustment for clinical cardiovascular risk score, coronary artery calcium score and the presence of obstructive coronary artery disease. Our results also establish the complementary predictive value of low-attenuation plaque burden and PCAT attenuation for 5-year risk of fatal or non-fatal myocardial infarction.
In our study, we assessed clinical, coronary and systemic determinants of PCAT attenuation. Consistent with previous studies,(18),(19) we demonstrate that men have higher PCAT attenuation than women. This may reflect the higher volume of epicardial adipose tissue(20) as well as sex-related differences of pericardial adipokines(21) and adipose tissue biology.(22) In contrast, we and others(23) found no differences in PCAT attenuation with age or individual clinical risk factors including hypertension, smoking, diabetes and hyperlipidaemia, although PCAT attenuation was lower in leaner individuals. Thus, only a few of systemic factors appear to have a weak influence on PCAT attenuation.
The anatomical location of the coronary artery was an important determinant of PCAT attenuation. Several previous reports(18,23,24) have highlighted that the left anterior descending coronary artery has lower PCAT attenuation, an observation that we have confirmed in the current study. This may reflect partial volume effects caused by the close proximity of the left coronary artery to low attenuation signal from the lungs but this cannot be readily explained in terms of vessel vulnerability. The Multi-Ethnic Study of Atherosclerosis (MESA)(25) established that up to 42% of newly characterised plaques are formed within the left anterior descending coronary artery and therefore we would expect that PCAT attenuation would perhaps be higher not lower in the left anterior descending coronary artery compared to other coronary arteries. In addition, PCAT has only been shown to be discriminative on contrast enhanced images, and this raises the possibility that it is representative of changes in local or more general vessel flow. We did demonstrate that the volume of PCAT was the greatest for the right coronary artery which also appeared to have the strongest associations with plaque burden, high risk plaques and clinical outcomes. This suggests that the PCAT attenuation signal may be most representative and predictive with higher volumes of pericoronary fat for analysis. Our univariable and multivariable regression analysis suggests that is the PCAT attenuation rather than the total volume of PCAT that is predictive of future events. Similar, to our study, Diemen et al.(26) showed that PCAT-RCA are predictive of future deaths and myocardial infarction, whereas PCAT-LAD/ PCAT-LCx are not. We believe that the most likely reason for this is that there is more fat surrounding the RCA compared to the other vessels and that there are fewer side branches to affect analysis of fat around the RCA.
We report that the burdens of total plaque, non-calcified plaque and low attenuation plaque were weakly associated with the PCAT-RCA attenuation whereas there were no associations with calcium artery calcium score or calcium burden. Moreover, PCAT-RCA attenuation was higher in patients with obstructive coronary artery disease or plaque with high-risk plaque features anywhere in the coronary tree. This suggests that PCAT attenuation is indicative of the global state of the coronary vasculature rather than a specific local effect representative of coronary artery disease in the immediate vicinity. Does this suggest that PCAT is indicative of systemic vascular inflammation? We assessed the relationship between PCAT attenuation and a range of cardiac and systemic inflammatory biomarkers including C-reactive protein and several inflammatory cytokines linked to cardiovascular disease. We found no relationship between PCAT attenuation and any of these biomarkers. Instead, the association of PCAT-RCA with markers of coronary plaque burden and morphology suggest it is specific to the coronary circulation and is not a surrogate measure of myocardial injury or strain, or global vascular inflammation. This is consistent with a smaller prior report of the absence of an association with C-reactive protein, cardiac troponin and brain natriuretic peptide.(19) These findings suggest that PCAT-RCA is potentially detecting overall coronary inflammatory status that is otherwise undetectable by systemic markers of inflammation.
Seminal work by Antonopoulos and colleagues has demonstrated that perivascular fat in close proximity to the coronary arteries has very different morphological and functional characteristics compared to epicardial fat related and that this could be measured using computed coronary tomography angiography.(6) Given its proximity to the adventitia of the coronary arteries, pericoronary adipose tissue interacts directly with the coronary vascular wall in a bidirectional way leading to coronary inflammation and atherosclerosis.(6) Inflammation of pericoronary adipose tissue is characterised by smaller adipose molecules with an increased aqueous component translating to higher CT attenuation. At the same time, the coronary inflammation leads to PCAT oedema due to increased permeability of the microcirculation in the vascular wall.(6) Although there are reports that PCAT attenuation can rapidly decline after acute myocardial infarction, the kinetics are not entirely understood or whether this is segment specific or a more general pan-coronary effect. Indeed, the latter seems more plausible given the dominance of the right coronary artery PCAT attenuation in our present and several previous studies(9,10,27,28) as well as the prediction of long-term outcomes that occur months or years later in all territories of the heart.
For clinical application and risk stratification, it is highly desirable to have a pre-defined threshold for PCAT attenuation. In their post-hoc analysis of two cohorts of over 4,000 patients, Oikonomou et al.(27), performed a more complex proprietary analysis which incorporates a number of other imaging and clinical factors. They found that a threshold of −70.1 HU for PCAT-RCA could predict a 9-fold increase in cardiac mortality in the derivation cohort and 5.62 in the validation cohort. In our analysis using a simpler assessment, we report a very similar threshold of −70.5 HU to predict a 2-fold increase in myocardial infarction suggesting a remarkable consistency of PCAT-RCA of both the thresholds and magnitude of risk prediction.
We have previously reported that low-attenuation plaque burden is a major and independent predictor of myocardial infarction, outperforming risk scores and other measures of coronary artery disease severity including stenosis severity and coronary calcium score(3). The correlation of low-attenuation plaque with a lipid-rich necrotic core likely underlies this strong association and its superior ability to predict subsequent myocardial infarction. PCAT-RCA attenuation was only weakly associated with low attenuation plaque and, in multivariable models, provided additive risk discrimination, something that no other measure was able to achieve. This suggests that PCAT attenuation provides additional complementary information regarding risk of coronary events; this is consistent with the hypothesis that it may represent a surrogate marker of vascular inflammation. In patients with non-obstructive coronary artery disease, higher risk of future myocardial infarction was noted in patients with RCA-PCAT ≥ −70.5 HU. Patients with non-obstructive disease and RCA-PCAT< −70.5 HU were not at an increased risk (p=0.39). The present study includes both qualitative and quantitative plaque assessment and we have demonstrated here that the only predictors of future events are the low-attenuation plaque burden and PCAT attenuation, while the presence of qualitative high-risk plaques are not significant.
Finally, in our study we have focused primarily on the assessment of pericoronary fat attenuation measured directly from CT rather than incorporate other complex radiomics features(7,29). This approach takes approximately 5 minutes per CT scan for direct measurement of PCAT attenuation and avoids the requirement for more complex modelling software (19).
Study Limitations
Our study has several limitations. We acknowledge that we observed only a modest number of myocardial infarctions and further validation may be required. Due to the relatively low-event rate there is a risk of model overfitting. In particular, the trend for the PCAT-RCA attenuation to be the best PCAT discriminator of downstream clinical events requires further confirmation, and the identification of the underlying mechanism needs to be more definitively established. Unfortunately, we did not have information regarding the territory involved in the subsequent myocardial infarction events to correlate our local and regional PCAT attenuation measures. However, these are often distant or fatal events and precise correlation would be challenging. Finally, in the SCOT-HEART trial, treatment was implemented based on CCTA findings, and this may have influenced our findings although this is likely to make our findings conservative and perhaps underestimate the strengths of these associations.
In conclusion, this post-hoc analysis of a large multicentre study shows that both PCAT-RCA attenuation and low-attenuation plaque burden are independent predictors of myocardial infarction, over and above other established markers of cardiovascular risk, including coronary artery stenosis severity and calcium score. Moreover, while low-attenuation plaque burden is the strongest predictor of myocardial infarction, PCAT-RCA attenuation and low-attenuation plaque burden provide complementary predictive value for 5-year risk of fatal or non-fatal myocardial infarction.
Supplementary Material
Central Illustration. Pericoronary Adipose Tissue Attenuation and Low Attenuation Plaque Burden Provide Complementary Predictive Value for 5-year Risk of Fatal or Nonfatal Myocardial Infarction in Patients with Suspected Coronary Artery Disease.
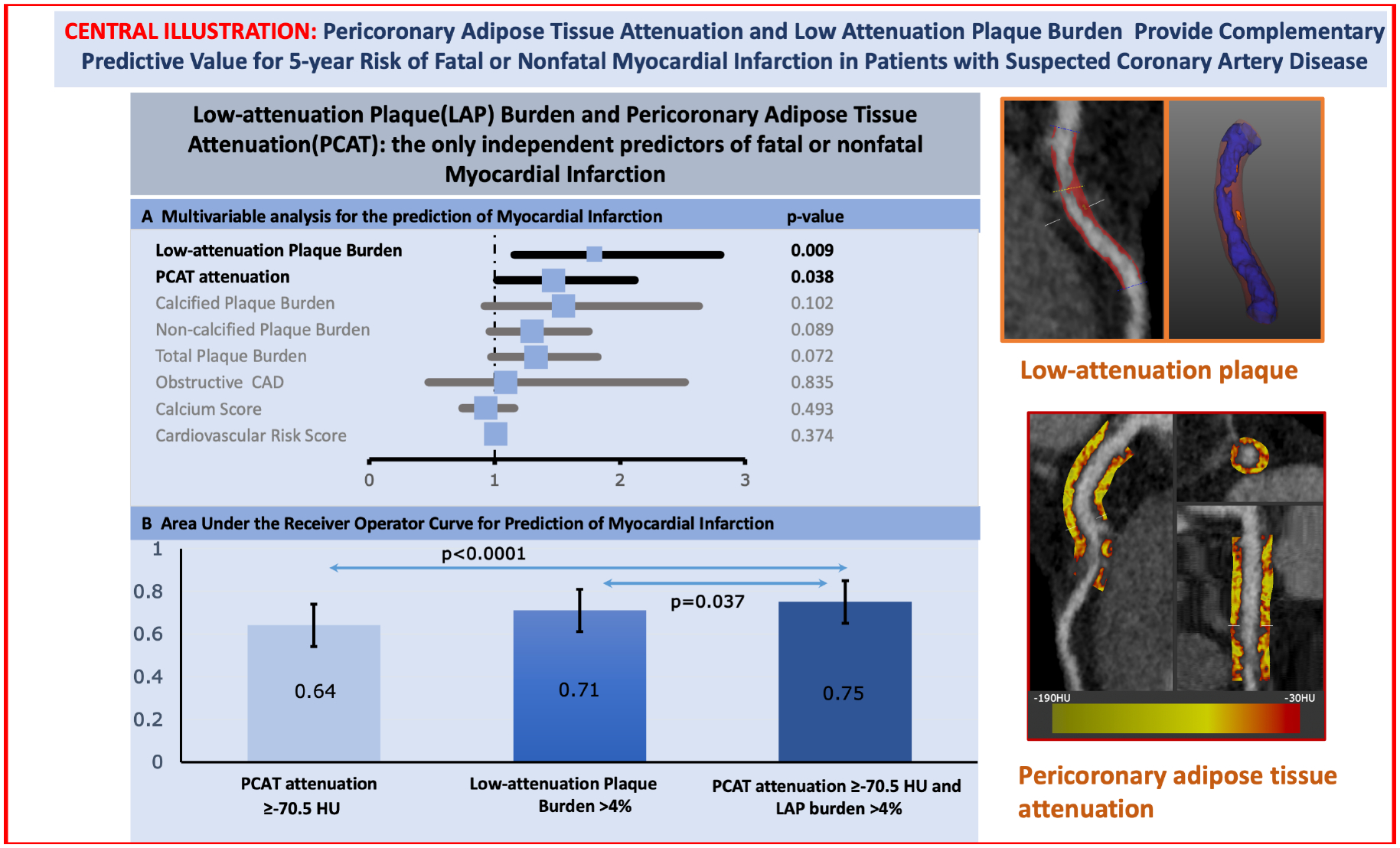
(Left – Top) Forrest plot of the multivariable analysis for the prediction of myocardial infarction. Low-attenuation Plaque (LAP) Burden and Pericoronary Adipose Tissue Attenuation (PCAT) are the only independent predictors of fatal or nonfatal Myocardial Infarction. (Left-Bottom) Bar graph with 95% confidence interval error bars comparing the area under the receiver operator characteristic curve (AUC) for the prediction of myocardial. Low-attenuation plaque burden showed higher area-under-the-curve (AUC) compared to PCAT-RCA attenuation for the prediction for myocardial infarction [0.71 (0.62–0.80) versus 0.64 (0.54–0.74); p<0.001], with the combination of both parameters yielding the highest AUC [0.75 (0.65–0.85); p=0.037] (Right) Based on the Youden’s index of the ROC curves, the optimal cut-off of the right coronary artery PCAT attenuation was −70.5 HU for the primary endpoint of fatal or non-fatal myocardial infarction. Patients with PCAT-RCA above ≥−70.5 HU were nearly 2.5 times more likely to suffer a myocardial infarction (HR 2.45, 95% CI 1.23 to 4.80; p=0.001). Patients with low-attenuation plaque burden (greater than 4%) were nearly 5 times more likely to suffer a myocardial infarction (HR 4.87, 95% CI 2.03 to 11.78, p<0.0001). When the two metrics were combined, patients with both low-attenuation plaque burden >4% and PCAT-RCA ≥−70.5 HU were at the greatest risk of myocardial infarction (HR 11.7, 95% CI 3.3 to 40.9, p<0.0001)
Perspectives:
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS:
Noninvasively assessed PCAT-RCA predicts risk of future myocardial infarction adding complementary predictive value to low-attenuation plaque burden, the strongest predictor of future myocardial infarction.
Translational Outlook:
Additional prospective trials are necessary to assess the effect of intensifying medical treatment (statins. PCSK9 inhibitors) on PCAT attenuation and future risk of myocardial infarction.
Funding:
This trial was funded by The Chief Scientist Office of the Scottish Government Health and Social Care Directorates (CZH/4/588), with supplementary awards from Edinburgh and Lothian’s Health Foundation Trust and the Heart Diseases Research Fund. M.C.W. (FS/ICRF/20/26002, FS/11/014, CH/09/002), D.E.N. (CH/09/002, RG/16/10/32375, RE/18/5/34216), and M.R.D. (FS/14/78/31020) are supported by the British Heart Foundation. M.C.W. was supported by The Chief Scientist Office of the Scottish Government Health (PCL/17/04). D.E.N. is the recipient of a Wellcome Trust Senior Investigator Award (WT103782AIA). E.J.R.v.B. is supported by the Scottish Imaging Network: A Platform of Scientific Excellence (SINAPSE). NLM is supported by the BHF through the award of the Butler Senior Clinical Research Fellowship and a Programme Grant (FS/16/14/32023, RG/20/10/34966). P.D.A. is supported by a National Heart Foundation of New Zealand Senior Fellowship (1844). M.R.D. is supported by the Sir Jules Thorn Biomedical Research Award 2015 (15/JTA). The Royal Bank of Scotland supported the provision of 320-multidetector CT for NHS Lothian and the University of Edinburgh. The Edinburgh Imaging facility QMRI (Edinburgh) is supported by the National Health Service Research Scotland (NRS) through National Health Service Lothian Health Board. The Clinical Research Facility Glasgow and Clinical Research Facility Tayside are supported by National Health Service Research Scotland (NRS). This work is supported in part by National Institute of Health/National Heart, Lung, and Blood Institute grant 1R01HL148787-01A1 and 1R01HL151266. A.L, K.G., P.M. S.C and D.D. are supported by National Institute of Health/National Heart, Lung, and Blood Institute grant 1R01HL148787-01A1. This work is also supported in part by the Miriam and Sheldon G. Adelson Medical Research Foundation.
Abbreviations:
- CCTA
Coronary Computed tomography angiography
- CP
Calcified plaque
- LAD
Left anterior descending
- LCx
Left circumflex artery
- LAP
Low attenuation plaque
- NCP
Non-calcified plaque
- PCAT
Pericoronary adipose tissue
- RCA
Right coronary artery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: D.D., S.C., P.S., and D.S.B. received software royalties from Cedars-Sinai Medical Center. D.D., P.S., and D.S.B. hold a patent (US8885905B2 in U.S. and WO patent WO2011069120A1, Method and System for Plaque Characterization). The remaining authors have nothing to disclose.
References
- 1.Chang HJ, Lin FY, Lee SE et al. Coronary Atherosclerotic Precursors of Acute Coronary Syndromes. J Am Coll Cardiol 2018;71:2511–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maddox TM, Stanislawski MA, Grunwald GK et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA 2014;312:1754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams MC, Kwiecinski J, Doris M et al. Low-Attenuation Noncalcified Plaque on Coronary Computed Tomography Angiography Predicts Myocardial Infarction. Circulation 2020;141:1452–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross R. Atherosclerosis — An Inflammatory Disease. New England Journal of Medicine 1999;340:115–126. [DOI] [PubMed] [Google Scholar]
- 5.van der Wal AC, Becker AE, van der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 1994;89:36–44. [DOI] [PubMed] [Google Scholar]
- 6.Antonopoulos AS, Sanna F, Sabharwal N et al. Detecting human coronary inflammation by imaging perivascular fat. Science Translational Medicine 2017;9:eaal2658. [DOI] [PubMed] [Google Scholar]
- 7.Oikonomou EK, Williams MC, Kotanidis CP et al. A novel machine learning-derived radiotranscriptomic signature of perivascular fat improves cardiac risk prediction using coronary CT angiography. Eur Heart J 2019;40:3529–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoshino M, Zhang J, Sugiyama T et al. Prognostic value of pericoronary inflammation and unsupervised machine-learning-defined phenotypic clustering of CT angiographic findings. Int J Cardiol 2021;333:226–232. [DOI] [PubMed] [Google Scholar]
- 9.Goeller M, Achenbach S, Cadet S et al. Pericoronary Adipose Tissue Computed Tomography Attenuation and High-Risk Plaque Characteristics in Acute Coronary Syndrome Compared With Stable Coronary Artery Disease. JAMA Cardiol 2018;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin A, Nerlekar N, Yuvaraj J et al. Pericoronary adipose tissue computed tomography attenuation distinguishes different stages of coronary artery disease: a cross-sectional study. Eur Heart J Cardiovasc Imaging 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newby DE, Williams MC, Flapan AD et al. Role of multidetector computed tomography in the diagnosis and management of patients attending the rapid access chest pain clinic, The Scottish computed tomography of the heart (SCOT-HEART) trial: study protocol for randomized controlled trial. Trials 2012;13:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dey D, Cheng VY, Slomka, PJ, Nakazato, R, Ramesh A, Gurudevan, S, Germano, Berman DS. Automated Three-dimensional Quantification of Non-calcified and Calcified Coronary Plaque from Coronary CT Angiography. Journal of Cardiovascular Computed Tomography 2009:372–382. [DOI] [PubMed] [Google Scholar]
- 13.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–32. [DOI] [PubMed] [Google Scholar]
- 14.Goeller M, Achenbach S, Cadet S et al. Pericoronary adipose tissue CT attenuation and high-risk plaque characteristics in acute coronary syndrome compared to stable coronary artery disease. Society of Cardiovascular CT midwinter meeting 2018;(Abstract):in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tzolos E, McElhinney P, Williams MC et al. Repeatability of quantitative pericoronary adipose tissue attenuation and coronary plaque burden from coronary CT angiography. J Cardiovasc Comput Tomogr 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Computerised record linkage: compared with traditional patient follow-up methods in clinical trials and illustrated in a prospective epidemiological study. The West of Scotland Coronary Prevention Study Group. J Clin Epidemiol 1995;48:1441–52. [DOI] [PubMed] [Google Scholar]
- 17.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 2007;44:837–845. [PubMed] [Google Scholar]
- 18.Ma R, Ties D, van Assen M et al. Towards reference values of pericoronary adipose tissue attenuation: impact of coronary artery and tube voltage in coronary computed tomography angiography. Eur Radiol 2020;30:6838–6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugiyama T, Kanaji Y, Hoshino M et al. Determinants of Pericoronary Adipose Tissue Attenuation on Computed Tomography Angiography in Coronary Artery Disease. J Am Heart Assoc 2020;9:e016202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mancio J, Pinheiro M, Ferreira W et al. Gender differences in the association of epicardial adipose tissue and coronary artery calcification: EPICHEART study: EAT and coronary calcification by gender. Int J Cardiol 2017;249:419–425. [DOI] [PubMed] [Google Scholar]
- 21.Fei J, Cook C, Blough E, Santanam N. Age and sex mediated changes in epicardial fat adipokines. Atherosclerosis 2010;212:488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang E, Varghese M, Singer K. Gender and Sex Differences in Adipose Tissue. Curr Diab Rep 2018;18:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hell MM, Achenbach S, Schuhbaeck A, Klinghammer L, May MS, Marwan M. CT-based analysis of pericoronary adipose tissue density: Relation to cardiovascular risk factors and epicardial adipose tissue volume. J Cardiovasc Comput Tomogr 2016;10:52–60. [DOI] [PubMed] [Google Scholar]
- 24.Gaibazzi N, Martini C, Botti A, Pinazzi A, Bottazzi B, Palumbo AA. Coronary Inflammation by Computed Tomography Pericoronary Fat Attenuation in MINOCA and Tako-Tsubo Syndrome. J Am Heart Assoc 2019;8:e013235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alluri K, McEvoy JW, Dardari ZA et al. Distribution and burden of newly detected coronary artery calcium: Results from the Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr 2015;9:337–344 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Diemen PA, Bom MJ, Driessen RS et al. Prognostic Value of RCA Pericoronary Adipose Tissue CT-Attenuation Beyond High-Risk Plaques, Plaque Volume, and Ischemia. JACC Cardiovascular imaging 2021. [DOI] [PubMed] [Google Scholar]
- 27.Oikonomou EK, Marwan M, Desai MY et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. The Lancet 2018;392:929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goeller M, Tamarappoo BK, Kwan AC et al. Relationship between changes in pericoronary adipose tissue attenuation and coronary plaque burden quantified from coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging 2019;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin A, Kolossvary M, Yuvaraj J et al. Myocardial Infarction Associates With a Distinct Pericoronary Adipose Tissue Radiomic Phenotype: A Prospective Case-Control Study. JACC Cardiovascular imaging 2020;13:2371–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


