Abstract
Introduction:
Medications to treat opioid use disorder (MOUD) during pregnancy and in the postpartum period remain underutilized. A need exists to enhance our understanding of modifiable factors, facilitators, and barriers to MOUD utilization and adherence in the perinatal period to improve maternal and child outcomes.
Methods:
The study conducted semi-structured qualitative interviews with recently pregnant people with opioid use disorder (OUD) to explore experiences as a pregnant and/or parenting person with OUD, perceptions of enabling factors and barriers to treatment utilization, incentivizing factors for maintaining adherence, and acceptability of ongoing supports to sustain treatment adherence. The study team used constant comparative methods to analyze transcripts and develop the codebook. The team double coded the transcripts, with an overall kappa coefficient of 0.88.
Results:
The study team interviewed twenty-six women on average 10.1 months after delivery. All women had some prior experience using MOUD. Four unique themes emerged as barriers to medication utilization and adherence in the perinatal period: 1) Lack of agency and autonomy surrounding medication decisions because pregnancy or parenting status affected treatment adherence; 2) Hesitancy to use MOUD to minimize risk of newborn withdrawal; 3) Concern about increased scrutiny and potential loss of custody due to mandated child welfare reporting for opioid-exposure at delivery in Massachusetts; and 4) Perception that treatment environments, particularly methadone clinics, did not provide gender-responsive or equitable care, and standardized, inflexible visit regulations were particularly difficult to comply with in the early postpartum period.
Conclusions:
Women with OUD experienced a double bind when making perinatal treatment decisions, describing pressure to use MOUD with negative consequences after delivery. Key areas for possible intervention emerged from interviews. These areas include improving uptake of shared decision-making to increase patient autonomy and agency, particularly among those in the earliest stages of recovery during pregnancy; ongoing education around perinatal MOUD safety and efficacy; detangling MOUD and neonatal withdrawal signs from mandated child welfare reporting; and improving gender-responsive and equitable care in substance use disorder treatment programs, including incorporating the utilization of home visiting services for dosing assessments and administration in the early postpartum period.
Keywords: Perinatal, Opioid use disorder, Medications to treat opioid use disorder, Methadone, Buprenorphine, Pregnancy, Postpartum, Child welfare
1. Introduction:
The number of deliveries affected by opioid use disorder (OUD) has increased from 1.5/1000 births to 6.0/1000 births in the past two decades in the United States (Haight, Ko, Tong, Bohm, & Callaghan, 2018; Winkelman, Villapiano, Kozhimannil, Davis, & Patrick, 2018). Use of medication to treat OUD (MOUD), such as methadone or buprenorphine, in combination with behavioral therapy, is the recommended treatment for OUD in pregnancy (American College of Obstetricians and Gynecologists (ACOG), 2017; “Clinical Guidance for Treating Pregnant and Parenting Women With Opioid Use Disorder and Their Infants | SAMHSA Publications and Digital Products,” n.d.; Ecker et al., 2019), and research has shown that this combination improves obstetrical, addiction, and neonatal outcomes (American College of Obstetricians and Gynecologists (ACOG), 2017; Goler, Armstrong, Taillac, & Osejo, 2008; Goler et al., 2012). Pregnancy motivates some people to reduce non-prescribed substance use and engage in treatment (Forray & Foster, 2015; Forray, Merry, Lin, Ruger, & Yonkers, 2015), yet consistent medication utilization during pregnancy and in the postpartum period remains low (Lo-Ciganic et al., 2019), particularly in the 6–12 months following delivery when overdose risk is elevated (Martin, Longinaker, & Terplan, 2015; Schiff et al., 2021, 2020, 2018; C. Wilder, Lewis, & Winhusen, 2015).
Few prospective studies have tracked the treatment trajectories of birthing people with OUD across the perinatal continuum outside of a structured clinical trial or examined factors associated MOUD utilization and adherence (Wilder et al., 2015). Retrospective studies that have examined perinatal MOUD adherence have found that the receipt of higher methadone dose and earlier treatment engagement are associated with improved adherence in substance use treatment and/or perinatal care (O’Connor, Uhler, O’Brien, & Knuppel, 2018; Schiff et al., 2021; Wilder, Hosta, & Winhusen, 2017). Additionally, inequities in MOUD utilization exist across the perinatal period by race/ethnicity and by age (Peeler et al., 2020; Schiff et al., 2021, 2020). A mixed association exists of the impact of psychiatric diagnoses, including depression and anxiety, with treatment utilization, hindering prenatal care attendance in one study and increasing likelihood of receiving MOUD treatment in another (Hensley, Sulo, Kozmic, & Parilla, 2018; Schiff et al., 2020). Qualitative work has highlighted that pregnant and postpartum people with OUD also experience unique barriers and challenges to medication utilization, including difficulty accessing treatment while pregnant, shame/fear of disclosure of drug use while pregnant, potential loss of custody related to mandated reporting of in utero opioid exposure to child protective services at delivery, and the risk of their infant experiencing neonatal opioid withdrawal syndrome (NOWS) (Proulx & Fantasia, 2021; Phillippi et al., 2021; Leiner, Cody, Mullins, Ramage, & Ostrach, 2021; Ostrach & Leiner, 2018; Patrick et al., 2020; Paris, Herriot, Maru, Hacking & Sommer 2020).
Given mothers’ poor perinatal MOUD utilization despite the established benefits of MOUD adherence, a need remains to enhance our understanding of modifiable factors, facilitators, and barriers to MOUD utilization and adherence in the perinatal period to improve maternal and child outcomes. The primary aim of this study was to engage a diverse group of recently pregnant patients with OUD and use qualitative methods to identify individual beliefs, attitudes, and structural factors that impeded and supported OUD treatment in the medical and social services system, with the goal of identifying target areas for interventions to improve perinatal MOUD utilization and adherence.
2. Materials and methods
2.1. Study design
Our study used a model developed for perinatal HIV medication adherence (Momplaisir, Storm, Nkwihoreze, Jayeola, & Jemmott, 2018) that integrates the Behavioral Model for Vulnerable Populations (Gelberg, Andersen, & Leake, n.d.) and the Ecological Model of Health Behavior (Glanz, Rimer, & Viswanath, 2008) and adapted them (Figure 1) to inform the development of open-ended questions about substance use and recovery in the perinatal period. We aimed to assess individual beliefs and attitudes to understand interpersonal, community, and health system factors that contribute to medication utilization and adherence. The interview content was informed by our research team’s clinical experience caring for the mother-infant dyad as pediatricians, family physicians, obstetricians, addiction physicians and psychiatrists, psychologists, and nurses. Probes explored participants’ experiences as a pregnant and/or parenting person with OUD, their perceptions of enabling factors and barriers to treatment utilization, incentivizing factors for maintaining adherence, and acceptability of ongoing supports to sustain treatment adherence. The guide was iteratively updated, throughout the interview process, to allow further questioning of novel themes that emerged along the way. Supplemental File 1 includes the questions and probes. We used the CoreQ-32 checklist to ensure quality (Tong, Sainsbury, & Craig, 2007). The Partners Institutional Review Board reviewed and approved this study.
Figure 1.
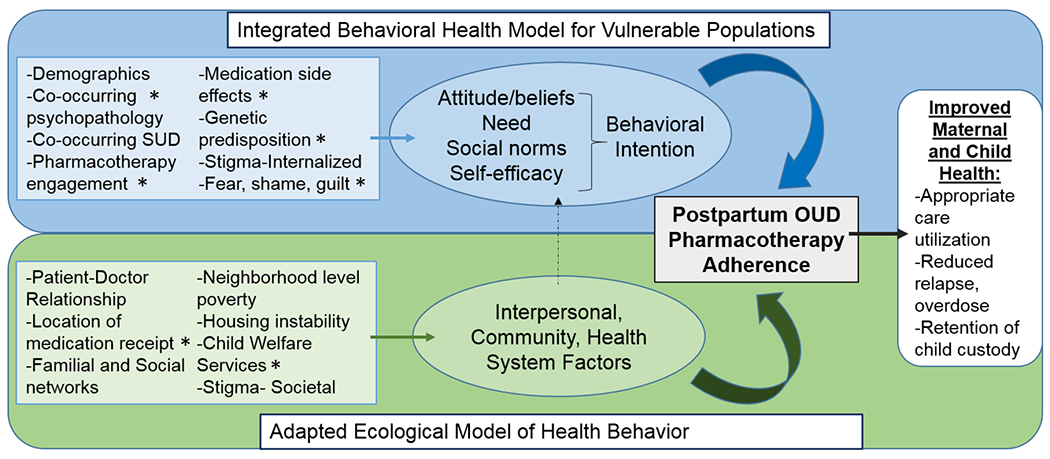
Adapted model for assessing medication to treat opioid use disorder initiation and adherence
Model adapted from Momplaiser et al. 2018, *represents inputs unique to perinatal OUD adaptation
2.2. Participants, recruitment, and data collection
We approached recently pregnant people with OUD, defined using the Diagnostic and Statistical Manual of Mental Disorders (DSM)-5 criteria. The study team identified potential participants who delivered a live birth in Massachusetts within the last three years through medical record review. Individuals were eligible to be included whether or not they received MOUD during pregnancy. Recruitment largely occurred at a single site, a multidisciplinary clinic caring for pregnant and postpartum people with substance use disorder and their families in an urban metropolitan area in the Northeast (n=24). The study used flyers, word-of-mouth, and snowball (“chain”) sampling to enhance recruitment efforts (n=2) (Given, 2008). The study also used purposive sampling to maximize the racial diversity of the sample, given the historic oversampling of non-Hispanic White individuals in qualitative research about OUD in the perinatal period (Atwood et al., 2016; H, 2016; Howard et al., 2018; Ostrach & Leiner, 2019; Schiff et al., 2022).
Participants were recruited by and interviewed with a research coordinator with no patient care responsibilities between October 2019 and December 2020. Interviews lasted 30–90 minutes, with a median time of 44 minutes. Recruitment continued until no new codes emerged and thematic saturation occurred using the iterative process described below. Participants received a $40 gift card as remuneration for their time and compensation for travel costs, and the study offered childcare during the interviews. Because the COVID-19 pandemic occurred in the middle of the study, the study team transitioned interviews from in-person to phone or video-conference call. All interviews were audio-recorded, transcribed verbatim by an outside transcription service, and deidentified for analysis.
2.3. Data analysis
We used constant comparative methods, an approach that involves an iterative process of updating interview questions, reviewing data collected, and analyzing codes all at the same time to identify, refine, and enrich themes and facilitate emergence of theory (Chun Tie, Birks, & Francis, 2019; Kolb, n.d.). Four investigators independently reviewed transcripts to generate a preliminary codebook of nodes and sub-nodes (SBP, DMS, ECW, JAB), reviewing as a group until no new codes were identified after review of nine transcripts. The study team used the final coding scheme to evaluate all transcripts using NVivo Qualitative software. Two independent coders performed a content analysis of all transcripts, including those reviewed to generate the codebook. The study team met frequently to discuss findings, review discrepancies, and monitor for thematic saturation. The team compared all double-coded transcripts using the Kappa statistic, with Kappa <0.8 used as a cut point for review and the team discussed until they reached consensus. The final kappa coefficient for all double-coded interviews was 0.88, indicating substantial agreement.
3. Results
3.1. Study sample
Table 1 lists the characteristics of the 26 women who completed interviews (5 declined to participate or were unable to schedule a time). The cohort’s mean age was 33 (SD 4.6). The average time since delivery was 10.1 months (range 3 weeks to 33.1 months). Twenty seven percent of women identified as non-White or of mixed-race; 19% identified as Hispanic or Latina. More than half of participants were single (54%), others were living with their partner (27%), dating or in partnered relationships (19%), or married (12%). One participant completed college, 31% had some college experience, 39% had a high school or equivalent degree, and 23% never received a high school degree. The average age of first substance use was 14 years, and the average age of first opioid use was 18 years. Prior use of MOUD was common; 92% had received buprenorphine and 77% had received methadone. Most were receiving MOUD at time of interview (35% methadone, 54% buprenorphine, and 11% no MOUD).
Table 1.
Study Participant Characteristics
| Characteristic | n (%) or mean (SD) |
|---|---|
| Age, mean (SD) | 33 years (4.6) |
| Months from delivery when interviewed, mean (range) | 10.1 months (3-33.1) |
| Race | |
| American Indian/Alaska Native | 1 (8.1%) |
| Black or African American | 3 (11.5%) |
| Mixed Race | 3 (11.5%) |
| White or Caucasian | 19 (73.1%) |
| Ethnicity | |
| Hispanic or Latina | 5 (19.2%) |
| Non-Hispanic or Latina | 21 (80.8%) |
| Sexual orientation | |
| Heterosexual | 24 (92.3%) |
| Lesbian/Bisexual | 2 (7.7%) |
| Relationship status | |
| Dating/Partnered | 5 (19.2%) |
| Single | 14 (53.8%) |
| Living with Partner | 7 (26.9%) |
| Married | 3 (11.5%) |
| Highest educational attainment | |
| Less than high school | 6 (23.1%) |
| High school/equivalent | 10 (38.5%) |
| Some college | 8 (30.8%) |
| College graduate/higher | 1 (3.8%) |
| Unknown | 1 (3.8%) |
| Living situation | |
| Residential treatment program/ sober house | 5 (19.2%) |
| Room, apartment, house that I own or rent | 13 (50.0%) |
| Shelter | 1 (4.0%) |
| Transitional Stabilization Services | 1 (3.8%) |
| With family or friends | 4 (15.4%) |
| Unknown | 1 (3.8%) |
| Age of first use of non-prescribed substances, Mean (SD) | 14 (5.1yrs) |
| Age of first use of opioids | 18.2 (5.7yrs) |
| History of injection drug use | 22 (84.6%) |
| Previously OUD treatment trials | |
| Methadone | 20 (76.9%) |
| Buprenorphine (Suboxone, Subutex) | 24 (92.3%) |
| Injectable buprenorphine | 4 (15.4%) |
| Naltrexone (oral) | 6 (23.1%) |
| Injectable naltrexone (Vivitrol) | 6 (23.1%) |
| MOUD at beginning of pregnancy | |
| Buprenorphine (Suboxone, Subutex) | 18 (69.2%) |
| Methadone | 7 (26.9%) |
| None | 1 (3.9%) |
| Current MOUD | |
| Buprenorphine (Suboxone, Subutex) | 12 (46.2) |
| Injectable buprenorphine | 2 (7.7%) |
| Methadone | 9 (34.6%) |
| None | 3 (11.5%) |
3.2. Overview of qualitative themes
We identified four key themes contributing to attitudes, beliefs, and structural factors affecting medication utilization and adherence that were unique to the perinatal period (Table 2). First, participants experienced a lack of agency and autonomy surrounding medication decisions because of their pregnancy or parenting status, hindering their treatment adherence. Second, participants were hesitant to use medications because they wanted to minimize perceived harms to their newborn from experiencing withdrawal symptoms at birth. Third, participants were concerned about increased scrutiny and potential loss of custody due to mandated child protective services reporting for opioid-exposure at delivery in Massachusetts. Fourth, participants reported that treatment environments, particularly methadone clinics, did not provide gender-responsive care, with standard inflexible visit regulations particularly difficult in the early postpartum period. We described below representative quotes and linkage to subthemes listed in Table 2.
Table 2:
Domains, themes, subthemes
| Domain | Theme | Subtheme |
|---|---|---|
| Attitudes/Beliefs | 1. Need for autonomy, agency, and choice in medication treatment decisions in the perinatal period | 1. Perceptions of being forced into prenatal medication decisions |
| 2. Lack of shared decision making in medication decisions | ||
| 3. Avoidance of care due to lack of autonomy | ||
| Attitudes/Beliefs | 2. Desire to minimize harm at delivery: fears and shame surrounding newborn withdrawal symptoms | 4. Fear around neonatal withdrawal severity |
| 5. Negative perceptions of mothers by newborn staff | ||
| Health System Factors / Structural barriers | 3. Concern about increased scrutiny and potential loss of custody due to mandated child welfare reporting for opioid-exposure at delivery due to medication use in Massachusetts. | 6. Experience of discrimination due to MOUD |
| 7. Desire to discontinue treatment to avoid mandated reporting | ||
| 8. Increased scrutiny due to MOUD | ||
| 9. Postpartum exhaustion blamed on MOUD | ||
| Health System Factors / Structural barriers | 4. Treatment environments did not provide gender-responsive, equitable care and regulations were not appropriate for early postpartum period or children | 10. Desire to shield children from experience of methadone clinic |
| 11. Treatment regulations at odds with physical recovery and parenting responsibilities | ||
| 12. Racial discrimination in treatment system |
3.2.1. Theme 1: Need for autonomy, agency, and choice in medication decisions in the perinatal period
When participants became pregnant, they commonly reported being told by medical providers that they had no alternative but to take methadone or buprenorphine. They interpreted these recommendations as admonishment that if they did not receive medication, they would harm the fetus. Fourteen of 26 participants endorsed experiencing pressure to initiate or continue MOUD:
When I got pregnant, I was told by the doctors at the hospital “Yon can’t come off of this, because you could harm the baby, or the baby could die. I’m not saying this to have an excuse. I really had no choice. I really couldn’t. If a doctor was telling me that I could hurt my baby by coming off of it, then I’m not gonna come off of it, so I stayed on it, and I came off all my other meds. (26-year-old White non-Hispanic mother, Subtheme 1)
At times, the recommendations of providers describing the benefits of medications to improve pregnancy outcomes were perceived by patients as coercive. Participants described feeling forced to stay on medications that they did not want or wanted to try to taper and discontinue:
I was forced to stay on buprenorphine my whole pregnancy. It was never my decision… I was talkin’ to my doctor about options, and I was tellin’ my doctor that I felt fine… [yet] I had to be on some kind of medication to prevent relapse. They still pressured me and put it in my head like it was the best decision or whatever… I just didn’t feel good bein’ on this medication. I did not. I was forced. I feel like I was forced. (28-year-old Latina mother, Subtheme 1)
Additionally, participants reported experiencing little in the way of discussion or shared decision-making with providers to help facilitate medication choice:
They basically just like, you have to go on something so you could stop using, and our options are this. I just, at that time I was like done. I’m like, alright, I’ll try the methadone. I’ve heard about it. The clinic was right down the street, so I just didn’t really realize it was so time consuming. They call it liquid handcuffs because you have to go every single day and wait in those lines and stuff. (35-year-old White non-Hispanic mother, Subtheme 2)
One participant even reported feeling pressure from a health care worker to terminate her pregnancy because she was on methadone:
The first thing [the caseworker] said to me was, “So I noticed you were on methadone. You realize that’s liquid heroin, correct?” … She said, “Are you sure you even wanna have this baby?… I know, right now, while you‘re pregnant, it seems like you can do it, but I’m really—I’m here to tell you, honey, it’s not easy … being a mom … and it-it’s really selfish to bring a child in the world when you‘re not ready” … She’s like, “You know, as of two years ago, Massachusetts passed a law where you could be up to five months to have an abortion.” I said, “So what the **** are you getting at?” And she’s like, “Well, I’m just saying there’s still time.” (31-year-old Black/mixed race mother, Subthemes 2, 12)
Another participant reported that her clinicians encouraged medication use despite her desire not to take it, and that the lack of medical support for discontinuing/tapering led to her avoidance of care:
Even my doctor was pushin’ me towards bein’ on it. I just feel like, if I’m saying I don’t wanna be on it, nobody should be forcing me or pressuring me to switch my decision or tell me things just to scare me. Which, a lot of the doctors did when I was pregnant. Which is a reason why I didn’t go—I stopped going to the clinic for a while. I didn’t go for a few months while I was pregnant because I just got tired of hearing certain things. (28-year-old Latina mother, Subtheme 3)
Further, one participant commented on an experience in which a clinician asked her if she would prefer a culturally concordant physician. She perceived this experience as her doctor not wanting to put in the work needed to relate to her as a patient:
Do you think you could relate better to an African American doctor?” What? Why would you think that? Why can’t you relate to me? I didn’t know heroin had a race … I didn’t know heroin picked people by their color. That was very offensive to me. It seems like [my doctor] since then hasn’t been able to fully help me or fully work with me because she can’t relate to me, and it’s not that it’s—I feel like she chooses not to. I feel like she chooses not to put in the work to relate to me as her patient. (31-year-old Black mother, Subtheme 3, 12)
A shared framework and the establishment of a trusting relationship facilitated the connection with individual providers and patients’ perceptions of a mutually agreed-upon treatment decision. Additionally, individuals who identified addiction as a chronic disease and MOUD as a necessary medication seemed to be most comfortable using medications:
I guess you have to find the right treatment, the right doctors ’cause I’ve had doctors that were very, very helpful… when I expressed how I don’t wanna be on medication for the rest of my life, they explain it to me in a different way. ‘Somebody with cancer or even diabetes, they might need a medication to stabilize that, and they still can do well throughout life’… letting a provider get to know you, not holding back, and being who you are is very helpful. (32-year-old White mother, Subtheme 1)
Finally, beyond the context of pregnancy and early parenting, participants endorsed more positive experiences with MOUD in supporting their recovery trajectories including security against cravings and feeling “normal.”
It allowed me to have a life. It allowed me to start to work on myself without having to have the daily waking up not well every day and starting my day off like that… It gave me the opportunity. That’s what it does. It gives you the opportunity. (33-year-old White mother)
3.2.2. Theme 2: Desire to minimize harm at delivery: Fears and shame surrounding newborn withdrawal symptoms
Participants most commonly cited hesitation to use medications because of concern for perceived harm to their infant due to risk of withdrawal symptoms, with 13 of 26 participants discussing concerns.
I expressed to [my doctor] when they told me that my daughter could become dependent on this medication, I didn’t wanna take it anymore. I said, “Is it more beneficial for me to not take it than to take it because I don’t need my—I don’t want my baby to become dependent on this or have withdrawals when she’s born?” They pretty much were like, “Well, it’s proven to be little this, little that… (28-year-old Latina mother, Subtheme 4)
Additionally, the uncertainty surrounding the degree of neonatal withdrawal leading up to delivery resulted in fear and anxiety during pregnancy for mothers taking their medications to treat their OUD:
My whole pregnancy I was terrified how she was gonna come out—and I was hearing all these horror stories about these babies that had to get morphine… because they were so sick from their mom being on methadone or suboxone, I was terrified. (26-year-old White mother, Subtheme 4)
Patients experienced fear and worry regarding dose changes that were physiologically necessary due to the perceived impact on their fetus and sedation following delivery:
I literally waited ‘til I was throwing up every day sick, sick, sick to go up on my methadone ‘cause I was trying so hard not to go up. I knew what it was gonna do to me after I had the baby … Because they don‘t just cut half your dose after you have the baby. You‘re still on that high dose, so how are you supposed to take care of a baby? (30-year-old White mother, Subtheme 4)
Following birth, participants expressed an increased scrutiny from inpatient care providers because of their medication use and endorsed feelings of blame and shame when their infants displayed symptoms of withdrawal:
There was maybe one nurse that was saying that the baby was withdrawing because of me. She was like, “The reason why you have to stay in the hospital longer is because you decided to be on Suboxone.” When the nurse said, “But since your baby is withdrawing from the Suboxone that you take,” it got me. Maybe it was the way she worded it. Maybe I’m takin’ it too personal. (30-year-old Latina mother, Sub theme 5)
3.2.3. Theme 3: Concern about increased scrutiny and potential loss of custody due to mandated child welfare reporting for opioid-exposure at delivery due to medication use in Massachusetts
The practice of mandated child abuse/neglect reporting in Massachusetts to the Department of Children and Families (DCF) for all mothers who receive MOUD (Commonwealth of Massachusetts, n.d.) was often feared by mothers with past DCF involvement, and described as surprising and frustrating for first-time mothers (reported by 12 of 26 participants):
But not one time did they tell me that DCF would be involved after my pregnancy. If I would have known that I was gonna get in trouble for takin’ a medication that’s supposed to help me, I would have never did it. I would have never did it. (28-year-old Latina mother, Subtheme 6)
Another mother commented on how this practice discriminates against people with substance use disorder:
It’s a flaw that the system just automatically assume[s] that because somebody is on Suboxone or methadone, that they’re doing the wrong thing. It really creates an awful stigma having the time that after you have your kid be interrupted by DCF. I got home from the hospital to a DCF worker. That’s horrifying. It’s not appropriate. They don’t do that to moms that are on any type of medication. (31-year-old White mother, Subtheme 7)
One woman who was in long-term recovery expressed that she considered stopping her medication because of the paradox around prescribed medication use and mandated reporting practices:
Even if you do everything right at the end of it, there’s still going to be system involvement. I can remember during certain pregnancies wanting to come off medication because I was afraid of that. (40-year-old White mother, Subtheme 6)
Similarly, following delivery, child protective services agencies’ perspectives impacted individuals’ views on medications, where five participants reported feeling judged and worried that there could be repercussions from their decisions around using medications to treat OUD:
Either way, with DCF, it was, basically, a no-win. I was [in recovery]. I was on Subutex while I was pregnant. Then they asked me, “Oh, why are you on Subutex?” I said, “I have to be on Subutex ’cause it’s keeping me [in recovery].” If I was using, they would be upset, or if they thought I was using. If I’m [in recovery] on Subutex, they ‘re still upset. (27-year-old Latina, mixed race mother, Subtheme 8)
Finally, mothers felt surveilled by residential programs and child protective services agencies who commonly blamed medications for a mother’s postpartum exhaustion:
Just because I’m on methadone and taking meds, it seemed like everything that happens is always because it’s methadone fault… I was tired, so that must be—not the baby getting up every night, but it must be my methadone. Everything had to do with my meds. That’s how they viewed it. (35-year-old White mother, Subtheme 9)
3.2.4. Theme 4: Treatment environments did not provide gender-responsive care and regulations were not appropriate for early postpartum period or children
Participants receiving methadone (n=8) viewed treatment programs as spaces that were difficult and challenging to get to immediately following birth. One participant described her experience bringing her child with her to a methadone clinic immediately after discharge from the delivery hospitalization:
I have an open wound.… My body is open, more open than it’s ever been, to a point where it itches. Now here I am in the clinic. Now this person—he’s got open sores all on his arms and his legs. I’m standin’ behind him, and I’ve got a[n] open body and a brand-new baby with hardly any immune system. I’ve gotta now go to the clinic every single day with my baby and hope that whatever this person’s coughin’ to the left and to the right of me isn’t somethin’ like pneumonia. Why is it that I just had a baby three days ago and they were able to dispense me my medication in the hospital, but yet I come home from the hospital and you expect me to waddle my open body with my child in this car seat and carry it, no doubt? (31-year-old Black mother, Subtheme 10)
They described the competing demands of parenting and attending daily medication visits, and fear of bringing children into the OUD clinical setting:
If there was a detox right now for methadone, I would go do it, even if it took three weeks out of my life to stay away from my child, I would do that ‘cause in the long run, it’s gonna benefit my child. I don’t wanna take him to a methadone clinic. I don’t want him to see that side of life. It’s sad what that life is and I just don’t want him ever to feel that pain that I had to feel or even look at somebody that’s in pain like that and feel their pain. (30-year-old White mother, Subtheme 10)
Even people who were more stable in their recovery, receiving take-home doses of methadone, described how the strict regulations and requirements made it difficult for them to be able to parent, work, and attend to their recovery:
I want a life where I don’t have to go—I get take-homes, but I still, it’s like you have to earn your take-homes. I call them take-aways because you’re more likely to get them taken away than to given… you‘re supposed to be a normal person of society and get a job and take care of your child and find a place to live, but recovery comes first. (30-year-old White mother, Subtheme 11)
Groups that parents attended at methadone clinics tolerated children, but one participant described the concerns of having no dedicated childcare on site:
It just exposes them to a different environment. When I was on methadone and I had my kids, I would have to bring them to the groups. It’s a good thing they were allowed to be there. At the same time, if you have an older child, you might not want them to know, or you might have not explained things to them yet or might not even plan to. (32-year-old White mother, Subtheme 11)
One participant highlighted ways that reducing federal regulations for substance use disorder treatment could increase satisfaction with a treatment that is highly effective by dispensing it like treatment for other chronic diseases:
They wanna do somethin’ with their life. Here they come, 5:00 in the mornin’. They get up. They come here every single day. I don’t see a line outside the insulin center. They get prescriptions to go to the pharmacy. (31-year-old Black mother, Subtheme 11)
Finally, one participant attributed her negative experiences with methadone to structural racism. She described another patient at the methadone clinic yelling racial slurs and physically assaulting her, which led to an emergency discharge when she defended herself:
This system is not built for African American people to be sober. It’s not. It’s not… This was an emergency discharge to the point where I had … to … get my methadone in the emergency room. Yet that [white]girl stayed on the clinic for the five times before this and assaulted people … Why would you kick somebody off that was assaulted and defended themselves? You mean to tell me that’s not racism at its finest? (31-year-old Black mother, Subtheme 12)
4. Discussion
Our analyses of patient perspectives highlight unique ways that pregnancy and parenting status interact with patients’ decisions to use MOUD. Over the last decade, clinicians have accepted MOUD as a gold standard treatment in pregnancy, but rates of prenatal utilization and retention in the postpartum period remain suboptimal (MacAfee, Sawyer, & Terplan, 2016; Schiff et al., 2021; Wilder et al., 2015). Clinician recommendations to use evidence-based medications to stabilize and support OUD recovery during pregnancy often conflicted with parents’ own desires for their treatment; participants reported lacking autonomy, agency, and choice around their treatment trajectories and at times felt forced into using medications when trusting relationships had not been established. Additionally, participants were deeply concerned about the potential postnatal consequences of medication and experienced guilt about risk of neonatal opioid withdrawal, concern surrounding the difficulties involved in caring for a newborn with withdrawal signs, and increased scrutiny following the delivery of an opioid-exposed newborn resulting from mandatory reporting to child welfare. Many women described feeling as though they were caught in a double bind in which they were punished for using the recommended treatment that they had only accepted because clinicians insisted it was the right thing to do.
With respect to participants’ attitudes and beliefs, we identified a disconnect between providers’ aims to offer evidence-based care and treatment and patients’ perceptions of those recommendations, highlighting the need for personalized, tailored approaches. The Substance Abuse and Mental Health Services Administration (SAMHSA) clinical guidance for caring for pregnant people with OUD highlights the importance of informed consent, shared decision-making, and individualized discussions with patients regarding their use of MOUD (“Clinical Guidance for Treating Pregnant and Parenting Women With Opioid Use Disorder and Their Infants | SAMHSA Publications and Digital Products,” n.d.; Guille, Jones, Abuhamad, & Brady, 2019; Krumholz, 2010; Terplan et al., 2018). Our participants’ experiences highlighted how use of MOUD in pregnancy requires discussions that are unique to the perinatal period, including: 1) reviewing known maternal, fetal, and newborn risks including NOWS; 2) discussing the risks and benefits associated with discontinuing or not initiating medications during pregnancy; 3) addressing MOUD dosing changes that can occur through pregnancy and after delivery; and 4) implications for child protective services reporting at delivery (Guille et al., 2019; Terplan et al., 2018).
Clinicians and researchers have used shared decision-making, a patient-centered health communications model by which treatment decisions are informed by patient values and improved knowledge of best practices using tools such as decision aids, coaching, and self-management in the treatment of OUD (Marshall et al., 2018; Thomas et al., 2021). Guille and colleagues developed a shared decision-making aid for MOUD use in pregnancy that includes these elements. In evaluating the feasibility of their aid, they recruited women who had been stable in recovery for at least four months pre-pregnancy but were considering tapering off MOUD when they became pregnant; they found strong satisfaction and high acceptability of shared decision-making to promote retention (Guille et al., 2019).
Women interviewed in our study shared that initial treatment decisions happened when they learned of an unexpected pregnancy, often when presenting for emergent care in the setting of complications from injection drug use. These patients may benefit the most from MOUD to decrease periods of cravings and withdrawal and be able to engage in prenatal care and address co-occurring medical and psychiatric issues, yet they may not have the benefit of having established a trusting relationship with a clinician. Research should help to determine best practices for engaging pregnant people in early recovery or determine a shared decision-making process around treatment decisions that can occur across care settings in which long-term, trusting relationships between provider and patient can be formed.
Our findings indicate that, critically, providers must be transparent about local child protective services reporting practices in discussions with pregnant individuals. Mandated reporting policies of substance use in pregnancy vary by state (Guttmacher Institute, 2017), with an increasing number of states adopting punitive policies toward substance use in pregnancy over the past two decades (Faherty et al., 2019; Jarlenski et al., 2017), despite guidance from all leading public health and medical organizations that punitive policies lead to avoidance of care and worse birth outcomes for women and their children (American College of Obstetricians and Gynecologists (ACOG), 2017; American Society of Addiction Medicine, 2017; Patrick & Schiff, 2017). Participants interviewed for this study delivered in a state that currently interprets use of MOUD as a reason for filing a report of abuse or neglect (Massachusetts Department of Public Health, 2017). Conflation of neonatal opioid dependence and withdrawal from methadone or buprenorphine with child abuse, especially when withdrawal results from prescribed therapy, serves to increase stigma and discrimination for patients considering initiating or continuing MOUD during pregnancy and fuels parental fears that transient and treatable withdrawal signs cause “physical injury.” Mandated reporting disrupts a parent’s ability to view the decision to take MOUD as best medical advice, highlighting the complexity and necessity of incorporating reporting information into shared decision-making. Yet until MOUD exposure is decoupled from mandated reporting, even comprehensive counseling may be insufficient, because the perceived threat is great. Individuals with OUD should be able to make treatment decisions based on medical risks and benefits, in the same way that other therapies such as antidepressants are recommended, without the fear of child protective services reporting and investigation.
Our findings also highlight the challenges people face adhering to opioid treatment program requirements in the early postpartum period, including daily or even twice daily dosing while recovering from a delivery, bringing newborns and/or older children into methadone clinic environments, and adjusting to postpartum hormonal changes affecting medication dosing. Terplan and colleagues have described the dearth of childcare services at addiction clinics and the lack of gender-specific treatment services across the country (Terplan, Longinaker, & Appel, 2015). Improved and accessible childcare or even brief respite care options are needed to enable parents of young children to attend parenting groups and counseling. Further research could explore the novel use of nurse home visiting programs with visits to parent-infant dyads following delivery to address environmental challenges inherent in most treatment environments. Nurses could be trained to perform assessments for maternal sedation and provide daily methadone dosing while allowing the mother and infant to heal and bond at home together during a brief “parental leave,” which would promote parental rest over increasing exhaustion. This outreach approach would require building an understanding that the primary goal is support for the dyad, not investigation for potential infractions.
Finally, our study highlighted specific instances where participants experienced interpersonal and structural racism, such as the experiences reported above of a Black non-Hispanic participant counseled to terminate her pregnancy and another’s narrative of discrimination at a methadone clinic. The intersection of stigma toward pregnant and parenting people who use drugs, coupled with pronounced racial/ethnic inequities in care and treatment, requires further focused investigation to elucidate the experiences of birthing people of color. In our study, one participant reported feeling disappointed and disrespected, when a clinician asked about preference for culturally concordant care. The patient interpreted (or misinterpreted) this query as the clinician’s unwillingness to relate to her. A shared decision-making framework to increase patient engagement in the communication process may enhance the patient-centeredness and partnership building that is critical to establishing trusting relationships (Shen et al., 2018).
4.1. Limitations
We performed this study in a state in which insurance and cost barriers to methadone and buprenorphine are fewer than other states in the United States (Patrick et al., 2020). As a result, themes surrounding cost and insurance barriers were likely not as common as they may have been in other settings. In addition, we primarily interviewed women who were engaged in care at a multidisciplinary clinic caring for families impacted by substance use disorder where two of the authors provide clinical care. While we ensured that clinicians had no role in study recruitment or the interview process, participants’ responses may have been impacted by our dual clinician-researcher roles. Additionally, parental age, living situation, and recovery capital may have influenced their experiences, yet the study did not specifically explore this. Finally, while we specifically developed a sampling strategy to increase the number of non-White participants in our sample, we still had a majority White non-Hispanic participant population. Further qualitative work with selective recruitment prioritizing the voices and treatment experiences of parents of color with substance use disorder will be important to address underlying inequities in treatment access and utilization.
5. Conclusion
To improve MOUD utilization and adherence, several key areas for possible intervention emerged from patient interviews. First, clinicians must incorporate shared decision-making into discussions to increase patient autonomy and agency, particularly among those in the earliest stages of recovery during pregnancy. Next, pregnant people with OUD will benefit from ongoing education around perinatal MOUD safety and efficacy. Third, we must detangle opioid exposure and withdrawal signs from mandated child welfare reporting. Fourth, improving gender-responsive care in substance use treatment programs could include incorporating the utilization of home visiting services for dosing assessments and administration in the early postpartum period. Finally, building trustworthy, antiracist systems of care delivery to ensure equitable care must be prioritized by treatment providers and policy-makers. Technical capacity exists to implement these changes but bringing everyday practice into closer alignment with the needs that patients expressed in our interviews will require shifts in the attitudes and perspectives of policy-makers, institutions, and clinicians.
Supplementary Material
Highlights:
Pregnant and parenting people endorse unique pressures when making MOUD decisions
Lack of autonomy and agency in pregnancy influence treatment decisions
Fear over risk of neonatal opioid withdrawal contributes to MOUD hesitancy
Mandated child welfare reporting of in-utero opioid exposure hinders MOUD receipt
Few opioid-treatment programs offer flexible gender-responsive postpartum services
Funding Support:
This work was supported by the National Institute on Drug Abuse (K12DA043490, K23DA048169, and UH3DA050252) and the National Institute on Alcohol Abuse and Alcoholism (K24AA022136).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Statement
Davida M. Schiff, MD, MSc conceptualized the study, received funding for the study, analyzed the data, drafted the initial manuscript, and reviewed and revised the manuscript.
Erin C. Work and Shayla Partridge participated in data collection, helped develop the codebook, analyzed the data and reviewed and revised the manuscript.
Serra Muftu and Kathryn De L. MacMillan helped analyze the data and supported theme development and reviewed and revised the manuscript
Jessica R. Gray participated in conceptualizing the study, facilitated data collection, and reviewed and revised the manuscript.
Bettina B. Hoeppner, John F. Kelly, Shelly F. Greenfield, MD, MPH, Hendrée E. Jones, Timothy E. Wilens, and Mishka Terplan, provided study supervision and mentorship to Dr. Schiff, analyzed the study findings and reviewed and revised the manuscript.
Judith Bernstein helped to conceptualize the study, refine the interview guide, establish a codebook, review the themes, and reviewed and revised the manuscript.
All authors have approved the final submission.
References:
- American College of Obstetricians and Gynecologists (ACOG). (2017). Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711 Summary. Obstetrics & Gynecology, 130(2), 488–489. 10.1097/AOG.0000000000002229 [DOI] [PubMed] [Google Scholar]
- American Society of Addiction Medicine. (2017). Public Policy Statement on Substance Use, Misuse, and Use Disorders During and Following Pregnancy, with an Emphasis on Opioids Background. Retrieved from https://www.asam.org/docs/default-source/public-policy-statements/substance-use-misuse-and-use-disorders-during-and-following-pregnancy.pdf?sfvrsn=644978c2_4
- Atwood EC, Sollender G, Hsu E, Arsnow C, Flanagan V, Celenza J, … Holmes AV (2016). A Qualitative Study of Family Experience With Hospitalization for Neonatal Abstinence Syndrome. Hospital Pediatrics, hpeds.2016--0024. 10.1542/hpeds.2016-0024 [DOI] [PubMed] [Google Scholar]
- Chun Tie Y, Birks M, & Francis K (2019). Grounded theory research: A design framework for novice researchers. SAGE Open Medicine, 7, 205031211882292. 10.1177/2050312118822927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical Guidance for Treating Pregnant and Parenting Women With Opioid Use Disorder and Their Infants | SAMHSA Publications and Digital Products. (n.d.). Retrieved July 12, 2021, from https://store.samhsa.gov/product/Clinical-Guidance-for-Treating-Pregnant-and-Parenting-Women-With-Opioid-Use-Disorder-and-Their-Infants/SMA18-5054
- Commonwealth of Massachusetts. (n.d.). 110 CMR 4: Intake | Mass.gov. Retrieved October 16, 2021, from https://www.mass.gov/regulations/110-CMR-4-intake
- D P, & HC F. (2021). The Lived Experience of Postpartum Women Attending Outpatient Substance Treatment for Opioid or Heroin Use. Journal of Midwifery & Women’s Health, 66(2), 211–217. 10.1111/JMWH.13165 [DOI] [PubMed] [Google Scholar]
- Ecker J, Abuhamad A, Hill W, Bailit J, Bateman BT, Berghella V, … Yonkers KA (2019). Substance use disorders in pregnancy: clinical, ethical, and research imperatives of the opioid epidemic: a report of a joint workshop of the Society for Maternal-Fetal Medicine, American College of Obstetricians and Gynecologists, and American Society of. American Journal of Obstetrics and Gynecology, 221(1), B5–B28. 10.1016/j.ajog.2019.03.022 [DOI] [PubMed] [Google Scholar]
- Faherty LJ, Kranz AM, Russell-Fritch J, Patrick SW, Cantor J, & Stein BD (2019). Association of Punitive and Reporting State Policies Related to Substance Use in Pregnancy With Rates of Neonatal Abstinence Syndrome. JAMA Network Open, 2(11), e1914078. 10.1001/jamanetworkopen.2019.14078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forray A, & Foster D (2015). Substance Use in the Perinatal Period. Current Psychiatry Reports, 77(11), 91. 10.1007/s11920-015-0626-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forray A, Merry B, Lin H, Ruger JP, & Yonkers KA (2015). Perinatal substance use: A prospective evaluation of abstinence and relapse. Drug and Alcohol Dependence, 150, 147–155. 10.1016/j.drugalcdep.2015.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelberg L, Andersen RM, & Leake BD (n.d.). Healthcare Access and Utilization The Behavioral Model for Vulnerable Populations: Application to Medical Care Use and Outcomes for Homeless People. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1089079/pdf/hsresearch00023-0019.pdf [PMC free article] [PubMed]
- Given LM (2008). The Sage encyclopedia of qualitative research methods.
- Glanz K, Rimer BK, & Viswanath K. (Kasisomayajula). (2008). Health behavior and health education: theory, research, and practice. Jossey-Bass. Retrieved from https://espace.library.uq.edu.au/view/UQ:175068 [Google Scholar]
- Goler NC, Armstrong MA, Taillac CJ, & Osejo VM (2008). Substance abuse treatment linked with prenatal visits improves perinatal outcomes: a new standard. Journal of Perinatology, 28(9), 597–603. 10.1038/jp.2008.70 [DOI] [PubMed] [Google Scholar]
- Goler Nancy C, Armstrong MA, Osejo VM, Hung Y-Y, Haimowitz M, & Caughey AB (2012). Early start: a cost-beneficial perinatal substance abuse program. Obstetrics and Gynecology 119(1), 102–110. 10.1097/AOG.0b013e31823d427d [DOI] [PubMed] [Google Scholar]
- Guille C, Jones HE, Abuhamad A, & Brady KT (2019). Shared Decision-Making Tool for Treatment of Perinatal Opioid Use Disorder. Psychiatric Research and Clinical Practice, 1(1), 27–31. 10.1176/appi.prcp.20180004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmacher Institute. (2017). Substance Use During Pregnancy | Guttmacher Institute. Retrieved November 25, 2017, from https://www.guttmacher.org/state-policy/explore/substance-use-during-pregnancy [Google Scholar]
- H H, (2016). Experiences of opioid-dependent women in their prenatal and postpartum care: Implications for social workers in health care. Social Work in Health Care, 55(1), 61–85. 10.1080/00981389.2015.1078427 [DOI] [PubMed] [Google Scholar]
- Haight SC, Ko JY, Tong VT, Bohm MK, & Callaghan WM (2018). Opioid Use Disorder Documented at Delivery Hospitalization — United States, 1999–2014. MMWR. Morbidity and Mortality Weekly Report, 67(31), 845–849. 10.15585/mmwr.mm6731a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley L, Sulo S, Kozmic S, & Parilla BV (2018). Opioid Addiction in Pregnancy: Does Depression Negatively Impact Adherence With Prenatal Care? Journal of Addiction Medicine, 12(1), 61–64. 10.1097/ADM.0000000000000364 [DOI] [PubMed] [Google Scholar]
- HM K (2010). Informed consent to promote patient-centered care. JAMA, 303(12), 1190–1191. 10.1001/JAMA.2010.309 [DOI] [PubMed] [Google Scholar]
- Howard MB, Wachman E, Levesque EM, Schiff DM, Kistin CJ, & Parker MG (2018). The Joys and Frustrations of Breastfeeding and Rooming-In Among Mothers With Opioid Use Disorder: A Qualitative Study. Hospital Pediatrics, 8(12), 761–768. 10.1542/hpeds.2018-0116 [DOI] [PubMed] [Google Scholar]
- Jarlenski M, Hogan C, Bogen DL, Chang JC, Bodnar LM, & Van Nostrand E (2017). Characterization of U.S. State Laws Requiring Health Care Provider Reporting of Perinatal Substance Use. Women’s Health Issues, 27(3), 264–270. 10.1016/j.whi.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- JC P, R S, K B, DD S, WO C, PR M, … SW P (2021). Reproductive-Age Women’s Experience of Accessing Treatment for Opioid Use Disorder: “We Don’t Do That Here.” Women’s Health Issues : Official Publication of the Jacobs Institute of Women’s Health. 10.1016/J.WHI.2021.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb SM (n.d.). Grounded Theory and the Constant Comparative Method: Valid Research Strategies for Educators. Journal of Emerging Trends in Educational Research and Policy Studies, 3(1). [Google Scholar]
- Leiner C, Cody T, Mullins N, Ramage M, & Ostrach BMM (2021). “The elephant in the room;” a qualitative study of perinatal fears in opioid use disorder treatment in Southern Appalachia. BMC Pregnancy and Childbirth, 21(1). 10.1186/S12884-021-03596-W [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo-Ciganic W-H, Donohue JM, Kim JY, Krans EE, Jones BL, Kelley D, … Jarlenski ΜP. (2019). Adherence trajectories of buprenorphine therapy among pregnant women in a large state Medicaid program in the United States. Pharmacoepidemiology and Drug Safety, 28(1), 80–89. 10.1002/pds.4647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAfee L, Sawyer A, & Terplan M (2016). Trends in Receipt of Medication Assisted Treatment for Pregnant Women with Opioid Use Disorder. In College on Problems of Drug Dependence. [Google Scholar]
- Martin CE, Longinaker N, & Terplan M (2015). Recent trends in treatment admissions for prescription opioid abuse during pregnancy. Journal of Substance Abuse Treatment, 48(1), 37–42. 10.1016/j.jsat.2014.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massachusetts Department of Public Health. (2017). 51A Reports regarding Substance Exposed Newborns (SENs). Boston. [Google Scholar]
- Momplaisir FM, Storm DS, Nkwihoreze H, Jayeola O, & Jemmott JB (2018, January 14). Improving postpartum retention in care for women living with HIV in the United States. AIDS. Lippincott Williams and Wilkins. 10.1097/QAD.0000000000001707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor AB, Uhler B, O’Brien LM, & Knuppel K (2018). Predictors of treatment retention in postpartum women prescribed buprenorphine during pregnancy. Journal of Substance Abuse Treatment, 86, 26–29. 10.1016/j.jsat.2017.12.001 [DOI] [PubMed] [Google Scholar]
- Ostrach B, & Leiner C (2018). “I didn’t want to be on Suboxone at first…”– Ambivalence in Perinatal Substance Use Treatment. Journal of Addiction Medicine, 1. 10.1097/ADM.0000000000000491 [DOI] [PubMed] [Google Scholar]
- Ostrach B, & Leiner C (2019). “I didn’t want to be on Suboxone at first…’– Ambivalence in Perinatal Substance Use Treatment. Journal of Addiction Medicine, 13(4), 264–271. 10.1097/adm.0000000000000491 [DOI] [PubMed] [Google Scholar]
- Patrick SW, Richards MR, Dupont WD, McNeer E, Buntin MB, Martin PR, … Cooper WO (2020). Association of Pregnancy and Insurance Status with Treatment Access for Opioid Use Disorder. JAMA Network Open, 3(8). 10.1001/JAMANETWORKOPEN.2020.13456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick SW, & Schiff DM (2017). A Public Health Response to Opioid Use in Pregnancy. Pediatrics, 139(3). 10.1542/peds.2016-4070 [DOI] [PubMed] [Google Scholar]
- Peeler M, Gupta M, Melvin P, Bryant A, Diop H, Iverson R, … Schiff DM (2020). Racial and Ethnic Disparities in Maternal and Infant Outcomes Among Opioid-Exposed Mother–Infant Dyads in Massachusetts (2017–2019). American Journal of Public Health, Epub ahead. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R P, AL H, M M, SE H, & AR S (2020). Secrecy Versus Disclosure: Women with Substance Use Disorders Share Experiences in Help Seeking During Pregnancy. Maternal and Child Health Journal, 24(11), 1396–1403. 10.1007/S10995-020-03006-1 [DOI] [PubMed] [Google Scholar]
- Schiff DM, Hoeppner BB, Terplan M, Hadland SE, Bemson D, SF G, … Wilens TE (2021). Methadone and buprenorphine discontinuation among postpartum women with opioid use disorder. American Journal of Obstetrics and Gynecology. 10.1016/J.AJOG.2021.04.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff DM, Nielsen T, Hoeppner BB, Terplan M, Hansen H, Bernson D, … Taveras EM (2020). Assessment of Racial and Ethnic Disparities in the Use of Medication to Treat Opioid Use Disorder Among Pregnant Women in Massachusetts. JAAA Network Open 3(5), e205734. 10.1001/jamanetworkopen.2020.5734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff DM, Nielsen T, Terplan M, Hood M, Bernson D, Diop H, … Land. (2018). Fatal and Nonfatal Overdose Among Pregnant and Postpartum Women in Massachusetts. Obstetrics & Gynecology, 132(2, 466–474. 10.1097/AOG.0000000000002734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff DM, Work EC, Foley B, Applewhite R, Diop H, Goullaud L, … Bryant AS (2022). Perinatal Opioid Use Disorder Research, Race, and Racism: A Scoping Review. Pediatrics, 149(3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MJ, Peterson EB, Costas-Muñiz R, Hernandez MH, Jewell ST, Matsoukas K, & Bylund CL (2018). The Effects of Race and Racial Concordance on Patient-Physician Communication: A Systematic Review of the Literature. Journal of Racial and Ethnic Health Disparities 5(1), 117. 10.1007/S40615-017-0350-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- T M, EN K, M H, S K-J, K O, A A-A, … S V. (2018). Patient engagement, treatment preferences and shared decision-making in the treatment of opioid use disorder in adults: a scoping review protocol. BMJ Open, 8(10). 10.1136/BMJOPEN-2018-022267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terplan M, Laird HJ, Hand DJ, Wright TE, Premkumar A, Martin CE, … Krans EE (2018). Opioid Detoxification During Pregnancy. Obstetrics & Gynecology, 131(5), 803–814. 10.1097/AOG.0000000000002562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terplan M, Longinaker N, & Appel L (2015). Women-Centered Drug Treatment Services and Need in the United States, 2002–2009. American Journal of Public Health, 105(11) e50–e54. 10.2105/AJPH.2015.302821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EC, Ben-David S, Treichler E, Roth S, Dixon LB, Salzer M, & Zisman-Ilani Y (2021). A Systematic Review of Shared Decision–Making Interventions for Service Users With Serious Mental Illnesses: State of the Science and Future Directions. Https://Doi-Org.Ezp-Prod1.Hul.Harvard.Edu/10.1176/Appi.Ps.202000429,appi.ps.2020004. 10.1176/APPI.PS.202000429 [DOI] [PMC free article] [PubMed]
- Tong A, Sainsbury P, & Craig J (2007). Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. International Journal for Quality in Health Care, 19(6), 349–357. 10.1093/intqhc/mzm042 [DOI] [PubMed] [Google Scholar]
- Wilder C, Lewis D, & Winhusen T (2015). Medication assisted treatment discontinuation in pregnant and postpartum women with opioid use disorder. Drug and Alcohol Dependence, 149, 225–231. 10.1016/j.drugalcdep.2015.02.012 [DOI] [PubMed] [Google Scholar]
- Wilder CM, Hosta D, & Winhusen T (2017). Association of methadone dose with substance use and treatment retention in pregnant and postpartum women with opioid use disorder. Journal of Substance Abuse Treatment, 80, 33–36. 10.1016/j.jsat.2017.06.005 [DOI] [PubMed] [Google Scholar]
- Winkelman TNA, Villapiano N, Kozhimannil KB, Davis MM, & Patrick SW (2018). Incidence and Costs of Neonatal Abstinence Syndrome Among Infants With Medicaid: 2004 – 2014. Pediatrics, 141(4), e20173520. 10.1542/peds.2017-3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


