Abstract
Objective
Successful retention on buprenorphine improves outcomes for opioid use disorder (OUD); however, we know little about associations between use of non–prescribed buprenorphine (NPB) preceding treatment intake and clinical outcomes.
Methods
The study conducted observational retrospective analysis of abstracted electronic health record (EHR) data from a multi-state nationwide office-based opioid treatment program. The study observed a random sample of 1,000 newly admitted patients with OUD for buprenorphine maintenance (2015–2018) for up to 12 months following intake. We measured use of NPB by mandatory intake drug testing and manual EHR coding. Outcomes included hazards of treatment discontinuation and rates of opioid use.
Results
Compared to patients testing negative for buprenorphine at intake, those testing positive (59.6%) had lower hazards of treatment discontinuation (HR=0.52, 95% CI: 0.44, 0.60, p<0.01). Results were little changed following adjustment for baseline opioid use and other patient characteristics (aHR: 0.60, 95% CI: 0.51, 0.70, p<0.01). Risk of discontinuation did not significantly differ between patients by buprenorphine source: prescribed v. NPB (reference) at admission (HR=1.15, 95% CI: 0.90, 1.46). Opioid use was lower in the buprenorphine positive group at admission (25.0% vs. 53.1%, p<0.0001) and throughout early months of treatment but converged after 7 months for those remaining in care (17.1% vs. 16.5%, p=0.89).
Conclusion
NPB preceding treatment intake was associated with decreased hazards of treatment discontinuation and lower opioid use. These findings suggest use of NPB may be a marker of treatment readiness and that buprenorphine testing at intake may have predictive value for clinical assessments regarding risk of early treatment discontinuation.
Keywords: Opioid use disorder, Buprenorphine, Medications for opioid use disorder
1. Introduction
Buprenorphine, the most frequently prescribed medication for opioid use disorder (OUD) (Olfson M, Zhang VS, Schoenbaum M, & King M, 2020), is the only form of opioid agonist treatment for OUD prescribed from a general office setting. It is highly effective at reducing opioid use, overdose, all-cause mortality, and improving quality of life (Sordo L, Barrio G, Bravo MJ et al., 2017; Santo Jr T, Clark B, Hickman M, et al., 2021; Bentzley BS, Barth KS, Back SE, & Book SW, 2015; Larochelle MR, Liebschutz JM, Zhang F, Ross-Degnan D, & Wharam JF, 2016). However, these protective effects mostly occur during care episodes and are lost once patients discontinue medication (Williams AR, Samples H, Crystal S, & Olfson M, 2019; Samples H, Williams AR, Crystal S, & Olfson M, 2020; and Krawczyk N, Mojtabai R, Stuart EA, et al., 2020).
Retention in care, a longtime primary outcome in addiction treatment studies (Samples H, Williams AR, Olfson M, Crystal S, 2018; Meinhofer A, Williams AR, Johnson P, Schackman B, & Bao Y, 2019), is critical to improving patient outcomes. Unlike other medications, buprenorphine prescribing has been subject to special training requirements, monitoring and reporting requirements, and patient caps, all of which have contributed to uneven access, underutilization, and disparities in retention (Bentzley BS, Barth KS, Back SE, & Book SW, 2015; Lin LA, Lowfall MR, Walsh SL, Gordon AJ, & Knudsen HK, 2018; Martin SA, Chiodo LM, Bosse JD, & Wilson A, 2018). Amid the largest year-over-year increase in overdose deaths following COVID-19, in April 2021 the U.S. Department of Health and Human Services announced liberalized training requirements for prescribing buprenorphine, following earlier emergency actions to expand treatment access (e.g., use of telemedicine, relaxed licensure requirements), with uncertainty about long-term continuation. Prescribers are now permitted a limited panel of up to 30 patients without specialized training by submitting a “Notice of Intent” to the Substance Abuse and Mental Health Services Administration (US DHHS April 27, 2021; Poorman E, 2021).
While substantial negative media and law enforcement attention have been devoted to buprenorphine diversion, use of non–prescribed buprenorphine (NPB) is prevalent among individuals trying to reduce opioid use or struggling to access treatment (Bazazi AR, Yokell M, Fu JJ, Rich JD, & Zaller ND, 2011; Schuman-Olivier Z, Albanese M, Nelson SE, et al., 2010; Yokell MA, Zaller ND, Green TC, & Rich JD, 2011). Qualitative research suggests that adults with a history of NPB do not seek or experience euphoric effects (Monico LB, Mitchell SG, Gryczynski J, et al., 2015; Allen B, Harocopos A. 2016) and studies indicate that approximately 70% to 90% of people report using NPB to prevent craving and withdrawal, suggesting use of NPB may be a stepping stone to formal treatment (Chilcoat HD, Amick HR, Sherwood MR, & Dunn KE., 2019; Schuman-Olivier Z, Albanese M, Nelson SE, et al., 2010; Genberg BL, Gillespie M, Schuster CR, et al., 2013). These findings are consistent with pre-clinical human trials that have found no preference for buprenorphine over other opioids for rewarding effects (Comer SD, Sullivan MA, Whittington RA, Vosburg Sk, & Kowalczyk WJ., 2008).
Limited literature exists regarding the association of NPB and clinical outcomes of patients with OUD. A 2011 study examining Office Based Opioid Treatment (OBOT) patients in Massachusetts found that 15.7% of patients (n=400) had used NPB and these patients were more likely to cease opioid use by 6 months and remain in care through the full 12 months of study (Alford DP, LaBelle CT, Kretsch N, et al., 2011). A follow-up study found that, among 87 interviewed OBOT patients, those with prior buprenorphine use (prescribed or nonprescribed) had significantly greater odds of treatment retention at 6 months, and self-reported opioid use did not differ between groups (Cunningham CO, Roose RJ, Starrels JL, Giovanniello A, & Sohler NL, 2013).
Finally, a study of publicly funded OBOT patients in Baltimore found double the retention at 6 months for those using NPB compared to those “buprenorphine naïve” (Monico LB, Mitchell SG, Gryczynski J, et al., 2015). However, this study did not assess opioid use during care. More recently, Carlson and colleagues found among a population in Ohio that more frequent self-reported use of NPB was associated with lower risk of overdose (Carlson RG, Daniulaityte R, Silverstein SM, Nahhas RW, Martins SS. 2020).
These studies predated widespread fentanyl penetration, were mainly single-site samples of limited generalizability, did not control for opioid use at presentation, and preceded an era of expanded buprenorphine prescribing. Increased adulteration of street drugs with fentanyl and co-use of psychostimulants are highly relevant as buprenorphine may be less effective at preventing overdose in these patient populations and fentanyl exposure complicates buprenorphine induction as patients are more likely to experience precipitated withdrawal. To date, prior work has not sufficiently characterized patients using NPB and related treatment outcomes, especially in recent years. Given that clinicians hesitation toward buprenorphine initiation may be motivated by concerns about misuse and fears about diversion, further research is needed (Schoenfield EM, Soares WE, Schaeffer EM, et al., 2021).
We present results of an observational analysis of new entrants to buprenorphine maintenance for OUD to assess associations of NPB at intake with treatment retention and opioid use while in care at a large multi-state, multi-site OBOT clinic system from 2015 to 2019. We hypothesized that use of NPB preceding treatment entry would be associated with lower hazards of treatment discontinuation and lower rates of opioid use during care.
2. Methods
2.1. Setting
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines (von Elm E, Altman DG, Egger M, et al., 2007).
We analyzed data from a multi-site OBOT clinic network, primarily managed by advanced practice professionals and supervising physicians across 8 states during the study period. The partner site is now the largest OBOT provider in the United States, having inducted more than 40,000 patients between 2009 and 2019 with patient diversity representative of buprenorphine patients nationwide, with a preponderance male and non-Hispanic white sample (Samples H, Williams AR, Crystal S, Olfson M. 2020; Williams AR, Samples H, Crystal S, Olfson M.). Clinical sites consistently implement evidence-based practices in addiction treatment, escalating frequencies of visits and urine testing as needed to retain patients in care, with coordination between nurse practitioners, physicians, behavioral health specialists, and care coordinators. A unified EHR system enabled characterization of patients at intake and ongoing monitoring. We collected longitudinal clinical data from the EHR. The New York State Psychiatric Institute IRB approved the study protocol and procedures and deemed them exempt from informed consent.
2.2. Study sample
Among 18,513 individuals initiating a new buprenorphine care episode between January 1, 2015, and April 1, 2018, we used SAS 9.4 procedure PROC SURVEYSELECT to randomly select 1,000 patients to examine manually in coded EHR data. We limited care episodes to patients completing their intake visit who were scheduled to initiate buprenorphine maintenance treatment, rather than xr-naltrexone, and who had not received care at any partner site in the preceding 90-day period to identify new care episodes.
2.3. Main exposure
We first categorized patients by the presence of buprenorphine in drug test results at presentation (positive/negative). We further used a protocol to distinguish the source of buprenorphine among those testing positive by patient self-report as documented in the EHR. Examples of sources used to classify buprenorphine as prescribed included: care transfer, outside prescriber, detoxification discharge. Examples of phrasing coded for NPB included: “was able to get street bupe”, “getting illicit Suboxone”, and “has been buying street Subx” (see Appendix B). Without documentation of any source, we assigned patients testing positive for buprenorphine to the NPB group given prior literature indicating high prevalence of NPB use in this population (Bazazi AR, Yokell M, Fu JJ, Rich JD, & Zaller ND, 2011; Schuman-Olivier Z, Albanese M, Nelson SE, et al., 2010; Yokell MA, Zaller ND, Green TC, & Rich JD, 2011, Chilcoat HD, Amick HR, Sherwood MR, & Dunn KE., 2019; Genberg BL, Gillespie M, Schuster CR, et al., 2013). We also performed a sensitivity analysis restricting the NPB group in the 3-group analysis (1. Buprenorphine positive [prescribed]; 2. Buprenorphine positive [NPB]; 3. Buprenorphine negative) to those with explicit mentions of NPB (i.e., removing those with unknown sources of procurement) to assess for the impact of possible misclassification.
2.4. Study outcomes
Our primary analysis investigated hazard of treatment discontinuation during the 365-day period following admission. We defined treatment discontinuation as a gap of 60+ days in visits, consistent with clinic policies and prior literature (Williams AR, Samples H, Crystal S, & Olfson M, 2019; Samples H, Williams AR, Crystal S, & Olfson M, 2020; and Krawczyk N, Mojtabai R, Stuart EA, et al., 2020). We attributed the last day in care to the final clinic visit date and associated drug test results.
Additional outcomes included rates of full-agonist opioid use (e.g., opiates, morphine, methadone, fentanyl) per urine toxicology results. We constructed one-month (30 day) intervals with the admission date defined as Day 1 (i.e. Month 1=Days 1–30). All patients had drug test results in the first month of care as staff obtained a drug screen during each clinic visit. Among patients remaining in care, we assessed the percentage of individuals with one or more positive opioid screening results per month by group.
2.5. Covariates
All covariates included in our adjusted models are contained in Table 1. EHR data contained patient age, sex, and drug test results at intake including opioids (excluding buprenorphine), cocaine, amphetamine, benzodiazepines, and cannabis. We additionally categorized patients by hepatitis C and HIV status at admission.
Table 1:
Patient Characteristics Based on Buprenorphine Use at Treatment Entry
| Covariate | Overall Sample n=971 (%) |
Buprenorphine positive at intake, n=579 (%) |
Buprenorphine negative at intake, n=392 (%) |
p value | |||
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age | |||||||
| - 18-29 | 296 | 30.5% | 177 | 30.6% | 119 | 30.4% | 0.37 |
| - 30-49 | 550 | 56.6% | 332 | 57.3% | 218 | 55.6% | |
| - 50-64 | 118 | 12.2% | 68 | 11.7% | 50 | 12.8% | |
| - >65 | 7 | 0.7% | 2 | 0.4% | 5 | 1.3% | |
| Sex | |||||||
| - Male | 566 | 58.3% | 326 | 56.3% | 240 | 61.2% | 0.13 |
| - Female | 405 | 41.7% | 253 | 43.7% | 152 | 38.8% | |
|
Drug Results (positive) at
Intake |
|||||||
| - Opioids | 353 | 36.4% | 145 | 25.0% | 208 | 53.1% | <0.0001 |
| - Cocaine | 197 | 20.3% | 115 | 19.9% | 82 | 21.1% | 0.64 |
| - Amphetamine | 53 | 5.5% | 36 | 6.2% | 17 | 4.4% | 0.22 |
| - Benzodiazepines | 130 | 13.4% | 84 | 14.5% | 46 | 11.8% | 0.23 |
| - Cannabis | 327 | 33.7% | 196 | 34.0% | 131 | 33.9% | 0.97 |
|
Manual Coding of HPI field
for history of: |
|||||||
| - IDU | 517 | 53.2% | 316 | 54.6% | 201 | 51.3% | 0.31 |
| - Heroin use | 747 | 76.9% | 441 | 76.2% | 306 | 78.1% | 0.49 |
| - Cocaine use | 519 | 53.5% | 309 | 53.4% | 210 | 53.6% | 0.95 |
| - Benzodiazepine use | 371 | 38.2% | 241 | 41.6% | 130 | 33.2% | 0.01 |
| - Criminal Justice involvement | 299 | 30.8% | 176 | 30.4% | 123 | 31.4% | 0.75 |
| Comorbidity | |||||||
| Hepatitis C | 225 | 23.2% | 134 | 23.1% | 91 | 23.2% | 0.98 |
| HIV | 5 | <1% | 1 | <1% | 4 | 1.0% | 0.65 |
IDU=Injection Drug Use. Data were collected from the EHR of a multi-site, multi-state buprenorphine treatment program for new admissions of patients with Opioid Use Disorder between January 1, 2015 and April, 2018.
A research assistant blinded to study hypotheses coded all EHR admission records manually for patients’ characteristics at baseline, including heroin use, injection drug use, cocaine use, benzodiazepine use, and criminal justice involvement (e.g., formerly incarcerated, on probation/parole, recently arrested). Admission records reviewed included the intake note, any subsequent induction visits (i.e., when the patient returned to be supervised in-person for starting buprenorphine), and any other clinical visit notes within the first 30 days of care. Among the 1,000 randomly selected individuals, 19.6% (n=196) had prior care episodes documented in the EHR. For this subset, the team also evaluated earlier intake notes. To assess reliability of manual coding by the research assistant (A.C.), an APBN board certified addiction psychiatrist (A.R.W.) double-coded a random subsample of 50 admissions to generate an inter-rater reliability score (kappa) for each item: injection drug use (κ=0.96), heroin use (κ=0.75), cocaine use (κ=0.96), benzodiazepine use (κ=0.81), and criminal justice involvement (κ=0.85).
2.6. Statistical methods
We assessed differences in demographic characteristics, drug testing results at intake, EHR characteristics, and hepatitis C and HIV status by exposure group using chi-squared tests for categorical variables and t-tests for continuous variables. We used Kaplan-Meier curves to assess the relationship between buprenorphine at admission and time to treatment discontinuation. First, an unadjusted Cox proportional hazards model assessed the presence of a relationship between exposure (drug test results positive for buprenorphine at presentation) and time to discontinuation and estimated the hazard ratio (HR) of treatment discontinuation. Second, a Cox proportional hazards model adjusted for baseline variables (Table 1), including opioid use in intake drug testing results. Study staff repeated survival methods using a three-level exposure group (buprenorphine negative, prescribed buprenorphine, non-prescribed buprenorphine). We assessed rates of opioid use by group using chi-squared tests for each 30-day interval in care. All hypothesis tests were two-sided and conducted at the 5% level of significance. We used SAS 9.4 for all analyses.
3. Results
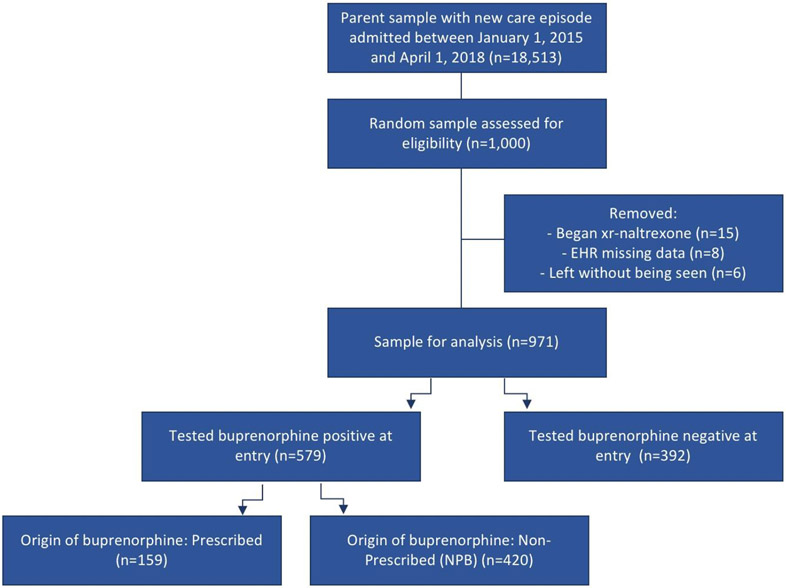
Among 1,000 individuals randomly selected from the parent sample, 971 met eligibility criteria. The study excluded fifteen for initiation on xr-naltrexone rather than buprenorphine, 8 for technical difficulties accessing full EHR data, and 6 patients left before completing their intake evaluation (Figure 1).
Figure 1:
STROBE Diagram for Construction of Patient Groups Based on Buprenorphine Use Preceding Treatment Intake
Among the study cohort (n=971), 58.3% were male with average age of 36.3 (SD=10.1) years, comparable to most OBOT patient populations (Sordo L, Barrio G, Bravo MJ et al., 2017). A majority tested positive for buprenorphine before receiving their first buprenorphine prescription (n=579, 59.6%). Most patients reported heroin use (76.9%), cocaine use (53.5%), and injecting drugs (53.2%). Roughly one-third had documented comorbid pain conditions (33.4%) and criminal justice involvement (30.8%) without significant variation across study groups.
Compared to buprenorphine-naiïve patients, those buprenorphine-positive at treatment entry were less likely to test positive for opioids (25.0% vs. 53.1%, p<0.0001) but more likely to self-report benzodiazepine use (41.6% vs. 33.2%, p=0.01; Table 1). Hepatitis C and HIV results were comparable. Among those buprenorphine positive, 420 (72.5%) had no indication of outside prescriptions (i.e. NPB) while 159 (27.5%) had documentation of using prescribed buprenorphine (i.e. transfers). Most of those with no indication of active outside prescriptions (81.4%, 342/420) had explicit documentation of obtaining buprenorphine through nonprescribed channels.
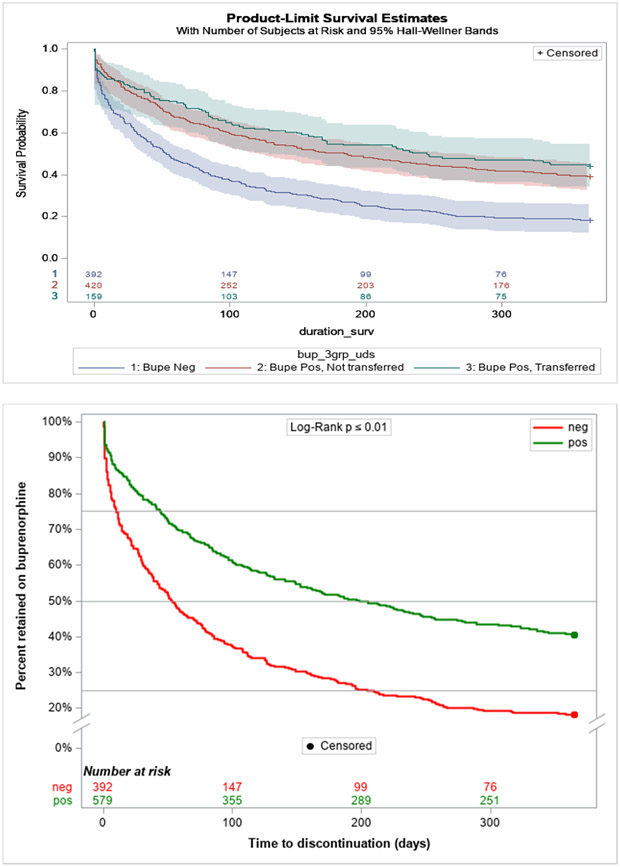
Treatment discontinuation rates were lower for patients testing positive for buprenorphine at treatment entry at all time points (Figure 2a), with a lower hazard for discontinuation (HR=0.52, 95% CI 0.44, 0.60, p<0.0001). Results were little changed after adjustment for baseline opioid use and other covariates (aHR=0.60, 95%CI:0.50, 0.71, p<0.0001).
Figure 2.
a: Treatment Retention Survival Curves Among Patients with and without Buprenorphine Positive Drug Tests at Treatment Intake Through Day 365
b: Sensitivity Analysis of Treatment Retention After Separating Patients by Source of Buprenorphine
Patients testing positive for buprenorphine at treatment entry were approximately half the cumulative probability of treatment discontinuation of patients testing negative for buprenorphine at treatment entry to discontinue (HR=0.52, 95% CI: (0.44, 0.60), p<0.0001). Results persisted after adjustment for baseline opioid use and other covariates (aHR=0.60, 95% CI: (0.51, 0.70), p<0.0001): a Cox model adjusting for baseline opioid use and other patient covariates (See Table 1) with the three groups demonstrated overall rates of discontinuation were lower for those transferring their prescription of buprenorphine (HR=0.47 95% CI: (0.37, 0.59), p<0.0001) and those with use of NPB (HR=0.54 95% CI: (0.45, 0.63), p<0.0001) compared to those testing buprenorphine negative at intake.
Similar results were observed after further partitioning the 579 buprenorphine-positive patients at intake into the 159 with documentation of prescribed buprenorphine versus the 420 who did not (Appendix A). Among those testing positive for buprenorphine but without any documentation of an outside prescriber, 81.4% (n=342) had explicit documentation of obtaining buprenorphine through nonprescribed channels while the remaining 18.6% (n=78) had indeterminate documentation. In this three-group analysis, treatment discontinuation in the NPB group closely resembled that of the prescribed buprenorphine group (HR=0.87, 95%CI:0.68, 1.11, p=0.26) (Figure 2b). Further, the three-group Cox model demonstrated that, compared to buprenorphine-negative patients at intake, hazards of discontinuation were lower for those transferring their buprenorphine prescription (HR=0.46, 95% CI:0.37, 0.59, p<0.0001) and for those with NPB use (HR=0.54, 95% CI:0.45, 0.63, p<0.0001). Results persisted after adjustment for opioid use at intake and other covariates with lower hazards of discontinuation for those using prescribed buprenorphine (aHR=0.60, 95% CI:0.46, 0.60, p=0.0004) and for those with NPB (aHR=0.59, 95% CI:0.50, 0.71, p<0.0001). In these adjusted analyses, the difference in hazards between groups with prescribed buprenorphine and NPB remained statistically nonsignificant (aHR=1.02, 95% CI:0.78, 1.34, p=0.87). Results from sensitivity analyses limiting the NPB group to those with explicit mentions of illicit procurement did not significantly change findings.
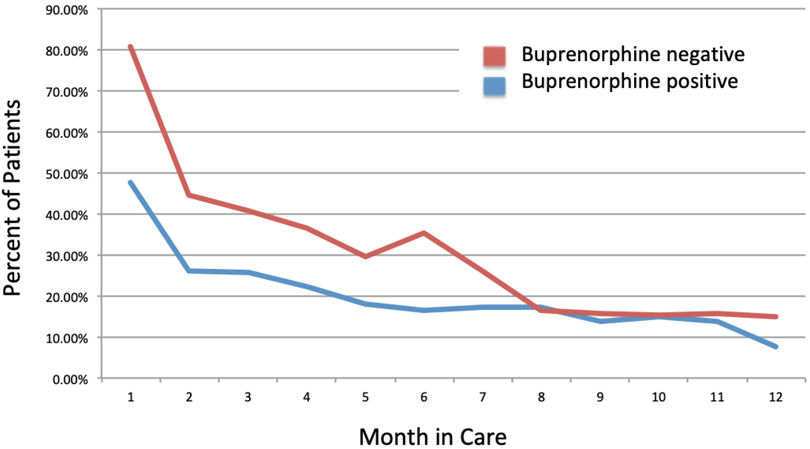
Positive tests for opioids (other than buprenorphine) at intake were higher in the buprenorphine negative group (53.1% v. 25.0%, p<0.0001) and remained higher during the first several months of treatment among patients continuing in care (Figure 3). Overall, the percentage of patients under care testing positive for opioids per month decreased for all groups during the first 6 months in treatment and converged around 15–17% per month after month 7 (17.1% v 16.5%, p=0.89) (Table 2).
Figure 3:
Percent of patients with any opioid use, by month retained in care
Table 2.
Percent of patients testing positive for opioids on a monthly basis following intake for buprenorphine maintenance treatment
| Month after intakea (30 day intervals) |
Buprenorphine positive at intake (n= 579) |
Buprenorphine negative at intake (n=392) |
p value | ||
|---|---|---|---|---|---|
| # positive/ # observed individualsb |
% | # positive/ # observed individualsb |
% | ||
| 1 | 276/579 | 47.7% | 316/392 | 80.6% | <0.0001 |
| 2 | 119/454 | 26.2% | 104/233 | 44.6% | <0.0001 |
| 3 | 102/400 | 25.5% | 74/182 | 40.7% | 0.0002 |
| 4 | 82/366 | 22.4% | 55/151 | 36.4% | 0.001 |
| 5 | 60/334 | 18.0% | 39/132 | 29.6% | 0.01 |
| 6 | 52/315 | 16.5% | 42/119 | 35.3% | <0.0001 |
| 7 | 51/297 | 17.2% | 28/108 | 25.9% | 0.05 |
| 8 | 48/281 | 17.1% | 16/97 | 16.5% | 0.89 |
| 9 | 37/267 | 13.9% | 14/90 | 15.6% | 0.69 |
| 10 | 39/258 | 15.1% | 12/79 | 15.2% | 0.99 |
| 11 | 34/248 | 13.7% | 12/76 | 15.8% | 0.66 |
| 12 | 18/241 | 7.5% | 11/74 | 14.9% | 0.05 |
Day of intake was coded as Day 1, therefore by definition all patients from both groups were observable in Month 1 (i.e. Day 1-30).
The # positive refers to the number of individual patients with one or more positive opioid drug test results among all tests performed in a given month.
4. Discussion
Among patients initiating buprenorphine maintenance treatment for OUD, over half tested positive for buprenorphine at intake, largely reflecting NPB rather than buprenorphine from an outside prescriber. Compared to patients testing negative for buprenorphine at admission, patients testing positive had higher rates of treatment retention and lower rates of opioid use while in care, including after controlling for opioid use at presentation and for other critical baseline patient variables. These findings suggest that use of NPB may independently contribute to increased treatment retention and lower rates of opioid use during the first 7 months of treatment. For patients remaining in care beyond 7 months, however, rates of opioid use per month converged across groups.
A positive buprenorphine drug test result at treatment entry may be a useful patient characteristic to help guide intake assessment and target clinical interventions, as it is a readily available biomarker in virtually all treatment settings. NPB at intake may reflect greater treatment readiness, motivation for reducing opioid use, or a favorable response to the medication. Existing evidence suggests that use of “diverted” buprenorphine is a marker of patient motivation for treatment (Bazazi AR, Yokell M, Fu JJ, Rich JD, & Zaller ND, 2011; Schuman-Olivier Z, Albanese M, Nelson SE, et al., 2010; Yokell MA, Zaller ND, Green TC, & Rich JD, 2011), and future studies may improve understanding of the relationship between use of NPB and the biopsychosocial factors that influence treatment initiation, retention, and response. Retention is critical to improving clinical outcomes and yet remains a common barrier. Unlike prior studies that have found marginal differences (~5-10%) in retention after adjusting for patient demographic characteristics (Williams AR, Samples H, Crystal S, & Olfson M, 2019), measures of addiction severity (Samples H, Williams AR, Crystal S, & Olfson M, 2020), and prior treatment episodes (Samples H, Williams AR, Crystal S, & Olfson M, 2020), we found robust effect sizes related to the use of NPB on outcomes.
Importantly, the use of NPB may itself be a pathway to entering professional care. Outcomes did not significantly differ whether the source of buprenorphine preceding intake was prescribed or nonprescribed. Superior clinical outcomes among patients using buprenorphine prior to treatment entry might reflect the medication’s large effect size for protective clinical benefits, exemplified by a 66–80% reduction in mortality (Sordo L, Barrio G, Bravo MJ et al., 2017; Santo Jr T, Clark B, Hickman M, et al., 2021; Morgan JR, Schackman BR, Leff JA, Linas BP, & Walley, AY, 2018). Further research should disentangle the relative impact of any retention in care, a longstanding primary endpoint for addiction treatment trials (Samples H, Williams AR, Olfson M, Crystal S, 2018; Meinhofer A, Williams AR, Johnson P, Schackman B, & Bao Y, 2019), versus opioid-free time in care to better determine the mechanism of buprenorphine treatment that reduces overdose and mortality (Sordo L, Barrio G, Bravo MJ et al., 2017; Santo Jr T, Clark B, Hickman M, et al., 2021; Larochelle MR, Liebschutz JM, Zhang F, Ross-Degnan D, & Wharam JF, 2016).
In line with prior insurance claims–based analyses (Williams AR, Samples H, Crystal S, & Olfson M, 2019; Samples H, Williams AR, Crystal S, & Olfson M, 2020), the current findings show favorable outcomes after a minimum 180 days of continuous pharmacotherapy (Samples H, Williams AR, Crystal S, & Olfson M, 2020), a quality measure for OUD treatment recently endorsed by the National Quality Forum (Williams AR, Samples H, Crystal S, & Olfson M, 2019). We found that rates of opioid use continued to decline for all groups in the first 180 days of care and then began to converge. Yet recent studies have also demonstrated that much longer care episodes, up to 18 months, may remain insufficient for reducing rates of adverse events postdiscontinuation, emphasizing the importance of long-term retention for this chronic disorder (Williams AR, Samples H, Crystal S, & Olfson M, 2019; Samples H, Williams AR, Crystal S, & Olfson M, 2020).
While 180 days may be a minimum duration necessary to stabilize drug use and reduce risks of adverse outcomes, most patients discontinue medication within a few weeks or months (Samples H, Williams AR, Crystal S, & Olfson M, 2020; and Krawczyk N, Mojtabai R, Stuart EA, et al., 2020; Morgan JR, Schackman BR, Leff JA, Linas BP, & Walley, AY, 2018), underscoring the need for strategies to improve retention. Peer support, care navigation, and other supportive interventions may be particularly beneficial for patients identified as buprenorphine negative at treatment entry to facilitate successful buprenorphine induction and maintenance while minimizing treatment dropout (Bassuk EL, Hanson J, Greene RN, Richard M, & Laudet A, 2016; Powell KG, Treitler P, Peterson NA, Borys S, & Hallcom D, 2019).
In the context of ongoing policy debates over requirements to prescribe buprenorphine (Poorman E, 2021; Fiscella K, Wakeman SE & Beletsky L, 2019), a shift has occurred in recent years toward removing barriers to buprenorphine access via lower-threshold care settings, aligned with principles of autonomy, harm reduction, equity, and distributive justice (Hayes BT, Jakubowski A, Fitzsimmons C, et al., 2021; Lister JJ, Weaver A, Ellis JD, Himle JA, & Ledgerwood DM, 2020). A study on buprenorphine initiation in the emergency department (ED) reported that patients given buprenorphine were more likely to initiate subsequent community-based care than those simply given referrals (D'Onofrio G, O'Connor PG, Pantalon MV, et al., 2015). Studies of pilot programs such as buprenorphine provision through alternative settings such as homeless outreach services (Carter J, Zevin B, Lum PJ. 2019), syringe exchange programs (Hood JE, Banta-Green CJ, Duchin JS, et al. 2020), and waitlist treatment (Sigmon SC, C Meyer A, Hruska B, et al. 2015) have found that patients receiving active medication typically have superior outcomes to those who were not provided interim medication, even in the absence of intensive in-person care.
Largely due to COVID-19 regulatory reforms, providers have expanded use of telemedicine for mental health and addiction treatment, bypassing initial in-person visits before prescribing controlled substances (Alegría M, Frank RG, Hansen HB, et al. 2021). These models, similar to ED-initiated medication “Bridge Clinics,” have many fewer requirements—such as group attendance, drug testing, and counseling—than historic standards of care. A shared theme across these models consistent with the current results is that lowering or removing barriers to buprenorphine access can improve treatment engagement, retention, and clinical outcomes, similar to literature on reducing counseling requirements and visit frequency for methadone maintenance (Brothers S, Viera A, & Heimer R, 2021; Kourounis G, Richards BD, & Kyprianou E, et al., 2016).
This study has several limitations. Foremost, the results are limited to patients entering buprenorphine maintenance treatment and do not generalize to those initiating treatment with other medications or in alternative care settings. Second, because the analysis included only patients who completed an intake session, results may not generalize to those who scheduled an intake but did not complete intake assessments. Third, patients did not systematically self-reported race/ethnicity nor did the EHR collect it, which limits our ability to detect disparities in retention. In addition, some patients may have been misclassified as not using NPB due to negative urine toxicology results at presentation. However, absence of buprenorphine in initial testing suggests these individuals were sufficiently infrequent users (i.e., went more than four days without use) to test negative and results were robust to a sensitivity analysis that excluded inferred classifications. Due to high levels of treatment attrition, the drug testing results do not represent opioid use among individuals who discontinued treatment. Additionally, while test results for benzodiazepines were available from intake visits, we were unable to determine which patients were prescribed benzodiazepines. Finally, as in all observational studies, we cannot exclude the possibility that unmeasured confounders may account for observed group differences in clinical outcomes.
4.1. Conclusion
NPB use before treatment intake is associated with higher treatment retention and lower opioid use, underscoring the importance of prioritizing continued expansion of access to buprenorphine. Buprenorphine has a significant clinical effect on stabilizing patient opioid use, retaining patients in care, and reducing overdose events and all-cause mortality (Sordo L, Barrio G, Bravo MJ et al., 2017; Santo Jr T, Clark B, Hickman M, et al., 2021; Samples H, Williams AR, Crystal S, & Olfson M, 2020). Yet many individuals with OUD struggle to find and afford buprenorphine treatment, especially in rural areas (Haffajee RL, Lin LA, Bohnert ASB, & Goldstick JE, 2019). Barriers to care may in part reflect entrenched concerns about buprenorphine diversion and a complex policy environment. Our results suggest that concerns regarding buprenorphine diversion due to misuse may be misplaced. Prescribing limits, which are unique to buprenorphine, are not required for higher risk schedule II opioids, and may contribute to misperceptions that buprenorphine maintenance treatment is not part of mainstream health care in general practice settings or best integrated with care for other conditions. Our findings also suggest that patients testing buprenorphine negative at treatment admission may require additional services and intervention, such as care coordination and comprehensive team approaches, to improve treatment retention and clinical outcomes.
Highlights.
Rates of non-prescribed buprenorphine preceding treatment entry seem to have increased in the past decade and the use of NPB may itself be a pathway to entering professional care.
Over half of new patients tested positive for buprenorphine at intake, largely reflecting NPB rather than buprenorphine from an outside prescriber, and compared to those testing negative had double the treatment retention and half the rate of opioid use while in care.
Outcomes did not significantly differ whether the source of buprenorphine preceding intake was prescribed or non-prescribed. This might reflect the medication’s large effect size for protective clinical benefits, which are typically associated with a 66-80% reduction in mortality.
Peer support, care navigation and other supportive interventions may be particularly beneficial for patients identified as buprenorphine negative at treatment entry in order to facilitate successful buprenorphine induction and maintenance while minimizing treatment dropout
Funding:
Financial support for this work was provided by grants from the National Institute on Drug Abuse (NIDA) [grant numbers K23 DA044342 and K01 DA049950], the Agency for Healthcare Research and Quality (AHRQ) [grant numbers R18 HS03258, U19 HS021112, and R18 HS02346], NCATS UL1TR003017, and the FORE Foundation.
Appendix A: Patient Characteristics Based on Buprenorphine Use at Treatment Entry Partitioning for Source of Buprenorphine
| Covariate | Overall Sample (n=971) |
% | Lab POS bup, Prescribed (n=159) |
% | Lab POS bup, Not Prescribed (NPB) (n=420) |
% | Lab NEG bup (n=392) |
% |
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Age | ||||||||
| - 18-29 | 296 | 30.5 | 41 | 25.8 | 136 | 25.8 | 119 | 30.4 |
| - 30-49 | 550 | 56.6 | 99 | 62.3 | 233 | 62.3 | 218 | 55.6 |
| - 50-64 | 118 | 12.2 | 18 | 11.3 | 50 | 11.3 | 50 | 12.8 |
| - >65 | 7 | 0.7 | 1 | 0.6 | 1 | 0.6 | 5 | 1.3 |
| Sex | ||||||||
| - Male | 566 | 58.3 | 78 | 49.1 | 248 | 49.1 | 240 | 61.2 |
| - Female | 405 | 41.7 | 81 | 50.9 | 172 | 50.9 | 152 | 38.8 |
| Drug Results (positive) at Intake | ||||||||
| Opioids | 353 | 36.4 | 26 | 16.4 | 119 | 16.4 | 208 | 53.1 |
| Cocaine | 197 | 20.4 | 17 | 10.7 | 98 | 10.7 | 82 | 21.1 |
| Amphetamine | 53 | 5.5 | 11 | 7.0 | 25 | 7.0 | 17 | 4.4 |
| Benzodiazepines | 130 | 13.4 | 18 | 11.3 | 66 | 11.3 | 46 | 11.8 |
| Cannabis | 327 | 33.9 | 40 | 25.3 | 156 | 25.3 | 131 | 33.9 |
| Manual Coding of EHR Intake Notes for history of |
||||||||
| IDU | 517 | 53.2 | 86 | 54.1 | 230 | 54.1 | 201 | 51.3 |
| Heroin use | 747 | 76.9 | 118 | 74.2 | 323 | 76.9 | 306 | 78.1 |
| Cocaine use | 519 | 53.5 | 75 | 47.2 | 234 | 55.7 | 210 | 53.6 |
| Benzodiazepine use | 371 | 38.2 | 59 | 37.1 | 182 | 43.3 | 130 | 33.2 |
| Criminal Justice history (current/past) | 299 | 30.8 | 38 | 23.9 | 138 | 32.9 | 123 | 31.4 |
POS=Positive, NEG=Negative, bup=buprenorphine, NPB=Non-prescribed buprenorphine, IDU=Injection Drug Use. Data were collected from the EHR of a multi-site, multi-state buprenorphine treatment program for new admissions of patients with Opioid Use Disorder between January 1, 2015 and April, 2018.
Appendix B: Study Protocol for Coding Source of Buprenorphine
| Categorization for Buprenorphine Source Prior to Treatment Entry |
Parameters for Coding | Example Language |
|---|---|---|
| Non-Prescribed Buprenorphine, NPB (i.e. diverted or illicit buprenorphine) | -Identify any language with references to generic buprenorphine mono or combo product or a trade name, typically a version of Suboxone (Subs, Subx, etc.) -Code for documentation of buying, trading, or informally receiving buprenorphine on the street prior to entering treatment |
"was able to get street bupe," "getting illicit Suboxone," "has been buying street Subx," “About 2 months ago started using street suboxone - anywhere between 4 mg and 8 mg daily" |
| Prescribed buprenorphine | Documentation of a care transfer, outside prescriber, or detoxification discharge | “patient recently moved from [town], had been attending [OBOT program] for two years,” “patient discharged from inpatient admission last week on bup/nal 16/4mg” |
| Unknown | No documentation regarding source of buprenorphine (i.e. no mention whatsoever) or vague and/or conflicting explanations which are indeterminate | “solely on Suboxone since last year,” “24 hours since last use of Suboxone,” "Last buprenorphine use a few days ago" |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial conflicts of interest: Arthur Robin Williams MD MBE receives equity, consulting fees, and travel expenses from Ophelia Health, Inc., a telehealth provider for opioid use disorder; Hillary Samples PhD has received consulting fees from the American Society for Addiction Medicine.
References:
- Alegría M, Frank RG, Hansen HB, Sharfstein JM, Shim RM, Tierney M. Transforming mental heath and addiction services. Health Affairs. 2021; 40(2): 226–234. [DOI] [PubMed] [Google Scholar]
- Alford DP, LaBelle CT, Kretsch N, Bergeron A, Winter M, Botticelli M, Samet JH. Collaborative care of opioid-addicted patients in primary care using buprenorphine: five-year experience. Archives of Internal Medicine. 2011; 171(5):425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen B, Harocopos A. Non-prescribed buprenorphine in New York City: Motivations for use, practices of diversion, and experiences of stigma. Journal of Substance Abuse Treatment. 2016; 70:81–86. [DOI] [PubMed] [Google Scholar]
- Bassuk EL, Hanson J, Greene RN, Richard M, Laudet A. Peer-Delivered Recovery Support Services for Addictions in the United States: A Systematic Review. Journal of Substance Abuse Treatment. 2016; Apr;63:1–9. [DOI] [PubMed] [Google Scholar]
- Bazazi AR, Yokell M, Fu JJ, Rich JD, & Zaller ND. Illicit use of buprenorphine/naloxone among injecting and noninjecting opioid users. Journal of Addiction Medicine. 2011; 5(3), 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Barth KS, Back SE, Book SW. Discontinuation of buprenorphine maintenance therapy: Perspectives and outcomes. Journal of Substance Abuse Treatment. 2015; 52:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers S, Viera A, Heimer R. Changes in methadone program practices and fatal methadone overdose rates in Connecticut during COVID-19. Journal of Substance Abuse Treatment. 2021; 131: 10.1016/j.jsat.2021.108449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson RG, Daniulaityte R, Silverstein SM, Nahhas RW, Martins SS. Unintentional drug overdose: Is more frequent use of non-prescribed buprenorphine associated with lower risk of overdose? International Journal of Drug Policy. 2020; Apr 17;79:102722. doi: 10.1016/j.drugpo.2020.102722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J, Zevin B, Lum PJ. Low barrier buprenorphine treatment for persons experiencing homelessness and injecting heroin in San Francisco. Addiction Science & Clinical Practice. 2019. May 6;14(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilcoat HD, Amick HR, Sherwood MR, Dunn KE. Buprenorphine in the United States: motives for abuse, misuse, and diversion. Journal of Substance Abuse Treatment. 2019; 104:148–157. [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Whittington RA, Vosburg Sk, Kowalczyk WJ. Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacology. 2008; 33(5):1179–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CO, Roose RJ, Starrels JL, Giovanniello A, Sohler NL. Prior buprenorphine experience is associated with office-based buprenorphine treatment outcomes. Journal of Addiction Medicine. 2013; 7(4):287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Human Services. HHS releases new buprenorphine practice guidelines, expanding access to treatment for opioid use disorder. April 27, 2021. (https://www.hhs.gov/about/news/2021/04/27/hhs-releases-new-buprenorphine-practice-guidelines-expanding-access-to-treatment-for-opioid-use-disorder.html)
- D'Onofrio G, O'Connor PG, Pantalon MV, Chawarski MC, Busch SH, Owens PH. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015; 313(16):1636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiscella K, Wakeman SE, Beletsky L. Buprenorphine deregulation and mainstreaming treatment for opioid use disorder: X the X waiver. JAMA Psychiatry. 2019; 76(3):229–230. [DOI] [PubMed] [Google Scholar]
- Genberg BL, Gillespie M, Schuster CR, Johanson CE, Astemborski J, Kirk GD, Mehta SH. Prevalence and correlates of street-obtained buprenorphine use among current and former injectors in Baltimore, Maryland. Addictive Behaviors. 2013; 38(12):2868–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffajee RL, Lin LA, Bohnert ASB, Goldstick JE. Characteristics of US Counties With High Opioid Overdose Mortality and Low Capacity to Deliver Medications for Opioid Use Disorder; JAMA Network Open. 2019; 2(6):e196373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes BT, Jakubowski A, Fitzsimmons C, Garcia B, Ramirez F, Fox AD. “The Doctor Says You Cannot Have [Buprenorphine]” Autonomy and Use of Prescribed or Non-Prescribed Buprenorphine, Substance Use & Misuse. 2021; 56:8,1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood JE, Banta-Green CJ, Duchin JS, Breuner J, Dell W, Finegood B, Glick SN, Hamblin M, Holcomb S, Mosse D, Oliphant-Wells T, Shim MM. Engaging an unstably housed population with low-barrier buprenorphine treatment at a syringe services program: Lessons learned from Seattle, Washington. Substance Abuse. 2020;41(3):356–364. [DOI] [PubMed] [Google Scholar]
- Kourounis G, Richards BD, Kyprianou E, Symeonidou E, Malliori MM, Samartzis L. Opioid substitution therapy: Lowering the treatment thresholds. Drug and Alcohol Dependennce. 2016; Apr 1;161:1–8. [DOI] [PubMed] [Google Scholar]
- Krawczyk N, Mojtabai R, Stuart EA, et al. Opioid agonist treatment and fatal overdose risk in a state-wide US population receiving opioid use disorder services. Addiction. 2020; 115(9):1683–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle MR, Liebschutz JM, Zhang F, Ross-Degnan D, Wharam JF. Opioid prescribing after nonfatal overdose and association with repeated overdose. Annals of Internal Medicine. 2016; 164:1–9. [DOI] [PubMed] [Google Scholar]
- Lin LA, Lowfall MR, Walsh SL, Gordon AJ, Knudsen HK. Perceptions and practices addressing diversion among US buprenorphine prescribers. Drug and Alcohol Dependence. 2018; 186:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister JJ, Weaver A, Ellis JD, Himle JA, Ledgerwood DM. A systematic review of rural-specific barriers to medication treatment for opioid use disorder in the United States. American Journal of Drug and Alcohol Abuse. 2020; May 3;46(3):273–288. PMID: 31809217. [DOI] [PubMed] [Google Scholar]
- Martin SA, Chiodo LM, Bosse JD, Wilson A. The Next Stage of Buprenorphine Care for Opioid Use Disorder; Annals of Internal Medicine. 2018; 169(9):628–635. [DOI] [PubMed] [Google Scholar]
- Meinhofer A, Williams AR, Johnson P, Schackman B, Bao Y. Prescribing decisions at buprenorphine treatment initiation: Do they matter for treatment discontinuation and adverse opioid-related events? Journal of Substance Abuse Treatment. 2019; 105:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monico LB, Mitchell SG, Gryczynski J, Schwartz RP, O'Grady KE, Olsen YK, Jaffe JH. Prior experience with non-prescribed buprenorphine: Role in treatment entry and retention. Journal of Substance Abuse Treatment. 2015; 57:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Schackman BR, Leff JA, Linas BP, & Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. Journal of Substance Abuse Treatment. 2018; 85,90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, Zhang VS, Schoenbaum M, King M. Trends in Buprenorphine Treatment in the United States, 2009-2018. JAMA. 2020; 323(3):276–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorman E. The number needed to prescribe- what would it take to expand access to buprenorphine? NEJM. 2021; 384(19):1783–1784. [DOI] [PubMed] [Google Scholar]
- Powell KG, Treitler P, Peterson NA, Borys S, Hallcom D. Promoting opioid overdose prevention and recovery: an exploratory study of an innovative intervention model to address opioid abuse. International Journal of Drug Policy. 2019; 64:21–29. [DOI] [PubMed] [Google Scholar]
- Samples H, Williams AR, Crystal S, Olfson M. Impact of long-term buprenorphine treatment on adverse health outcomes in Medicaid. Health Affairs. 2020; 39(5):747–755. PMID 32364847. PMC7531057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samples H, Williams AR, Olfson M, Crystal S. Risk Factors for Discontinuation of Buprenorphine Treatment for Opioid Use Disorders in a Multi-State Sample of Medicaid Enrollees. Journal of Substance Abuse Treatment. 2018; 95:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santo T Jr, Clark B, Hickman M, et al. Association of Opioid Agonist Treatment With All-Cause Mortality and Specific Causes of Death Among People With Opioid Dependence: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2021; Jun 2. doi: 10.1001/jamapsychiatry.2021.0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman-Olivier Z, Albanese M, Nelson SE, Roland L, Puopolo F, Klinker L, & Shaffer HJ. Self-treatment: Illicit buprenorphine use by opioid-dependent treatment seekers. Journal of Substance Abuse Treatment. 2010; 39(1), 41–50. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Meyer AC, Hruska B, et al. Bridging waitlist delays with interim buprenorphine treatment: initial feasibility. Addictive Behavior. 2015; Dec;51:136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordo L, Barrio G, Bravo MJ et al. : Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017; 357(j1550):1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Annals of Internal Medicine. 2007; 147(8):573–577. [DOI] [PubMed] [Google Scholar]
- Williams AR, Samples H, Crystal S, Olfson M. Retention on buprenorphine beyond six months and risk of acute care service utilization, opioid prescription use, and overdose, American Journal of Psychiatry. 2019; Feb 1;177(2):117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokell MA, Zaller ND, Green TC, Rich JD. Buprenorphine and buprenorphine/naloxone diversion, misuse, and illicit use: An international review. Current Drug Abuse Reviews. 2011; 4(1):28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]