Abstract
Tuft cells are sentinel chemosensory cells that monitor the lumen of hollow organs for noxious or infectious stimuli and respond with disease- and tissue-specific effectors. The discovery of critical tuft cell functions in intestinal type 2 immune responses and airway defense has sparked interest in the formation and function of this architecturally unique cell type. Recent advances in single cell transcriptomics and computational biology allow for new insights into the genetics and environmental cues underlying tuft cell formation and maturation. Here we summarize the most recent research on tuft cell development and function in various disease states and organ systems.
INTRODUCTION:
Tuft cells are solitary chemosensory cells found in most endoderm-derived organs, dispersed throughout the simple columnar epithelium (as reviewed in [1]). These architecturally unique cells have many aliases (e.g.: microvillus cells, brush cells), however the field has largely moved towards a consensus definition that TRPM5+, POU2F3-dependent epithelial cells expressing genes related to taste signaling and eicosanoid biosynthesis are tuft cells, regardless of tissue origin. Robust protein markers also include ChAT, DCLK1, and Advillin, though none are unique to tuft cells [1, 2]. Tuft cells are definitively identified by salient ultrastructural features, including tall, blunt microvilli (Figure 1) that project their actin rootlets deep into supranuclear cytoplasm.
Figure 1. Tuft cell identification by salient ultrastructural features.

SEM of the apical surface of the normal murine gallbladder (top panels) or pre-cancerous lesions in the pancreas (Panc neoplasia, bottom panels) highlighting tuft cell microvilli. Tuft cells may be identified by these physical features, despite tissue of origin or disease state. Scale bars, left panels, 1 μm; right panels, 2 μm. Red asterisks mark the location of the insets.
Despite morphological identification of tuft cells by microscopy as early as the 1950s, (as reviewed in [3]), the function of these enigmatic cells has only recently come to light. Only a decade ago, researchers identified a function for tracheal and nasal tuft cells in airway responses to noxious stimuli and bacteria through release of acetylcholine [4–6]. In 2016, another major role for tuft cells was uncovered in the small intestine, with several groups demonstrating that tuft cells drive activation of the immune response to helminth infection through production of cytokine interleukin (IL)-25 [7–9]. The emerging picture of tuft cells as potent cytokine producers and cholinergic cells has spurred major interest in understanding function at homeostasis and in disease states in the numerous tissues where tuft cells are found.
Over the last several years, there have been major advances in the characterization and function of tuft cells. New discoveries have been aided by transcriptomic analyses using high resolution single cell RNA sequencing (scRNA-seq), use of several in vivo tools (including genetic tools for tuft cell manipulation [10–12]), and recent methods focused on tuft cell isolation and characterization [11, 13, 14]. Notably, new roles for tuft cells have been uncovered in tumorigenesis. Here we review recent literature on tuft cell formation and function, highlighting cell of origin and the transcriptional programs that regulate this architecturally unique cell type.
Tuft cell formation in normal tissues
The origin of intestinal tuft cells has been the subject of intense study. Tuft cells are derived from LGR5+ crypt stem cells in both the small intestine and colon [15]. Unlike other secretory cells in the small intestine, tuft cells can differentiate in the absence of transcription factor ATOH1 in a SOX4-dependent manner, which may be driven by type 2 cytokine signaling [16, 17]. More recently, a third route of tuft cell differentiation was uncovered in the context of long-term muscarinic blockade or epithelial deletion of Chrm3, an acetylcholine receptor. In this model, tuft cells can differentiate from a PROX1 positive precursor, sharing a common lineage with enteroendocrine cells [18].
Tuft cells are found throughout the respiratory and alimentary tracts, including in the trachea, nasal, and olfactory epithelium. Extensive work on differentiation of epithelial cells in the trachea and anterior nasal cavity demonstrates that the pseudostratified epithelium, including tuft cells, arises from basal cells [19]. Tracheal tuft cells differentiate prenatally and expand significantly post-weaning [20]. Increased frequency of tracheal tuft cells was observed in response to fungal aeroallergens; however, the mechanism is unclear [21]. Although tracheal, nasal, and olfactory tuft cells have similar transcriptomes [21], olfactory tuft cells are distinct in that they share a common progenitor with neurons, arising from globose basal cells [22]. Tuft cells in the nasal epithelium are a particularly active area of study, due to the increased prevalence of a tuft-like gene signature in human nasal polyps [23, 24].
In other gastrointestinal tissues, the cellular provenance of tuft cells remains unclear. In the extrahepatic biliary tree O’Leary et al used fate-mapping tools to demonstrate that the abundant tuft cell compartment has very limited turnover in the adult, but that tuft cells turnover rapidly in neonatal mice; additionally, bile acids negatively regulate tuft cell frequency through as-yet unknown mechanisms [12]. Consistent with the distinct fetal origins of the two tissues, no tuft cells were observed among the intrahepatic biliary epithelium, even after cholestatic injury. Inducible deletion of tuft cells results in slow repopulation, suggesting a local progenitor population [12]. However, an extrahepatic biliary stem cell has yet to be identified, despite the relative ease of culturing this tissue in vitro [25]; as such, how tuft cells develop in the biliary tree remains an open question and additional work is needed to understand how tuft cells arise here and elsewhere in the gastrointestinal tract (e.g.: stomach).
Tuft cells arising in response to injury or tumorigenesis
While studies in normal tissues are consistent with a resident stem or progenitor cell source for tuft cell formation, recent studies in the pancreas demonstrate that tuft cells can arise from the transdifferentiation of terminally differentiated lineages. By lineage tracing, oncogenic mutation or chronic injury have been shown to induce tuft cell formation from acinar cells, digestive enzyme-producing cells of the pancreas [26, 27]. The appearance of tuft cells beyond their homeostatic niches in response to injury and tumorigenesis has also been found in the respiratory [28] and digestive tracts [29, 30], however cell-of-origin studies were not done. Interestingly, irrespective of tissue origin or disease state, tuft cell structure is retained, suggesting an intimate link to development and function (Figure 1).
Transcriptomic requirements for tuft cell formation
The sophistication of single cell RNA sequencing (scRNA-seq) and computational biology approaches in recent years has provided tools to predict the transcriptomic requirements for the formation of rare cell types like tuft cells. To this end, Ma et al., used lineage tracing and scRNA-seq to capture the transitional states between acinar and tuft cells in a murine model of pancreatitis [31]. Enrichment strategies allowed for the identification of putative tuft cell progenitor populations and the cell states associated with differentiation (Figure 2A–B). Analysis of regulons, transcription factor-directed transcriptional programs or operons, in this dataset identified known tuft cell master regulator Pou2f3, but also Spib, Ehf, and Ascl1, where activity increases with tuft cell maturation (Figure 2C and Ma et al.)[31]. Insight into the mechanisms by which these transcription factors control tuft cell formation may be gleaned from target genes, including known markers such as Trpm5, Ptgs1, and Il25, but also genes related to tuft cell structure such as those associated with microtubule polymerization (Dclk1, Tuba1a, Tubb2a), actin bundling (Espn), and microvilli formation (Avil, Cdhr2). Importantly, comparisons to scRNA-seq studies conducted in human pancreatitis suggest that these programs are conserved [32].
Figure 2. Transcriptional regulation of tuft cell formation.

(A) UMAP of scRNA-seq data of tuft cells in multiple stages of maturation forming from pancreatic acinar cells under conditions of chronic injury in murine pancreatitis. Acinar cells first form a mucin/ductal progenitor-like population which can seed tuft cells. (B) Monocle3 pseudotime overlaid on the UMAP in (A), consistent with tuft cell formation from an induced progenitor population and moving through several stages of maturation. (C) Regulon scores for tuft cell master regulator Pou2f3 or predicted regulator Spib overlaid on the UMAP in (A). Figure modified from Ma et al.
Tuft cell heterogeneity - towards a new paradigm of spatio-temporal driven phenotypes
Transcriptomic profiling by scRNA-seq has revolutionized our understanding of cell states, revealing previously unappreciated heterogeneity among populations previously thought to be homogenous. In one of the earliest single-cell atlases of the small intestine, Haber et al observed two transcriptional programs in tuft cells, dubbing these intestinal “tuft-1” and intestinal “tuft-2”, associated with neuronal and immune transcripts, respectively [33]. Recent work in the small intestine has dissected positional impact on tuft cell transcriptional phenotypes, suggesting that spatial and temporal drivers of gene expression may underlie observations of tuft cell heterogeneity. Crypt-villus “zonation” was recently described using scRNA-seq and laser capture microscopy to dissect epithelial cell function along the crypt-villus axis of the small intestine [34]. The markers used to delineate these zones were subsequently leveraged in an approach called “ClumpSeq”, which improves capture of rare cells by sequencing small cell aggregates [35]. Manco et al describe “tuft-2” (immune) transcripts at the villus tip, with the Sox4+ “tuft-1” (neuronal) transcriptional signature found toward the bottom of the villus, consistent with differentiation states of the same population. This maturation model is also supported by a study using a reporter for “mature” small intestinal tuft cells, GPR46, in combination with TRPM5, which is expressed in all tuft cells [36]
In the context of injury, the ontogeny of tuft cell heterogeneity has been best explored in the transdifferentiation of tuft cells from acinar cells during pancreatitis, suggesting temporal regulation of tuft cell gene expression programs. Recent work from the DelGiorno laboratory using a battery of informatic analyses on scRNA-seq data from murine pancreatitis identifies tuft cell differentiation states that align with the original “tuft-1” (immature tuft) and “tuft-2” (mature tuft) classifications, confirming that these designations reflect differentiation status (Figure 3) [31]. Interestingly, tuft cell maturation and the shift from “tuft-1” to “tuft-2” is accompanied by the expression of genes necessary for the formation of salient tuft cell structural features, such as microvillus formation (e.g. Avil, Cdhr5)[31].
Figure 3. Tuft cell heterogeneity reflects maturation status.

Intestinal “tuft-1” and “tuft-2” signatures plotted against monocle3 pseudotime generated from scRNA-seq data on tuft cell formation in pancreatitis. The intestinal “tuft-1” signature is enriched early in tuft cell formation, while the intestinal “tuft-2” signature is concentrated in mature tuft cells. These data and prior studies suggest a model where “tuft-1” and “tuft-2” represent stages in tuft cell formation and not necessarily different functional states. Figure generated from data in Ma et al.
Advances in tuft cell function
The prevailing model of tuft cell function is that this sentinel cell type senses noxious stimuli, inflammation, signaling from microbiota, and/or harmful bacteria in the lumen of hollow organs and responds with signaling molecules that modulate the tissue microenvironment. Small intestine: Advances in functional characterization include the notable discoveries that tuft cells sense the protist metabolite succinate (recently reviewed in [37]), which can control ileal inflammation and may be relevant in human disease [38]. Intestinal tuft cell-derived leukotrienes are critical for ILC2 activation and anti-helminth immunity, along with the canonical tuft cell cytokine, IL-25[10]. Intestinal research continues to focus on the role of tuft cells in activating ILC2s, and recent studies reveal deleterious effects, with Kotas et al demonstrating that acute IL-25 treatment impairs anti-bacterial immunity during Salmonella typhimurium infection [39], while Desai et al., found that acute tuft cell-dependent type 2 cytokine signaling leads to increased pathogenesis of flavivirus [40]. In contrast to these “activating” conditions, PGD2, a known tuft cell effector [41], was recently identified as a negative regulator of type 2 cytokine responses and the tuft cell–ILC2 circuit [42]. Airway: While earlier work in the airways focused on tuft cell-derived ACh, recent studies demonstrate similar effector functions to those described in the small intestine, with P2Y2-dependent activation of tuft cells driving a cysteinyl leukotriene response to Alternaria challenge in tracheal and nasal epithelium [21]. Tuft cell-derived leukotrienes in the context of exogenously administered IL-25 leads to lung eosinophilia and type 2 inflammation [43]. Biliary tree: Optogenetic activation of murine gallbladder tuft cells revealed that biliary tuft cells also produce measurable cysteinyl leukotrienes and acetylcholine, which, respectively, drive gallbladder contraction and release of mucin granules [11]. In vivo, loss of tuft cells resulted in influx of highly activated neutrophils and inflammatory gene expression in non-tuft epithelial cells [12]; this phenotype was microbiome-dependent [12], consistent with the ability of microbiome-derived short chain fatty acids to activate defensive tissue responses (contraction, mucin release) via tuft cells [11]. Tumorigenesis: POU2F3-expressing small cell lung cancers and thymomas, likely tuft cell-derived, have been identified. Expression of neuroendocrine markers is consistent with the maturation paradigm described here whereby immature tuft cells are more neuronal-like [44, 45]. Ablation of tuft cell master regulator transcription factor POU2F3 or proteins required for tuft cell signaling (HPGDS, GNAT3) in the pancreas results in accelerated tumorigenesis, identifying an anti-tumorigenic function for tuft cells [41, 46].
CONCLUSIONS
Implications for structure-function relationships.
While significant advances have been made in terms of tuft cell formation, regulation, and function (summarized in Figure 4), studies linking the unique tuft cell architecture to these roles in homeostasis and disease are lacking. It is tempting to speculate that the tall blunt microvilli have evolved to reach through the mucus layer that coats many tuft-cell containing organs to sense noxious stimuli or microbiota-derived metabolites, such as succinate. Microvilli increase surface area, and therefore the availability of key receptors allowing for the detection of small quantities of ligand. We predict that disruption of microvillus formation would impact the sensory function of these cells and potentially blunt secretion. Secretion is universal among tuft cells and may be linked to the deep actin rootlets that are similarly conserved. The tubulovesicular network likely collaborates with the rootlets to deliver secretory products to the cell surface. We speculate that the microvilli-actin rootlet unit functions in mechanosensation. While there is currently little evidence for mechanosensation in tuft cells, it has been linked to the secretion of eicosanoids, which is a universal tuft cell function. Further studies are required to determine how tuft cell form follows function, and how disruption of tuft cell architecture affects tissue homeostasis and disease progression.
Figure 4. Model(s) of tuft cell formation.
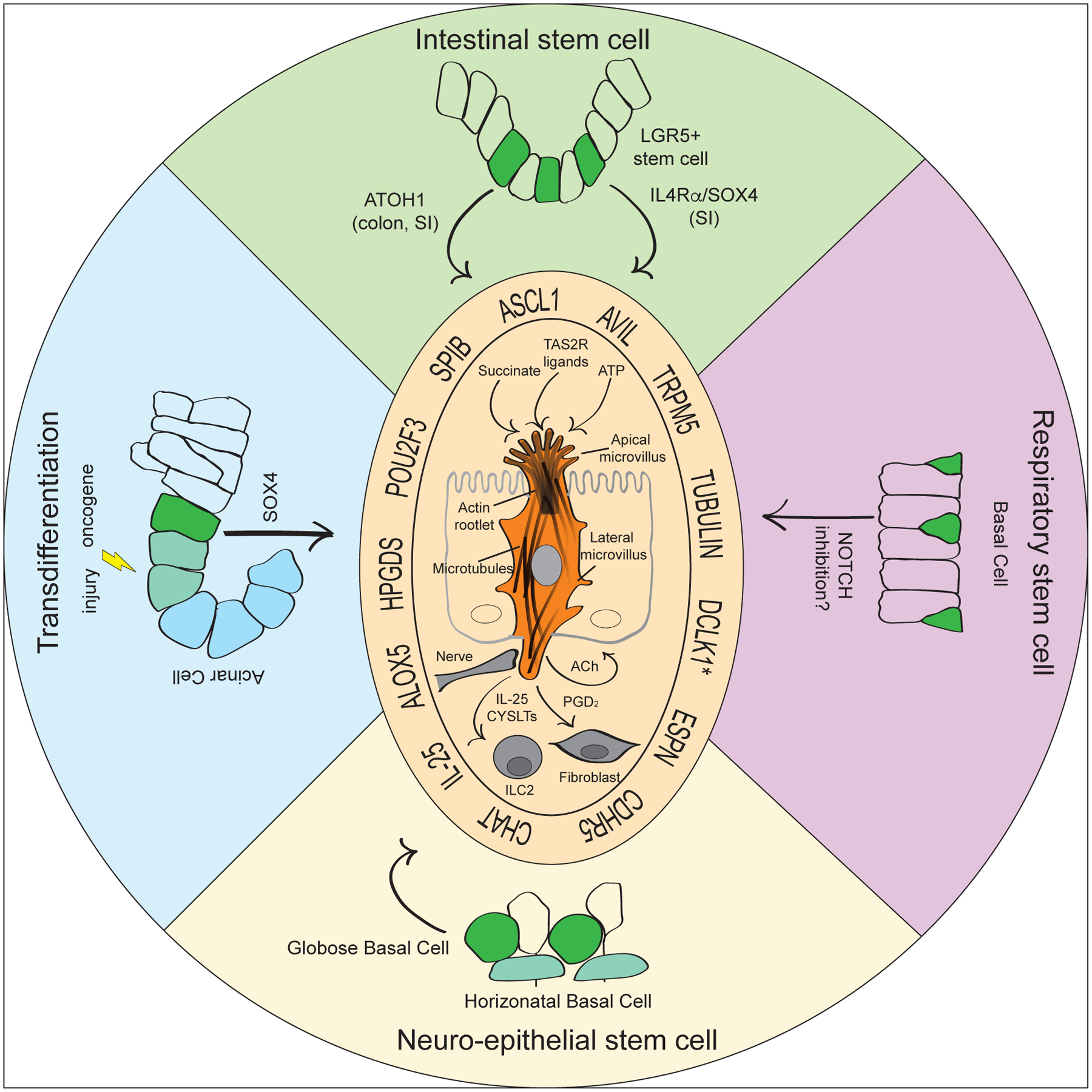
Tuft cells form from stem or progenitor cells in many organs throughout the respiratory and digestive tracts and have been shown to arise from transdifferentiation of differentiated cells, such as pancreatic acinar cells, under conditions of chronic injury or oncogenic mutation. Despite formation in different organs and from different cell types, tuft cell formation and maturation require core transcriptional changes under all conditions, such as expression of key transcription factors and transition through a neuronal-like “tuft-1” state before maturing into an inflammatory-like “tuft-2” state. This maturation is accompanied by the formation of salient tuft cell structural features, such as characteristic microvilli and deep actin rootlets (*Dclk1 is not expressed in all tuft cells). While studies are lacking, we hypothesize that these structures are required for the function of tuft cells as sentinel cells that sense specific stimuli (succinate, ATP, TAS2R ligands) and respond with disease-specific effectors (IL-25, CYSLTs, ACh, PGD2).
ACKNOWLEDGEMENTS:
The authors thank Richard Locksley, Maya Kotas, Uri Manor, and Olivia Ben-Levy for critical reading of the manuscript, and Leonardo R. Andrade, and Olivia Ben-Levy for Figure generation. Funding: Claire O’Leary is supported by the National Institutes of Health (5F32DK121476-02) and the following grants to Richard M. Locksley: National Institutes of Health (R01AI026918, R01HL128903), Sandler Asthma Basic Research Center, and the Howard Hughes Medical Institute. Zhibo Ma is supported by the National Institutes of Health (R35 CA197687) and the Freeberg Foundation to Geoffrey Wahl. Sammy Weiser Novak is supported by the following grants to the Manor laboratory: the Waitt Foundation, the Chan-Zuckerberg Initiative Imaging Scientist Award, the National Institutes of Health (R21DC018237, CA0141950), the L.I.F.E. Foundation, and by the National Science Foundation NeuroNex Award (2014862). The DelGiorno laboratory is supported by the National Institutes of Health (NIH/NCI 5P30 CA068485-26, NIH/NIDDK 5P30 DK058404-20, NIH/NIAID 5R01 AI145848-02, NIH/NIGMS 1R35 GM142709-01), and an American Gastroenterological Association Research Scholar Award (AGA2021-13-02). Declaration of Interest: none.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
REFERENCES:
- 1.O’Leary CE, Schneider C, and Locksley RM, Tuft Cells-Systemically Dispersed Sensory Epithelia Integrating Immune and Neural Circuitry. Annu Rev Immunol, 2019. 37: p. 47–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schutz B, et al. , Distribution pattern and molecular signature of cholinergic tuft cells in human gastro-intestinal and pancreatic-biliary tract. Sci Rep, 2019. 9(1): p. 17466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Moltke J, Chapter 31 - Intestinal Tuft Cells A2 - Said, Hamid M, in Physiology of the Gastrointestinal Tract (Sixth Edition). 2018, Academic Press. p. 721–733. [Google Scholar]
- 4.Krasteva G, et al. , Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci U S A, 2011. 108(23): p. 9478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tizzano M, et al. , Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci U S A, 2010. 107(7): p. 3210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saunders CJ, et al. , Cholinergic neurotransmission links solitary chemosensory cells to nasal inflammation. Proc Natl Acad Sci U S A, 2014. 111(16): p. 6075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerbe F, et al. , Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature, 2016. 529(7585): p. 226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Moltke J, et al. , Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature, 2016. 529(7585): p. 221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howitt MR, et al. , Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science, 2016. 351(6279): p. 1329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGinty JW, et al. , Tuft-Cell-Derived Leukotrienes Drive Rapid Anti-helminth Immunity in the Small Intestine but Are Dispensable for Anti-protist Immunity. Immunity, 2020. 52(3): p. 528–541 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]; **-This study identifies disease-specific roles for tuft cell-derived eicosanoids, leukotrienes.
- 11.Keshavarz M, et al. , Cysteinyl leukotrienes and acetylcholine are biliary tuft cell cotransmitters. Sci Immunol, 2022. 7(69): p. eabf6734. [DOI] [PubMed] [Google Scholar]
- 12.O’Leary CE, et al. , Bile acid-sensitive tuft cells regulate biliary neutrophil influx. Sci Immunol, 2022. 7(69): p. eabj1080. [DOI] [PMC free article] [PubMed] [Google Scholar]; *-This paper demonstrates a role for tuft cells in limiting microbiome-dependent inflammation in the biliary tree.
- 13.O’Leary CE, et al. , Interrogating the Small Intestine Tuft Cell-ILC2 Circuit Using In Vivo Manipulations. Curr Protoc, 2021. 1(3): p. e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ualiyeva S, et al. , Isolation and Quantitative Evaluation of Brush Cells from Mouse Tracheas. J Vis Exp, 2019(148). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerbe F, et al. , Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol, 2011. 192(5): p. 767–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gracz AD, et al. , SOX4 Promotes ATOH1-independent Intestinal Secretory Differentiation Toward Tuft and Enteroendocrine Fates. Gastroenterology, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herring CA, et al. , Unsupervised Trajectory Analysis of Single-Cell RNA-Seq and Imaging Data Reveals Alternative Tuft Cell Origins in the Gut. Cell Syst, 2018. 6(1): p. 37–51.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Middelhoff M, et al. , Prox1-positive cells monitor and sustain the murine intestinal epithelial cholinergic niche. Nat Commun, 2020. 11(1): p. 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaragosi LE, Deprez M, and Barbry P, Using single-cell RNA sequencing to unravel cell lineage relationships in the respiratory tract. Biochem Soc Trans, 2020. 48(1): p. 327–336. [DOI] [PubMed] [Google Scholar]
- 20.Perniss A, et al. , Development of epithelial cholinergic chemosensory cells of the urethra and trachea of mice. Cell Tissue Res, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ualiyeva S, et al. , Airway brush cells generate cysteinyl leukotrienes through the ATP sensor P2Y2. Sci Immunol, 2020. 5(43). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fletcher RB, et al. , Deconstructing Olfactory Stem Cell Trajectories at Single-Cell Resolution. Cell Stem Cell, 2017. 20(6): p. 817–830.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sell EA, et al. , Tuft cells in the pathogenesis of chronic rhinosinusitis with nasal polyps and asthma. Ann Allergy Asthma Immunol, 2021. 126(2): p. 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bankova LG and Barrett NA, Epithelial cell function and remodeling in nasal polyposis. Ann Allergy Asthma Immunol, 2020. 124(4): p. 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rimland CA, et al. , Regional Differences in Human Biliary Tissues and Corresponding In VitroDerived Organoids. Hepatology, 2021. 73(1): p. 247–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delgiorno KE, et al. , Identification and manipulation of biliary metaplasia in pancreatic tumors. Gastroenterology, 2014. 146(1): p. 233–44 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DelGiorno KE, et al. , Tuft Cell Formation Reflects Epithelial Plasticity in Pancreatic Injury: Implications for Modeling Human Pancreatitis. Front Physiol, 2020. 11: p. 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rane CK, et al. , Development of solitary chemosensory cells in the distal lung after severe influenza injury. Am J Physiol Lung Cell Mol Physiol, 2019. 316(6): p. L1141–l1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang Y, Li W, and Chen X, P63 Deficiency and CDX2 Overexpression Lead to Barrett’s-Like Metaplasia in Mouse Esophageal Epithelium. Dig Dis Sci, 2021. 66(12): p. 4263–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saqui-Salces M, et al. , Gastric tuft cells express DCLK1 and are expanded in hyperplasia. Histochem Cell Biol, 2011. 136(2): p. 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Z, et al. , Single-Cell Transcriptomics Reveals a Conserved Metaplasia Program in Pancreatic Injury. Gastroenterology, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]; **-This study captures and sequences over 1200 pancreatitis tuft cells, generating transcriptomic signatures for all stages of formation and maturation.
- 32.Tosti L, et al. , Single nucleus and in situ RNA sequencing reveals cell topographies in the human pancreas. Gastroenterology, 2020. [DOI] [PubMed] [Google Scholar]
- 33.Haber AL, et al. , A single-cell survey of the small intestinal epithelium. Nature, 2017. 551(7680): p. 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moor AE, et al. , Spatial Reconstruction of Single Enterocytes Uncovers Broad Zonation along the Intestinal Villus Axis. Cell, 2018. 175(4): p. 1156–1167 e15. [DOI] [PubMed] [Google Scholar]
- 35.Manco R, et al. , Clump sequencing exposes the spatial expression programs of intestinal secretory cells. Nat Commun, 2021. 12(1): p. 3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grunddal KV, et al. , Adhesion receptor ADGRG2/GPR64 is in the GI-tract selectively expressed in mature intestinal tuft cells. Mol Metab, 2021. 51: p. 101231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Billipp TE, Nadjsombati MS, and von Moltke J, Tuning tuft cells: new ligands and effector functions reveal tissue-specific function. Curr Opin Immunol, 2021. 68: p. 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banerjee A, et al. , Succinate Produced by Intestinal Microbes Promotes Specification of Tuft Cells to Suppress Ileal Inflammation. Gastroenterology, 2020. 159(6): p. 2101–2115 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]; *-This paper demonstrates that induction of tuft cell expansion in models of intestinal inflammation can resolve disease.
- 39.Kotas ME, et al. , CISH constrains the tuft-ILC2 circuit to set epithelial and immune tone. Mucosal Immunol, 2021. 14(6): p. 1295–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desai P, et al. , Enteric helminth coinfection enhances host susceptibility to neurotropic flaviviruses via a tuft cell-IL-4 receptor signaling axis. Cell, 2021. 184(5): p. 1214–1231.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DelGiorno KE, et al. , Tuft Cells Inhibit Pancreatic Tumorigenesis in Mice by Producing Prostaglandin D2. Gastroenterology, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; *-This paper identifies a tumor suppressive role for pancreatic tuft cells through modulation of the microenvironment.
- 42.Oyesola OO, et al. , PGD2 and CRTH2 counteract Type 2 cytokine-elicited intestinal epithelial responses during helminth infection. J Exp Med, 2021. 218(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ualiyeva S, et al. , Tuft cell-produced cysteinyl leukotrienes and IL-25 synergistically initiate lung type 2 inflammation. Sci Immunol, 2021. 6(66): p. eabj0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang YH, et al. , POU2F3 is a master regulator of a tuft cell-like variant of small cell lung cancer. Genes Dev, 2018. 32(13–14): p. 915–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamada Y, et al. , A Tuft Cell-Like Signature Is Highly Prevalent in Thymic Squamous Cell Carcinoma and Delineates New Molecular Subsets Among the Major Lung Cancer Histotypes. J Thorac Oncol, 2021. 16(6): p. 1003–1016. [DOI] [PubMed] [Google Scholar]
- 46.Hoffman MT, et al. , The Gustatory Sensory G-Protein GNAT3 Suppresses Pancreatic Cancer Progression in Mice. Cell Mol Gastroenterol Hepatol, 2021. 11(2): p. 349–369. [DOI] [PMC free article] [PubMed] [Google Scholar]


