Abstract
The release of biosurfactants by adhering microorganisms as a defense mechanism against other colonizing strains on the same substratum surface has been described previously for probiotic bacteria in the urogenital tract, the intestines, and the oropharynx but not for microorganisms in the oral cavity. Two Streptococcus mitis strains (BA and BMS) released maximal amounts of biosurfactants when they were grown in the presence of sucrose and were harvested in the early stationary phase. The S. mitis biosurfactants reduced the surface tensions of aqueous solutions to about 30 to 40 mJ m−2. Biochemical and physicochemical analyses revealed that the biosurfactants released were glycolipids. An acid-precipitated fraction was extremely surfactive and was identified as a rhamnolipidlike compound. In a parallel-plate flow chamber, the number of Streptococcus mutans NS cells adhering to glass with and without a salivary conditioning film in the presence of biosurfactant-releasing S. mitis BA and BMS (surface coverage, 1 to 4%) was significantly reduced compared with the number of S. mutans NS cells adhering to glass in the absence of S. mitis. S. mutans NS adhesion in the presence of non-biosurfactant-releasing S. mitis BA and BMS was not reduced at all. In addition, preadsorption of isolated S. mitis biosurfactants to glass drastically reduced the adhesion of S. mutans NS cells and the strength of their bonds to glass, as shown by the increased percentage of S. mutans NS cells detached by the passage of air bubbles through the flow chamber. Preadsorption of the acid-precipitated fraction inhibited S. mutans adhesion up to 80% in a dose-responsive manner. These observations indicate that S. mitis plays a protective role in the oral cavity and protects against colonization of saliva-coated surfaces by cariogenic S. mutans.
Dental plaque is a complex, multispecies biofilm that forms on oral surfaces in which bacteria are embedded in bacterial and salivary polymers. Many species and strains have been identified in dental plaque; some of these organisms (29, 32) are now recognized as early colonizers, and others (16, 34) are considered cariogenic organisms or periodontopathogens (36). However, no real ecological role has been defined for most strains and species in dental plaque, and it is possible that these organisms should be considered part of the normal, indigenous, healthy microflora in the oral cavity.
Similarly, normal healthy microfloras have been identified for the gastrointestinal tract (31), the urogenital tract (17, 24), the skin (15), and the eyes (18). In a healthy host, a normal microflora effectively competes with invading pathogens. The mechanisms employed by the healthy microflora to interfere with the adhesion of invading pathogens include competitive exclusion (26) and displacement (20), production of antibacterial compounds, such as lactic acid, hydrogen peroxide, bacteriocins, and bacteriocin-like substances, by lactobacilli (13, 19), coaggregation (7, 25), and release of biosurfactants (8).
Recently, it has been demonstrated that biosurfactants released by lactobacilli can be adsorbed to catheter materials in order to discourage uropathogen adhesion (37). Microorganisms in certain dairy products also release biosurfactants that inhibit adhesion of yeasts, most notably to voice prostheses in the oropharynx (1). Several years ago, it was pointed out that Streptococcus mitis BMS released substances, which were later recognized as biosurfactants (33), that discourage adhesion of Streptococcus mutans (23). This observation has never been followed up on, but such a process is of considerable interest as a mechanism that could be used to prevent dental caries, since S. mutans is an important etiological agent of coronal (16, 34) and root surface caries (30).
The aim of this study was twofold: (i) to determine the surface tensions of biosurfactant solutions and their chemical characteristics when they are released by two oral S. mitis strains grown on different carbohydrate sources and (ii) to determine the effect of biosurfactant-releasing S. mitis cells adhering to an artificial (glass) substratum with and without a salivary conditioning film on the subsequent adhesion of a S. mutans strain. In addition, surface properties of S. mitis cells before and after biosurfactants were released were measured in order to rule out the possibility that the compounds released were cell surface compounds whose release affected the adhesive properties of the cell surface.
MATERIALS AND METHODS
Microorganisms.
S. mutans NS and S. mitis BA and BMS were originally isolated from the human oral cavity and were stored in Todd-Hewitt broth (THB) (Oxoid, Basingstoke, England) supplemented with 0.5% sucrose and 7% (vol/vol) dimethyl sulfoxide at −60°C. Streptococci from the frozen stock preparations were streaked every 2 weeks onto blood agar plates and incubated at 37°C. After 2 days the plates were stored at 5°C.
For adhesion assays and surface characterization studies the bacteria from a blood agar plate were grown overnight at 37°C in 10 ml of THB supplemented with 0.5% sucrose. The resulting culture was used to inoculate a second culture, which was grown for 16 h and then harvested by centrifugation at 4,000 × g, washed twice with adhesion buffer (2 mM potassium phosphate, 50 mM potassium chloride, 1 mM calcium chloride; pH 6.8), and resuspended in adhesion buffer.
To break bacterial chains, a bacterial suspension was sonicated five times for 10 s at 30 W (model 375 Vibra Cell sonicator; Sonics and Materials Inc., Danbury, Conn.). Sonication was performed intermittently while the preparation was cooling in an ice-water bath. Finally, the cells were suspended in adhesion buffer. The cell concentration was fixed at 3 × 108 cells ml of buffer−1 by using a Bürker-Türk counting chamber.
Biosurfactant release.
To release biosurfactant from S. mitis BA and BMS, subcultures (10 ml) from blood agar plates were prepared by inoculating THB supplemented with 0.5% (wt/vol) glucose, 0.5% (wt/vol) glycerol, 0.5% (wt/vol) galactose, or 0.5% (wt/vol) sucrose and incubating the preparations overnight at 37°C.
An overnight subculture was used to inoculate 1,400 ml of a second culture. Cells were harvested in the mid-exponential, early-stationary, and stationary phases by centrifugation at 4,000 × g, washed twice in adhesion buffer, and resuspended in 200 ml of water. Crude biosurfactant was produced by gently stirring the suspension for 2 h at room temperature. Subsequently, the microorganisms and the biosurfactants released were separated by centrifugation at 10,000 × g. To ensure that all of the cell remnants were removed, the supernatant was centrifuged twice at 10,000 × g. After the final centrifugation, both the cellular pellet and the crude biosurfactant were freeze-dried and weighed, and the crude biosurfactant was stored at −20°C until it was used.
In a separate experiment, the freeze-dried crude biosurfactant of S. mitis BMS was resuspended in water and subsequently acid precipitated with concentrated HCl at pH 2.0. After the supernatant was decanted, the precipitate was washed twice with acidic water (pH 2) and collected by centrifugation at 4,000 × g. After the acid precipitate was redissolved, it was freeze-dried. The supernatant was adjusted to pH 7 with KOH and also freeze-dried.
Surface tension measurements.
Axisymmetric drop shape analysis by profile (ADSA-P) was performed as described by Noordmans and Busscher (22) in order to determine the surface tensions of biosurfactant solutions. Briefly, ADSA-P involves digitizing the circumference of a liquid droplet on a solid surface. The circumference of the droplet is fitted to the Laplace equation of capillarity (27), which yields the surface tension of the biosurfactant solution. Droplets containing biosurfactant dissolved in water (volume, approximately 100 μl) were placed on a clean piece of fluoroethylenepropylene (Teflon). Measurements for one solution droplet were obtained after 2 h in order to allow equilibration of the interface in an enclosed chamber at room temperature. One liquid profile was recorded twice with a minimal time interval (<0.5 s) between measurements, and the ADSA-P surface tensions were averaged. This procedure was performed in duplicate with separate liquid droplets.
ADSA-P surface tension measurements were obtained for crude biosurfactant solutions in water as a function of biosurfactant concentration, as well as for the freeze-dried acid-precipitated fraction and supernatant of crude S. mitis BMS biosurfactant.
Biochemical assay.
The protein content of the crude biosurfactant was determined by the Bio-Rad protein assay; bovine albumin was used as the standard.
XPS.
For X-ray photoelectron spectroscopy (XPS), 100-μl droplets of crude stationary-phase biosurfactants and the acid-precipitated fraction dissolved in water (approximately 10 mg ml−1) were placed on gold-coated glass slides (1 by 1 cm). After air drying, the glass slides were inserted into the chamber of a spectrometer (Surface Science Instruments, S-probe, Mountain View, Calif.). The residual pressure in the spectrometer during operation was approximately 10−9 Pa. A magnesium anode was used to produce X-rays (10 kV, 22 mA) with a spot size of 250 by 1,000 μm. After scans of the overall spectrum in the binding energy range from 1 to 1,200 eV at low resolution (150-eV pass energy) were obtained, peaks over a 20-eV binding energy range were recorded at high resolution (50-eV pass energy) in the following order: C1s (four scans), O1s (four scans), N1s (eight scans), P2p (eight scans), and C1s again in order to account for contamination or deterioration under X rays of the samples.
The carbon peak was split by a least-squares fitting program into four Gaussian components at 284.8, 286.2, 287.8, and 289.2 eV by imposing a constant full width at a half-maximum of 1.35 eV, and these components were thought to be representative of carbon involved in C—C, C—O and C—N, C⩵O and O—C—O, and O⩵C—OH bonds, respectively. The oxygen peak was split into two components at 531.0 and 532.4 eV by imposing a constant full width at a half-maximum of 1.70 eV, and these components were thought to be representative of oxygen involved in O⩵C and C—O bonds, respectively.
Cell surface properties.
The bacterial cell surface hydrophobicity of the S. mitis strains was assessed by measuring water contact angles on bacterial lawns (35) on membrane filters (pore diameter, 0.45 μm). Wet filters with deposited organisms were fixed on sample holder plates with double-sided sticky tape and air dried. Water contact angles were measured after 20 min by using image analysis techniques at 25°C and sessile droplets of water. At least three different filters containing samples from separate cultures were prepared.
The zeta potentials of the S. mitis strains were determined in adhesion buffer from the speed of suspended bacteria in a 150-V applied electric field by using the Helmholtz-Smoluchowski equation (12). The instrument used, a model 501 Lazer Zee meter (PenKem, Bedford Hills, N.Y.), was equipped with an image analysis option for tracking and zeta sizing (38).
Saliva.
Human whole saliva was collected from 10 healthy volunteers of both sexes in ice-chilled cups. Saliva production by the volunteers was stimulated by chewing Parafilm (3M Company, Minneapolis, Minn.). After the saliva was pooled and centrifuged at 10,000 × g for 5 min at 10°C, phenylmethylsulfonyl fluoride (0.2 M; Merck, Darmstadt, Germany) was added to a final concentration of 1 mM as a protease inhibitor. The solution was centrifuged again, dialyzed overnight at 4°C against water, and freeze-dried for storage. A 1.5-mg ml−1 solution of freeze-dried stock in adhesion buffer (see above) was designated reconstituted human whole saliva.
Adhesion experiments.
The flow chamber (length, 7.6 cm; width, 3.8 cm; height, 0.06 cm) and image analysis system used have been described in detail previously (28). Images were obtained from the bottom glass plate (5.8 by 3.8 cm) of the parallel-plate flow chamber. The top plate of the chamber was also made of glass.
Prior to each experiment, all tubes and the flow chamber were filled with adhesion buffer, and care was taken to remove air bubbles from the system. Flasks containing microbial suspensions, buffer, and saliva when appropriate were placed at the same height with respect to the chamber, so that immediately after the flow was switched, all of the fluids would circulate through the chamber under the influence of hydrostatic pressure at a shear rate of 20 s−1 (0.05 ml s−1; well within the limits of laminar flow), which represented a shear rate between the shear rates exerted by stimulated and unstimulated salivary flow at the level of the tooth surface (4). First, the flow was switched to saliva (when appropriate) for 1.5 h in order to create a salivary conditioning film, and then the flow was switched for 15 min to buffer to remove all remnants of saliva from the tubing and the flow chamber. After this, the flow was switched (when appropriate) to an S. mitis BMS or BA suspension until the desired surface coverage by adhering S. mitis BMS or BA cells (between 1 and 4%) was attained, as measured in real time with the image analysis system. In the experiments carried out to determine the effects of preadsorbed biosurfactants, biosurfactants in a 20-mg ml−1 solution were adsorbed overnight to the glass plate. Between flow steps, buffer was run through the system to remove unbound material from the tubes and chamber. Finally, a S. mutans suspension was circulated through the system for 4 h.
The initial increase in the number of adhering S. mutans NS cells with time was expressed by a so-called initial deposition rate; this rate was the number of microorganisms that adhered initially per unit of time and area. The number of bacteria adhering after 4 h was considered an estimate of microbial adhesion at a more advanced stage of the adhesion process.
Finally, after 4 h, air bubbles were passed through the chamber in order to obtain an indication of the adhesive forces (14). The passage of an air-liquid interface (i.e., an air bubble) over adhering micron-sized particles is accompanied by a detachment force of about 10−7 N per adhering microorganism. After the passage of each air bubble, the mean percentage of bacteria detached by the high removal force was determined at five different spots on the substratum surface with respect to the mean number of organisms adhering at five spots prior to the introduction of the first air bubble.
All adhesion experiments were performed in triplicate with separately cultured organisms at room temperature.
RESULTS
Table 1 shows the amounts of biosurfactant released by S. mitis BA and BMS grown on different carbohydrates in various growth phases. The amount of biosurfactant released per gram of dry cell weight was largest for bacteria in the mid-exponential growth phase and decreased as the organisms entered the stationary growth phase. Adding glycerol to the medium increased the biosurfactant yield of S. mitis BMS in the stationary phase compared to the other carbohydrates used. S. mitis BA in the stationary growth phase released similar amounts of biosurfactants irrespective of the carbohydrate present.
TABLE 1.
Cell dry weights and amounts of biosurfactant released in different growth phases by S. mitis strains grown in media supplemented with different carbohydrate sources, expressed as averages of data from three experiments performed with separately cultured bacteriaa
| Strain | Growth phase | Carbohydrate source | Cell dry wt (g liter of medium−1) | Biosurfactant yield (mg g−1) |
|---|---|---|---|---|
| S. mitis BA | Stationary | Glucose | 0.50 | 36 |
| Glycerol | 0.25 | 32 | ||
| Galactose | 0.38 | 26 | ||
| Sucrose | 0.58 | 31 | ||
| Early stationary | Sucrose | 0.50 | 52 | |
| Mid-exponential | Sucrose | 0.08 | 125 | |
| S. mitis BMS | Stationary | Glucose | 0.43 | 28 |
| Glycerol | 0.18 | 56 | ||
| Galactose | 0.35 | 37 | ||
| Sucrose | 0.40 | 30 | ||
| Early stationary | Sucrose | 0.38 | 42 | |
| Mid-exponential | Sucrose | 0.10 | 70 |
In all cases the standard deviation was less than 15%.
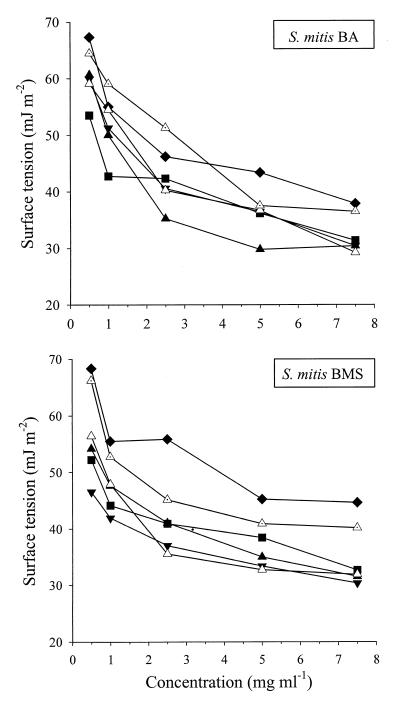
Figure 1 shows that the S. mitis biosurfactants could reduce the surface tension of an aqueous solution to around 30 to 40 mJ m−2 irrespective of the growth phase or the carbohydrate present. When glucose was used as the carbohydrate, the surface activity of the compounds released was less than the surface activities after growth on the other carbohydrates used.
FIG. 1.
Plots of surface tensions of biosurfactant solutions in water versus concentrations of freeze-dried biosurfactants released by S. mitis BA and BMS grown on medium supplemented with glucose (⧫), glycerol (■), galactose (▾), or sucrose (▴, ▵, and ◬) and harvested in the mid-exponential phase (◬), early-stationary phase (▵), and stationary phase (⧫, ■, ▾, and ▴). The results are averages of data obtained from three separately grown cultures with standard deviations of less than 10%.
The protein contents of the biosurfactants released by both S. mitis BA and BMS were extremely low (less than 1%). Also, XPS physicochemical analyses (Table 2) revealed that the biosurfactants contained little nitrogen (the N/C ratios were between 0.101 and 0.172) compared with protein reference compounds (the N/C ratio is 0.270 for the average protein). The nitrogen contents of the biosurfactants were too high, however, for association of the biosurfactants with lipoteichoic acid, which was also ruled out by relatively low oxygen and phosphorus contents. The absence of proteins and lipoteichoic acids in the biosurfactants was confirmed by the low fractions of carbon doubly bound to oxygen (C⩵O). C—(C,H) functionalities were abundant in the biosurfactants released by the S. mitis strains, as they are in rhamnolipids.
TABLE 2.
Chemical composition data (as determined by XPS) for S. mitis BMS and BA biosurfactants released in the stationary growth phase and for reference compounds
| Crude biosurfactant or compound | Elemental concn ratios
|
Fractions of carbon and oxygen involved in various chemical functions
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N/C | O/C | P/C | C—(C,H) | C—(O,N) | C⩵O | O⩵C—OH | (O⩵C)/C | (OH)/C | |
| Biosurfactant released by: | |||||||||
| S. mitis BA | 0.172 | 0.407 | 0.019 | 0.58 | 0.24 | 0.18 | 0 | 0.342 | 0.065 |
| S. mitis BMS | 0.101 | 0.426 | 0.016 | 0.64 | 0.20 | 0.13 | 0.04 | 0.268 | 0.158 |
| Acid-precipitated S. mitis BMS biosurfactant | 0.071 | 0.366 | 0.016 | 0.61 | 0.24 | 0.11 | 0.05 | 0.121 | 0.244 |
| Reference compounds | |||||||||
| Proteina | 0.270 | 0.32 | 0 | 0.41 | 0.32 | 0.28f | 0.275 | 0.045 | |
| Glycosidic residueb | 0 | 0.83 | 0 | 0 | 0.83 | 0.17f | 0 | 0.833 | |
| Rhamnolipid R1c | 0 | 0.406 | 0 | 0.56 | 0.31 | 0.06 | 0.06 | 0.061 | 0.345 |
| Rhamnolipid R2c | 0 | 0.346 | 0 | 0.65 | 0.23 | 0.04 | 0.08 | 0.076 | 0.269 |
| Phospholipidd | 0.009 | 0.21 | 0.034 | 0.82 | 0.13 | 0.05 | 0.01 | 0.077 | 0.132 |
| LTAe | 0.031 | 0.63 | 0.066 | 0.41 | 0.44 | 0.44 | 0.10 | 0.151 | 0.478 |
Average protein, calculated based on a collection of bacterial, fungal, and mammalian proteins (21).
Glycosidic residue C6H10O5 (21).
Calculated for rhamnolipids synthesized by Pseudomonas aeruginosa. R1 contains two rhamnose units, and R2 contains one rhamnose unit (6, 11).
l-α-Phosphatidyl-dl-glycerol dimyristoyl.
LTA, Lipoteichoic acid from S. mutans (Sigma).
C⩵O and O⩵C—OH together.
Table 3 shows the adhesion of S. mutans NS in the presence of adhering, biosurfactant-releasing S. mitis strains grown on sucrose. As Table 3 shows, when the surface coverage by S. mitis BMS on bare glass was up to 4%, the initial deposition rate of S. mutans NS was reduced from 1,043 to 394 cm−2 s−1. Also, the number of S. mutans cells adhering after 4 h decreased from 130 × 105 to 50 × 105 cells cm−2. The effects of biosurfactant-releasing S. mitis BA on S. mutans NS adhesion were far less obvious than the effects of S. mitis BMS. Whereas surface coverage by S. mitis BA of up to 2% resulted in similar or sometimes even greater reductions in S. mutans adhesion than the reductions observed in the presence of S. mitis BMS, the effect of S. mitis BA on S. mutans adhesion completely disappeared when the surface coverage was 4%. Finally, S. mitis BA and BMS biosurfactants decreased the strength of S. mutans NS bonds to glass, as the percentage of adhering S. mutans cells that were detached by air bubble passage increased as the surface coverage by the S. mitis strains increased.
TABLE 3.
Adhesion of S. mutans NS to glass in competition with adhering biosurfactant-releasing and nonreleasing S. mitis BA and BMS and to glass with preadsorbed stationary-phase S. mitis BA and BMS biosurfactants
| Prepn | Surface coverage | Initial deposition rate (cm−2 s−1) | No. of bacteria adhering after 4 h (105 cells cm−2) | Detachment due to air bubbles (%)a
|
||
|---|---|---|---|---|---|---|
| First bubble | Second bubble | Third bubble | ||||
| Glass without salivary conditioning film | None | 1,043 ± 160b | 130 ± 18 | 14 ± 16 | 20 ± 11 | 32 ± 9 |
| 1% S. mitis BA | 798 ± 148 | 111 ± 5 | 0 ± 10 | 5 ± 16 | 0 ± 16 | |
| 2% S. mitis BA | 577 ± 331 | 73 ± 43 | 0 ± 19 | 7 ± 12 | 19 ± 20 | |
| 4% S. mitis BA | 1,096 ± 127 | 100 ± 4 | 5 ± 12 | 27 ± 13 | 47 ± 19 | |
| S. mitis BA biosurfactant alone | 48 ± 49 | 4 ± 3 | 47 ± 8 | 74 ± 9 | 67 ± 16 | |
| 4% S. mitis BA−c | 977 ± 279 | 111 ± 26 | 1 ± 6 | 8 ± 18 | 11 ± 9 | |
| 1% S. mitis BMS | 774 ± 65 | 100 ± 15 | 15 ± 13 | 16 ± 20 | 20 ± 15 | |
| 2% S. mitis BMS | 895 ± 293 | 92 ± 21 | 42 ± 19 | 17 ± 17 | 39 ± 50 | |
| 4% S. mitis BMS | 394 ± 83 | 50 ± 11 | 45 ± 21 | 35 ± 15 | 43 ± 9 | |
| S. mitis BMS biosurfactant alone | 106 ± 37 | 9 ± 4 | 59 ± 11 | 57 ± 21 | 68 ± 16 | |
| 4% S. mitis BMS− | 789 ± 168 | 113 ± 28 | 12 ± 25 | 0 ± 11 | 0 ± 15 | |
| Glass with salivary conditioning film | None | 965 ± 100 | 83 ± 9 | 72 ± 20 | 70 ± 22 | 56 ± 39 |
| 1% S. mitis BA | 495 ± 331 | 37 ± 11 | 72 ± 19 | 77 ± 15 | 77 ± 21 | |
| 1% S. mitis BMS | 657 ± 277 | 49 ± 20 | 72 ± 15 | 92 ± 5 | 91 ± 7 | |
| 2% S. mitis BMS | 573 ± 262 | 43 ± 19 | 70 ± 22 | 78 ± 9 | 82 ± 12 | |
| 4% S. mitis BMS | 349 ± 35 | 30 ± 8 | 60 ± 16 | 67 ± 8 | 61 ± 18 | |
Total percentages of S. mutans NS cells that were detached by the passage of three consecutive air bubbles through the flow chamber.
The data are means ± standard deviations based on data from three experiments performed with separately cultured cells.
The minus sign denotes nonreleasing cells.
In order to determine whether the decreases in S. mutans NS adhesion in the presence of adhering S. mitis strains were due to simple geometrical effects or to the biosurfactants released by the adhering S. mitis strains, we performed experiments with two non-biosurfactant-releasing preparations, S. mitis BA− and BMS−. Nonreleasing bacteria were prepared by allowing the S. mitis strains to release their biosurfactants overnight; after this they were used in adhesion experiments with S. mutans NS. Using ADSA-P surface tension measurements, we determined that the BA− and BMS− bacteria had lost the ability to reduce the surface tension of an aqueous suspension (Δγ = 0 and 2 mJ m−2, respectively) and thus their ability to release biosurfactants. Furthermore, we observed no significant changes in the cell surface hydrophobicities and zeta potentials of the organisms that might affect S. mutans adhesion. The water contact angle on S. mitis BMS was 99° before and after the biosurfactant was released, while the water contact angle on S. mitis BA was 104°. Similarly, the zeta potential of S. mitis BMS in adhesion buffer was not altered by biosurfactant release; it remained −9 mV. For S. mitis BA, there was an insignificant change in the bacterial zeta potential from −8 to −5 mV that occurred when the biosurfactant was released.
Table 3 shows that there was not a significant difference between adhesion of S. mutans NS in the presence of adhering, nonreleasing S. mitis BA− and BMS− (surface coverage, 4%) and adhesion to bare glass. Table 3 also shows that preadsorption of crude biosurfactants released by S. mitis BA, as well as by S. mitis BMS, significantly reduced the adhesion of S. mutans to glass. The initial deposition rates and the numbers of S. mutans cells adhering after 4 h to biosurfactant-coated glass were almost 10-fold lower in the presence of S. mitis BMS biosurfactant and more than 20-fold lower in the presence of S. mitis BA biosurfactant. Also, detachment of adhering S. mutans cells by air bubble passage increased drastically in the presence of preadsorbed crude biosurfactants.
The acid-precipitated fraction of S. mitis BMS biosurfactant was extremely surface active compared with the crude biosurfactant, and an aqueous solution of the acid precipitate containing only 1 mg ml−1 had a surface tension of 35 mJ m−2 (compare with Fig. 1) while an aqueous solution of the freeze-dried supernatant (the supernatant left after acid precipitation) hardly decreased the surface tension of water in the same concentration range. Moreover, substratum coverage by adsorption of the acid-precipitated fraction from an aqueous solution (between 0.1 and 1 mg ml−1) resulted in up to 80% inhibition of S. mutans NS adhesion in a dose-responsive manner. XPS analysis of the acid-precipitated fraction revealed a reduced nitrogen content compared to the crude biosurfactant (Table 2), while the O/C elemental concentration ratio was between the ratios for rhamnolipids R1 and R2.
Table 3 also shows the adhesion of S. mutans NS to glass with a salivary conditioning film in the presence of stationary-phase biosurfactant-releasing S. mitis BA and BMS grown on sucrose. This table shows that biosurfactant-releasing S. mitis strains interfered not only with S. mutans adhesion to glass but also with S. mutans adhesion to salivary conditioning films.
DISCUSSION
In this study we found that release of biosurfactants by adhering S. mitis strains interferes with adhesion of a cariogenic S. mutans strain. A chemical characterization study suggested that glycolipids were present in the crude biosurfactant, while further purification revealed that the active component was rhamnolipidlike. It was found previously that S. mitis ATCC 9811 released extracellular substances that were surface active (10, 33). However, these extracellular substances were identified as an exohemagglutinin, a lectinlike substance that is responsible for interaction with saliva (10).
The most commonly isolated biosurfactants are glycolipids (e.g., rhamnolipids produced by Pseudomonas aeruginosa [9]) and lipopeptides (e.g., surfactin released by Bacillus subtilis [3]). The yields of both of these types of biosurfactants are relatively high (approximately 2.5 g liter of medium−1), and these biosurfactants reduce the surface tension of spent culture supernatant to less than 30 mJ m−2. Streptococcus thermophilus B (2) and Lactobacillus species (37) are also biosurfactant-releasing strains. The biosurfactants of these organisms decrease the surface tension of water to around 37 mJ m−2, but the amounts released per liter of culture medium were orders of magnitude smaller than the amounts of the rhamnolipids and lipopeptides released (approximately 100 mg liter−1 for Lactobacillus species and 20 mg liter−1 for S. thermophilus B). A simple calculation revealed that small amounts of biosurfactants may have substantial effects on adhesion to substratum surfaces. If it is assumed that the biosurfactant is a small molecule with a molecular weight of about 1,000, it can be estimated based on a biosurfactant yield of around 10−8 mg per cell that a substratum surface coverage value of around 8% for biosurfactant-releasing S. mitis BA or BMS cells results in 100% coverage of the surface by biosurfactants (2). Indeed, the adhesion experiments performed with S. mitis BA and BMS grown on medium supplemented with sucrose showed that the presence of biosurfactant-releasing S. mitis BA and BMS cells on glass and on glass with a salivary conditioning film effectively reduced the adhesion of S. mutans NS even with the influence of geometrical effects (i.e., physical collisions between suspended and adhering organisms which resulted in increased deposition) (Table 3). Moreover, preadsorption of the biosurfactants to glass also drastically reduced S. mutans NS adhesion.
With regard to the oral cavity, the increased detachment of adhering S. mutans cells in the presence of biosurfactants after air bubble passage is very important, as air-liquid interfaces frequently pass over the enamel surface and the adhering microorganisms during eating and swallowing (i.e., adhering microorganisms are exposed to high detachment forces). Our results indicate that even if S. mutans NS adheres to bare surfaces or to a salivary conditioning film, biosurfactants effectively stimulate detachment of this organism by the dynamic shear forces that occur in the oral cavity.
In conclusion, adhering S. mitis BA and BMS cells decrease the adhesion of S. mutans NS to glass and to glass with a salivary conditioning film through the release of rhamnolipidlike biosurfactants. Thus, we propose that S. mitis plays an ecological role in the oral cavity.
REFERENCES
- 1.Busscher H J, Bruinsma G, van Weissenbruch R, Leunisse C, van der Mei H C, Dijk F, Albers F W J. The effect of buttermilk consumption on biofilm formation on silicone rubber prostheses in an artificial throat. Eur Arch Otorhinolaryngol. 1998;255:410–413. doi: 10.1007/s004050050088. [DOI] [PubMed] [Google Scholar]
- 2.Busscher H J, van Hoogmoed C G, Geertsema-Doornbusch G I, van der Kuijl-Booij M, van der Mei H C. Streptococcus thermophilus and its biosurfactants inhibit adhesion by Candida spp. on silicone rubber. Appl Environ Microbiol. 1997;63:3810–3817. doi: 10.1128/aem.63.10.3810-3817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper D G, MacDonald C R, Duff J J B, Kosaric W. Enhanced production of surfactin from Bacillus subtilis by continuous product removal and metal cation addition. Appl Environ Microbiol. 1981;42:408–412. doi: 10.1128/aem.42.3.408-412.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawes C, Watanabe S, Biglow-Lecomte P, Dibdin G H. Estimation of the velocity of the salivary film at some different locations in the mouth. J Dent Res. 1989;68:1479–1482. doi: 10.1177/00220345890680110201. [DOI] [PubMed] [Google Scholar]
- 5.Desai J D, Desai A J. Production of biosurfactants. In: Kosaric N, editor. Biosurfactants. Production, properties, applications. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 65–97. [Google Scholar]
- 6.Edwards J, Hayashy J A. Structure of a rhamnolipid from Pseudomonas aeruginosa. Arch Biochem Biophys. 1965;111:415–421. doi: 10.1016/0003-9861(65)90204-3. [DOI] [PubMed] [Google Scholar]
- 7.Ganeshkumar N, Hughes C V, Weiss E I. Coaggregation in dental plaque formation. In: Busscher H J, Evans L V, editors. Oral biofilms and plaque control. India: Harwood Academic Publishers; 1998. pp. 125–144. [Google Scholar]
- 8.Gerson D F. The biophysics of microbial surfactants: growth on insoluble substrates. In: Kosaric N, editor. Biosurfactants. Production, properties, applications. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 269–286. [Google Scholar]
- 9.Hisatsuka K, Nakahara F, Sano N, Yamada K. Formation of rhamnolipid by Pseudomonas aeruginosa and its function in hydrocarbon fermentation. Agric Biol Chem. 1971;33:686–692. [Google Scholar]
- 10.Hsieh C C, Iwakura K, Takagaki M, Shibata S. Exohemagglutinin derived from Streptococcus mitis ATCC9811. J Osaka Univ Dent Sch. 1984;24:67–76. [PubMed] [Google Scholar]
- 11.Itoh S, Suzuki T. Effect of rhamnolipids on growth of Pseudomonas aeruginosa mutant deficient in n-paraffin utilizing ability. Agric Biol Chem. 1972;36:2233–2235. [Google Scholar]
- 12.James A M. Electrophoresis of particles in suspension. Surf Colloid Sci. 1979;11:121–185. [Google Scholar]
- 13.Klaenhammer T R. Bacteriocins of lactic acid bacteria. Biochimie. 1988;70:337–349. doi: 10.1016/0300-9084(88)90206-4. [DOI] [PubMed] [Google Scholar]
- 14.Leenaars A F M. A new approach to the removal of sub-micron particles from solid (silicon) substrates. In: Mittal K L, editor. Particles on surfaces: detection, adhesion and removal. New York, N.Y: Plenum Press; 1989. pp. 361–372. [Google Scholar]
- 15.Leyden J J, McGinley K J, Nordstrom K M, Webster G F. Skin microflora. J Invest Dermatol. 1987;88:65s–72s. doi: 10.1111/1523-1747.ep12468965. [DOI] [PubMed] [Google Scholar]
- 16.Loesche W J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrie T J, Swantee C A, Hartlen M. Aerobic and anaerobic urethral flora of healthy females in various physiological age groups and of females with urinary tract infections. J Clin Microbiol. 1980;11:654–659. doi: 10.1128/jcm.11.6.654-659.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McBride M E. Evaluation of microbial flora of the eye during wear of soft contact lenses. Appl Environ Microbiol. 1979;37:233–236. doi: 10.1128/aem.37.2.233-236.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGroarty J A, Reid G. Detection of a Lactobacillus substance that inhibits Escherichia coli. Can J Microbiol. 1988;34:974–978. doi: 10.1139/m88-171. [DOI] [PubMed] [Google Scholar]
- 20.Millsap K W, Reid G, van der Mei H C, Busscher H J. Displacement of Enterococcus faecalis from hydrophobic and hydrophilic substrata by Lactobacillus and Streptococcus spp. as studied in a parallel plate flow chamber. Appl Environ Microbiol. 1994;60:1867–1874. doi: 10.1128/aem.60.6.1867-1874.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mozes N, Lortal S. X-ray photoelectron spectroscopy and biochemical analysis of the surface of Lactobacillus helveticus ATCC 12046. Microbiology. 1995;141:11–19. [Google Scholar]
- 22.Noordmans J, Busscher H J. The influence of the droplet volume and contact angle on liquid surface tension measurements by axisymmetric drop shape analysis-profile. (ADSA-P) Colloids Surf. 1991;58:239–249. [Google Scholar]
- 23.Pratt-Terpstra I H, Weerkamp A H, Busscher H J. Microbial factors in a thermodynamic approach of oral streptococcal adhesion to solid substrata. J Colloid Interface Sci. 1989;129:568–574. [Google Scholar]
- 24.Redondo-Lopez V, Cook R L, Sobel J D. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis. 1990;12:856–872. doi: 10.1093/clinids/12.5.856. [DOI] [PubMed] [Google Scholar]
- 25.Reid G, McGroarty J A, Angotti R, Cook R L. Lactobacillus inhibitor production against Escherichia coli and coaggregation ability with uropathogens. Can J Microbiol. 1988;34:344–351. doi: 10.1139/m88-063. [DOI] [PubMed] [Google Scholar]
- 26.Reid G, Tieszer C. Preferential adhesion of urethral bacteria from a mixed population to a urinary catheter. Cells Mater. 1993;3:171–176. [Google Scholar]
- 27.Rotenberg Y, Boruvka L, Neumann A W. Determination of surface tension and contact angle from the shape of axisymmetric fluid interfaces. J Colloid Interface Sci. 1983;93:169–183. [Google Scholar]
- 28.Sjollema J, Busscher H J, Weerkamp A H. Real-time enumeration of adhering microorganisms in a parallel plate flow cell using automated image analysis. J Microbiol Methods. 1989;9:73–78. [Google Scholar]
- 29.Socransky S S, Manganielo A D, Propas D, Oram V, van Houte J. Bacteriological studies of developing supragingival dental plaque. J Periodontal Res. 1977;12:90–106. doi: 10.1111/j.1600-0765.1977.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 30.Syed S A, Loesche W J, Pape H L, Jr, Grenier E. Predominant cultivable flora isolated from human root surface caries plaque. Infect Immun. 1975;11:727–731. doi: 10.1128/iai.11.4.727-731.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tannock G W. The microecology of lactobacilli inhabiting the gastrointestinal tract. Adv Microb Ecol. 1990;11:147–171. [Google Scholar]
- 32.Theilade E, Theilade J, Mikkelsen L. Microbiological studies on early dento-gingival plaque on teeth and mylar strips in humans. J Periodontal Res. 1982;17:12–25. doi: 10.1111/j.1600-0765.1982.tb01127.x. [DOI] [PubMed] [Google Scholar]
- 33.Van der Vegt W, van der Mei H C, Noordmans J, Busscher H J. Assessment of bacterial biosurfactant production through axisymmetric drop shape analysis by profile. Appl Microbiol Biotechnol. 1991;35:766–770. [Google Scholar]
- 34.Van Houte J. Bacterial specificity in the etiology of dental caries. Int Dent J. 1980;30:305–326. [PubMed] [Google Scholar]
- 35.Van Oss C J, Gillman C F. Phagocytosis as a surface phenomenon. I. Contact angles and phagocytosis of non-opsonized bacteria. J Reticuloendothel Soc. 1972;12:283–292. [PubMed] [Google Scholar]
- 36.Van Palenstein-Helderman W H. Microbial aetiology of periodontal disease. J Clin Periodontol. 1981;8:261–280. doi: 10.1111/j.1600-051x.1981.tb02038.x. [DOI] [PubMed] [Google Scholar]
- 37.Velraeds M M C, van der Mei H C, Reid G, Busscher H J. Inhibition of initial adhesion of uropathogenic Enterococcus faecalis by biosurfactants from Lactobacillus isolates. Appl Environ Microbiol. 1996;62:1958–1963. doi: 10.1128/aem.62.6.1958-1963.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wit P J, Noordmans J, Busscher H J. Tracking of colloidal particles using microscopic image sequence analysis. Application to particulate microelectrophoresis and particle deposition. Colloids Surf A Physicochem Eng Aspects. 1997;125:85–92. [Google Scholar]