Abstract
Inflammatory bowel disease (IBD) refers to disorders involving chronic inflammation of the gastrointestinal tract. Well-established treatments for IBD have not yet to be suggested. To address this gap, we investigated the effects of co-administration of Lactobacillus gasseri (L. gasseri) KBL697 and infliximab (IFX), the first approved tumor necrosis factor (TNF)-alpha inhibitor, on the dextran sodium sulfate-induced colitis mouse model. 2 × 109 colony-forming units/g of L. gasseri KBL697 were administered to seven-week-old female C57BL/6J mice daily by oral gavage. On day three, IFX (5 mg/kg) suspended in 1 × PBS (200 µL) was intravenously injected in the IFX-treated group and all mice were sacrificed on day nine. Co-administration of L. gasseri KBL697 and IFX improved colitis symptoms in mice, including body weight, disease activity index, colon length, and histology score. Additionally, pro-inflammatory cytokines, such as interferon-gamma, interleukin (IL)-2, IL-6, IL-17A, and TNF were significantly decreased, while IL-10, an anti-inflammatory cytokine, was increased. Expression levels of tight junction genes and CD4 + CD25 + Foxp3 + T regulatory cells in the mesenteric lymph nodes were synergistically upregulated with the combined treatment. Furthermore, co-administered mice displayed altered cecum microbial diversity and composition with increases in the genus Prevotella. Related changes in the predicted amino and nucleic acid metabolic pathways were also evident, along with increased acetate and butyrate level. Therefore, the synergistic effect of L. gasseri KBL697 and IFX co-administration is a possible method of prevention and treatment for IBD.
Subject terms: Immunology, Microbiology, Health care
Introduction
Inflammatory bowel disease (IBD) is an umbrella term describing chronic inflammatory disorders in the gastrointestinal tract1. Well-established treatments for IBD have not yet to be suggested2. Recent evidence has indicated that disruption of the homeostasis between the mucosal immune system in gut and gut microbiota correlates with increased susceptibility to IBD3. Gut microbiota can affect integrity of the gut mucosal barrier and immunomodulation of host4,5. Additionally, short-chain fatty acids (SCFAs) synthesized by gut microbiota have key roles in adjusting host metabolism and regulating immune response6. Previous studies have suggested that changes in bacterial composition of the gut can lead inflammation7.
Tumor necrosis factor-alpha (TNF-α), an important pro-inflammatory cytokine, is a major immune-cell activator and involved in immunopathology of IBD8. Therefore, inhibition of TNF-α has been suggested as a novel treatment for IBD. For example, infliximab (IFX), a chimeric mouse–human monoclonal antibody, can effectively neutralize human TNF-α and has been used in alleviating IBD symptoms9. Another suggested treatment for IBD is probiotics. Probiotics have been shown to be useful in the prevention and treatment for various acute and chronic human diseases10. Probiotics can interact with the intestinal epithelium and modulate immune system of host11–13. Previous studies have been especially suggested that Lactobacillus spp. can alleviate IBD symptoms via modulation of gut microbiota compositions, metabolic pathways and immune systems of host12,14.
Tight junction proteins have important roles for gut permeability. During the colon inflammation, the expression of tight junction proteins can be altered and epithelial mucosa is disrupted, which can cause invasion of pathogens15,16. Lactobacillus spp. can affect the improvement of mucus layer through the recovery of intestinal microbiota17,18. Moreover, our previous studies have been suggested that administration of various isolates of Lactobacillus spp., including Lactobacillus fermentum and Lactobacillus paracasei, have strong immunomodulation effects, can improve tight junction protein expression and maintain the diversity of gut microbiota in the colitis-induced mice4,11.
Therefore, in this study, we investigated the effect of co-administration of Lactobacillus gasseri (L. gasseri) KBL697, isolated from the vaginal samples of healthy donors, and IFX for treatment in dextran sodium sulfate (DSS)-induced colitis mouse model. First, we induced the acute colitis in mice and co-treated L. gasseri KBL697 and IFX. We assessed amelioration of IBD symptoms and abnormal immune responses due to L. gasseri KBL697 and IFX. Moreover, we confirmed microbial compositions and their predicted metabolic pathways in colon of mice with colitis due to co-treatment of L. gasseri KBL697 and IFX. We also evaluated the levels of acetate and butyrate, important SCFAs produced by genus Lactobacillus, to elucidate of possible mechanisms of co-administration of L. gasseri KBL697 and IFX for IBD treatment.
Methods
Bacteria strain preparation
Lyophilized L. gasseri KBL697 [2 × 1010 colony-forming unit (CFU)/g], isolated from vaginal samples of healthy Korean adults, were provided by KoBioLabs, Inc (Seoul, Republic of Korea). We confirmed superior resistances of L. gasseri KBL697 to high concentration of bile salts and low pH (data not shown). The bacteria were diluted appropriately using 1 × phosphate-buffered saline (PBS) before administration.
In vivo colitis mouse model
We developed the in vivo colitis mouse model as previously described11,12. Briefly, seven week-old female C57BL/6J mice (Central Lab Animals Inc., Seoul, Republic of Korea) were purchased for the colitis model. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC: SNU-190528-1) of Seoul National University, Republic of Korea and in compliance with ARRIVE guidelines. All procedures were carried out in accordance with relevant guidelines and regulations. Five groups of mice (n = 8) [(1) Water + PBS, (2) DSS + PBS, (3) DSS + IFX, (4) DSS + KBL697, and (5) DSS + KBL697 + IFX (DSS + Combine)] were kept in air-conditioned cages and fed the water containing 2% DSS (molecular mass 36,000–50,000 Da; MP Biomedical, LLC., Santa Ana, CA, USA) for seven days to induce acute colitis. The group treated with water + PBS were used as the negative control. Simultaneously, 200 μL of 1 × PBS with 2 × 109 CFU of L. gasseri KBL697 were administered daily by oral gavage. On day three, IFX (5 mg/kg) suspended in 1 × PBS (200 µL) was intravenously injected for the IFX-treated group. Disease activity index (DAI) of mice were measured daily using the following criteria: (1) weight loss (%), (2) stool consistency and (3) blood in feces, as previously described (Table S1)11,12. On day nine, the mice were sacrificed and organs, including colon, cecum, and mesenteric lymph nodes (MLNs), and stool samples were collected for further experiments.
Histological analysis
Distal colon samples were fixed in 10% formaldehyde and stained with hematoxylin and eosin, as described previously19. Tissues were analyzed using a panoramic viewer (3DHISTECH, Ltd., Budapest, Hungary). Histological scores were measured using the following criteria: (1) loss of epithelium, (2) crypt damage, (3) depletion of goblet cells and (4) inflammatory cell infiltration (Table S2)20. Three individual researches separately evaluated all samples and averaged scores were suggested.
Myeloperoxidase (MPO) measurement
Colon samples were weighed and homogenized in 1 × radioimmunoprecipitation assay (RIPA) buffer (Thermo Fisher Scientific, Waltham, MA, USA) with the Halt protease inhibitor cocktail (Thermo Fisher Scientific) using a Mixer Mill MM 400 homogenizer (Retsch, GmbH, Haan, Germany), as described previously11,12. Homogenates were centrifuged at 4 °C for 10 min at 15,000×g and supernatants were collected. MPO concentration in the supernatant was measured using an ELISA kit (Hycult Biotech. Inc., Plymouth Meeting, PA, USA) according to the manufacturer’s instructions.
Cytokine measurement
Levels of various cytokines in the supernatant, including interferon (IFN)-γ, interleukin (IL)-2, IL-6, IL-10, IL-17A, and TNF, were measured using a BD Cytometric Bead Array Mouse T helper cell (Th)1/Th2/Th17 Cytokine Kit (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions.
mRNA measurement related to tight junction
Total RNA was extracted from distal colon sample using an easy-spin total RNA Extraction Kit (Intron, Seoul, Republic of Korea) according to the manufacturer’s instructions. RNA samples were quantified using a Nanodrop ND 1000 (Thermo Fisher Scientific) and reverse-transcribed using a High-Capacity RNA-to-cDNA kit (Thermo Fisher Scientific). cDNA samples were amplified using a Power SYBR Green PCR Master Mix (Thermo Fisher Scientific) and specific primers designed for the tight junction-related genes, including zonula occludens (ZO)-1, ZO-2, and Claudin4 (Table 1)7,21. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) were performed using a Step-One-Plus Real-Time PCR System (Applied Biosystems, Forster, CA USA) with the following conditions: initially denatured at 95 °C for 10 min; 40 cycles of 95 °C for 15 s and 60 °C for 60 s. mRNA levels were normalized using hypoxanthine–guanine phosphoribosyltransferase (Hprt) gene22.
Table 1.
Primers used in this study.
| Gene | Sequence (5' → 3') | References |
|---|---|---|
| ZO-1 | Fw: 5′-ACC CGA AAC TGA TGC TGT GGA TAG-3′ | 21 |
| Rv: 5′-AAA TGG CCG GGC AGA ACT TGT GTA-3′ | ||
| ZO-2 | Fw: 5′-CTA GAC CCC CAG AGC CCC AGA AA-3′ | 21 |
| Rv: 5′-TCG CAG GAG TCC ACG CAT ACA AG-3′ | ||
| Claudin4 | Fw: 5′-ACT TTT TGT GGT CAC CGA CT-3′ | 21 |
| Rv: 5′-GCG AGC ATC GAG TCG TAC AT-3′ | ||
| Hprt | Fw: 5′-TTA TGG ACA GGA CTG AAA GAC-3′ | 7 |
| Rv: 5′-GCT TTA ATG TAA TCC AGC AGG T-3′ |
Fw Represents sequences of a forward primer.
Rv Represents sequences of a reverse primer.
T regulatory cell analysis
To analyze T regulatory cells (Tregs), flow cytometry analysis was performed as described previously11,12. MLNs were smashed carefully and filtered using a cell strainer (100 μm pore diameter, SPL Life Science Co., Ltd., Pocheon-Si, Gyeonggi-do, Republic of Korea). Live cells were stained with a Fixable Viability Stain 510 (FVS510; BD Biosciences, San Jose, CA, USA) and the cell surface was stained using anti-CD3 fluorescein isothiocyanate (145-2C11; BD Biosciences), anti-CD4 Percep-Cyanine5.5 (RM4-5; BD Biosciences), and anti-CD25 phycoerythrin (PC61; BD Bioscience), according to the manufacturer’s instructions. Cells were permeabilized with a fixation/permeabilization buffer (eBioscience, San Diego, CA, USA), and subjected to intracellular staining using an Alexa Fluor 647 anti-Foxp3 antibody (MF23; BD Biosciences). Finally, CD4 + CD25 + Foxp3 + Tregs were measured using a BD FACSVerse Flow Cytometer (BD Biosciences). Immunoglobulin G isotypes (BD Biosciences) were used as a control.
Cecal microbiota analysis
Analysis for cecal microbiota was performed as described previously11,12. Briefly, total bacterial DNA in cecum was extracted using a QIAamp Fast DNA Stool Mini Kit (Qiagen), according to the manufacturer’s instructions, and the V4-5 region of 16S rRNA gene was amplified using the universal primers (515F and 926R)23. PCR amplicons were sequenced appropriately using an Illumina Miseq platform (Illumina Inc., San Diego, CA, USA). The Quantitative Insights into Microbial Ecology (QIIME)2 version 2022.2 (QIIME 2 Development Team; https://qiime2.org/) with Greengenes version 13_8 database (http://greengene.secondgenome.com) were used for data analysis24,25. Low-quality or duplicated, and chimeric sequences were removed using Divisive Amplicon Denoising Algorithm 2 (DADA2). Sequences were classified into operational taxonomic units at the 99% similarity level. Alpha-diversity was indicated using Faith’s phylogenetic diversity (PD) whole tree index while beta-diversity was calculated using unweight UniFrac distance and demonstrated three-dimentional plots. The Linear discriminant analysis (LDA) Effect Size analysis (LEfSe) were performed using Galaxy ver. 2.1.1 (Hutlab; http://huttenhower.org/galaxy) as described previously11,12. Phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt)2 analysis (version 2.4.1) was also performed using QIIME 1.8.0 (QIIME Development Team; http://qiime.org) with Greengenes version 13_8 database and the Kyoto Encyclopedia of Genes and Genomes pathway database (GenomeNet; https://www.genome.jp/kegg/pathway.html)11,12.
Short-chain fatty acid measurement
SCFAs in cecum samples were analyzed as described previously11,12. Briefly, cecum was well-dispersed in 1 mL distilled water by vigorous vortexing and the supernatant was collected by centrifugation for 5 min at 13,000 ×g. 2-methylpentanoic acid (1%) was added as the internal standard. Then, ethyl ether was applied as the extraction solvent. After centrifugation for 5 min at 13,000 ×g, the organic layer was collected and analyzed using an Agilent 7890A gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) as described previously11,12. SCFA standards were used as references for retention time analysis and quantification of sample peak areas26.
Statistical analysis
Data are expressed as means ± standard deviation of the experimental groups in three independent experiments. GraphPad Prism ver. 5.04 (GraphPad Software, Inc., La Jolla, CA, USA) was applied for statistical analyses, including one-way analysis of variance (ANOVA) with Dunnett’s post hoc test and visualizations. P-values (P) < 0.05 were considered statistically significant.
Results
Effects of L. gasseri KBL697 and IFX co-administration on colitis symptoms of mice
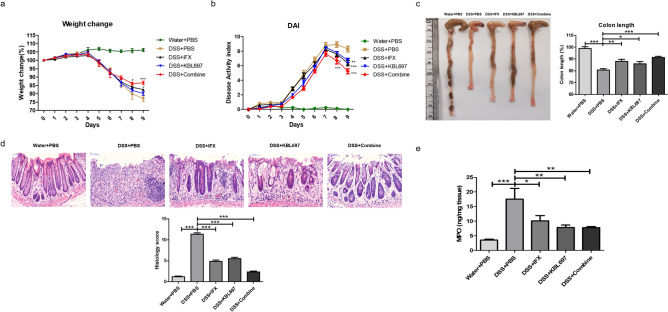
Figure 1 shows improvements of colitis symptoms via co-administration of L. gasseri KBL697 and IFX. Compared to the DSS + PBS group, body weights and DAI scores of DSS + Combine-treated mice were clearly restored after treatment (P < 0.001) (Fig. 1a,b). Moreover, DSS + Combine-treated mice showed significantly longer colon lengths (P < 0.001) and higher histology scores (P < 0.001) compared to DSS + PBS-treated mice (Fig. 1c,d). MPO in colon tissues collected from DSS + Combine and DSS + KBL697-treated mice were also significantly decreased compared to the DSS + PBS group (P < 0.01) (Fig. 1e).
Figure 1.
Improvements of colitis symptoms in mice via co-administration of L. gasseri KBL697 and Infliximab (IFX). (a,b) Body weights and DAI scores measured during experimental period. (c,d) Colon lengths and histology scores. (e) MPO levels. Statistical analysis was performed using one-way ANOVA with Dunnett’s post hoc test (*P < 0.05; **P < 0.01; ***P < 0.001).
Differences in the cytokine levels of mice with DSS-induced colitis using co-administration
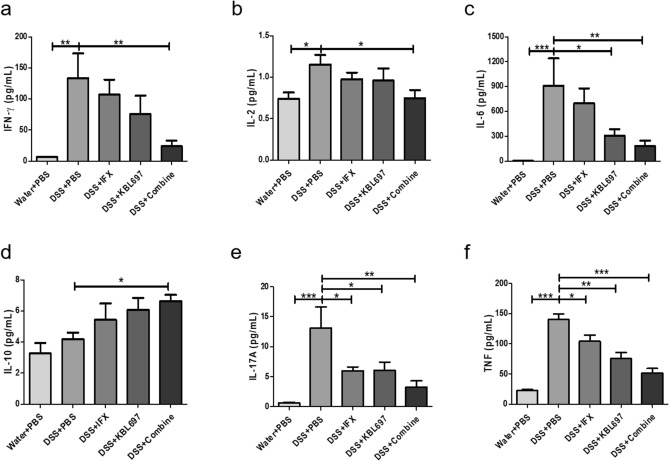
Figure 2 shows the effects of co-administration on cytokine levels of the mice with DSS-induced colitis. Overall, only the co-administered group showed significant decrease of IFN-γ, IL-2 and IL-6 levels compared to the DSS + PBS group (P < 0.05) (Fig. 2a–c). Moreover, high levels anti-inflammatory cytokine IL-10 in DSS + Combine-treated mice was discovered compared to the DSS + PBS group (P < 0.05) (Fig. 2d). Additionally, the co-administered group had the lowest IL-17A (P < 0.01) and TNF (P < 0.001) levels among all experimental groups (Fig. 2e,f).
Figure 2.
Effect of the different treatments on colon cytokine levels. (a) IFN-γ. (b) IL-2. (c) IL-6. (d) IL-10. (e) IL-17A. (f) TNF. Statistical analysis was performed using one-way ANOVA with Dunnett’s post hoc test (*P < 0.05; **P < 0.01; ***P < 0.001).
Effects of co-administration in the mRNA expressions related to tight junction
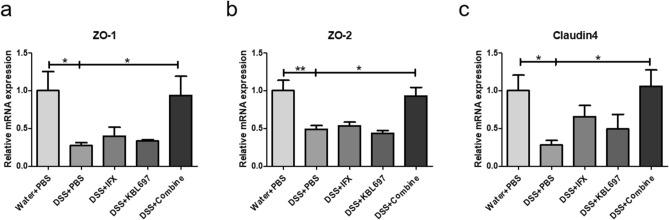
DSS + Combine-treated mice showed significant restoration of mRNA expressions for ZO-1, ZO-2 and Claudin4 compared to the DSS + PBS group (P < 0.05) (Fig. 3). DSS + IFX and DSS + KBL697-treated mice showed higher mRNA level of Claudin4 than the DSS + PBS group, however, there were no statistical significance discovered (Fig. 3c).
Figure 3.
Effects of the different treatments on the mRNA expressions related to tight junction. (a) ZO-1. (b) ZO-2. (c) Claudin4. Statistical analysis was performed using one-way ANOVA with Dunnett’s post hoc test (*P < 0.05; **P < 0.01; ***P < 0.001).
Effects of co-administration on CD4 + CD25 + Foxp3 + Tregs in MLNs of mice with DSS-induced colitis
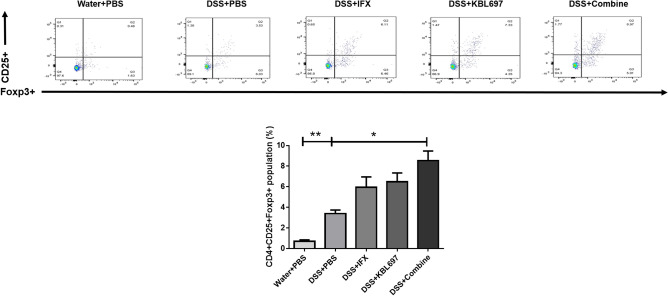
Figure 4 shows that CD4 + CD25 + Foxp3 + Tregs were significantly increased in DSS + Combine-treated mice compared to the DSS + PBS group (P < 0.05) (Fig. 4). DSS + IFX and DSS + KBL697-treated mice also showed higher CD4 + CD25 + Foxp3 + Tregs than the DSS + PBS group, however, there were no statistical significance discovered (Fig. 4).
Figure 4.
Effect of the different treatments on CD4 + CD25 + Foxp3 + Tregs. Statistical analysis was performed using one-way ANOVA with Dunnett’s post hoc test (*P < 0.05; **P < 0.01).
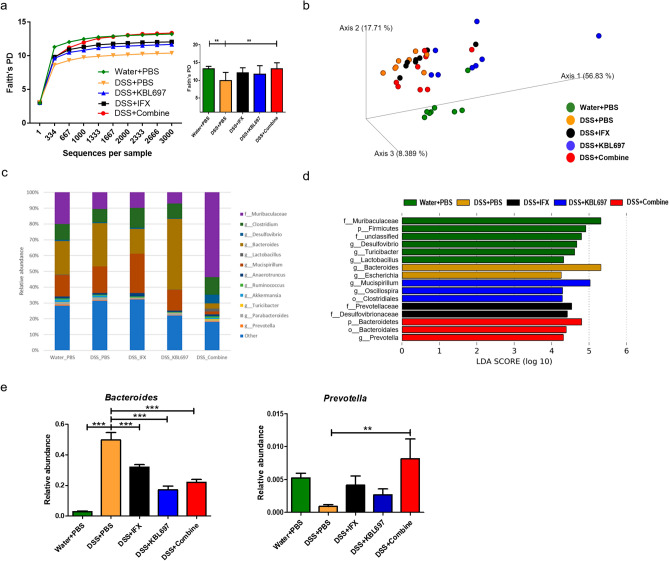
Effects of L. gasseri KBL697 and IFX co-administration on cecal microbiota
DSS + Combine-treated mice have significantly increased bacterial diversities compared to the DSS + PBS group (P < 0.01). The bacterial communities of DSS + Combine-treated mice only showed similarity (did not shown significantly differences) with the DSS + IFX group (Fig. 5a,b). Unlike the Water + PBS group, which has family Muribaculaceae as their major cecal microbiota, genus Bacteroides was the dominant in DSS + PBS-treated mice (Fig. 5c,d). However, the microbial profiles for DSS + Combine-treated mice showed the clear increase in family Muribaculaceae and decrease in genus Bacteroides (Fig. 5c,d). Additionally, the higher relative abundances of genus Prevotella (P < 0.01) were discovered in DSS + Combine-treated mice compared to the DSS + PBS group (Fig. 5e).
Figure 5.
Effect of the different treatments on cecal microbiota composition. (a) Rarefaction plots using Faith’s phylogenetic diversity (PD) whole tree indices. (b) Principal coordinates analysis with unweighted UniFrac distances. (c) Taxonomic structures of cecal microbiota. (d) LEfSe results in experimental groups (threshold > 2.5). (e) Relative abundances of significantly different microbial taxa in experimental groups at the genus level. When appropriate, statistical analysis was performed using one-way ANOVA with Dunnett’s post hoc test (*P < 0.05; **P < 0.01; ***P < 0.001).
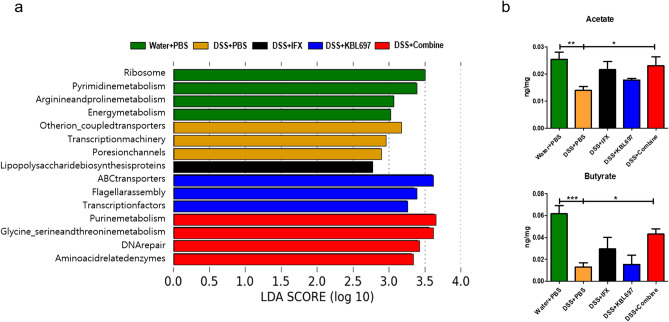
Differences in predicted profiles of functional alteration related to cecal microbiota and SCFAs via co-administration
Figure 6a presents the predicted functional alterations due to cecal microbial composition in the various treatment groups. The microbiota in Water + PBS-treated mice displayed strong correlations with metabolic pathways related to amino acid, energy, and nucleic acid. Similar to the Water + PBS group, the microbiota in the DSS + Combine-treated mice were highly correlated with metabolic pathways related to amino acid and nucleic acid and they had correlation with DNA repair pathway. Moreover, significant increases of acetate and butyrate (P < 0.05) were discovered in DSS + Combine-treated mice (Fig. 6b).
Figure 6.
Differences in profiles of functional alteration related to cecal microbiota and SCFAs via the different treatments. (a) PICRUSt results in experimental groups (threshold > 2.5). (b) Acetate and butyrate levels. When appropriate, statistical analysis was performed using one-way ANOVA with Dunnett’s post hoc test (*P < 0.05; **P < 0.01; ***P < 0.001).
Discussion
In this study, we preliminarily confirmed that co-administration of L. gasseri KBL697 and IFX, a commercialized TNF-α inhibitor, can significantly alleviate colitis symptoms in the DSS-induced colitis mouse model. Moreover, levels of MPO, which is the important inflammatory mediator of innate immunity, were clearly restored after co-administration11,12. Especially, the effects for co-administration in colitis symptoms were clearly higher than single treatment of L. gasseri KBL697 or IFX, indicating that co-administration has synergistic effects for prevention and treatment of colitis. Activation of innate immune cells lead to the development of antigen-specific T cells, such as Th1 and Th17, during the acute stage of DSS-induced colitis and can exacerbate colitis severely27–29. We observed that the levels of pro- or inflammatory cytokines, such as IFN-γ, IL-2, IL-6, IL-17A, and TNF, were appropriately controlled with co-administration. Moreover, co-administration resulted in a significant increase in the anti-inflammatory cytokine IL-10, which can aid in the treatment of colitis. However, in this study, we applied PBS as the negative control, hereafter, further studies should be applied using a suitable control of IFX, such as an equivalent intravenous injection of human immunoglobulin G1.
Tight junction proteins are important for establishing the gut epithelial barrier. ZO-1 and ZO-2 are intracellular tight junction proteins, which are the important linkers between cell cytoskeleton and transmembrane tight junction proteins21. Claudin4, an important transmembrane epithelial protein, also have a role in establishing integrity of the intestinal barrier21. Previous studies have been reported that probiotics or IFX treatment can improve intestinal permeability and promote colonic recovery11,21. Gene expression of tight junction proteins ZO-1, ZO-2, and Claudin4, were significantly up-regulated in DSS + Combine-treated mice. Therefore, co-administration of L. gasseri KBL697 and IFX can synergistically alleviate colitis by improving intestinal permeability. Further researches should be performed with cross-validation of various house-keeping genes to secure the accuracy of gene expression data. Moreover, to fully elucidate the KBL697 effects in attenuation of colitis, various experiments such as protein expression or localization related to tight junction need to be suggested.
Activated Tregs can suppress the progression of colitis effectively via production of inflammatory cytokines such as IL-10 and regulation of Th17s29. Co-administration of L. gasseri KBL697 and IFX increased CD4 + CD25 + Foxp3 + Tregs in MLNs of DSS-treated mice. Especially, our results suggested that the effects of co-administration are significantly higher than those of either single treatment of L. gasseri KBL697 or IFX, indicating that synergistic effects can be occurred.
A previous study suggested that the perturbation of microbial diversity and composition can accelerate inflammation in IBD patients30. In this study, we confirmed cecal microbiota composition, which is generally used to gut microbiome studies for mouse because mouse cecum is relatively larger than human and has digestion function for food components31,32. Feces is not suitable for our study because only watery-type feces can be collected from mice with DSS-induced colitis. We administered high concentration (2 × 109 CFU/day) of L. gasseri KBL697 by oral gavage to mice and only mice in the Water + PBS group showed high abundance of Lactobacillus spp. in their cecal microbiome. Therefore, we can confirm indirectly that most administered L. gasseri KBL697 were excreted from digestive system of mouse with DSS-induced colitis. Our results showed that the microbial diversity was significantly different between the DSS + Combine-treated group and the DSS + PBS-treated group. The relative abundances of several bacterial species, including genus Prevotella, were also significantly increased in DSS + Combine-treated mice. Genus Prevotella can induce Tregs and regulate cytokines for suppression of diseases such as inflammation of central nervous system and multiple sclerosis33. Differences in microbial composition due to co-administration of L. gasseri KBL697 and IFX in mice with DSS-induced colitis can affect the predicted functional alterations of cecal microbiota, including metabolic pathways related to amino acid and nucleic acid, and production of important SCFAs such as acetate and butyrate. Acetate has various crucial roles for regulating epithelial cell differentiation, Treg stimulation, and amelioration of mucosal inflammation. Additionally, butyrate can exert beneficial effects on colitis treatment via induction of Tregs34. However, further longitudinal studies with various colitis models should be performed to fully elucidate the combination effects of L. gasseri KBL697 and IFX on gut microbiota and their functional alterations from IBD patients. Also, recent study was reported that mucus microbiota of mice has distinctive compositions compared to cecal samples or feces35, therefore, further studies using various gut samples with other reference microbiome databases such as SILVA rRNA database project (https://www.arb-silva.de/) or The Ribosomal Database Project (RDP; http://rdp.cme.msu.edu/) can be suggested important information for colonization or function of L. gasseri KBL697 in gut.
In conclusion, our study suggested that the co-administration of L. gasseri KBL697 and IFX exhibits synergistic effects for alleviation of colitis in mice via immunomodulation and restoration of gut microbiota. Further, additional studies for comparisons with widely used probiotic strains, such as L. rhamnosus GG14, need to be performed for development of the promising method of prevention and treatment of IBD.
Supplementary Information
Acknowledgements
This work was supported by National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIT) (No. 2018R1A2A1A05078258).
Author contributions
D.H.H., W.-K.K., and G.K. designed the study. Other authors contributed to data acquisition and manuscript preparation. G.K. supervised this study.
Competing interests
G.K. is the founder of KoBioLabs, Inc., and S.P., K.L., and S.J.J. are employees by KoBioLabs, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Dae Hee Han and Woon-ki Kim.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-13753-6.
References
- 1.Abraham C, Cho JH. Inflammtory bowel disease. N. Engl. J. Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lügering A, Lebiedz P, Koch S, Kucharzik T. Apoptosis as a therapeutic tool in IBD? Ann. N. Y. Acad. Sci. 2006;1072:62–77. doi: 10.1196/annals.1326.013. [DOI] [PubMed] [Google Scholar]
- 3.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 4.Kim WK, et al. Administration of Lactobacillus paracasei strains improves immunomodulation and changes the composition of gut microbiota leading to improvement of colitis in mice. J. Funct. Foods. 2019;52:567–575. [Google Scholar]
- 5.Kim WK, et al. Administration of Lactobacillus fermentum KBL375 causes taxonomic and functional changes in gut microbiota leading to improvement of atopic dermatitis. Front. Mol. Biosci. 2019;6:1–12. doi: 10.3389/fmolb.2019.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vonk RJ, Priebe M, Meijer K, Venema K, Roelofsen H. The interaction of short-chain fatty acids (SCFA) with adipose tissue; relevance for systemic inflammation. Gastroenterology. 2011;140:S860. doi: 10.1016/S0016-5085(11)63571-3. [DOI] [Google Scholar]
- 7.Kim W, et al. Lactobacillus paracasei KBL382 administration attenuates atopic dermatitis by modulating immune response and gut microbiota. Gut Microbes. 2020;12:1–14. doi: 10.1080/19490976.2020.1819156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macdonald TT, Monteleone G, Pender SLF. Recent developments in the immunology of inflammatory bowel disease. Scand. J. Immunol. 2000;51:2–9. doi: 10.1046/j.1365-3083.2000.00658.x. [DOI] [PubMed] [Google Scholar]
- 9.Scallon BJ, Moore MA, Trinh H, Knight DM, Ghrayeb J. Chimeric anti-tnf-α monoclonal antibody ca2 binds recombinant transmembrane tnf-α and activates immune effector functions. Cytokine. 1995;7:251–259. doi: 10.1006/cyto.1995.0029. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Tran DQ, Rhoads JM. Probiotics in disease prevention and treatment. J. Clin. Pharmacol. 2019;58:S164–S179. doi: 10.1002/jcph.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang YJ, Kim WK, Han DH, Lee K, Ko G. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes. 2019;10:696–711. doi: 10.1080/19490976.2019.1589281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim W, et al. Alleviation of DSS-induced colitis via Lactobacillus acidophilus treatment in mice. Food Funct. 2020;12:340–350. doi: 10.1039/D0FO01724H. [DOI] [PubMed] [Google Scholar]
- 13.Han DH, Kim WK, Park SJ, Jang YJ, Ko G. Lactobacillus paracasei treatment modulates mRNA expression in macrophages. Biochem. Biophys. Rep. 2020;23:100788. doi: 10.1016/j.bbrep.2020.100788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghouri YA, et al. Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in infammatory bowel disease. Clin. Exp. Gastroenterol. 2014;9:473–487. doi: 10.2147/CEG.S27530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poritz LS, et al. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J. Surg. Res. 2007;140:12–19. doi: 10.1016/j.jss.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 16.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 17.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Baarlen P, Wells JM, Kleerebezem M. Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol. 2013;34:208–215. doi: 10.1016/j.it.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Ghaleb AM, McConnell BB, Kaestner KH, Yang VW. Altered intestinal epithelial homeostasis in mice with intestine-specific deletion of the Krüppel-like factor 4 gene. Dev. Biol. 2011;349:310–320. doi: 10.1016/j.ydbio.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akgun E, et al. Effects of N-acetylcysteine treatment on oxidative stress in acetic acid-induced experimental colitis in rats. J. Int. Med. Res. 2005;33:196–206. doi: 10.1177/147323000503300207. [DOI] [PubMed] [Google Scholar]
- 21.Bischoff SC, et al. Intestinal permeability—a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodríguez-Nogales A, et al. Differential intestinal anti-inflammatory effects of Lactobacillus fermentum and Lactobacillus salivarius in DSS mouse colitis: Impact on microRNAs expression and microbiota composition. Mol. Nutr. Food Res. 2017;61:1700144. doi: 10.1002/mnfr.201700144. [DOI] [PubMed] [Google Scholar]
- 23.Walters W, et al. Improved bacterial 16S rRNA gene (V4 and V4–5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. MSystems. 2015;1:e00009–e00015. doi: 10.1128/mSystems.00009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caporaso JG, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.David LA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan ME, et al. New perspective on dextran sodium sulfate colitis: Antigen-specific T cell development during intestinal inflammation. PLoS ONE. 2013;8:e69936. doi: 10.1371/journal.pone.0069936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brand S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: New immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut. 2009;58:1152–1167. doi: 10.1136/gut.2008.163667. [DOI] [PubMed] [Google Scholar]
- 29.Boehm F, et al. Deletion of Foxp3+ regulatory T cells in genetically targeted mice supports development of intestinal inflammation. BMC Gastroenterol. 2012;12:97. doi: 10.1186/1471-230X-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni J, Wu GD, Albenberg L, Tomov VT. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017;14:573–584. doi: 10.1038/nrgastro.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen TL, Vieira-Silva S, Liston A, Raes J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 2015;8:1–16. doi: 10.1242/dmm.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alessandro T, et al. Metaproteogenomics reveals taxonomic and functional changes between cecal and fecal microbiota in mouse. Front. Microbiol. 2017;8:391. doi: 10.3389/fmicb.2017.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mangalam A, et al. Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Rep. 2017;20:1269–1277. doi: 10.1016/j.celrep.2017.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machiels K, et al. A decrease of the butyrate-producing species roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63:2–9. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 35.Kozik AJ, Nakatsu CH, Chun H, Jones-Hall YL. Comparison of the fecal, cecal, and mucus microbiome in male and female mice after TNBS-induced colitis. PLoS ONE. 2019;14:e0225079. doi: 10.1371/journal.pone.0225079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.