Abstract
Most cases of acute leukemia (AL) with KMT2A rearrangement (KMT2A-r) have a dismal prognosis. Detection of this aberration in Chinese adult patients relies on reverse transcription polymerase chain reaction (RT-PCR) and chromosome banding analysis (CBA). The fluorescence in situ hybridization (FISH) probe for KMT2A detects KMT2A-r and copy number variation (CNV) but is not routinely used as a detection technique. This study investigated the potential value of FISH in the treatment of AL by performing FISH along with CBA and RT-PCR in 269 de novo cases of AL. The three detection techniques were compared in identification of KMT2A-r, and the applicability of FISH for detecting KMT2A CNV was evaluated. Twenty-three samples were identified as positive for KMT2A-r (20 using FISH, 15 using RT-PCR, 16 using CBA, and eight according to all three). FISH also identified 17 KMT2A CNV, 15 with gains and two with deletions. Ten patients with acute myeloid leukemia (AML) harboring KMT2A CNV had a complex karyotype, a negative prognostic factor in AML. Adding FISH of KMT2A to routine detection leads to more accurate detection of KMT2A-r and improved identification of KMT2A CNV, which would benefit patients by improving the risk stratification in AL.
Subject terms: Genetics, Biomarkers, Molecular medicine, Oncology, Risk factors
Introduction
Translocations involving the lysine (K)-specific methyltransferase 2A gene (KMT2A), previously known as mixed lineage leukemia (MLL), are frequently associated with acute lymphoid leukemia (ALL), acute myeloid leukemia (AML), and mixed-phenotype acute leukemia (MPAL)1. According to the World Health Organization (WHO) 2016 classification, leukemia with KMT2A rearrangements (KMT2A-r) is an independent subtype that often leads to a dismal prognosis, except for the intermediate prognostic factor t(9;11) involving KMT2A-MLLT32. KMT2A-chimeric genes that remain stable during clonal evolution of tumors can be used for molecular monitoring of minimal residual disease, which is a reliable predictor of clinical outcome3. Accordingly, timely and accurate identification of KMT2A aberrations is of great clinical significance. To date, a total of 135 types of KMT2A-r have been identified, of which 94 translocation partner genes have been characterized at the molecular level4. In addition to the wide range of partner genes, the location of the KMT2A breakpoint varies within different chimeric genes5. Therefore, confirmation of chromosomal rearrangements in KMT2A-associated acute leukemia (AL) remains a difficult diagnostic task owing to the high heterogeneity of the rearrangements.
Currently, chromosomal banding analysis (CBA) and commercial kits in multiplex reverse transcription polymerase chain reaction (RT-PCR) are routinely used in China as the main methods of detecting KMT2A-r. However, both methods have inherent limitations. CBA may miss KMT2A-r, possibly owing to the low mitotic index of leukemic cells and the presence of complex or cryptic chromosomal abnormalities6. Meanwhile, multiplex RT-PCR kits can detect only a limited number of the most frequent KMT2A-r7. A commercially available dual-color fluorescence in situ hybridization (FISH) break apart probe is only used as an auxiliary method to detect KMT2A-r when there is a discrepancy in CBA and RT-PCR results; there is a lack of comprehensive understanding of KMT2A-r heterogeneity and widespread underestimation of the value of the FISH test.
Although uncommon, amplification of the 11q23 region is recognized as a recurrent event in both AML and myelodysplastic syndrome (MDS) and is associated with older age, a complex karyotype (CK), and a very poor prognosis8–10. In addition to detecting KMT2A-r, the KMT2A probe can detect the copy number variation (CNV) of KMT2A. However, we are unaware of any studies that aimed to elucidate the value of the FISH technique in the identification of both rearrangement and numerical abnormality of KMT2A. In this study, FISH analysis of KMT2A was performed in each newly diagnosed adult patient with AL at a single institution. The study analyzed the distribution of KMT2A-r in Chinese adults with AL using a combination of different testing methods, including RT-PCR, CBA, and FISH, and it evaluated the clinical significance of including the KMT2A probe in KMT2A CNV detection methods in routine diagnostic workups.
Materials and methods
Patients
A total of 269 patients with de novo AL from Tongji Hospital at Tongji Medical College of Huazhong University of Science and Technology (Wuhan, China) between January 2019 and November 2020 were selected for the study. All cases were categorized according to the 2016 WHO classification2. Bone marrow (BM) or peripheral blood (PB) samples were collected from the patients at diagnosis, and their medical records were retrospectively reviewed.
Conventional cytogenetics analysis
CBA of BM or PB cells was performed using short-term (24 h) and unstimulated cultures, according to standard G-banding procedures. The G-banded metaphase chromosomes were obtained by treatment of the cells with trypsin, and they were then stained with Leishman’s stain. At least 20 metaphase cells were analyzed in detail for each sample. Karyotypes were interpreted according to the 2016 International System for Human Cytogenetic Nomenclature11.
FISH analysis
FISH analysis for KMT2A-r was performed using fixed cells and the LSI KMT2A dual-color, break apart rearrangement probe (BAP; Abbott Laboratories, Abbott Park, IL, USA) according to the manufacturer’s instructions. A minimum of 200 nuclei or metaphase chromosomes per sample were evaluated for KMT2A-r. The probe produced two fusion signals of orange and green when hybridized to normal nuclei; green (5′-KMT2A), orange (3′-KMT2A), and orange/green fusion signals were detected in nuclei containing typical KMT2A-r. Cases with either a single copy or ≥ 3 KMT2A copies were considered as numerically abnormal KMT2A.
RT-PCR
The RNA samples were prepared from mononuclear cells using a RNeasy Mini Kit (Qiagen, Redwood City, CA, USA) according to standard protocols. Next, RT-PCR was conducted for the 43 fusion genes (FGs) using a Leukemia-Related Fusion Gene Detection Kit (Yuanqi Bio-Pharmaceutical Co., Ltd., Shanghai, China) according to the manufacturer’s instructions. The screening panel tested for partial tandem duplications (PTD) specific for KMT2A (KMT2A-PTD) and 10 relatively common types of FGs involving KMT2A (KMT2A-FG): KMT2A-MLLT10, KMT2A-MLLT6, KMT2A-EPS15, KMT2A-MLLT11, KMT2A-AFF1, KMT2A-MLLT4, KMT2A-MLLT3, KMT2A-SEPT6, KMT2A-ELL, and KMT2A-MLLT1.
Flow cytometric immunophenotyping
After RT-PCR, PB or BM blast cells were analyzed using flow cytometry. A panel of monoclonal antibodies against CD45, CD34, CD38, CD33, CD56, CD3, CD2, CD5, CD7, CD10, CD8, CD19, CD20, CD138, CD24, CD22, CD28, kappa, lambda, TdT, HLA-DR, and CD79a was used as previously described to determine the immunophenotypes of the leukemia cells12.
Statistical analysis
IBM SPSS Statistics software (version 16.0; SPSS Inc., Chicago, IL, USA) was used to perform all statistical analyses. Comparisons between groups were performed using χ2 tests. The statistical significance was set at P < 0.05.
Compliance with ethical standards
Appropriate informed consent was obtained from all donors prior to specimen collection in accordance with the Declaration of Helsinki and the research protocol approved by the ethics committees of Tongji Hospital.
Results
Patient characteristics
In total, 269 newly diagnosed adult AL patients were evaluated. The median patient age was 45 years (range 18–86) and the male: female ratio was 1.56 (164:105). Of the 269 patients, 185 had AML, 74 had ALL, and 10 had MPAL. A total of 23 cases exhibited KMT2A-r based on a combination of cytogenetic and molecular techniques, including FISH, CBA, and specific RT-PCR methods. Of the 23 KMT2A-r cases, samples from eight patients were positive according to all three tests. Meanwhile, 17 cases were identified as having KMT2A CNV. The clinicopathological features of the 269 AL patients are listed in Table 1.
Table 1.
The clinical and pathological features of the 269 AL patients.
| Variable | Number of cases | Percentage |
|---|---|---|
| Age | ||
| 18–40 | 107 | 39.8 |
| 41–60 | 95 | 35.3 |
| ≥ 60 | 67 | 24.9 |
| Sex | ||
| Male | 164 | 61.0 |
| Female | 105 | 39.0 |
| Diagnosis | ||
| AML | 185 | 68.8 |
| ALL | 74 | 27.5 |
| MPAL | 10 | 3.7 |
| Cytogenetic abnormality | ||
| KMT2A rearrangement | 23 | 8.6 |
| KMT2A copy number variation | 17 | 6.3 |
| Complex karyotype | 37 | 13.8 |
FISH analysis
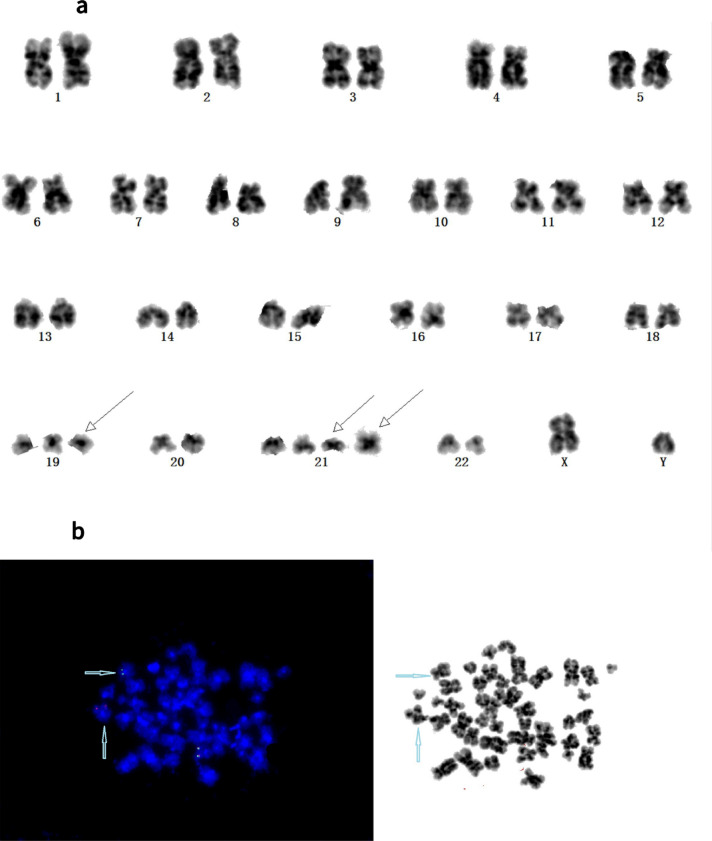
FISH analysis revealed that 37 of the 269 AL patients (14.9%) exhibited aberrant KMT2A signals, including 20 cases with a KMT2A-r signal (14 with AML, 5 with ALL, and 1 with MPAL). The samples with a KMT2A-r signal included 17 cases of typical positive signals and three cases of atypical abnormal signals (Patient 20: one fusion and one green signal, Patient 3: two fusion signals with one exhibiting a diminished red signal, and Patient 2: two fusion signals with one extra green signal). The clinical and laboratory characteristics of these patients are listed in Table 2. The remaining 17 cases had KMT2A CNV, including multiple copy numbers of KMT2A in 15 patients and a single copy in the other two patients. As shown in Fig. 1, the multiple copy numbers of KMT2A varied, with three copies in each of seven cases (Patients 31, 32, 33, 34, 35, 36, and 40), four copies in four cases (Patients 28, 29, 30, and 39), five copies in two cases (Patients 26 and 27), and more than five copies in two cases (Patients 24 and 25). The clinical and laboratory characteristics of these patients are listed in Table 3.
Table 2.
Clinical, cytogenetic and molecular results of 23 patients with KMT2A rearrangements.
| Patient number | Sex | Age | Diagnosis | Karyotype | FISH signal | Fusion transcript |
|---|---|---|---|---|---|---|
| 1 | M | 19 | AML | 49,XY, + 19, + 21, + add(21)(p11) [10] | 1F1R1G | N |
| 2 | F | 22 | AML | 46,XX [20] | 2F1G | KMT2A-MLLT4 |
| 3 | F | 25 | AML | 46,XX [25] | 2F(1F with diminished red signal) | KMT2A-MLLT4 |
| 4 | M | 30 | AML | 46,XY [20] | N | KMT2A-SEPT6 |
| 5 | F | 72 | AML | 46,XX [20] | N | KMT2A-PTD |
| 6 | M | 41 | AML | 51,XY, + 3, + 6, + 8, + 18, + 19[7] /46,XY [13] | N | KMT2A-MLLT10 |
| 7 | M | 52 | AML | failure | 1F1R1G | KMT2A-MLLT1 |
| 8 | M | 46 | AML | 47,XY, + 3,t(10;11)(p12;q22) [8]/46,XY [2] | 1F1R1G | N |
| 9 | F | 52 | AML | 46,XX,t(2;11)(p21;q23) [15] | 1F1R1G | N |
| 10 | F | 62 | AML | 48,XX, + 8, + 11,t(5;11)(q31;q23) [10] | 2F1R1G | N |
| 11 | M | 21 | AML | 46,XY,t(11;19)(q23;p13.1) [9]/46,XY [11] | 1F1R1G | N |
| 12 | F | 62 | AML | 50,XX, + 8, + add(9)(p24),t(11;17)(q23;q25), + 13, + 21 [20] | 1F1R1G | N |
| 13 | M | 32 | AML | 46,XY,t(11;17)(q23;q21) [5]/46,XY [8] | 1F1R1G | N |
| 14 | M | 18 | ALL | 46,XY,t(4;11)(q21;q23) [9]/46,XY [1] | 1F1R1G | N |
| 4 | F | 50 | ALL | 46,XX,t(11;19)(q23;p13.3) [10] | 1F1R1G | KMT2A-MLLT1 |
| 16 | M | 49 | ALL | 47,XY, + X,t(11;19)(q23;p13.3) [5]/46,XY [5] | 1F1R1G | KMT2A-MLLT1 |
| 17 | M | 49 | AML | 46,XY,t(6;11)(q27;q23) [8]/46,XY [2] | 1F1R1G | KMT2A-MLLT4 |
| 18 | F | 22 | AML | 46,XX,t(9;11)(p21;q23) [5]/47,idem, + 8 [5] | 1F1R1G | KMT2A-MLLT3 |
| 19 | F | 26 | AML | 46,XX,t(11;19)(q23;p13.1) [8] | 1F1R1G | KMT2A-ELL |
| 20 | M | 28 | AML | 46,XY,t(11;19)(q23;p13.1) [6]/46,XY [5] | 1F1G | KMT2A-ELL |
| 21 | M | 18 | MPAL | 46,XY,t(9;11)(p21;q23) [5]/46,XY [5] | 1F1R1G | KMT2A-MLLT3 |
| 22 | F | 54 | ALL | 47,XX, + X,t(1;11)(p32;q23) [10] | 1F1R1G | KMT2A-EPS15 |
| 23 | M | 31 | ALL | 47,XY,add(1)(p36),t(4;11)(q21;q23), + 8 [10] | 1F1R1G | KMT2A-AFF1 |
F female, M male, ALL acute lymphoid leukemia, AML acute myeloid leukemia, MPAL mixed-phenotype acute leukemia, N negative.
Figure 1.
(a) Chromosome banding analysis of patient 25 showing a complex karyotype: 45,XY,del(5)(q13q32),del(11)qdp(11)(q21q23)r(11)(p15;q25),der(16;17)(p10;q10),add(19)(q13),del(20)(q11). (b) Fluorescence in situ hybridization (FISH) analysis of Patient 25 using the KMT2A probe revealed multiple KMT2A fusion signals present on derivative chromosome 11 (blue arrow).
Table 3.
Clinical, cytogenetic and molecular results of 17 patients with KMT2A copy number variation.
| Patient number | Sex | Age | Diagnosis | FISH | Gene | Karyotype |
|---|---|---|---|---|---|---|
| 24 | F | 56 | AML | > 5F | N | 46,XX,add(1)(p36),del(3)(p11),add(5)(p15),add(5)(q23),r(11)(p15q25), del(13)(q21),add(14)(q23),del(15)(q22) [9]/46,XX [9] |
| 25 | M | 79 | AML | > 5F | N | 45,XY,del(5)(q13q32),der(11)qdp(11)(q21q23)r(11)(p15;q25), der(16;17)(p10;q10),add(19)(q13),del(20)(q11) [10] |
| 26 | M | 61 | AML | 5F | N | 41–44,XY,del(2)(p13),-4,del(5)(q13), -7,add(9)(q34),?add(19)(q13),del(20)(q11),-22, + mar[cp7]/46,XY [2] |
| 27 | F | 72 | AML | 5F | N | failure |
| 28 | F | 28 | AML | 4F | N | 45,XX,del(5)(q11q21),del(6)(q11),-7,add(8)(q24),add(12)(p11) [14]/46,XX [1] |
| 29 | M | 73 | AML | 4F | N | 47,XY, + mar [2] |
| 30 | F | 60 | AML | 4F | N | 60,XX,-X,-3,-4,del(5)(q21),del(5)(q21),-6,-7,-9, + 11, -12,-14,-17,-21[cp13] |
| 31 | M | 65 | AML | 3F | N | 47,XY, + 11,i(17)(q10),?del(16)(q11) [10] |
| 32 | M | 27 | AML | 3F | N | 53,XY, + 4,del(6)(q22), + 9, + 11, + 13, + 19, + 21, + 21 [3]/46,XY [4] |
| 33 | M | 30 | AML | 3F | N | 49–51,XY, + del(3)(p11), + 4, + del(6)(q23), + del(11)(q24), + 14,i(17)(q10)[cp10] |
| 34 | F | 66 | AML | 3F | N | 44–45,XX,del(3)(p21),del(5)(q21),?-7, t(12;14)(p10;q10),add(10)(q25)[cp6] |
| 35 | M | 63 | AML | 3F | N | 47,XY, + 11 [8]/46,XY [2] |
| 36 | F | 75 | AML | 3F | N | 47,XX, + 11 [10] |
| 37 | M | 38 | AML | 1F | N | 46,XY,-2,add(11)(q23), + mar [10] |
| 38 | F | 76 | AML | 1F | N | 46,XX,add(11)(q23) [5]/46,idem,del(5)(q22q34) [5] |
| 39 | M | 18 | ALL | 4F | N | 56–59,X, + X,-Y, + 1, + 5, + 6, + 7, + 8, + 8, + 10, + 11, + 11, + 14, + 19, + 21, + 21[cp10] |
| 40 | F | 30 | ALL | 3F | N | 47,XX,add(9)(q34), + 11 [2]/46,XX [11] |
F female, M male, ALL acute lymphoid leukemia, AML acute myeloid leukemia, N negative, F fusion.
Conventional cytogenetic results
Successful CBA was obtained in 255 patients. A total of 120 (44.6%) patients demonstrated an abnormal karyotype, including 16 (6.3%) cases with KMT2A-r (10 AML, 5 ALL, and 1 MPAL). Of the 185 AML cases, 28 (15.1%) showed CK with at least three chromosomal abnormalities, including 10 that had KMT2A CNV identified via FISH analysis. The incidence rate of CK in the group with KMT2A CNV was significantly higher than that in the group with a normal KMT2A copy number (10/15 vs. 18/170, respectively, P < 0.01; Table 4). Six patients of CK with KMT2A CNV had either del (5q) or other structural aberrations involving 5q. Among the 74 ALL patients, CK was detected in nine cases without numerical abnormality of KMT2A.
Table 4.
The results of KMT2A copy number and complex karyotype in AML.
| CK | Non-CK | Total | p | |
|---|---|---|---|---|
| KMT2A CNV | 10 (66.7%) | 5 (33.3%) | 15 | < 0.01 |
| KMT2A CNN | 18 (10.6%) | 152 (89.4%) | 170 |
KMT2A CNV KMT2A copy number variation, KMT2A CNN normal KMT2A copy number, CK complex karyotype.
Metaphase FISH after karyotype
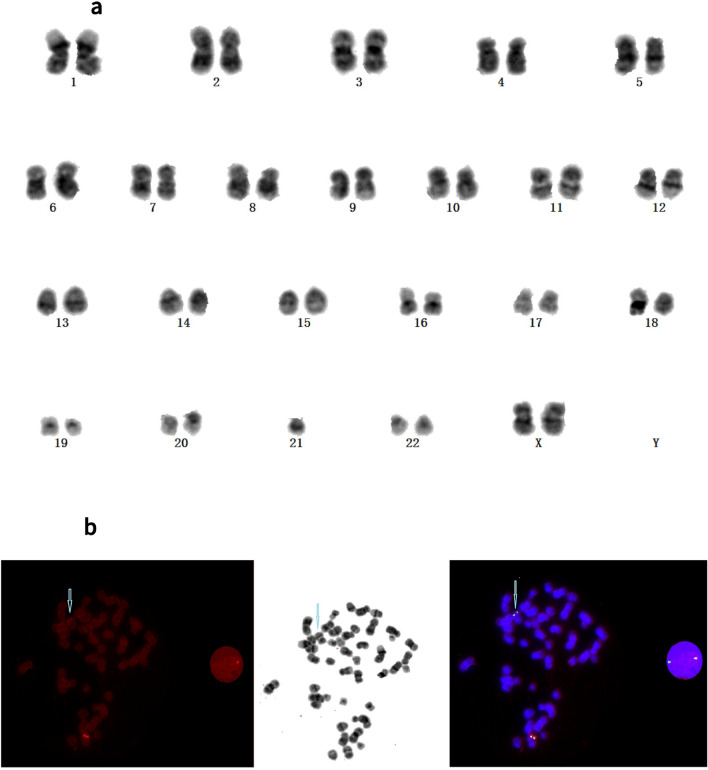
Repeated G-banding karyotype analysis (with the assistance of the metaphase FISH of KMT2A analysis) confirmed three additional cases harboring atypical KMT2A-r (Patients 1, 2, and 3). Initially, Patient 1 showed no structural or numeric abnormalities involving chromosomes 10 or 11 and typical rearrangement signals of one fusion, one green, and one orange signal in the nuclei. However, metaphase FISH analysis using the KMT2A BAP showed a 5′-green signal on the short arm of chromosome 10, whereas the 3′-orange signal of the KMT2A probe on the long arm of chromosome 11 was retained. Therefore, submicroscopic insertion of the 5′ region of KMT2A into 10p had occurred (Fig. 2). The RT-PCR failed to detect the KMT2A-MLLT10 fusion transcript in leukemic cells, most likely because of inappropriate primers being used. Meanwhile, Patient 2 had a normal karyotype but showed the atypical result of an extra 5′-KMT2A green signal in interphase FISH analysis using KMT2A BAP. FISH analysis following metaphase revealed that the extra 5′-KMT2A green signal was located in the long arm of chromosome 6 and was due to the partial duplication and insertion of KMT2A into MLLT4, resulting in the KMT2A-MLLT4 FG-positive finding. Karyotype analysis of Patient 3 showed an initial normal karyotype. However, KMT2A BAP FISH analysis revealed two intact KMT2A FISH signals, with one of the two KMT2A fusion signals demonstrating a significantly diminished 3′-KMT2A orange signal in nuclei during metaphase (Fig. 3), suggesting an atypical KMT2A-r.
Figure 2.
(a) Karyotype of Patient 1 showing 49,XY, + 19, + 21, + add(21)(p10). (b) Metaphase fluorescence in situ hybridization (FISH) analysis of patient 1 using the KMT2A probe, with chromosome 11 presenting a normal KMT2A gene signal (fusion-yellow FISH signal), a translocation presenting splatted signals, a green KMT2A signal in the short arm of a derivative chromosome 10, and an orange signal indicating the long arm of derivative chromosome 11 (blue rows).
Figure 3.
(a) Karyotype of Patient 3 showing 46,XX. (b) Fluorescence in situ hybridization (FISH) analysis of a metaphase cell of Patient 3 using the KMT2A probe. Two intact KMT2A copies were revealed, and one of the two KMT2A fusion signals (3′-KMT2A orange signal) was significantly diminished (blue arrow).
RT-PCR results
Among the 269 patients, there were 15 cases that were multiplex RT-PCR–positive for FGs produced by KMT2A-r, including one each of KMT2A-AFF1, KMT2A-EPS15, KMT2A-SEPT6, KMT2A-PTD, and KMT2A-MLLT10; two each of KMT2A-MLLT3 and KMT2A-ELL; and three each of KMT2A-MLLT1 and KMT2A-MLLT4. Of the 15 positive cases, KMT2A FISH and karyotype analyses failed to reveal any aberration involving KMT2A-r in Patients 4, 5, and 6, who were positive for KMT2A-SEPT6, KMT2A-PTD, and KMT2A-MLLT10, respectively. Finally, in the eight patients demonstrating a negative FG involving KMT2A, FISH or karyotype analyses revealed positive results for KMT2A-r (Patients 1, 8, 9, 10, 11, 12, 13, and 14).
Discussion
This study simultaneously identified and described KMT2A-r and KMT2A CNV alterations in AL using FISH analysis. KMT2A, which is 92 kb in size, is located on chromosome 11, band 23 (11q23) and includes at least 37 exons13. The accurate detection of KMT2A-r remains a challenge in clinical practice because of the complex mechanisms involved, including reciprocal translocations, complex chromosomal rearrangements, gene internal duplications, deletions, inversions on chromosome 11q, insertion of chromatin material into KMT2A, and KMT2A insertions into other chromosomes or vice versa14. It has been reported that the occurrence of a 3′-KMT2A deletion is associated with KMT2A-r15.
Currently, the methods routinely used for detecting KMT2A-r in AL patients in China include CBA and RT-PCR. However, FISH analysis using a dual-color BAP is used to further confirm the existence of KMT2A-r when these two primary methods yield inconsistent results.
In the current study, KMT2A FISH was performed for samples from every patient in the AL cohort, as were CBA and RT-PCR analyses. Through a combination of the three methods, a total of 23 cases were identified as being KMT2A-r, with eight being positive using all three methods. Methods of FG detection, karyotype analysis, and FISH analysis yielded positive results in 15, 16, and 20 cases, respectively (Table 2). Conventional karyotype analysis, which can reveal structural and numerical abnormalities of chromosomes, may miss some instances of KMT2A-r, probably owing to poor metaphase division of leukemic cells and poor detection of subtle chromosomal changes caused by complex or cryptic abnormalities6. Use of the LSI KMT2A BAP allows for the recognition of complex rearrangements, notably cryptic insertions of KMT2A segments into other chromosomes or the disruption of KMT2A by the insertion of other chromosomal segments, which improves the detection rate of KMT2A-r16. Three cases in our cohort (Patients 1, 2, and 3) were confirmed to have cryptic KMT2A insertions after a second review of karyotype analysis, which was based on the metaphase FISH analysis of KMT2A. This illustrates the need for FISH analysis using the KMT2A probe as an important auxiliary method to the CBA of AL patients. Furthermore, beyond the typical separation of signals in FISH detection of KMT2A, atypical FISH signal patterns are worth studying in clinical practice, especially changes in the number and intensity of orange and green signals. When an unusual KMT2A signal pattern is observed, metaphase FISH should be implemented to further clarify the mechanism of complex rearrangement of KMT2A. Several cases of insertions involving band 11q23 into chromosomes 2, 4, 5, 6, 9, and 10 and the X-chromosome have been previously reported16–20.
Compared to cytogenetic analysis, RT-PCR is a more sensitive and rapid technique. Furthermore, commercially available multiplex RT-PCR kits that can reveal KMT2A-r with frequently involved partner genes have been used worldwide. However, these kits do not contain primer sets for detecting rare or unknown partner genes, which may result in missed diagnosis of some patients with KMT2A-r. The RT-PCR panel used in the present study may not have included primers to detect the FG of KMT2A-r caused by t(2;11), t(5;11), and t(11;17), which was demonstrated by the karyotype analysis of Patients 9, 10, 12, and 13. However, the remaining sample volumes were insufficient to further confirm the presence of this FG by RNA sequencing. Additionally, fusion transcripts cannot be found in cases with such widespread aberrations as t(4;11) (Patient 14), t(10;11) (Patient 8), or t(11;19) (Patient 11). We speculated that there are several potential causes of the negative RT-PCR result. First, in addition to the major breakpoint cluster region (BCR) of the KMT2A gene (KMT2A exons 8–14), the other BCR is novel and minor (KMT2A intron 21–23), which was verified by Meyer et al. in 20195. The commercial multiplex RT-PCR kits are not always compatible and may only cover the major BCR, so if KMT2A-r occurred in minor BCR or other special mechanisms, including in the cases of 11q deletion, inversion, or three-way translocation, which can all cause KMT2A-r breakpoint outside of the major BCR. In these situations, the results were both beyond the range of the panel designed for the commercial kit. Second, in addition to AFF1, MLLT10, MLLT1, and ELL, different partner genes can be involved in 4q, 10p, and 19p, including SEPT11 and ARGBP2 (4q), ABI1 and NEBL (10p), and ASAH3, EEN and MYO1F (19p)4. However, these different partner genes all demonstrate the same t(4;11), t(10;11), and t(11;19) in the karyotype, which cannot be clearly distinguished by CBA. Patients 4 and 6 showed the FG of KMT2A-SEPT6 and KMT2A-MLLT10 by RT-PCR without any aberration involving the rearrangement of KMT2A in FISH and karyotype analyses. Similar cases have been previously described by other groups, and mechanisms that involve either a fragment of KMT2A being inserted elsewhere into the genome or a fragment of a locus being inserted proximally to KMT2A have been identified6,16. Both circumstances create a copy-neutral KMT2A-fusion oncogene that is not be detectable by BAP FISH testing. In addition, KMT2A-PTDs that are undetectable using currently available FISH probes or conventional karyotype analysis were identified in one case of the current study (Patient 5) and confirmed by RT-PCR. Therefore, the combined use of FISH, CBA, and multiplex RT-PCR analyses can improve the detection of KMT2A-r, particularly in unusual, complex, or cryptic chromosomal rearrangements that are frequently observed in AL.
A total of 17 patients with KMT2A CNV, including 15 with AML and two with ALL, were identified in the current cohort. Moreover, 10 of 15 (66.7%) AML cases with KMT2A CNV detected by FISH were CK, which was higher than that for the group with normal FISH results (66.7% vs. 10.6%; Table 4). Amplification of KMT2A could be defined as the presence of at least two extra gene copies as seven of 10 patients with AML and CK demonstrated ≥ 4 copies of KMT2A. Tang et al. reported that AML/MDS with KMT2A amplification is associated with a CK and high frequency of TP53 mutations8. It has been demonstrated by Zatkova et al. that patients with AML and CK, including 11q/KMT2A amplification, respond poorly to therapy and have a poor prognosis with an extremely short overall survival9. Therefore, the application of KMT2A FISH may have an additional benefit of shedding light on the identification of CK, especially when conventional karyotype analysis fails. The sample from Patient 27 of the current study failed in CBA, but FISH analysis revealed five copies of KMT2A. Three months after AML diagnosis, the patient had not achieved remission and died of infectious shock. Of the 10 AML cases with CK, including KMT2A amplification, recurrent chromosomal aberrations of 5q deletions were observed in six cases, which was similar to previous findings of 5q deletions being the most frequently associated with 11q amplifications in AML with CK9,21.
The mechanisms underlying the KMT2A-r and KMT2A CNV pathogenesis differ in that some KMT2A-r are driver abnormalities that require very few cooperate mutations to induce tumorigenesis22, while KMT2A CNV is easily accompanied by CK, meaning it must act in coordination with other genetic abnormalities to cause leukemia. Thus, detection and distinction of these two types of pathogenesis are important for development and management of treatment protocols. Several studies have demonstrated the potential use of KMT2A inhibitors as promising targeted therapies for KMT2A-r leukemia23. However, the effect on KMT2A CNV was not yet known at the time of this study.
Our study has several limitations. First, the commercial multiplex RT-PCR kits did not disclose primers sequences for trademark restrictions, which hindered us in distinguishing whether there were some defects in their primer design leading to the false negative in our cohort. Second, because of the insufficient sample and unaffordable cost, the cases with discrepant results from the three detection techniques cannot be further clarified using the complex mechanism of KMT2A-r by taking long distance inverse PCR (LDI-PCR) or next generation sequencing (NGS) technologies, such as RNA sequencing, which were both previously considered as robust ways to identify KMT2A-r.
Despite these limitations, we have shown that using multiplex nested RT-PCR, karyotype analysis, and FISH techniques in combination can improve the detection rate of KMT2A-r. We think the results show that KMT2A FISH detection should become a routine component of diagnostic and prognostic workups to identify cryptic KMT2A-r in AL patients. Furthermore, analysis may reveal a set of different KMT2A CNVs, which is highly associated with poor prognosis in AL, especially for patients in which there is a failure to obtain adequate levels of evaluable metaphase cells in cytogenetic analysis.
Author contributions
Q.L. collected clinical sample and related data and wrote the manuscript. Y.W. designed the study, performed data analysis, and revised the manuscript. S.X. performed the FISH experiments and gathered related data. H.Z. conducted chromosome banding analysis. X.M. performed the flow cytometry test. M.X. performed RT-PCR. All authors contributed to the article and approved the submitted version.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rubnitz JE, Behm FG, Pui CH, Evans WE, Relling MV, Raimondi SC, et al. Genetic studies of childhood acute lymphoblastic leukemia with emphasis on p16, MLL, and ETV6 gene abnormalities: Results of St Jude total therapy study XII. Leukemia. 1997;11(8):1201–1206. doi: 10.1038/sj.leu.2400779. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4. International Agency for Research on Cancer; 2017. [Google Scholar]
- 3.Grimwade D, Vyas P, Freeman S. Assessment of minimal residual disease in acute myeloid leukemia. Curr. Opin. Oncol. 2010;22(6):656–663. doi: 10.1097/CCO.0b013e32833ed831. [DOI] [PubMed] [Google Scholar]
- 4.Meyer C, Burmeister T, Groger D, Tsaur G, Fechina L, Renneville A, et al. The MLL recombinome of acute leukemias in 2017. Leukemia. 2018;32(2):273–284. doi: 10.1038/leu.2017.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer C, Lopes BA, Caye-Eude A, Cavé H, Arfeuille C, Cuccuini W, et al. Human MLL/KMT2A gene exhibits a second breakpoint cluster region for recurrent MLL-USP2 fusions. Leukemia. 2019;33(9):2306–2340. doi: 10.1038/s41375-019-0451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko K, Kwon MJ, Woo HY, Park H, Park CH, Lee ST, et al. Identification of mixed lineage leukemia gene (MLL)/MLLT10 fusion transcripts by reverse transcription-PCR and sequencing in a case of AML with a FISH-negative cryptic MLL rearrangement. Ann. Lab. Med. 2015;35(4):469–471. doi: 10.3343/alm.2015.35.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lomov N, Zerkalenkova E, Lebedeva S, Viushkov V, Rubtsov MA. Cytogenetic and molecular genetic methods for chromosomal translocations detection with reference to the KMT2A/MLL gene. Crit. Rev. Clin. Lab. Sci. 2021;58(3):180–206. doi: 10.1080/10408363.2020.1844135. [DOI] [PubMed] [Google Scholar]
- 8.Tang G, DiNardo C, Zhang L, Ravandi F, Khoury JD, Huh YO, et al. MLL gene amplification in acute myeloid leukemia and myelodysplastic syndromes is associated with characteristic clinicopathological findings and TP53 gene mutation. Hum. Pathol. 2015;46(1):65–73. doi: 10.1016/j.humpath.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Zatkova A, Merk S, Wendehack M, Bilban M, Muzik EM, Muradyan A, et al. AML/MDS with 11q/MLL amplification show characteristic gene expression signature and interplay of DNA copy number changes. Genes Chromosomes Cancer. 2009;48(6):510–520. doi: 10.1002/gcc.20658. [DOI] [PubMed] [Google Scholar]
- 10.Sarova I, Brezinova J, Zemanova Z, Izáková S, Lizcová L, Malinová E, et al. Cytogenetic manifestation of chromosome 11 duplication/amplification in acute myeloid leukemia. Cancer Genet. Cytogenet. 2010;199(2):121–127. doi: 10.1016/j.cancergencyto.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Shaffer LG, Slovak ML, Campbell LJ, editors. ISCN 2016. An International System for Human Cytogenetic Nomenclature. Karger; 2016. [Google Scholar]
- 12.Bacher U, Haferlach C, Schnittger S, Kern W, Ott MM, Haferlach T. Diagnostics of acute leukemia: Interaction of phenotypic and genetic methods. Pathologe. 2012;33(6):528–538. doi: 10.1007/s00292-012-1653-1. [DOI] [PubMed] [Google Scholar]
- 13.Tamai H, Inokuchi K. 11q23/MLL acute leukemia: Update of clinical aspects. J. Chin. Exp. Hematopathol. 2010;50(2):91–98. doi: 10.3960/jslrt.50.91. [DOI] [PubMed] [Google Scholar]
- 14.Marschalek R. Mechanisms of leukemogenesis by MLL fusion proteins. Br. J. Haematol. 2011;152(2):141–154. doi: 10.1111/j.1365-2141.2010.08459.x. [DOI] [PubMed] [Google Scholar]
- 15.Lim JH, Jang S, Park CJ, Chi HS, Lee JO, Seo EJ. FISH analysis of MLL gene rearrangements: Detection of the concurrent loss or gain of the 3' signal and its prognostic significance. Int. J. Lab. Hematol. 2014;36(5):571–579. doi: 10.1111/ijlh.12192. [DOI] [PubMed] [Google Scholar]
- 16.Peterson JF, Sukov WR, Pitel BA, Smoley SA, Pearce KE, Meyer RG, et al. Acute leukemias harboring KMT2A/MLLT10 fusion: A 10-year experience from a single genomics laboratory. Genes Chromosomes Cancer. 2019;58(8):567–577. doi: 10.1002/gcc.22741. [DOI] [PubMed] [Google Scholar]
- 17.Deveney R, Chervinsky DS, Jani-Sait SN, Grossi M, Aplan PD. Insertion of MLL sequences into chromosome band 5q31 results in an MLL-AF5Q31 fusion and is a rare but recurrent abnormality associated with infant leukemia. Genes Chromosomes Cancer. 2003;37(3):326–331. doi: 10.1002/gcc.10224. [DOI] [PubMed] [Google Scholar]
- 18.Bruch J, Wilda M, Teigler-Schlegel A, Harbott J, Borkhardt A, Metzler M. Occurrence of an MLL/LAF4 fusion gene caused by the insertion ins (11;2)(q23;q11.2q11.2) in an infant with acute lymphoblastic leukemia. Genes Chromosomes Cancer. 2003;37(1):106–109. doi: 10.1002/gcc.10187. [DOI] [PubMed] [Google Scholar]
- 19.De Braekeleer E, Meyer C, Douet-Guilbert N, Morel F, Le Bris MJ, Berthou C, et al. Complex and cryptic chromosomal rearrangements involving the MLL gene in acute leukemia: A study of 7 patients and review of the literature. Blood Cells Mol. Dis. 2010;44(4):268–274. doi: 10.1016/j.bcmd.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Matveeva E, Kazakova A, Olshanskaya Y, Tsaur G, Shelikhova L, Meyer C, et al. A new variant of KMT2A(MLL)-FLNA fusion transcript in acute myeloid leukemia with ins(X;11)(q28;q23q23) Cancer Genet. 2015;208(4):148–151. doi: 10.1016/j.cancergen.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Schoch C, Kern W, Kohlmann A, Hiddemann W, Schnittger S, Haferlach T. Acute myeloid leukemia with a complex aberrant karyotype is a distinct biological entity characterized by genomic imbalances and a specific gene expression profile. Genes Chromosomes Cancer. 2005;43(3):227–238. doi: 10.1002/gcc.20193. [DOI] [PubMed] [Google Scholar]
- 22.Forgione MO, McClure BJ, Eadie LN, Yeung DT, White DL. KMT2A rearranged acute lymphoblastic leukemia: Unravelling the genomic complexity and heterogeneity of this high-risk disease. Cancer Lett. 2020;469:410–418. doi: 10.1016/j.canlet.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Krivtsov AV, Evans K, Gadrey JY, Eschle BK, Hatton C, Uckelmann HJ, et al. A menin-MLL inhibitor induces specific chromatin changes and eradicates disease in models of MLL-rearranged leukemia. Cancer Cell. 2019;36(6):660–673. doi: 10.1016/j.ccell.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.