Abstract
The primary cause of the increase in melanoma incidence in the United States has been suggested to be overdiagnosis. We used SEER data from 1975 to 2017 to examine epidemiological trends of melanoma incidence and mortality and better characterize overdiagnosis in white Americans. Over the 43-year period, incidence and mortality showed discordant temporal changes across population subgroups; trends most suggestive of overdiagnosis alone were present in females aged 55-74. Other groups showed mixed changes suggestive of overdiagnosis plus changes in underlying disease risk (decreasing risk in younger individuals and increasing risk in older males). Cohort effects were identified for male and female mortality and male incidence but were not as apparent for female incidence, suggesting that period effects have had a greater influence on changes in incidence over time in females. Encouraging trends included long-term declines in mortality in younger individuals and recent stabilization of invasive incidence in individuals aged 15-44 and males aged 45-54. Melanoma in-situ incidence, however, has continued to increase throughout the population. Overdiagnosis appears to be relatively greater in American females and for melanoma in-situ.
Introduction
Overdiagnosis refers to the detection of asymptomatic disease that would not have otherwise become clinically apparent during a patient’s life. It can occur due to more sensitive or intensive screening or from changing the disease classification threshold or nomenclature (Brodersen et al., 2018). Overdiagnosis is problematic because the patient derives no benefit and can be potentially harmed from both the diagnosis and resultant treatment. Growing evidence suggests that overdiagnosis may be particularly common for some cancers in the United States (US) (Welch and Black, 2010). Welch et al examined incidence and mortality trends of common cancers and identified “epidemiologic signatures” that may indicate overdiagnosis (Welch et al., 2019). In particular, the discordant combination of rising incidence and stable mortality, which was identified in thyroid cancer, kidney cancer, and cutaneous melanoma, was interpreted to primarily indicate overdiagnosis. This has also led to a re-evaluation of the potential causes of the increase in melanoma incidence as well as the efficacy of prevention efforts on mortality (Welch et al., 2021).
There are few published reports examining epidemiologic trends of melanoma in the US through the lens of overdiagnosis considering demographic factors, period effects, and cohort effects. Such an analysis is particularly relevant to melanoma as ultraviolet radiation (UVR) exposure, dermatologic care, and public awareness have changed over time and are heterogenous throughout the population. In addition, effective therapies for metastatic disease have only been available since 2011. We present an analysis of incidence and mortality trends in melanoma stratified by age and sex and consider period and cohort effects to help elucidate relative differences in overdiagnosis among subgroups of the population. These data might allow appropriate changes in prevention strategies that could improve the benefit-to-harm trade-off.
Results
Patient Characteristics
The nine SEER registries reported 268,109 first cases of melanoma in white individuals (55.2% male) from 1975-2017. There were 175,442 first cases of invasive melanoma (55.3% male) and 105,385 first cases of in-situ melanoma (56.3% male). During this same period, there were 291,214 deaths (63.0% male) among white individuals across the entire US attributed to melanoma.
Overall Period Trends
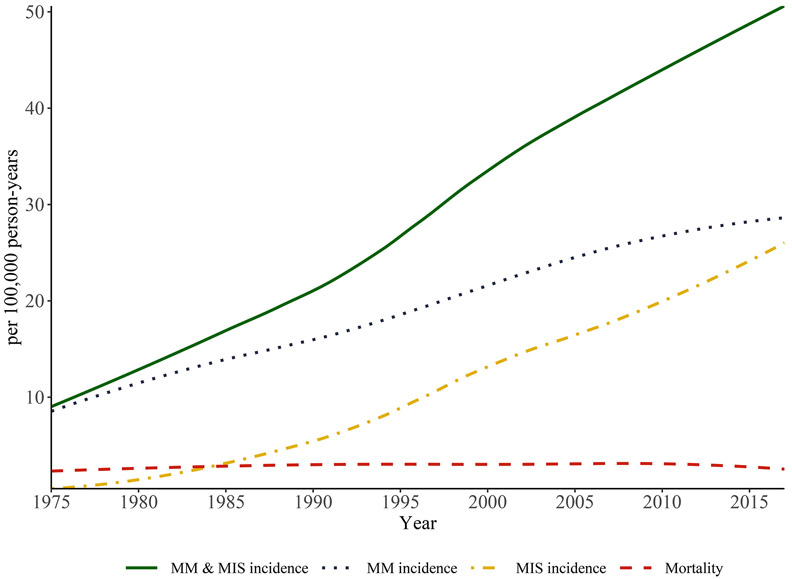
Melanoma incidence increased 41.6 per 100,000 (+459.0%) from 1975-2017; invasive and in-situ incidence increased 20.1 per 100,000 (+235.8%) and 25.4 per 100,000 (+4,675.0%), respectively (Figure 1 and Supplementary Table S1). Mortality increased 0.8 per 100,000 (+34.2%) from 1975-2010 and decreased 0.6 per 100,000 (−20.0%) from 2010-2017.
Figure 1: Age-adjusted rates of melanoma incidence and mortality in the United States, 1975-2017.
Incidence data are from the SEER Program, SEER 9 Registries (five states [Connecticut, Hawaii, Iowa, New Mexico, and Utah] and four metropolitan areas [Atlanta, Detroit, San Francisco, and Seattle]). All ages are included, and all rates are age-adjusted to the 2000 U.S. standard population. Mortality data are from the National Vital Statistics System maintained by the National Center for Health Statistics. Incidence = cases of invasive and in-situ melanoma. MM incidence = cases of invasive melanoma. MIS incidence = cases of in-situ melanoma. Mortality = cases of melanoma death.
Discordant Age-Sex Trends in Incidence and Mortality
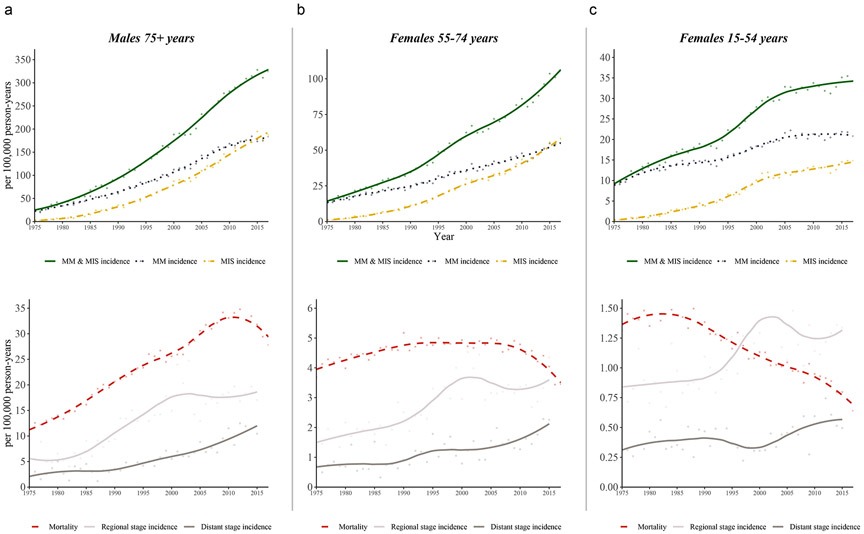
Not all age-sex groups (Supplementary Figure S1 and Supplementary Table S1) demonstrated rising incidence and stable mortality from 1975-2017 evident in the population-level overall period analysis. Three distinct signatures were identified: (1) rising incidence and stable mortality (for example, females aged 55-74), (2) a disproportionate rise in incidence compared to an increase in mortality (for example, males aged 75+), and (3) a rise in incidence and a decrease in mortality (for example, females aged 15-44) (Figure 2).
Figure 2: Representative epidemiological signatures in age-sex groups.
The age-sex group that showed trends most suggestive of melanoma overdiagnosis alone were in females, aged 55-74 years (B). Other groups showed mixed effects, suggestive of overdiagnosis plus changes in underlying disease risk. For example, males aged 75+ years (A) had a disproportionate increase in incidence compared to the increase in mortality (increase in true melanoma risk plus overdiagnosis). Females aged 15-44 years (C) had rising incidence but declining mortality (decrease in true melanoma risk plus overdiagnosis). Changes in the incidence of regional and distant cases over time are most likely due to differences in staging practices over time (that is, “up-staging” due to increased use of imaging and sentinel lymph node biopsy).
Trends in incidence by Breslow thickness from 1988-2017 across age-sex groups showed that most of the increase in incidence occurred in diagnoses of in-situ and thinly invasive (<1mm) disease (Supplementary Figure S2). The only age-sex group with a decline in >1-2mm and >2mm melanoma incidence occurred in males aged 15-44. In all other age-sex groups, it either remained stable or increased. The greatest increase in thick melanoma incidence occurred in males aged 55+ and females aged 65+.
Although invasive incidence continuously increased from 1975-2017 in the overall population, it has plateaued in some age-sex groups: males aged 15-44 (beginning in 1985), males aged 45-54 (beginning in 1995), males aged 55-64 (beginning in 2005), and females aged 15-54 (beginning in 2005). The melanoma in-situ incidence rate, however, has not stabilized in any age-sex group.
Age- and Sex-Related Effects on Mortality, Incidence, and the Incidence-to-Mortality Ratio
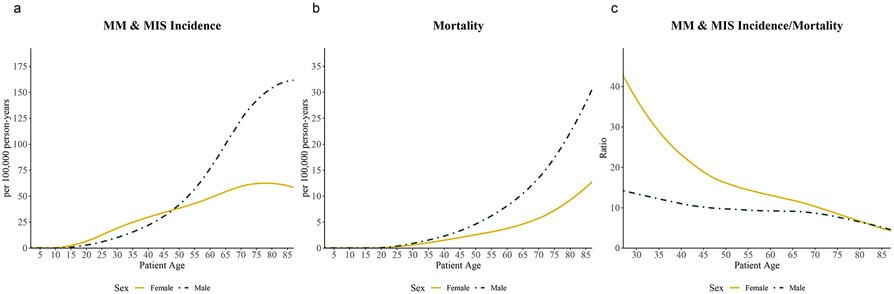
Mortality rates increase exponentially with age (Figure 3). These rates are similar in magnitude in males and females until age 25, after which rates are higher in males. Incidence rates also increase with age; however, incidence increases exponentially in males and linearly in females. Unlike mortality, incidence is higher in females than males until age 50. After 50 years, incidence sharply increases in males and is double that of females by age 70. Thus, the ratio of incidence-to-mortality in females is two to three times as high as in males from ages 20 to 40; the difference in ratios becomes smaller with increasing age until it is approximately equal in males and females 80+ years. Age effects were similar over stratified time periods and for melanoma in-situ and invasive melanoma separately, respectively (data not shown).
Figure 3: Effect of age on average melanoma incidence and mortality rates from 1975-2017.
Incidence rate (A), mortality rate (B), and incidence-to-mortality ratio (C) stratified by sex and 5- year-grouped age categories, averaged from 1975-2017. Curves fit with LOESS regression.
The incidence-to-mortality ratio increased from 3.9 to 14.0 (+261.4%) from 1975-2010. From 1975-1995, this ratio increased at a similar rate in females [4.7 to 11.0 (+132.5%)] and males [3.3 to 7.4 (+126.2%)] (Supplementary Figure S1). From 1995-2010, the incidence-to-mortality ratio increased more in females [11.0 to 19.1 (+73.9%)] than in males [7.4 to 11.5 (+53.8%)]. This disproportionate temporal increase in the incidence-to-mortality ratio was driven by a comparatively greater increase in melanoma incidence in females vs. males aged 15-54, despite similar declines in the mortality rate.
Birth Cohort Effects on Incidence and Mortality
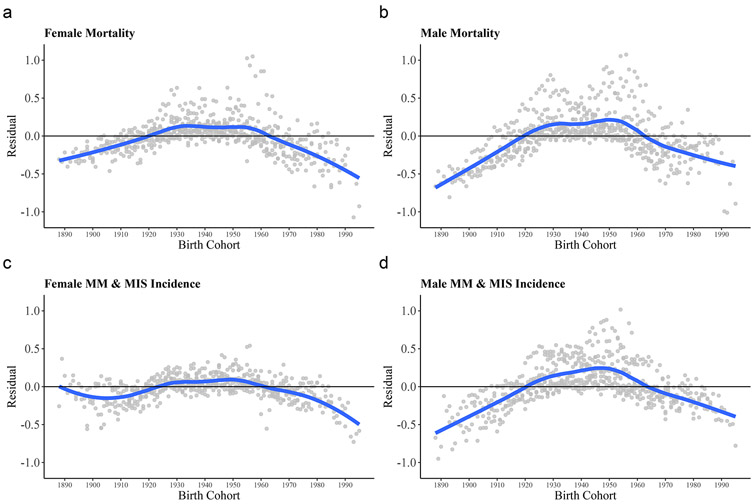
Non-parallel changes in age-specific incidence and mortality rates plotted by sex across years of birth suggested that age and period effects alone do not fully account for the trends in these rates and that the variation includes cohort effects (Supplementary Figure S3). The birth cohort residuals and estimated rate ratios for the effect of birth cohort on melanoma incidence and mortality are shown in Figure 4 and Supplementary Table S1. Strong cohort effects on mortality rates among males and females were observed, but the effects were relatively greater in males. After removing the effects of age and period, cohorts born during 1890-1920 and 1960-2000 had lower mortality than those born from 1920-1960, with the highest risk being those born at 1950. Cohort effects were also evident for male incidence. Female incidence showed less pronounced evidence of cohort effects until generations beginning with 1990, at which the risk of diagnosis had declined (independent of age/period effects) compared to those born in 1950.
Figure 4: Birth cohort residuals of the median polish analysis.
Dots represent the residuals from the median polish procedures plotted against year of birth. Four median polish procedures modelled absolute age-adjusted male and female mortality (A, B), and incidence (C, D) by adult (≥ 20 years) 5-year-grouped age categories and year of occurrence from 1975-2017. The curve fit of the residuals is produced from LOESS regression. Rates were transformed by taking natural logarithms prior to fitting the median polish models to analyze the interaction of age and period on the multiplicative scale. Systematic deviation from 0.0 suggests the presence of a birth cohort effect.
Discussion
We present an analysis of trends in melanoma incidence and mortality rates in the US from 1975-2017. Overall, there were complex patterns in the trends of these rates. We identified evidence to suggest overdiagnosis, which appeared relatively greater in middle-aged and younger females. We also identified evidence of a true epidemic of disease, which was most apparent in older males. Positive findings include the success in reducing deaths in contemporary cohorts and stabilization of invasive incidence in younger age groups. Of concern, the increase in melanoma in-situ incidence was particularly high and it has not yet stabilized or decreased in any age-sex group.
From 1975 to 2011, females aged 55-74 most clearly demonstrated rising incidence and stable mortality. Overdiagnosis alone could account for these discordant trends, as a true increase in cancer occurrence should be accompanied by an increase in mortality. For mortality to remain stable, a synchronous annual counterbalancing of improved treatment and/or detection would be required to prevent additional deaths. As there was no effective systemic therapy for melanoma prior to 2011 and fewer than 20% of US adults have ever received a screening total body skin examination in their lifetime (Lakhani et al., 2014), a true rise in cancer occurrence appears unlikely. Although the rise in incidence of regional and distant metastatic cases in these individuals could be interpreted as a true increase in cancer occurrence, “up-staging” is a more likely cause due to temporal changes in staging (i.e., use of sentinel lymph node biopsy and whole-body imaging with computed tomography).
Prior to 2011, males aged 75+ had both rising incidence and mortality, suggesting an increase in true cancer occurrence. In line with this observation, the incidence of thicker tumors ≥1mm also substantially increased in older males. However, the relative increase in incidence compared to mortality was disproportionate, suggesting additional overdiagnosis. Although an incongruent rise in incidence vs. mortality over time might be due to an increase in true cancer occurrence plus effective secondary prevention mitigating the rise in the observed mortality or causing lead time bias, these factors appear unlikely. First, the penetrance of screening total body skin examinations in the population remains low. Second, the efficacy of physician-based melanoma screening examinations in reducing melanoma-related deaths remains unproven. Although low-to-moderate quality data (Aitken et al., 2010, Bibbins-Domingo et al., 2016, Schneider et al., 2008) suggests that screening could reduce melanoma mortality, this has not yet been proven through a randomized trial, and at present the United States Preventive Task Force considers there to be insufficient evidence to support physician-based screening in the general population.
Females aged 15-54 years had rising incidence but declining mortality. Such a relationship is most commonly found after introduction of effective screening, but young Americans are the least likely to have ever received a total body skin examination (Lakhani et al., 2014). In addition, the magnitude of the decline in mortality (~50%) parallels or surpasses mortality declines found in cancers with widely implemented and effective screening (i.e., breast cancer in women >40 years of age, colon cancer in individuals >50 years of age) (Welch et al., 2019). If effective secondary prevention was the primary factor leading to a decrease in mortality, one would additionally expect a decrease in the incidence of thicker melanomas due to earlier diagnoses. Among females aged 15-54 years, however, the incidence of thicker melanomas increased. Young females may be at particular risk of having a Spitz nevus/tumor be misdiagnosed as melanoma, which could contribute to overdiagnosis of thicker tumors. These lesions are most prevalent in younger females, present as dermal nodules and are associated with false-positive melanoma diagnoses (Dika et al., 2017, Orchard et al., 1997). The absence of improving medical therapy suggests that a decrease in true occurrence risk plus overdiagnosis may be the most likely explanation for these discordant trends. A decline in true occurrence risk could be due to effective primary prevention and possibly the successful removal of potential melanoma precursors (that is, congenital and dysplastic nevi).
The non-concordant temporal changes in incidence and mortality in older and younger individuals suggested that the variance included cohort effects (that is, factors that uniquely affect a birth generation through age-specific exposure or susceptibility). After removing the effects of age and period, male and female generations born in the US from 1920-1960 were found to be at a relatively increased risk of melanoma mortality, consistent with previous analyses (Roush et al., 1992, Scotto et al., 1991). Autier et al (Autier et al., 2015) identified that cohort effects explained changes in melanoma mortality over time better than period effects and postulated that excessive UVR exposure of children and adolescents from 1900-1960 was probably responsible for the epidemic of fatal melanoma (Albert and Ostheimer, 2003). In particular, the 1920-1940s was characterized by a zealous enthusiasm for UVR exposure as a panacea for health (Albert and Ostheimer, 2003, Sorene, 2015) and the skin of young children was not uncommonly exposed to ultraviolet radiation (UVR) lamps by the medical community (Sorene, 2015). Childhood is thought to be a particularly susceptible window for the long-term harmful effects of UVR on melanoma risk (Green et al., 2011).
Cohort effects were similarly present for incidence in males but were not apparent for incidence in females until generations born after 1980. The presence of cohort effects on female mortality and absence of cohort effects on female incidence suggests that changes in female incidence over time are predominantly explained by period effects (that is, factors that affect the entire population during the same period time). A possible explanation is that there is a greater degree of overdiagnosis in females vs. males, which would appear as a period effect. This could result from more scrutiny for melanoma due to higher rates of overall health care use, total body skin examinations, and skin self-examinations in females (Berwick et al., 1996, Lakhani et al., 2014, Manuel, 2018, Xu and Borders, 2003).
The incidence-to-mortality ratio was higher in younger women vs. men; with increasing age, the ratios became more similar until equivalency at ages 80+. The primary reason for this discrepancy is a higher incidence, but lower mortality, in younger females vs. younger males. Multiple factors could contribute to these observations. First, there may be a paradoxical age-dependent sex difference (Natale et al., 2018) in melanoma risk and survival. Indeed, higher overall melanoma survival in females compared to males (Hieken et al., 2020, Scoggins et al., 2006) has been suggested to be related to intrinsic biologic sex differences (Natale et al., 2018). Unique age-related differences in melanoma risk by sex could be due to indoor tanning, which is more prevalent in young females (2012). An alternative explanation is that there is a greater degree of overdiagnosis in females vs. males.
There are likely multiple contributing factors to the disproportionate rise of in-situ melanoma. First, the diagnostic criteria used by pathologists have changed over time (1992, Davis and Little, 1977, Dubow and Ackerman, 1990, Elder et al., 2020, Hirst, 1977). Second, population-based ecological studies have shown that increased skin biopsies are associated with increased diagnoses of in-situ, but not invasive, melanoma (Weinstock et al., 2017, Welch et al., 2005). Third, newer diagnostic technologies have allowed detection of clinically featureless tumors (Brouha et al., 2021, Carli, 2007, Kittler et al., 2006). Concerningly, a large study of pathologists in the US demonstrated that the diagnosis of in-situ melanoma is neither reproducible nor accurate (Elmore et al., 2017).
There are limitations to this study. First, mortality and incidence data were drawn from unique datasets that differ in geographical coverage of the country. To mitigate race/ethnicity accounting for disparate trends in incidence and mortality we limited analyses to white individuals. Analyses assumed that the completeness of case reporting has been similar over time. Reporting of incident cases of melanoma to registries has previously shown to be sub-optimal and there has been a recent trend toward electronic reporting (Cockburn et al., 2008, Raji et al., 2015). If the reporting of incident melanoma cases to registries improved over time, it could lead to the appearance of an artificial rise in incidence and the false interpretation of overdiagnosis. Inferences made from examining trends in incidence and mortality should be cautiously interpreted; as this study was descriptive, we can only speculate about potential explanations for the observed melanoma trends. Ultimately, the most reliable method to identify overdiagnosis is through a randomized trial (Carter et al., 2015, Duffy et al., 2010).
In conclusion, long-term trends in melanoma incidence and mortality vary among subsets of the population, suggesting an interplay of age, sex, period, and cohort effects. There is evidence to suggest overdiagnosis throughout the population. Time-varying factors, however, make it challenging to precisely quantify overdiagnosis but it appears greater in females. Further research is needed to identify how to limit overdiagnosis. A re-evaluation of the benefits and harms of diagnosing and treating melanoma in-situ may be a starting point. Taken together, these data argue for the need to refocus detection pressure to groups at highest risk of death from melanoma and to improve diagnosis of potentially lethal disease, perhaps through the use of more objective triage and diagnostic tests (Fried et al., 2020, Marchetti et al., 2021). Refining the ability to risk-stratify patients diagnosed with melanoma may also limit overtreatment (Grossman et al., 2020, Marchetti et al., 2020).
Materials and Methods
The study was exempt from Institutional Review Board review under federal regulation because the data were publicly available. All data were obtained from the Surveillance, Epidemiology, and End Result Program (SEER). Incidence data were drawn from SEER 9, which includes the states of Connecticut, Hawaii, Iowa, New Mexico, and Utah, and the cities of Detroit, Atlanta, San Francisco-Oakland, and Seattle-Puget Sound (~9.8% of the US population). New instances of cutaneous melanoma were defined from International Classification of Diseases (ICD)-0-3 histology codes 8720-8799 with ‘in-situ’ or ‘malignant’ behavior codes and primary sites C44.0-C44.9, and only those with a known patient age were included. Distinctly for each reported outcome, if a patient had more than one instance in the registry, only the first record was included. Data for mortality attributed to ‘Melanoma of the Skin’ is provided by the National Center for Health Statistics and covers the entire US population. Year-, age-, and sex-specific incidence and mortality rates were extracted and age-adjusted to the 2000 US standard population. Additionally for each recorded case of melanoma, the year and age (19-category in 5 year age groups) at diagnosis, sex, tumor staging, and Breslow thickness were extracted. Tumor staging was defined according to SEER historic stage A, which is derived from various schemas used during the period. Breslow thickness data was not available prior to 1988. Instances of in-situ melanoma by ICD-0-3 codes were considered in-situ even when a thickness of >0 mm was indicated (2.2% of cases). The analyses were limited specifically to white individuals, the more susceptible population, to account for potential racial-demographic shifts in the overall US population (Crombie, 1979, Hobbs and Stoops, 2002).
Relationships between melanoma incidence rates [invasive, in-situ, and combined invasive or in-situ], mortality rates, and the combined incidence-to-mortality ratio were assessed over the period of 1975-2017. Estimated rates were stratified by sex and five age classes as previously recommended (Corazziari et al., 2004) for standardized cancer survival analysis (15-44, 45-54, 55-64, 65-74, and 75+). Rates are reported in terms of per 100,000 individuals per year. The presence of overdiagnosis was estimated by qualitatively examining temporal trends in incidence and mortality for previously described epidemiological signatures attributed to cancer (Oke et al., 2018, Welch et al., 2019). In addition, five-year recorded ages were used to analyze birth cohort effect as well as continuous age-specific effects. Given the identification problem with age-period-cohort analyses, birth cohort effects were conceptualized as a partial interaction between age and period rather than an independent effect (Keyes and Li, 2010). Median polish was used to remove the log-additive components of age and period effects (Keyes and Li, 2010). The resulting residuals were modelled by 10-year period birth cohorts using linear regression (ordinary least squares). Relative birth cohort rate ratios were derived by exponentiating the resulting coefficients from the linear regression model.
Breslow thickness was undefined for 6.6% of cases in SEER (ranging from 19% of cases in 1988 to <4% of cases in 2017) and imputed using multivariable imputation with chained equations (MICE). Similar imputation methods were used for regional and distant staging (undefined in 12% of cases in 1975 to <2% of cases in 2015). Both tumor staging and thickness were defined as ordinal categorical variables, and a proportional odds model was selected as the MICE imputation method, which controlled for year, sex, and age (19-category in 5-year age groups) as independent factors.
Data were exported from SEER and statistical analyses were performed in R using base, stats, dplyr, tidyr, readxl, ggplot2, mice, wesanderson, extrafont, grid, gridExtra, and reshape2 packages. Periodic trend was approximated with locally estimated scatterplot smoothing, using a smoothing parameter of ½ and reported rates and relative rates estimated from the smoothed trends.
Supplementary Material
Funding:
This work was supported by the MSKCC institutional National Cancer Institute Cancer Center Support Grant P30 CA008748.
Role of the funder:
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations:
- SEER
Surveillance, Epidemiology, and End Result Program
- US
United States
- UVR
ultraviolet radiation
- ICD
International Classification of Diseases
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
R. H: Funding for other research projects from the Melanoma Research Foundation and the Veterans Affairs Integrated Service Network 1. Scientific Officer, Evereden (makes personal care products, including sunscreen).
V.R.: Expert Advisor, Inhabit Brands, Inc
All remaining authors report no potential conflicts of interest for this work.
Data availability:
All data used in the preparation of this manuscript are publicly available.
References:
- NIH Consensus conference. Diagnosis and treatment of early melanoma. Jama 1992;268(10):1314–9. [DOI] [PubMed] [Google Scholar]
- Use of indoor tanning devices by adults--United States, 2010. MMWR Morb Mortal Wkly Rep 2012;61(18):323–6. [PubMed] [Google Scholar]
- Aitken JF, Elwood M, Baade PD, Youl P, English D. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer 2010;126(2):450–8. [DOI] [PubMed] [Google Scholar]
- Albert MR, Ostheimer KG. The evolution of current medical and popular attitudes toward ultraviolet light exposure: part 2. Journal of the American Academy of Dermatology 2003;48(6):909–18. [DOI] [PubMed] [Google Scholar]
- Autier P, Koechlin A, Boniol M. The forthcoming inexorable decline of cutaneous melanoma mortality in light-skinned populations. Eur J Cancer 2015;51(7):869–78. [DOI] [PubMed] [Google Scholar]
- Berwick M, Begg CB, Fine JA, Roush GC, Barnhill RL. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst 1996;88(1):17–23. [DOI] [PubMed] [Google Scholar]
- Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Ebell M, Epling JW Jr., et al. Screening for Skin Cancer: US Preventive Services Task Force Recommendation Statement. Jama 2016;316(4):429–35. [DOI] [PubMed] [Google Scholar]
- Brodersen J, Schwartz LM, Heneghan C, O'Sullivan JW, Aronson JK, Woloshin S. Overdiagnosis: what it is and what it isn't. BMJ Evid Based Med 2018;23(1):1–3. [DOI] [PubMed] [Google Scholar]
- Brouha B, Ferris LK, Skelsey MK, Peck G, Rock J, Nguyen A, et al. Genomic Atypia to Enrich Melanoma Positivity in Biopsied Lesions: Gene Expression and Pathology Findings From a Large U.S. Registry Study. J of Skin 2021;[Internet]. 2021 Jan.1 [cited 2021Jun.15];5(1):13–8. Available from: https://jofskin.org/index.php/skin/article/view/1146. [Google Scholar]
- Carli P Identification of incipient tumors by means of sequential dermoscopy imaging: a new way to inflate the "epidemic" of melanoma? Archives of dermatology 2007;143(6):805; author reply -6. [DOI] [PubMed] [Google Scholar]
- Carter JL, Coletti RJ, Harris RP. Quantifying and monitoring overdiagnosis in cancer screening: a systematic review of methods. BMJ (Clinical research ed) 2015;350:g7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn M, Swetter SM, Peng D, Keegan TH, Deapen D, Clarke CA. Melanoma underreporting: why does it happen, how big is the problem, and how do we fix it? Journal of the American Academy of Dermatology 2008;59(6):1081–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer 2004;40(15):2307–16. [DOI] [PubMed] [Google Scholar]
- Crombie IK. Racial differences in melanoma incidence. Br J Cancer 1979;40(2):185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N, Little JH. Malignant melanoma in situ. Aust N Z J Surg 1977;47(3):379–80. [DOI] [PubMed] [Google Scholar]
- Dika E, Ravaioli GM, Fanti PA, Neri I, Patrizi A. Spitz Nevi and Other Spitzoid Neoplasms in Children: Overview of Incidence Data and Diagnostic Criteria. Pediatr Dermatol 2017;34(1):25–32 [DOI] [PubMed] [Google Scholar]
- Dubow BE, Ackerman AB. Ideas in pathology. Malignant melanoma in situ: the evolution of a concept. Mod Pathol 1990;3(6):734–44. [PubMed] [Google Scholar]
- Duffy SW, Tabar L, Olsen AH, Vitak B, Allgood PC, Chen TH, et al. Absolute numbers of lives saved and overdiagnosis in breast cancer screening, from a randomized trial and from the Breast Screening Programme in England. J Med Screen 2010;17(1):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder DE, Bastian BC, Cree IA, Massi D, Scolyer RA. The 2018 World Health Organization Classification of Cutaneous, Mucosal, and Uveal Melanoma: Detailed Analysis of 9 Distinct Subtypes Defined by Their Evolutionary Pathway. Arch Pathol Lab Med 2020;144(4):500–22. [DOI] [PubMed] [Google Scholar]
- Elmore JG, Barnhill RL, Elder DE, Longton GM, Pepe MS, Reisch LM, et al. Pathologists' diagnosis of invasive melanoma and melanocytic proliferations: observer accuracy and reproducibility study. BMJ (Clinical research ed) 2017;357:j2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried L, Tan A, Bajaj S, Liebman TN, Polsky D, Stein JA. Technological advances for the detection of melanoma: Advances in diagnostic techniques. Journal of the American Academy of Dermatology 2020;83(4):983–92. [DOI] [PubMed] [Google Scholar]
- Green AC, Wallingford SC, McBride P. Childhood exposure to ultraviolet radiation and harmful skin effects: epidemiological evidence. Prog Biophys Mol Biol 2011;107(3):349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman D, Okwundu N, Bartlett EK, Marchetti MA, Othus M, Coit DG, et al. Prognostic Gene Expression Profiling in Cutaneous Melanoma: Identifying the Knowledge Gaps and Assessing the Clinical Benefit. JAMA dermatology 2020;156(9):1004–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieken TJ, Glasgow AE, Enninga EAL, Kottschade LA, Dronca RS, Markovic SN, et al. Sex-Based Differences in Melanoma Survival in a Contemporary Patient Cohort. J Womens Health (Larchmt) 2020;29(9):1160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst E Comments on the histological staging of melanoma. Aust N Z J Surg 1977;47(3):377–8. [DOI] [PubMed] [Google Scholar]
- Hobbs F, Stoops N. Demographic Trends in the 20th Century, http://www.census.gov/prod/2002pubs/censr-4.pdf; 2002. [accessed October 29.2021].
- Keyes KM, Li G. A multiphase method for estimating cohort effects in age-period contingency table data. Ann Epidemiol 2010;20(10):779–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler H, Guitera P, Riedl E, Avramidis M, Teban L, Fiebiger M, et al. Identification of clinically featureless incipient melanoma using sequential dermoscopy imaging. Archives of dermatology 2006; 142(9):1113–9. [DOI] [PubMed] [Google Scholar]
- Lakhani NA, Saraiya M, Thompson TD, King SC, Guy GP Jr. Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev Med 2014;61:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel JI. Racial/Ethnic and Gender Disparities in Health Care Use and Access. Health Serv Res 2018;53(3):1407–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti MA, Coit DG, Dusza SW, Yu A, McLean L, Hu Y, et al. Performance of Gene Expression Profile Tests for Prognosis in Patients With Localized Cutaneous Melanoma: A Systematic Review and Meta-analysis. JAMA dermatology 2020;156(9):953–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti MA, Nanda JK, Mancebo SE, Dusza SW. Real-World Application of a Noninvasive Two-Gene Expression Test for Melanoma Diagnosis. The Journal of investigative dermatology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale CA, Li J, Zhang J, Dahal A, Dentchev T, Stanger BZ, et al. Activation of G protein-coupled estrogen receptor signaling inhibits melanoma and improves response to immune checkpoint blockade. Elife 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oke JL, O'Sullivan JW, Perera R, Nicholson BD. The mapping of cancer incidence and mortality trends in the UK from 1980-2013 reveals a potential for overdiagnosis. Sci Rep 2018;8(1):14663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard DC, Dowling JP, Kelly JW. Spitz naevi misdiagnosed histologically as melanoma: prevalence and clinical profile. The Australasian journal of dermatology 1997;38(1):12–4. [DOI] [PubMed] [Google Scholar]
- Raji KO, Payne L, Chen SC. Reporting Melanoma: A Nationwide Surveillance of State Cancer Registries. Journal of skin cancer 2015;2015:904393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roush GC, McKay L, Holford TR. A reversal in the long-term increase in deaths attributable to malignant melanoma. Cancer 1992;69(7):1714–20. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Moore DH 2nd, Mendelsohn ML. Screening program reduced melanoma mortality at the Lawrence Livermore National Laboratory, 1984 to 1996. Journal of the American Academy of Dermatology 2008;58(5):741–9. [DOI] [PubMed] [Google Scholar]
- Scoggins CR, Ross MI, Reintgen DS, Noyes RD, Goydos JS, Beitsch PD, et al. Gender-related differences in outcome for melanoma patients. Ann Surg 2006;243(5):693–8; discussion 8-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotto J, Pitcher H, Lee JA. Indications of future decreasing trends in skin-melanoma mortality among whites in the United States. Int J Cancer 1991;49(4):490–7. [DOI] [PubMed] [Google Scholar]
- Sorene P Light Therapy fo Naked Children, Delicate Adults, Sick Pigs, and Quacks (Photos: 1900-1950), https://flashbak.com/light-therapy-for-naked-children-delicate-adults-sick-pigs-and-quacks-photos-1900-1950-41389/; 2015. [accessed April 15.2021].
- Weinstock MA, Lott JP, Wang Q, Titus LJ, Onega T, Nelson HD, et al. Skin biopsy utilization and melanoma incidence among Medicare beneficiaries. The British journal of dermatology 2017;176(4):949–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst 2010;102(9):605–13. [DOI] [PubMed] [Google Scholar]
- Welch HG, Kramer BS, Black WC. Epidemiologic Signatures in Cancer. N Engl J Med 2019;381(14):1378–86. [DOI] [PubMed] [Google Scholar]
- Welch HG, Mazer BL, Adamson AS. The Rapid Rise in Cutaneous Melanoma Diagnoses. N Engl J Med 2021;384(1):72–9. [DOI] [PubMed] [Google Scholar]
- Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ (Clinical research ed) 2005;331(7515):481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu KT, Borders TF. Gender, health, and physician visits among adults in the United States. Am J Public Health 2003;93(7):1076–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in the preparation of this manuscript are publicly available.