Abstract
The NACHT, leucine-rich repeat (LRR), and pyrin domain (PYD)-containing protein 3 (NLRP3) inflammasome is an intracellular sensing protein complex that plays a major role in innate immunity. Following tissue injury, activation of the NLRP3 inflammasome results in cytokine production, primarily interleukin(IL)-1β and IL-18, and, eventually, inflammatory cell death – pyroptosis. While a balanced inflammatory response favors damage resolution and tissue healing, excessive NLRP3 activation causes detrimental effects. A key involvement of the NLRP3 inflammasome has been reported across a wide range of cardiovascular diseases (CVDs). Several pharmacological agents selectively targeting the NLRP3 inflammasome system have been developed and tested in animals and early phase human studies with overall promising results. While the NLRP3 inhibitors are in clinical development, multiple randomized trials have demonstrated the safety and efficacy of IL-1 blockade in atherothrombosis, heart failure and recurrent pericarditis. Furthermore, the non-selective NLRP3 inhibitor colchicine has been recently shown to significantly reduce cardiovascular events in patients with chronic coronary disease. In this review, we will outline the mechanisms driving NLRP3 assembly and activation, and discuss the pathogenetic role of the NLRP3 inflammasome in CVDs, providing an overview of the current and future therapeutic approaches targeting the NLRP3 inflammasome.
Keywords: NLRP3, interleukin-1, interleukin-18, inflammation, cardiovascular diseases, thrombosis, heart failure, pericarditis, COVID-19
1. Introduction
Innate immunity is a conserved stereotyped response that is necessary for the prompt identification of infectious pathogens [1]. The innate immune response is largely based on pattern recognition. Infectious pathogens share a variety of molecules, called pathogen-associated molecular patterns (PAMPs), that possess similar chemical properties. A large variety of pathogens can be recognized by a small set of pattern recognition receptors (PRRs) [1]. Activation of PRRs induces a rapid and robust local and systemic inflammatory response resulting in production of several pro-inflammatory cytokines including interleukin (IL)-1β, IL-6 and IL-18. While lymphoid-mediated acquired immunity prepares antibody- and cell-mediated defenses during the first days of infection, these cytokines crucially activate myeloid-mediated innate immunity. Adequate cooperation of the innate and acquired immune responses, finely orchestrated by cytokines, ultimately leads to confinement and eradicatation of the pathogen [2].
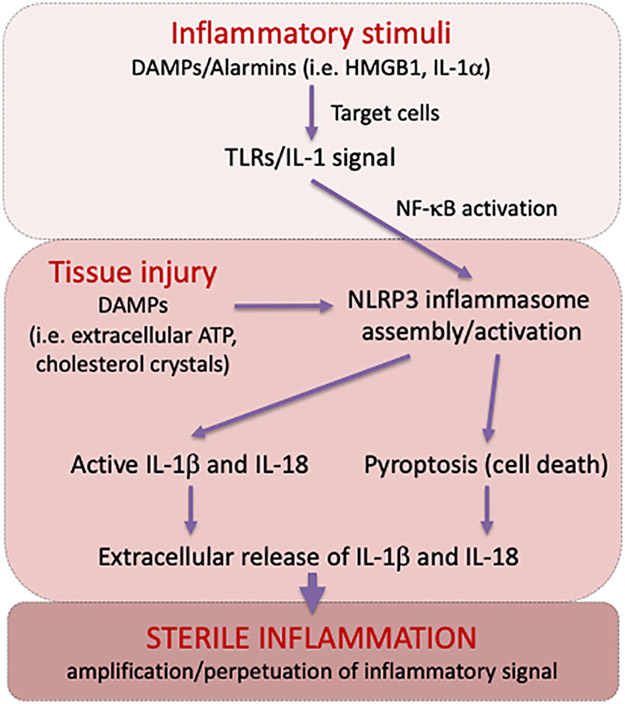
PRRs also recognize self-derived molecules, defined as damage-associated molecular patterns (DAMPs) (Figure 1) [1-2]. DAMPs are released following tissue stress or injury, and serve as ‘alarmins’ to boost the inflammatory process, clear dead cells and initiate tissue healing [3]. DAMPs thus enable differential innate immune responses to commensal and non-commensal organisms [2,3].
Figure 1.
Overview of the key molecular events driving the inflammatory response following tissue injury.
This primordial innate immune response is considered an essential first-line defense to infections. Indeed, invididuals with inherited or acquired defects in the innate immunity are subject to deadly infections [1]. On the other hand, an exaggerated innate immune response exacerbates tissue damage and worsens prognosis [1]. In recent times, this adverse consequence has been evident in coronavirus disease 19 (COVID-19), in which hyperinflammation secondary to severe acute respiratory syndrome (SARS-CoV-2) infection significantly contributes to worse disease manifestations [4].
In the setting of chronic degenerative diseases, overactivation of PRRs drives chronic inflammation, which aggravates disease progression and outcomes. While it is well-established that inflammation plays a causal role in rheumatic disorders, the contribution of dysregulated innate immunity is now recognized in the development and progression of a wide range of non-rheumatic diseases such as cardiovascular, onco-hematological and neurodegenerative conditions [5-10].
The nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) are PRRs that recognize a wide range of pathogens and danger-associated products [8]. The different members of the NLR family form multimolecular complexes named inflammasomes [8]. An inflammasome is a multiprotein complex that leads to the activation of a pro-inflammatory caspase (e.g. caspase-1) and promotes the release of cytokines of the IL-1β family [8]. The NACHT, leucine-rich repeat (LRR), and pyrin domain (PYD)-containing protein 3 (NLRP3) is the most extensively studied inflammasome, and its involvement has been demonstrated in several rheumatic and non-rheumatic diseases [9]. Besides the NLRP3 inflammasome, several other inflammasomes, including NLRP1, NLRC4 and AIM2, have been well characterized, and their role in health and disease has been reviewed elsewhere [8]. Within the cardiovascular field, recent clinical trials have validated the inflammatory hypothesis of atherosclerosis, and ongoing work has elucidated a role of the NLRP3 inflammasome following ischemic and non-ischemic, acute and chronic insults to the cardiovascular system [10]. In this review, we outline the processes beyond NLRP3 inflammasome formation and activation, and discuss the role of the NLRP3 in cardiovascular diseases (CVDs). In this context, current and future therapeutic approaches targeting the NLRP3 inflammasome will be also discussed.
2. The NLRP3 Inflammasome
NLRP3 acts as a receptor for immune/damage surveillance [8,9]. Activation of the NLRP3 inflammasome transforms the cell into a powerhouse for the production and release of inflammatory cytokines belonging to the IL-1 family. NLRP3 activity also determines cell fate, by potentially inducing, through the formation of gasdermin D (GSDMD) channels in the membrane, pyroptosis, an inflammatory cell death.
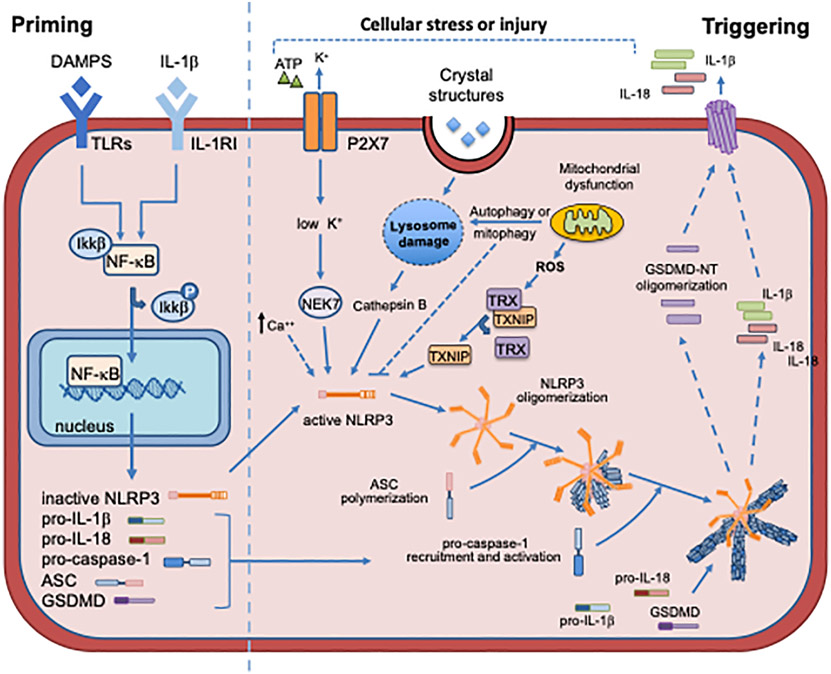
NLRP3 is a multi-domain protein. It has a leucine-rich repeats (LRRs) domain at the C-terminus, that is the “sensing” component for PAMPs and DAMPs. It also possesses a nucleotide-binding and oligomerization domain (NOD, also known as NACHT), which contains the active ATPase site through the Walker A motif (ATP-binding site) and the Walker B motif (ATPase activity). Once activated, the LRR domain induces NLRP3 to oligomerize through their NACHT domain (Figure 2) [10-24]. The effector pyrin domain (PYD) at the N-terminus is responsible for the downstream pro-inflammatory effects, binding to the adaptor protein ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain or CARD) through a PYD-PYD interaction [10,25-27]. This central oligomeric structure catalizes the polymerization of ASC into filamentous structures [25-27]. ASC then binds to the CARD of pro-caspase-1 to form the inflammasome [25-27]. Pro-caspase-1 auto-cleavage releases active caspase-1, which in turn activates pro-IL-1β, IL-18 and GSDMD. While IL-1β and IL-18 exert classical pro-inflammatory effects, the activated N-terminal GSDMD (NT-GSDMD) forms membrane pores that facilitate the extracellular release of IL-1β and IL-18 as well as inflammatory cell death - pyroptosis [10,28-30].
Figure 2.
Priming and triggering signals regulating the assembly and activation of the NLRP3 inflammasome. Primining signals result in the transcription, primarily through NF-κB, of the NLRP3 inflammasome components. Triggering signals result in: NLRP3 inflammasome assembly; caspase-1 activation: cleavage of pro-IL-1β and pro-IL-18 into the mature forms; cleavage of GSDMD which forms pores on the cell membrane, allowing secretion of active IL-1β and IL-18.
Two independent but concomitant signals are required to induce inflammasome formation (Figure 2). This represents a mechanism of control, in order to regulate the potent inflammatory and pyroptotic consequences of NLRP3 activation [31-34]. A ‘priming’ signal is necessary to induce the transcription of the components of the inflammasome (NLRP3, pro-IL-1β and pro-IL-18). A ‘triggering’ signal, instead, leads to the structural changes in NLRP3 domains necessary for the assembly of the inflammasome. An additional regulatory level of NLRP3 activity is provided by post-translational modifications such as ubiquitination and phosphorylation of inflammasome proteins, as outlined below [35-38].
The priming signal can be induced by PRRs, including toll-like receptors (TLRs) and NOD2, by cytokine receptors, inducing nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)-mediated transcriptional upregulation of the inflammasome components and substrates, as well as by pathways involved in cardiovascular regulation, such as the angiotensin receptor type 1 (AT1) or adrenergic receptors (Figure 2) [15,20,21,35].
The activation of the NLRP3 inflammasome signaling pathway is finely modulated by complex regulatory networks that include post-translational and post-transcriptional modifications. Numerous microRNAs (miRs) and long non-coding RNAs (lncRNAs) have been found to control the post-translational expression of the NLRP3 inflammasome proteins and substrates, by either reducing (miRs) or increasing (lncRNAs) NLRP3 expression [8,9,24,25]. Among these, miR22 reduces NLRP3 mRNA expression in rat coronary artery endothelial cells, whereas the lncRNA MALAT1 was shown to increase NLRP3 expression in the setting of acute myocardial infarction (AMI) [39,40]. Protein phosphorylation and ubiquitination also regulate post-translational priming and triggering of the NLRP3 inflammasome [41]. Cell experiments have shown that NLRP3, ASC and caspase-1 are targets of several kinases, phosphatases and ubiquitin ligases [41,42], although the role of these modifications in development and progression of CVDs has not been thoroughly investigated.
3. Mechanisms of Cardiac NLRP3 activation
Multiple alarmins and DAMPs can contribute to the priming phase in the heart. During AMI, the ischemic insult promotes the release of cellular debris and alarmins. In addition, chronic conditions such as obesity, hypertension or diabetes promote the priming through metabolites and neurohormonal activation (e.g. angiotensin II, fatty acids and glucose) [43-48]. For example, diabetic mice display basally increased levels of NLRP3 inflammasome components compared to their normoglycemic counterparts, and this exacerbates tissue damage in case of insults such as ischemia-reperfusion [49]. Numerous intracellular and extracellular signals activate NLRP3 [10]. These signals are unrelated and include intracellular pathways (e.g. reactive oxygen species [ROS], mitochondrial dysfunction, lysosome rupture) and extracellular signals (e.g. ATP-mediated activation of the purinergic-type 2 receptor X7 [P2X7], potassium efflux and calcium influx) (Figure 2).
3.1. ATP and K+ efflux
Extracellular ATP has been described as one of the signals capable of inducing NLRP3 activation [50,51]. The binding of ATP to the P2X7 opens the channel pore causing K+ efflux that leads to conformational changes of NLRP3, allowing the interaction with ASC (Figure 2) [50]. During ischemia, P2X7 activation is one of the main mechanisms driving the formation of the NLRP3 inflammasome in cardiomyocytes [52]. Bacterial toxins like nigericin form membrane pores, allowing K+ efflux independent of P2X7 activation [50,52]. The mitotic serine/threonine kinase NEK7, a member of the mammal NIMA-related kinases (NEK proteins) family, acts downstream of K+ efflux, and directly binds NLRP3, thereby regulating its oligomerization and activation (Figure 2) [53]. NEK7 is highly expressed in the heart, making this protein a possible target to inhibit NLRP3 inflammasome activity [54].
3.2. Ca2+ mobilization
Different stimuli as extracellular ATP, nigericin and particulates can induce Ca2+ mobilization from the endoplasmic reticulum (ER) or the extracellular space, leading to mitochondrial damage and subsequent NLRP3 activation [55]. One of the sensors of extracellular Ca2+ is the calcium-sensing receptor (CaSR), that seems to mediate the increase in intracellular Ca2+ and the decrease in cellular cyclic AMP (cAMP). Both events are associated with NLRP3 activation [56]. In rats, CaSR mediates NLRP3 activation in the setting of hypertension and AMI [57,58].
3.3. Lysosomal rupture
Cytoplasmatic leak of lysosomal content has been shown to activate NLRP3 [59]. Incomplete phagocytosis of crystals causes lysosome swelling and instability, leading to lysosomal rupture with release the of cathepsin B, a lysosomal enzyme able to activate NLRP3 (Figure 2) [59-63]. This mechanism is common to different inflammasome-activating stressors, such as monosodium urate crystals, calcium phosphate crystals and cholesterol crystals [59-63]. In particular, these last two have been correlated with the development of atherosclerotic plaques [63].
3.4. Autophagy
Autophagy comprises several cellular pathways that synergistically lead to compartmentalization and digestion of cellular protein and/or organelles [64]. In cells in culture, abrogation of the physiological autophagy, either by deletion of autophagy-inducer proteins (e.g. microtubule-associated proteins 1A/1B light chain 3B [LC3B], beclin 1, autophagy related [ATG] 5, ATG7) or treatment with autophagy inhibitors (e.g. 3-Methyladenine), is associated with induction of the NLRP3 inflammasome [65]. On the contrary, in cells already expressing the inflammasome, induction of autophagy through rapamycin or starvation results in reduced NLRP3-mediated signaling [65]. In acute myocardial infarction, autophagy limits cardiac damage, while its suppression can worsen cardiac remodeling [66,67]. In diabetic rats, induction of autophagy with rapamycin reduces the expression of the NLRP3 inflammasome components, which parallels with reduced infarct size after myocardial ischemia-reperfusion [68]. In vitro, exposure of rat cardiac myocytes H9C2 to high glucose or hypoxia induces the NLRP3 inflammasome, which is reduced by rapamycin treatement. In rats lacking NLRP3, experimental ischemia-reperfusion stimulates the autophagic flux together with reduced myocardial damage and infarct size [69].
Saturated fatty acids also promote inflammasome activation through autophagy and increase ROS production [70].
3.5. Reactive oxygen species and mitochondrial dysfunction
Mitochondria are the major source of DAMPS, including ROS, and are involved in the control of different types of cell death (necrosis/necroptosis, apoptosis as well as pyroptosis) [71]. Mitochondrial dysfunction and impaired mitochondrial autophagy (i.e. mitophagy) can cause increased ROS generation and cytosolic accumulation of dysfunctional mitochondria or mitochondrial DNA (mtDNA) leakage, which are potent NLRP3 activators (Figure 2) [71-74]. Other mitochondrial-derived molecules can mediate the activation of NLRP3. Among these, cardiolipin can directly bind NLRP3 through its LRR domain allowing its activation [75]. Also MAVS, a mitochondrial adaptor protein activated by ATP and nigericin, promotes NLRP3 oligomerization and ASC recruitment [76,77]. Mitochondrial dysfunction seems to play an important role in atherosclerosis, ischemia-reperfusion injury and pressure overload [78-80]. In addition, thioredoxin-interacting protein (TXNIP) plays a critical role in cellular redox control by binding to the oxidoreductase thioredoxin (TRX) [81]. In the setting of hyperglycemia and hypercholesterolemia, excessive ROS production and the presence of unfolded proteins can cause the dissociation of TXINP from TRX [81-83]. In this state, TXINP can bind and activate NLRP3 [84]. TXINP inhibition by siRNA has been shown to protect the heart after ischemia-reperfusion [85].
4. The NLRP3 inflammasome in cardiovascular diseases
Activation of the NLRP3 inflammasome has been importantly implicated in several CVDs. The dynamics of NLRP3 activation differs also between acute and chronic injury. Acute injury is associated with rapid and robust upregulation of the NLRP3 inflammasome [10,12,29,34]. In this setting, the therapeutic window to target the inflammasome tends to be narrower (i.e. hours-days) depending on the nature of the injury. On the contrary, in chronic conditions such as atherosclerosis, hypertension, diabetes, obesity, heart failure [HF], where low-grade basal activation of the NLRP3 inflammasome contributes to the disease progression, inhibition of NLRP3 inflammasome at different stages may alleviate worsening of the chronic condition, the therapeutic window is significantly wider (i.e. days-months-years)[10,12,29]. The impact may also be different in acute versus chronic setting. In the acute conditions, the inhibition of the NLRP3 inflammasome activity may lead to the rescue of viable tissue and provide ensuing large beneficial effects, but the efficacy will depend also by the nature of the injury and the time of the intervention [10,12,29]. In chronic conditions, the inhibition of the NLRP3 inflammasome can change the rate of progression of the degenerative illness, and the efficacy may depend also by the activity of the NLRP3 inflammasome as compared with the chronic nature of the condition and injury already performed, and the ability of the treatment to ameliorate and change trajectory of conditions that are likely to progress and/or recur over time [12,15,29]. We herein provide an overview of the potential role of NLRP3 in CVDs including atherosclerosis, ischemic heart disease, diabetic cardiomyopathy, hypertensive cardiomyopathy, dilated cardiomyopathy, drug-induced cardiotoxicity, myocarditis, cardiac sarcoidosis, pericarditis, venous thromboembolism and COVID-19.
4.1. Atherosclerosis
Atherosclerosis is a chronic inflammatory disorder and a well-known cause of several CVDs [86]. Macrophage infiltration in the vascular wall is the main characteristic of atherosclerosis [87]. In macrophages, cholesterol and calcium phosphate crystals lead to lysosomal instability causing cathepsin B release that, in turn, activates the NLRP3 inflammasome with IL-1β release [62,63]. The importance of NLRP3 inflammasome has been highlighted in several animal models of atherosclerosis. Decreased atherosclerosis was demonstrated in low density lipoprotein receptor knock-out mice (Ldlr−/−) transplanted with bone marrow from Nlrp3−/−, Asc−/− and Il-1β−/− mice [63]. These pieces of evidence make the NLPR3 inflammasome a promising therapeutic target for atherosclerosis.
4.2. Ischemic heart disease.
The inhibition of NLRP3 and other inflammasome components in animal model of ischemic cardiac injury showed beneficial effects in terms of reduced infarct size and improved cardiac function [52,85,88]. NLRP3 activation after ischemia is a time-dependent mechanism [89]. DAMPs and alarmins released from cells damaged by ischemia strongly stimulate the inflammatory response, with recruitment of highly active inflammatory cells at the site of injury. This feed-forward mechanism exacerbates the initial ischemic damage [90,91]. In experimental models of reperfused and non-reperfused AMI, peak NLRP3 inflammasome activation occurs respectively 1 and 3 days after ischemia [52,85,91]. NLRP3 inflammasome specks can be detected in leukocytes, endothelial cells, fibroblasts and cardiomyocytes after AMI [52,85,88]. However, when the healing phase starts, ASC aggregates prevalently localize in fibroblasts and cardiomyocytes [52,85,88], The response to inflammasome activation seems to be cell-type specific. Leukocytes, fibroblasts and endothelial cells mainly respond with IL-1β production. In cardiomyocytes, NLRP3 activation tend to result in caspase-1 activation and culminate with pyroptotic cell death [92]. After ischemia-reperfusion in mice, genetic or pharmacological inhibition of the NLRP3 inflammasome reduces infarct size and preserves cardiac function. This is recapitulated in studies with mice lacking caspase-1 or ASC (reviewed below) [52,85,88,93].
4.3. Dilated cardiomyopathy
Several pieces of evidence suggest an important inflammatory component in the pathogenesis of dilatated cardiomyopathy. Circulating levels of NLRP3 inflammasome seem to clinically correlate with cardiac function, NT-pro BNP levels and cumulative rehospitalization rate at 6 months [94]. At autopsy, markedly increased pyroptotic cell death was demonstrated in the hearts of patients with dilatated cardiomyopathy [95].
4.4. Hypertensive heart disease
Hypertensive cardiac damage promotes myocardial hypertrophy and fibrosis leading to left ventricular remodeling and development of HF. Cardiac upregulation of NLRP3 and IL-1β has been found in two different mouse models of hypertension: transverse aortic constriction, a pressure overload model inducing myocardial fibrosis and remodeling, and the hypertensive angiotensin II-infusion model [96-100]. In both models, inhibition or deletion of NLRP3 ameliorated cardiac remodeling, reducing inflammation and fibrosis [96,97]. However, the mechanisms of inflammasome activation in absence of ischemic damage and cell death are not completely elucidated. A recent study indicates that, in response to pressure overload, priming and activation of cardiac NLRP3 is mediated by Ca2+/calmodulin-dependent protein kinase II δ (CaMKIIδ) [100].
4.5. Cancer therapy-associated cardiac injury
Several therapies employed to treat different types of cancer (i.e. radiation therapy and chemotherapy) are associated with the development of cardiomyopathy both in animals and humans. Mice injected with doxorubicin develop left ventricular dilatation, reduced cardiac function and increased cardiac fibrosis [101,102]. The decline in cardiac function parallels with increased cardiomyocyte expression of NLRP3, caspase-1, IL-1β and IL-18, and abudant pyroptosis [95,103]. Blocking NLRP3 by pharmacological inhibition or gene deletion attenuates cardiac dysfunction and myocardial damage induced by pyroptosis [95,104]. Given the beneficial effects of IL-1β and IL-18 blockade in radiation-induced cardiomyopathy, a direct involvement of the NLRP3 inflammasome in the initiation and progression of radiation-induced cardiac damage has been proposed [105-107].
4.6. Metabolic disorders and diabetic cardiomyopathy
In the context of cardiometabolic disorders, IL-1β and IL-18 are key mediators of the detrimental effects of obesity and aging [108,109]. Adipose tissue actively contributes to the systemic pro-inflammatory state, characterized by increased plasma levels of pro-inflammatory cytokines [110]. Upregulation of the NLRP3 inflammasome was reported in the adipose tissue of obese patients and animals [47,110-113]. In animal models of aging and obesity, NLRP3 inhibition was associated with improved metabolic profile [111-113]. Sustained metabolic abnormalities may lead to diabetic cardiomyopathy [114], in which cardiac NLRP3 directly contributes to organ dysfunction [115,116]. Cardiomyocyte death seems to be the first step in initiating the structural remodeling leading to diabetic cardiomyopathy [117]. Glucotoxicity and lipotoxicity represent potent stimuli for the NLRP3 inflammasome [118-120]. In particular, in several cell types, high glucose levels induce ROS production and subsequent activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and TXNIP, thereby acting as priming and triggering signals to the inflammasome [118-121].
4.7. Pericarditis
Acute pericarditis is characterized by an intense inflammatory response due to an acute injury of the mesothelial cells in the pericardium [122-124]. A key role of NLRP3 in pericarditis has been attested in several pre-clinical and clinical studies [122,123]. Furthermore, the efficacy of colchicine, an anti-inflammatory agent with NLRP3 inflammasome inhibitory activity (reviewed below), in the treatment of acute pericarditis further supports a direct role of NLRP3 in this condition [122,123]. A recent study revealed the presence of inflammasome components (NLRP3, ASC and caspase-1) in pericardial samples of patients with chronic pericarditis experiencing an acute flare [123]. Consistent findings were obtained in a novel mouse model in which pericarditis was induced by zymosan intrapericardial injection [123]. In this model, inhibition of the inflammasome or IL-1α/β reduced pericardial effusion and thickening [123]. These observations are in line with the clinical efficacy of rilonacept, which inhibits both IL-1α and IL-1β, in patients with recurrent pericarditis [124,125]. In the phase III, RHAPSODY trial, rilonacept monotherapy was associated with a 96% reduction in recurrences as compared with placebo [125]. Similar benefits had been seen in the smaller AIRTRIP trial with anakinra, a recombinant IL-1 receptor antagonist, in patients with recurrent pericarditis resistant to colchicine [126]. IL-1 blockers are now considered standard of care for the treatment of recurrent pericarditis in patients who failed initial therapy. A potential role for interleukin-1 blockade in acute pericarditis is under investigation [127].
4.8. Myocarditis
The presence of the NLRP3 inflammasome has been shown in endomyocardial biopsies of patients with acute myocarditis [128]. Infection with coxsackievirus B3 (CVB3), one of the most common viruses causing myocarditis, is associated with enhanced NLRP3 activation together with increased ASC, caspase-1 and IL-1β expression observed within 7 days from the infection in mice [129]. Inhibition of caspase-1 or IL-1β ameliorates cardiac function and reduces the release of myocardial enzymes [129]. Of note, in experimental CVB3-induced myocarditis, inflammasome activation and subsequent pyroptosis seem to be mediated by cathepsin B [130]. A peculiar form of myocarditis, namely cardiac sarcoidosis, is characterized by the formation of giant cell granulomas in the heart, which progressively lead to cardiac failure and arrhythmias [131]. Recently, intense expression of the NLRP3 inflammasome and its products in the granulomas has been described [132]. A clinical trial with anakinra in cardiac sarcoidosis is currently ongoing [133].
4.9. Venous thromboembolism
Venous thromboembolism (VTE), comprising deep vein thrombosis and pulmonary embolism, is the third leading cause of cardiovascular mortality worldwide [134]. In addition, post-thrombotic syndrome (PTS), a chronic inflammatory condition complicating VTE, accounts for considerable morbidity, affecting 20-40% of patients following VTE [134].
Initiation and propagation of venous thrombosis is a multifactorial process involving a complex sequence of events, in which inflammation directly promotes activation of the coagulation system and the endothelium, as well as the recruitment of leukocytes and platelets forming aggregates and aggravating thrombosis [135,136]. Several lines of evidence indicate that the NLRP3 inflammasome is implicated in the regulation of these events [135-141]. Blood flow restriction and hypoxia following experimental venous thrombosis have been shown to induce the NLRP3, caspase-1 and IL-1β [137-139]. In mice, genetic deletion of the NLRP3 inflammasome and pharmacological inhibition of caspase-1 or IL-1β significantly ameliorate venous thrombosis [137-139]. Recently, deficiency of caspase-1 or GSDMD has been shown to protect against experimental venous thrombosis [140]. Furthermore, tissue factor released from monocytes and macrophages following NLRP3 activation trigger the coagulation cascade [141]. In patients with VTE, elevated NRLP3 activity, as measured by high concentrations of caspase-1, IL-1β, IL-6 or C-reactive protein, were found from days to months after the index event, and correlated with thrombus extent and incomplete thrombus resolution [137,142]. Surrogate biomarkers of NLRP3 inflammasome activation seemed to predict the development PTS, as well as the recurrence of thrombotic events [143,144]. Other studies have also found an association between basal levels of inflammation and the occurrence of a first event of VTE [145]. Despite no clinical trial specifically addressing inflammation in VTE exists, althogether, these data may allow to speculate that strategies selectively targeting the NLRP3 inflammasome may lead to the development of novel therapeutics against VTE, which may maximize the benefits of anticoagulation.
4.10. COVID-19
Viruses, including severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), have the ability to trigger the NLRP3 inflammasome [146]. In recent times, a role of the NLRP3 inflammasome has been demonstrated in the pathophysiology of COVID-19. Severe forms of COVID-19 are characterized by systemic hyperinflammation which contributes to diffuse organ damage, including the lungs, heart and vasculature, and worsens clinical outcomes [4]. Upon autopsy, abundant presence of NLRP3, ASC and caspase-1 were found in the lungs of patients with fatal COVID-19 [147,148]. Analysis of circulating myeloid cells isolated from COVID-19 patients indicated that NLRP3 and caspase-1 are highly active and produce large amounts of mature IL-1β [148-150]. In addition, higher concentrations of inflammasome-derived products such as caspase-1 and IL-18 in the sera of patients with COVID-19 correlated with disease severity and predicted worse evolution [148-150]. Mechanistic in vitro studies have consistently demonstrated that the interaction of SARS-CoV-2 spike protein with the virus receptor entry angiotensin-converting enzyme 2 induces abundant cytokine release including IL-1β, IL-6, IL-8 and IL-18, through activation of NLRP3 and caspase-1 [151-153]. Of note, abrogation of NLRP3-mediated signaling with different selective NLRP3 inhibitors resulted in significantly reduced cytokine production in cultured peripheral blood mononuclear cells (PBMCs) exposed to recombinant SARS-CoV-2 spike protein [152]. It has been hypothesized that IL-1β and IL-18 resulting from NLRP3 signaling activate monocytes, which in turn produce several other cytokines such as IL-6, IL-8 and tumor necrosis factor (TNF) responsible for hyperinflammation and, through multiple mechanisms, subsequent local (i.e. in the lungs) and systemic damage associated with severe forms of COVID-19 [4,148-150]. These mechanisms include abundant recruitment of neutrophils to the lungs, generation of neutrophil extracellular traps (NETs) through GSDMD and ROS, as well as cell death (e.g. through pyroptosis) [147-152]. NETs recruit platelets, facilitate tissue factor release from pyroptotic monocytes and promote the hypercoagulable state associated with COVID-19. In addition, multiple cytokines such as IL-1α, IL-1β and IL-6 increase vascular permeability and activate the endothelium, which further predispose to thrombosis [135,153].
Besides regulating the activation of leukocytes, platelets, endothelial cells and the coagulation cascade following SARS-CoV-2 infection, the NLRP3 inflammasome has been found in hematopoietic stem cells, opening to the possibility that SARS-CoV-2, by means of NLRP3, may also remotely affect tissue proliferation and regeneration [154]. Altogether, these observations indicated that hyperactivation of the NLRP3 inflammasome contributes in initiating and propagating hyperinflammation associated with COVID-19, and suggested that selectively targeting of the NLRP3 inflammasome may represent a promising therapeutic strategy in COVID-19. This has led to the initiation of a phase II randomized trial with dapansutrile, an oral NLRP3 inhibitor (NCT04540120). Nevertheless, IL-1 inhibition with anakinra or canakinumab has been already evaluated in multiple observational studies with overall encouraging results [155-157]. In the SAVE-MORE double-blind, randomized controlled trial of anakinra, IL-1 receptor antagonist, in 594 patients with COVID-19 pneumonia, significantly reduced the risk as compared with placebo of clinical worsening and reduced twenty-eight-day mortality [158]. In the phase III CAN-COVID trial randomizing 454 patients with severe COVID-19 pneumonia and systemic hyperinflammation, treatment with canakinumab was safe and associated with a trend towards improved survival without invasive mechanical ventilation and COVID-19-related mortality at day 29 compared to placebo [159]. Although results were not statistically significant for the primary endpoint, early treatment with canakinumab was associated with a reduction of death, need for invasive mechanical ventilation or use of other IL-1/IL-6 blockers for worsening disease [159]. The efficacy of anti-cytokine treatments in COVID-19 has been proven for strategies targeting IL-6, a potent pleiotropic cytokine induced by the NLRP3 inflammasome [160]. During the early phases of the pandemic, different IL-6 antagonists including tocilizumab, sarilumab and siltuximab, already approved for rheumatic and oncological diseases, have been repurposed for COVID-19 [160]. After initial promising experiences with tocilizumab, several randomized trials were launched worldwide [156,161-165]. A meta-analysis including 10,930 hospitalized COVID-19 patients from 27 randomized controlled trials, found that administration of either tocilizumab or sarilumab, two monoclonal antibodies directed against the IL-6 receptor, significantly reduced 28-day all-cause-mortality compared to standard of care or placebo [166]. Early administration of tocilizumab in combination with dexamethasone has become standard-of-care in hospitalized COVID-19 patients exhibiting rapid respiratory decompensation [167,168].
5. Pharmacological targeting of the NLRP3 inflammasome
Due to the broad role of the NLRP3 inflammasome in several types of diseases as well as the direct and indirect evidences showing the benefits of NLRP3 blockade, several NLRP3 inflammasome inhibitors are in preclinical and clinical development (Table 1). While some of these selectively block the NLRP3 inflammasome, others have broader effects which indirectly result in NLRP3-mediated signaling inhibition (Figure 3). This review will focus on the NLRP3 inhibitors that have been tested in CVDs.
Table 1.
Clinical trials targeting the NLRP3 inflammasome, IL-1 and IL-6 in cardiovascular diseases.
| Study (year) |
Pharmacological target |
Disease (Total No. of patients enrolled) |
Study design |
Main findings |
Ref. |
|---|---|---|---|---|---|
| OLATEC-HF (2021) | NLRP3 | Stable HFrEF (NYHA Class II-III) (30) | Randomization: Dapansutrile (OLT1177) or placebo 4:1 (500, 1000, or 2000 mg/day) | Treatment with dapansutrile for 14 days was safe and well tolerated in patients with stable HFrEF. Improvements in left ventricular EF and in exercise time were observed in cohort of patients receiving dapansutrile at a dose of 2000 mg/day | 202 |
| COLchicine Cardiovascular Outcomes Trial (COLCOT) (2019) | NLRP3 (non selective) | AMI < 30 days (4745) | Randomization: colchicine or placebo 1:1 (0.5 mg/day) | Colchicine led to a significantly lower risk of ischemic cardiovascular events as compared to placebo | 207 |
| Low Dose Colchicine Trial (LoDoCo) (2013) | NLRP3 (non selective) | Stable CAD (532) | Randomization: colchicine or no colchicine 1:1 (0.5 mg/day) | Colchicine on top of standard therapy was effective in preventing cardiovascular events in patients with established stable CAD | 208 |
| Colchicine in Patients with Chronic Coronary Disease (LoDoCo2) (2020) | NLRP3 (non selective) | Chronic CAD (5522) | Randomization: colchicine or placebo 1:1 (0.5 mg/day) | Colchicine significantly reduced the risk of cardiovascular events in patients with chronic CAD | 210 |
| COlchicine in Patients with ACS (COPS) (2020) | NLRP3 (non selective) | ACS (795) | Randomization: colchicine or placebo 1:1 (0.5 mg twice a day for 1 month, 0.5 mg/day for 11 months) | Colchicine in addition to standard care did not significantly affect cardiovascular outcomes at 12 months in patients with ACS and it was associated with a higher rate of mortality | 213 |
| Colchicine Therapy and Plaque Stabilization in Patients With ACS (2018) | NLRP3 (non selective) | ACS < 30 days (80) | Non-randomized: Colchicine or no colchicine (0.5 mg/day) | Low-dose colchicine therapy improved plaque morphology and reduced hsCRP | 214 |
| Colchicine in acute ST-segment elevation myocardial infarction (2015) | NLRP3 (non selective) | ACS <12 hours (151) | Randomization: Colchicine or placebo 1:1 (loading dose 2 mg followed by 1 mg/day for 5 days) | Reduction in infarct size measured by area-under-the-curve for CK-MB | 211 |
| Colchicine in acute ST-segment elevation myocardial infarction (2021) | NLRP3 (non selective) | ACS <12 hours (192) | Randomization: Colchicine or placebo 1:1 (loading dose 2 mg followed by 1 mg/day for 5 days) | No effect on infarct size, trend toward reduction in CRP at 48 hours, trend toward lower heart failure events at 3 months | 212 |
| COlchicine for Recurrent Pericarditis (CORP) (2011) | NLRP3 (non selective) | First recurrence of pericarditis (120) | Randomization: colchicine or placebo 1:1 (0.5 to 1.0 mg/day) | Colchicine reduced the risk of recurrence and at 18 months | 216 |
| Efficacy and Safety of Colchicine for Treatment of Multiple Recurrences of Pericarditis: a Multicentre, Double-Blind, Placebo-Controlled Randomised Trial (CORP-2) (2014) | NLRP3 (non selective) | Recurrent pericarditis (240) | Randomization: colchicine or placebo 1:1 (0.5 to 1.0 mg/day) | Colchicine in combination with conventional anti-inflammatory therapy significantly reduced the rate of subsequent recurrences of pericarditis | 217 |
| Effects of Interleukin-1β Inhibition with Canakinumab on Hemoglobin A1c, lipid, C-reactive protein, Interleukin-6, and Fibrinogen: a Phase IIb randomized, placebo-controlled trial (CANTOS pilot trial) (2012) | IL-1β | Subjects with well-controlled diabetes mellitus at high cardiovascular risk (556) | Randomization: Canakinum ab or placebo 1:1:1:1:1 (5, 15, 50, 150 mg monthly) | Canakinum ab significantly reduced inflammation without major effects on low-density lipoprotein cholesterol or high-density lipoprotein cholesterol | 240 |
| Canakinumab ANti-inflammatory Thrombosis Outcomes Study (CANTOS) (2017) | IL-1β | AMI > 30 days (10061) | Randomization: Canakinum ab or placebo 1:1:1:1.5 (50,150 or 300 mg monthly) | Canakinum ab significantly reduced hsCRP, the incidence of recurrent AMI and the rate of atherothrom botic events in patients with established atherosclerotic disease | 241 |
| Virginia Commonwealth University Anakinra Remodeling Trial (VCUART/VCU ART2) (2010, 2015) | IL-1β | Acute STEMI <12 hrs (40) | Randomization: Anakinra or placebo 1:1 (100 mg/day) | Anakinra reduced hsCRP and the incidence of death or new onset HF | 253,254,256 |
| Virginia Commonwealth University Anakinra Remodeling Trial 3 (VCUART3) (2020) | IL-1β | Acute STEMI < 12 hrs (99) | Randomization: Anakinra or placebo 1:1:1 (100 or 200 mg/day) | Anakinra significantly reduced the area under the curve for hsCRP at 14 days, and reduced the incidence of death or new onset HF and of death or HF hospitalization | 255,256 |
| Effects of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes (MRC-ILA Heart Study) (2015) | IL-1β | NSTEMI < 48 hrs (182) | Randomization: Anakinra or placebo 1:1 (100 mg/day) | Anakinra significantly reduced systemic inflammator y markers without improving clinical outcomes | 257 |
| Safety and Efficacy of Anakinra in Heart Failure (AIR-HF) (2012) | IL-1β | Stable HFrEF (NYHA Class II-III) (7) | Non-randomized: anakinra (100 mg/day) | Anakinra significantly reduced systemic inflammatory markers and improved peak aerobic exercise capacity and ventilatory efficiency | 260 |
| Interleukin-1 Blockade with Canakinumab to Improve Exercise Capacity in Patients with Chronic Systolic Heart Failure and hsCRP (CANTOS sub-study) (2018) | IL-1β | AMI > 30 days, Stable HFrEF (NYHA Class II-III) (15) | Randomization: Canakinum ab or placebo 1:1:1:1.5 (50,150 or 300 mg) | Canakinum ab improved peak aerobic exercise capacity and reduced the rate of hospitalizations for HF | 261 |
| REcently Decompensate d Heart failure Anakinra Response Trial (REDHART) (2017) | IL-1β | HFrEF < 14 days post-discharge (60) | Randomization: anakinra or placebo 1:1:1 (100 mg/day for 2 or 12 weeks) | Anakinra reduced hsCRP, and improved peak aerobic exercise capacity and quality of life | 262 |
| REcently Decompensated Heart failure Anakinra Response Trial II (REDHART2) | IL-1β | HFrEF < 14 days post-discharge (estimated enrollment 102) | Randomization: anakinra or placebo 1:1 (100 mg/day 24 weeks) | Ongoing study | 263 |
| Diastolic Heart failure Anakinra Response Trial (D-HART) (2014) | IL-1β | Stable HFpEF (12) | Randomization: anakinra or placebo 1:1 (100 mg/day 14 days) | Anakinra reduced hsCRP, and improved peak exercise capacity and quality of life score | 264 |
| Diastolic Heart failure Anakinra Response Trial 2 (D-HART2) (2018) | IL-1β | Stable HFpEF (31) | Randomization: anakinra or placebo 1:1 (100 mg/day 12 weeks) | Anakinra treatment reduced hsCRP, increased treadmill exercise time and improved quality of life, albeit in absence of a significant change in peak oxygen consumption | 265 |
| Effects of Interleukin-1 Inhibition on Vascular and Left Ventricular Function in Rheumatoid Arthritis Patients (2008) | IL-1β | RA (23) | Randomization: anakinra or placebo 1:1 (150 mg/day) | Acute and chronic anakinra treatment reduced nitrooxidative stress and improves vascular and Left ventricular function in RA patients | 270 |
| Effects of Interleukin-1 Inhibition on Vascular and Left Ventricular Function in Rheumatoid Arthritis Patients with CAD (262) | IL-1β | RA and chronic stable CAD (80) | Randomization: anakinra or placebo 1:1 (100 mg) | Anakinra treatment improved endothelial and coronary aortic function, ameliorated left ventricular myocardial deformation and twisting | 271 |
| Effect of Anakinra on Recurrent Pericarditis among Patients with Colchicine Resistance and Corticosteroid Dependence (AIRTRIP) (2016) | IL-1β | Recurrent pericarditis (21) | Open-label anakinra followed by a double-blind withdrawal step with anakinra or placebo until pericarditis occurred (2 mg/kg per day, up to 100 mg) | Anakinra compared to placebo reduced the risk of recurrence | 126 |
| Efficacy and Safety of Rilonacept for Recurrent Pericarditis: Results from a Phase II Clinical Trial (2020) | IL-1α/IL-1β | Recurrent pericarditis (25) | Rilonacept 320 mg loading dose, followed by 160 mg weekly maintenance dose for at least 6 weeks | Rilonacept was safe, reduced background corticosteroid therapy, and led to a rapid and sustained improvement in pain, inflammation and health-related quality of life | 124 |
| Rilonacept Inhibition of Interleukin-1 Alpha and Beta for Recurrent Pericarditis (RHAPSODY) (2021) | IL-1α/IL-1β | Recurrent pericarditis (86) | Randomization after a 12-week run-in period with Rilonacept: Rilonacept or placebo 1:1 (320 mg loading dose, followed by 160 mg weekly maintenance dose) | Rilonacept was associated with rapid resolution of recurrent pericarditis episodes and to a significantly lower risk of pericarditis recurrence compared to placebo | 125 |
| ASSessing the effect of Anti-IL-6 treatment in Myocardial Infarction (ASSAIL-MI) (2021) | IL-6 | STEMI < 6 hours from symptoms (199) | Randomization: tocilizumab or placebo 1:1 (280 mg) | Tocilizumab treatment reduced systemic inflammation and increases myocardial savage index | 291 |
| Trial to Evaluate Reduction in Inflammation in Patients with Advanced Chronic Renal Disease Utilizing Antibody Mediated IL-6 Inhibition (RESCUE) (2021) | IL-6 | Chronic Kidney Disease and hsCRP >2 mg/L (264) | Randomization: ziltivekimab or placebo 1:1:1:1 (7.5, 15 or 30 mg) | Ziltivekimab treatment reduced biomarkes of inflammation and thrombosis | 292 |
| Effects of Ziltivekimab Versus Placebo on Cardiovascular Outcomes in Participants with Established Atherosclerotic Cardiovascular Disease, Chronic Kidney Disease and Systemic Inflammation (ZEUS) | IL-6 | Atherosclerotic Cardiovascular Disease, Chronic Kidney Disease and Systemic Inflammation (estimated enrollment 6200) | Randomization: ziltivekimab or placebo 1:1 (15 mg/month) | Ongoing study | 290 |
Abbreviations: HF = Heart Failure, HFrEF = Heart Failure with reduced Ejection Fraction, NYHA = New York Heart Association, AMI = Acute Myocardial Infarction, CAD = Coronary Artery Disease, hsCRP = high-sensitive C-Reactive Protein, ACS = Acute Coronary Syndrome, STEMI = ST-segment elevation myocardial infarction, NSTEMI = non ST-segment elevation myocardial infarction, HFpEF = Heart Failure with Preserved Ejection Fraction, RA = rheumatoid arthritis.
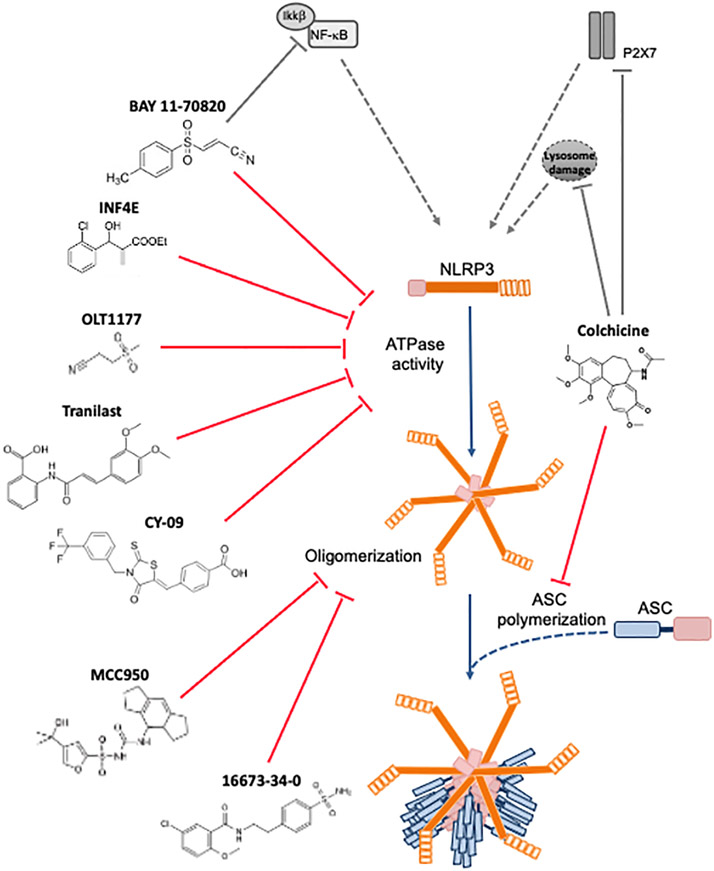
Figure 3.
NLRP3 inhibitors under clinical development in cardiovascular diseases: chemical structure, molecular target and mechanisms of action.
5.1. Glyburide derivates
Glyburide is an oral sulfonylurea used in the treatment of type 2 diabetes mellitus. It promotes insulin release from pancreatic β-cells through a cyclohexylurea moiety [169]. It was the first drug shown to inhibit NLRP3 at high doses in vitro [170]. Nevertheless, high-dose glyduride induce severe hypoglycemia in vivo [169,170]. For this reason, a glyburide derivative, named 16673-34-0, which lacks the cyclohexylurea moiety but retains the inhibitory activity against NLRP3, was developed [171]. The 16673-34-0, at a dose of 100 mg/Kg, inhibited cardiac caspase-1 activity, and reduced infarct size in mice subjected to myocardial ischemia followed by 24 hours reperfusion [89,171]. Comparable beneficial effects were obtained even when treatment with 16673-34-0 was administered in a clinically relevant scenario (i.e. 60 minutes after reperfusion) [89,171]. The 16673-34-0 improved cardiac function also in a model of permanent coronary artery ligation, independently from infarct size reduction [104]. In rats in which circulatory death was induced through, pre-treatment with 16673-34-0 before cardiac arrest or addition of 16673-34-0 to the buffer used for ex-vivo reanimation of the heart limited the ischemic damage and improved contractility [172]. In a rat model of donation after circulator death (DCD), consisting of heterotopic heart transplantation to a recipient rat after circulatory death of the donor is induced, the 16673-34-0 was administered with cardioplegia to the donor rat heart at the moment of heart procurement as well as to the recipient rat one hour before heart transplantation. After 24 hours, the transplanted heart displayed reduced myocardial ASC staining and improved contractility [173]. In mice with experimental pericarditis, 16673-34-0 reduced pericardial effusion and thickening [123]. In mouse models of doxorubicin-induced or Western diet-induced cardiomyopathy, 16673-34-0 improved cardiac function and reduced interstitial fibrosis [104,174]. JC-124, a compound derived from 16673-34-0 developed at Virginia Commonwealth University, was shown to be more powerful than 16673-34-0 in inhibiting the inflammasome and reducing infarct size in a mouse model of ischemia-reperfusion [175]. Similarly to 16673-34-0, JC-124 is specific for NLRP3 and shows no inhibitory activity on the NLRP1 or NLRC4 inflammasomes, while it remains active against NLRP3 mutants associated with genetic forms of cryopyrin-associated diseases [104,175].
5.2. MCC950 (CP-456,773 or CRID3)
MCC95 is a small-molecule that non-covalently binds near the Walker B motif and blocks NLRP3 ATPase activity, potently inhibiting NLRP3 both in vivo and in vitro [176-182]. MCC950 specifically targets NLRP3 and lacks inhibitory effects againts NLRP1, NLRC4 or AIM2 inflammasome activity [176-182]. In pigs, a 7-day treatment of MCC950, at a dose of 3-6 mg/Kg, reduced neutrophil infiltration and myocardial IL-1β expression, and reduced infarct size and cardiac dysfunction [183]. Similar results have been shown in mice [184,185]. Additionaly, in a rat model of cardiac arrest and cardiopulmonary resuscitation, MCC950 reduced cardiac troponin I release and reduced mitochondrial damage, ameliorating cardiac function [186]. MCC950 also possesses cardioprotective benefits in non-ischemic cardiomyopathy, as evidenced by reductions in myocardial fibrosis and IL-1β levels in angiotensin II-induced hypertension [96]. When administered for 8 weeks (10 mg/kg, 3 times/week) in a mouse model of post-menopausal heart disease, MCC950 limited hypertrophic remodeling, improved systolic and diastolic function and reduced cardiac ANF and BNP mRNA levels [187]. The long-term use of MCC950 (20 mg/kg/daily) for 15 weeks improved autophagy flux and reduced cardiac apoptosis [188]. Mice with cardiomyocyte-specific expression of constitutively active NLRP3 spontaneously develop premature atrial contractions and are particularly prone to atrial fibrillation. These NLRP3-induced arrhytomogenic effects were attenuated by MCC950 [189]. MCC950 prevents atherosclerotic plaque development by decreasing the expression of adhesion molecules in the plaque and the number of infiltrating macrophages [182]. Twenty-five days of MCC950 treatment (10 mg/kg) can reduce blood pressure and renal inflammation in hypertensive mice [190]. In mice fed with high-fat, high-cholesterol diet or treated with angiotensin II, MCC950 inhibited the dilatation of the aorta, the dissection and rupture of aortic segments in the thoracic and abdominal tract [191].
5.3. Bay 11-7082
The synthetic kappa B kinase β (IKKβ) inhibitor, Bay 11-7082, structurally related to vinyl sulfone, alkylates the cysteine residues in the NLRP3 ATPase region, thus leading to NF-κB pathway inhibition [192]. However, independently from its IKKβ inhibitory activity, Bay 11-7082 can specifically block the NLRP3 inflammasome, without affecting other inflammasome receptors (NLRP1 and NLRC4) [192]. In experimental myocardial ischemia-reperfusion in the mouse, administration of Bay 11-7082 10 minutes before coronary artery reperfusion reduces leukocyte infiltration in the infarcted area and improves cardiomyocyte apoptosis and infarct size [193]. Likewise, pre-treatment with Bay 11-7082 alleviates myocardial damage, preserves contractility and limits ensuing fibrosis [194]. In diabetic rats undergoing cardiac ischemia-repurfusion, Bay 11-7082 attenuated NLRP3 activation, caspase-1 and IL-1β expression and pyroptosis [49]. Overall, these findings indicate that Bay 11-7082 exerts cardioprotective effects and inhibit NLRP3-mediated signaling inflammasome in multiple rodent models of ischemia-reperfusion injury. Nevertheless, since Bay 11-7082 inhibits both IKKβ and NLRP3, it remains unclear whether its beneficial effects are due to the inhibiton of NF-κB-dependent signaling or the blockade of the NLRP3 inflammasome.
5.4. OLT1177
OLT1177, is an orally available beta-sulfonyl nitrile small molecule that specifically inhibits NLRP3 by blocking its ATPase activity (Figure 3) [195-198]. Notably, OLT1177 demonstrates efficacy against constitutively active mutants of NLRP3 such as those observed in patients with cryopyrin-associated periodic syndrome [195]. In an animal models of myocardial ischemia-reperfusion, OLT1177 dose-dependently reduced the infarct size and preserved cardiac function at 24 hours and 7 days after reperfusion [199]. In permanent coronary artery occlusion models, OLT1177 preserved left ventricular contractile reserve and end diastolic pressure [200]. Importantly, OLT1177 is effective when administered up to 60 minutes after reperfusion, a scenario which resembles clinical practice in which patients receive treatment with a delay after reperfusion [199]. OLT1177 was safe and effective in reducing target joint pain in patients with gout enrolled in an open-label phase 2A study [201]. In a phase 1B, double-blind trial in patients with heart failure with reduced ejection fraction (HFrEF), 14-day treatment with OLT1177 was safe. Interestingly, in the cohort receiving the highest dose tested, an increase in the left ventricular ejection fraction and treadmill exercise time was observed (Table 1) [202].
5.5. Colchicine
Colchicine is a tricyclic alkaloid used in the treatment of gout, familial mediterranean fever, and acute and recurrent pericarditis [203,204]. Colchicine concentrates in circulating leukocytes where it interferes with NLRP3 and ASC approximation through microtubule disruption, among other effects [205]. Colchicine also inhibits neutrophil chemotaxis and diapedesis, possibly by inducing hepatic synthesis of growth differentiation factor 15 (GDF-15) without directly interfering with leukocyte function [206]. Colchicine improved survival and preserved left ventricular systolic function in mice at 4 weeks after permanent coronary artery ligation, and reduced myocardial mRNA of NLRP3 inflammasome components [207]. Among the NLRP3 inflammasome inhibitors, colchicine is the one that has been most investigated. Data from multiple clinical trials have consistently suggested that colchicine reduces the risk of ischemic cardiovascular events in patients with acute or chronic coronary artery disease (Table 1) [208-215]. Among 5522 patients with chronic coronary disease randomized to colchicine at a dose of 0.5 mg once daily or placebo, colchicine significantly reduced adverse cardiovascular events [210]. When initiated in-hospital in patients with acute coronary syndromes, colchicine may reduce the risk of recurrent coronary events - [209]. An earlier study with colchicine in patients with ST-segment elevation myocardial infarction had shown a reduction in infarct size measured with plasma biomarkers [211]. A recent randomized clinical trial of 192 patients with ST-segment elevation myocardial infarction colchicine failed to meet the primary endpoint of infarct size reduction measured at cardiac magnetic resonance [212]. While colchicine in patients with stable or chronic coronary disease has been thoroughly explored in clinical trials, further investigation is warranted to establish the effects of colchicine in the acute setting. In addition to its beneficial effects on coronary artery disease, colchicine reduces the risk of recurrence in adults with acute or recurrent pericarditis, possibly through NLRP3 inflammasome inhibition [123,216,217].
5.6. H2S, CY-09 and IFN4E
Endogenous hydrogen sulfide (H2S) is a gasotransmitter that exerts important physiological functions [218]. In vivo, administration of H2S protects the cardiovascular system in several models of disease [219]. Na2S, a H2S donor, reduces NLRP3-dependent caspase-1 activation and pyroptosis in primary cardiomyocytes, and reduces caspase-1 activity and infarct size in mice undergoing ischemia-reperfusion injury [220]. H2S appears to reduce inflammasome activity by acting both on the priming and trigger signals [221,222], CY-09, binds directly to the ATP-binding motif of the NLRP3 NACHT domain [223]. In mice, Cy-09 was able to protect from cardiac dysfunction associated with diabetic ischemic stroke [223]. In ex-vivo experiments using Langendorff-perfused rat hearts subjected ischemia-reperfusion, pre-treatment with IFN4E reduced infarct size and improved left ventricular pressure. Furthermore, IFN4E treatment of these hearts reduced expression of NLRP3 system components and concomitantly activated the protective RISK pathway and improved mitochondrial function [224,225]. Other structurally similar compounds have been developed but not yet tested in models of CVDs [226].
6. The role of the other inflammasome components in myocardial damage
Inhibition of the other components of the inflammasome machinery (i.e. ASC, caspase-1, IL-1β and IL-18) can block NLRP3 activation. However, since these components and substrates are shared by different inflammasomes, their inhibition may result in less specific but wider effects due to potential inhibition of the activity of other inflammasomes.
6.1. Specific role of ASC, caspase-1, and GSDMD in cardiovascular diseases
ASC knock-out mice are protected from ischemia-reperfusion injury, and display reduced levels of IL-1β [88]. ASC-deficient mice display attenuated neointimal formation after vascular injury [227]. ASC and caspase-1 knock-out mice are protected from the development of atherosclerosis, however, the reproducibility of these effects seems dependent on the atherosclerosis model used [228,229]. In humans, methylation of the Asc gene affects the ASC protein expression. The degree of methylated of CpG sites in the promoter region of exon 1 of Asc is inversely proportional to the ASC mRNA and protein expression [230]. In patients with HF (NYHA class II and III), the levels of Asc methylation measured in PBMCs were inversely correlated to the levels of plasma IL-1β (i.e. higher degrees of methylation corresponded to higher IL-1β levels), and directly correlated with peak VO2 [230]. In HF patients, physical exercise increased the methylation of ASC in PBMCs, and this was associated with a significant decrease in IL-1β [231].
The clinical effects of ASC inhibition on the cardiovascular system have not been tested yet. IC100 is a monoclonal antibody of the IgG4 class that inhibits ASC polymerization in cell extracts, resulting in inhibition of the inflammasome pathway. Since the monoclonal antibody needs to cross the cell membrane to exert its inhibitory activity on ASC, IC100 efficacy should be carefully examined when used in vivo. The data from a mouse model of multiple sclerosis suggested that IC100 reduced disease severity, and the number of CD4+, CD8+ and active myeloid cells [232]. Nevertheless, since IgG4 antibodies themselves have immunomodulatory effects [233], the absence of a non-IgG4 antibody control together with the lack of direct evidence ASC/inflammasome inhibition limit the findings of the this study [232].
Cell specific overexpression of active caspase-1 in cardiomyocytes is sufficient to induce a heart failure in the mouse. In fact, caspase-1 transgenic mice develop dilated cardiomyopathy, increased ventricular fibrosis and increased expression of genes associated with cardiac failure [234]. In accordance with the observations in NLRP3- and ASC-deficient mice, caspase-1 knock-out mice display reduced levels of IL-1β and are protected from ischemia-reperfusion injury [88]. In vitro, caspase-1 inhibition on myocardial tissue strips exposed to hypoxic conditions, improves tissue contractility compared to controls [235]. Another caspase-1 inhibitor, VX795, protected rat hearts from ex-vivo ischemia-reperfusion injury [236,237].
GSDMD is responsible for forming pores into the cell membrane necessary for the release of the active IL-1β and IL-18 and then mediating inflammatory cell death [28]. There are two distinct pathways responsible for GSDMD activation, one through the NLRP3/caspase-1 pathway and one through the toll-like receptor/caspase-4 pathway (caspase-11 in the mouse) [28,238]. In mice, cardiomyocyte-restricted conditional deletion of GSDMD significantly ameliorates infarct size and cardiac function [238]. Targeting of GSDMD may therefore represent another prominisg approach to counteract excessive inflammasome activation.
6.2. Role of IL-1β in atherosclerosis, myocardial damage and heart failure
The role of IL-1 in the development of atherosclerosis and plaque instability is well substantiated. Deletion of the IL-1 receptor type I (IL-1RI), as well as deletion of IL-1β and IL-1α, reduces the size of the plaque in mice [63]. Vromann and colleagues elegantly showed that, while IL-1α determines arterial remodeling during early experimental atherogenesis, IL-1β regulates progression of atheromas [239]. In the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) pilot trial, canakinumab, a monoclonal antibody against IL-1β, was shown to blunt inflammation without affecting low-densitiy lipoprotein cholesterol or high-density lipoprotein cholesterol [240]. These preliminary findings were confirmed by the CANTOS trial, in which canakinumab compared to placebo significantly reduced the rate of atherothrombotic events among 10061 patients with established atherosclerotic disease (Table 1) [241,242].
Modulation of IL-1 signaling following permanent coronary artery occlusion modulates the process of scar formation and ventricular remodeling. IL-1RI-deficient mice display attenuated infarct scarring and more favorable ventricular remodeling compared to wild-type mice [243]. On the other hand, deletion of IL-1 receptor antagonist (IL-1Ra), an endogenous antagonist of IL-1 signaling, worsens post-ischemic ventricular remodeling and produces a dysfunctional scar [243]. Treatment with anakinra, a recombinant form of the human IL-1Ra, or with IL-1 trap, a chimeric protein that neutralizes bot IL-1α and IL-β, reduces the myocardial remodeling in mice undergoing permanent coronary artery ligation [244-246]. Anakinra also exerts cardioprotective effects in reperfused mouse models of AMI [247]. Follow-up studies demonstrated that while the selective IL-1β inhibition using a antibody reduces the infarct size in mice that undergo ischemia-reperfusion injury, the inhibition of IL-1α has no effect on infarct size in the same model [248,249]. However, IL-1β inhibition using two different monoclonal antibodies reduces adverse remodeling and improves left ventricular contractility following permanent coronary ligation [250-252]. Three sequential double-blinded placebo-controlled phase II clinical studies (The VCUART-Virginia Commonwealth University Anakinra Remodeling Trials) tested the efficacy of anakinra in patients with ST-segment elevation myocardial infarction, and proved that the anakinra is safe and blunts the acute inflammatory response following AMI, thereby reducing the rate of new onset HF and HF hospitalization when compared to placebo [253-256]. In the MRC-ILA-Heart study, however, patients with smaller non-ST-segment elevation myocardial infarction, anakinra also reduced the acute systemic inflammatory response during AMI, but it did not result in improved clinical outcomes (Table 1) [257].
One potential advantage of using anakinra as a therapeutic strategy is the blockade of both IL-1β and IL-1α [258]. IL-1α is a member of the IL-1 family that shares high homology with IL-1β but lacks the cleavage domain for caspase-1 and therefore is not activated in the inflammasome [258]. IL-1α is active however already in its pro-form inducing a pro-inflammatory signal through the IL-1 type I receptor, and priming the cell for the formation of the inflammasome and as such it functions as an alarmin [258]. Strategies targeting specifically IL-1α reduce inflammatory injury and infarct size [248]. IL-1α is also expressed on the cell membrane of leukocytes and regulates the systemic inflammatory response after ischemic injury [259].
Experimental studies in vitro and in vivo have shown that IL-1β impairs myocardial relaxation and contractility and alters the response to β-adrenergic stimulation in mice, even in absence of AMI or other types of acute myocardial injury [258]. Compared to the placebo, anakinra reduced the acute inflammatory response and improved the ejection fraction in hospitalized patients hospitalized with acute decompensated systolic HF [260]. In a subgroup of patients enrolled in the CANTOS trial, canakinumab reduced the rate of hospitalizations for HF [261]. In the REDHART study (Recently Decompensated Heart Failure Anakinra Response Trial), anakinra improved cardiorespiratory fitness (peak oxygen consumption), reduced levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP) and increased the patient quality of life [262]. The REDHART2 study is currently ongoing [263]. In the D-HART (Diastolic Heart Failure Anakinra Response Trial), anakinra was administered to patients with heart failure with preserved ejection fraction (HFpEF) and promoted significant improvements in peak oxygen consumption [264]. The D-HART2 study, conducted in the same patient population, albeit with a higher degree of obesity, showed a significant increase in treadmill exercise time, lower plasma levels of NT-proBNP, and improved quality of life measures, in absence of a significant change in peak oxygen consumption (Table 1) [265].
Rheumatic diseases have been linked to an increased risk of developing HF [266]. In these diseases, the activity of the NLRP3 inflammasome in the site of inflammation is increased and, it is associated with a systemic increase of pro-inflammatory markers, including those of the IL-1 pathway [267]. Experimental induction of arthritis promotes, indeed, remodeling of the heart in mice [268,269]. A strong link between cardiac dysfunction and the pro-inflammatory effects of IL-1β has been found in patients with rheumatoid arthritis (RA) [270]. In this patient population, recombinant IL-1Ra improved cardiovascular function within three hours after the first dose, and the effects lasted for 30 days (Table 1) [270]. Besides, independent of the presence of coronary artery disease, RA patients treated with recombinant IL-1Ra had reduced oxidative stress and improved LV contractility and relaxation. These pieces of evidence point out that extra-cardiac (or systemic) inflammasome activation affects the heart as well as the inflammasome activation in cardiac cells [270,271]. This notion is of utmost importance since systemic upregulation of the NLRP3 inflammasome and its downstream cytokines observed in several chronic conditions such obesity, diabetes, hypertension or aging, may directly impact the heart structure and function with detrimental consequences.
6.3. Role of IL-18 in cardiovascular diseases
Together with IL-1, IL-18 is one of the main NLRP3-derived cytokines. IL-18 has been implicated in several cardiovascular diseases, in which elevated plasma levels of IL-18 are found, and correlate with disease severity [272]. Following AMI, circulating IL-18 concentrations increase and predict dire outcomes [272-274]. Decompensation of HF is associated with increased levels of IL-18 [275]. In mouse models, pretreatment with a neutralizing antibody against IL-18 reduces the infarct size [276]. Recombinant IL-18 binding protein (IL-18BP) reduces myocardial damage and inflammation following ischemia-reperfusion in a mouse model of heterotopic heart transplantation [277]. IL-18BP improves the heart function and reduced myocardial damage in a mouse model of heart donation after circulatory death ex vivo [278]. Furthermore, IL-18BP improves cardiomyocyte contractility in cultured human heart strips exposed to ischemia in vitro [235]. IL-18BP also ameliorates right ventricular function in mice exposed to chronic hypoxia for 2 weeks [279]. In a mouse model of β-adrenergic receptor overstimulation induced by high-dose injections of isoproterenol, genetic deletion of IL-18 or neutralization through anti-IL-18 antibodies reduced damage to the heart, ameliorating cardiac function and remodeling [280]. Like IL-1, IL-18 is involved in the progression of atherosclerosis in multiple models of atherogenesis in mice [281-284]. In fact, IL-18 administration to atherosclerosis-prone mice accelerates plaque development, whereas deletion of IL-18 reduces plaque development [282,283]. In contrast with these observations, one study reported increased atherosclerotic lesions in proatherogenic lacking the IL-18 gene [284]. However, another study found that IL-18 deletion prevented the development of cardiomyopathy in mice fed with a high-fat and high-sugar diet [285].
IL-18 plays a crucial role in homeostasis. Even when fed with a regular diet, mice lacking IL-18 are hyperphagic and become diabetic and obese [285,286]. When fed with a high-fat and high-sugar diet, deficiency of IL-18 induces significant gain in body weight compared to wild-type mice [283,284]. In addition, IL-18 controls the appetite in the central nervous system and the NLRP1 inflammasome regulates IL-18 physiological production [286,287].
6.4. Role of IL-6 in cardiovascular diseases
IL-6 production is induced by IL-1β, therefore IL-6 is an indirect product of the NLRP3 inflammasome [160,288]. Nevertheless, several other cytokines can control IL-6 expression and secretion [160,288]. IL-6 has powerful pro-inflammatory and pro-thrombotic effects, strongly contributing to augment cardiovascular risk [160,288]. Increased IL-6 levels predict worse outcomes across health and disease, and several IL-6 inhibitors are clinically available [289,290]. The preclinical evidence supporting the beneficial effects of IL-6 blockade in ischemia-reperfusion provided the basis for the phase II trial with the IL-6 receptor inhibitor tocilizumab [291]. In the ASSAIL-MI trial, treatment with tocilizumab was associated with a significant reduction in the systemic inflammatory response and an improvement in the myocardial salvage index in patients with ST-segment elevation myocardial infarction [291]. The positive results of the recently completed phase II RESCUE trial with ziltivekimab, an anti-IL-6 antibody, prompted the launch of a larger cardiovascular outcome trial [292]. The ZEUS trial will test ziltivekimab in patients with low-grade chronic inflammation, established atherosclerotic disease and chronic renal disease (Table 1) [290]. IL-6 directly activates membrane-bound IL-6 receptors (classical signaling), but also can complex with soluble IL-6 receptors, which then bind membrane-bound gp130 to initiate IL-6 signaling (trans-signaling) [160,288,290]. Initial evidence suggests that trans-, but not classical, IL-6 signaling contributes to atherosclerosis [293]. Whether specific inhibitors of IL-6 trans-signaling offer better efficacy or more targeted anti-inflammatory effects in humans remains unclear.
7. Cell-specific effects of the inflammasome in the myocardium
Circulating monocytes, in which the NLRP3 inflammasome is constitutively expressed at high levels, are proptly recruited in the heart following injury [32]. On the contrary, the most abundant cardiac resident cells, such as cardiomyocytes, fibroblasts and endothelial cells, display low basal expression of the inflammasome constituents, which are strongly upregulated after priming [10,30,33]. Activation of the NLRP3 inflammasome induces secretion of IL-1β in leukocytes, endothelial cells and fibroblasts. In cardiomyocytes, the inflammasome primarily activates caspase-1 leading to pyroptotic cell death [92]. GSDMD permits release of IL-1β and IL-18 from cells after activation of the inflammasome, and mediates pyroptosis independently from cytokine production [238]. The type of response to injury depends on the nature of insult, and it appears to be cell-type-specific in the heart [92]. In the heart of patients who died from AMI, ASC was expressed in infiltrating cells [88]. However, in animal models, NLRP3 inflammasome expression also localizes in cardiomyocytes, endothelial cells, and fibroblasts [88,93,84]. Activation of the inflammasome is responsible for the pyroptotic death in cardiomyocytes [52,104]. Endothelial cells and fibroblasts exposed to ischemia release IL-1β [92-94]. In these cells, the inflammasome promotes the production of active IL-1β and IL-18. IL-18 modulates myocardial contractility, collagen deposition by fibroblasts and endothelial function [92-94]. However, except for the production of IL-1β, the specific contribution of fibroblast and endothelial NLRP3 in the early phases following acute myocardial injury has not been fully elucidated yet.
8. Conclusions
The formation and activation of the NLRP3 inflammasome with the production of IL-1β and IL-18 and inflammatory cell death – pyroptosis - have a central role in the pathogenesis of acute and chronic cardiovascular diseases, ranging from atherosclerosis to acute myocardial infarction, heart failure to pericarditis. The recent development of specific NLRP3 inflammasome inhibitors has opened the way to testing the hypothesis that targeting the NLRP3 inflammasome may improve clinical outcomes. Early phase clinical trials with targeted NLRP3 inflammasome inhibitors show promising results awaiting validation. Phase II-III clinical trials targeting cytokines downstream of the inflammasome like IL-1β and IL-6 have shown efficacy across a variety of cardiovascular conditions, and are currently under further investigation. IL-1 inhibition with rilonacept has recently become standard-of-care for the treatment of recurrent pericarditis.
Funding:
ST and AA are supported by an NIH grant (R01HL150115). LFB is supported by NIH/NHLBI K23HL150311. AA received support from the “Sapienza Visiting Professor Programme 2020” of the Sapienza University of Rome, Italy.
Abbreviations:
- AMI
acute myocardial infarction
- AT1
angiotensin receptor type 1
- ATP
adenosine triphosphate
- cAMP
cyclic AMP
- CaMKIIδ
Ca2+/calmodulin-dependent protein kinase II δ
- CaSR
Ca2+ is the calcium-sensing receptor
- COVID-19
coronavirus disease 19
- CVB3
coxsackievirus B3
- CVDs
cardiovascular diseases
- DAMPs
damage-associated molecular patterns
- DCD
donation after circulator death
- ER
endoplasmic reticulum
- GDF-15
growth differentiation factor 15
- GSDMD
gasdermin D
- HF
heart failure
- HFpEF
preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- H2S
hydrogen sulfide
- IKKb
inhibitor of kappa B kinase b
- IL
interleukin
- IL-1Ra
IL-1 receptor antagonist
- IL-18BP
IL-18 binding protein
- lncRNAs
long non-coding RNAs
- LRR
leucine-rich repeat
- MAVS
Mitochondrial antiviral-signaling protein
- MERS-CoV
Middle East respiratory syndrome coronavirus
- miRs
microRNAs
- mtDNA
mitochondrial DNA
- NETs
neutrophil extracellular traps
- NEK
NIMA-related kinases
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NLRs
nucleotide-binding oligomerization domain (NOD)-like receptors
- NLRP3
NACHT, leucine-rich repeat (LRR), and pyrin domain (PYD)-containing protein 3
- NOD
nucleotide-binding oligomerization domain
- NT-GSDMD
N-terminal GSDMD
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- PAMPs
pathogen-associated molecular patterns
- PBMCs
peripheral blood mononuclear cells
- PRRs
pattern recognition receptors
- PYD
pyrin domain
- PTS
post-thrombotic syndrome
- P2X7
purinergic-type 2 receptor X7
- RA
rheumatoid arthritis
- ROS
reactive oxygen species
- SARS-CoV
severe acute respiratory syndrome coronavirus
- SARS-CoV-2
severe acute respiratory syndrome
- TNF
tumor necrosis factor
- TRX
thioredoxin
- TXNIP
thioredoxin-interacting protein
- VTE
venous thromboembolism
Footnotes
Conflict of interest statement:
ST has received research grant funding from Kiniksa, Olatec and Serpin Pharma. AA has received research grant funding and has served as a paid scientific advisor to Cromos Pharma, Kiniksa, Lilly, Merck, Novartis, Novo Nordisk, Olatec, Serpin Pharma, and Swedish Orphan Biovitrum. LFB has served as a consultant to Kiniksa Pharmaceuticals. GBZ has served as a consultant to Cardionovum, CrannMedical, Meditrial, Optisense Medical, and Replycare. All other authors declare no conflict of interest related to the content of the present work to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Mogensen TH Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 2009, 22, 240–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeuchi O; Akira S Pattern recognition receptors and inflammation. Cell 2010, 140, 805–20. [DOI] [PubMed] [Google Scholar]
- 3.Di Virgilio F; Sarti AC; Coutinho-Silva R Purinergic signaling, DAMPs, and inflammation. Am J Physiol Cell Physiol 2020, 318, C832–C835. [DOI] [PubMed] [Google Scholar]
- 4.Potere N; Valeriani E; Candeloro M; Tana M; Porreca E; Abbate A; Spoto S; Rutjes AWS; Di Nisio M; Acute complications and mortality in hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Crit Care 2020, 24, 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullen LM; Chamberlain G; Sacre S Pattern recognition receptors as potential therapeutic targets in inflammatory rheumatic disease. Arthritis Res Ther 2015, 17, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmer S; Grebe A; Latz E Danger signaling in atherosclerosis. Circ Res 2015, 116, 323–40. [DOI] [PubMed] [Google Scholar]
- 7.Salminen A; Ojala J; Kauppinen A; Kaarniranta K; Suuronen T Inflammation in Alzheimer's disease: amyloid-beta oligomers trigger innate immunity defence via pattern recognition receptors. Prog Neurobiol 2009, 87, 181–94. [DOI] [PubMed] [Google Scholar]
- 8.Broz P; Dixit VM Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 2016, 16, 407–20. [DOI] [PubMed] [Google Scholar]
- 9.Swanson KV; Deng M; Ting JP The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 2019, 19, 477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toldo S; Abbate A The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol 2018, 15, 203–214. [DOI] [PubMed] [Google Scholar]
- 11.Motta V; Soares F; Sun T; Philpott DJ NOD-like receptors: versatile cytosolic sentinels. Physiol Rev 2015, 95, 149–78. [DOI] [PubMed] [Google Scholar]
- 12.Toldo S; Mezzaroma E; Mauro AG; Salloum F; Van Tassell BW; Abbate A The inflammasome in myocardial injury and cardiac remodeling. Antioxid Redox Signal 2015, 22, 1146–61. [DOI] [PubMed] [Google Scholar]
- 13.Yin Q; Fu TM; Li J; Wu H Structural biology of innate immunity. Ann Rev Immunol 2015, 33, 393–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tattoli I; Travassos LH; Carneiro LA; Magalhaes JG; Girardin SE The Nodosome: Nod1 and Nod2 control bacterial infections and inflammation. Semin Immunopathol 2007, 29, 289–301. [DOI] [PubMed] [Google Scholar]
- 15.Mezzaroma E; Abbate A; Toldo S The inflammasome in heart failure. Curr Opinion Physiol 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy AJ; Kraakman MJ; Kammoun HL; Dragoljevic D; Lee MK; Lawlor KE; Wentworth JM; Vasanthakumar A; Gerlic M; Whitehead LW; DiRago L; Cengia L; Lane RM; Metcalf D; Vince JE; Harrison LC; Kallies A; Kile BT; Croker BA; Febbraio MA; Masters SL IL-18 Production from the NLRP1 Inflammasome Prevents Obesity and Metabolic Syndrome. Cell Metab 2016, 23, 155–64. [DOI] [PubMed] [Google Scholar]
- 17.Hausmann A; Böck D; Geiser P; Berthold DL; Fattinger SA; Furter M; Bouman JA; Barthel-Scherrer M; Lang CM; Bakkeren E; Kolinko I; Diard M; Bumann D; Slack E; Regoes RR; Pilhofer M; Sellin ME; Hardt WD Intestinal epithelial NAIP/NLRC4 restricts systemic dissemination of the adapted pathogen Salmonella Typhimurium due to site-specific bacterial PAMP expression. Mucosal Immunol 2020, 13, 530–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludlow LE; Johnstone RW; Clarke CJ The HIN-200 family: more than interferon-inducible genes? Exp Cell Res 2005, 308, 1–17. [DOI] [PubMed] [Google Scholar]
- 19.Komada T; Chung H; Lau A; Platnich JM; Beck PL; Benediktsson H; Duff HJ; Jenne CN; Muruve DA Macrophage Uptake of Necrotic Cell DNA Activates the AIM2 Inflammasome to Regulate a Proinflammatory Phenotype in CKD. J Am Soc Nephrol 2018, 29, 1165–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavillard LE; Cañadas-Lozano D; Alcocer-Gómez E; Marín-Aguilar F; Pereira S; Robertson AAB; Muntané J; Ryffel B; Cooper MA; Quiles JL; Bullón P; Ruiz-Cabello J; Cordero MD NLRP3-inflammasome inhibition prevents high fat and high sugar diets-induced heart damage through autophagy induction. Oncotarget 2017, 8, 99740–99756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arterbery AS; Yao J; Ling A; Avitzur Y; Martinez M; Lobritto S; Deng Y; Geliang G; Mehta S; Wang G; Knight J; Ekong UD Inflammasome Priming Mediated via Toll-Like Receptors 2 and 4, Induces Th1-Like Regulatory T Cells in De Novo Autoimmune Hepatitis. Front Immunol 2018, 19, 1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y; Lv J; Jiang S; Ma Z; Wang D; Hu W; Deng C; Fan C; Di S; Sun Y; Yi W The emerging role of Toll-like receptor 4 in myocardial inflammation. Cell Death Dis 2016, 7, e2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar H; Kawai T; Akira S Toll-like receptors and innate immunity. Biochem Biophys Res Commun 2009, 388, 621–5. [DOI] [PubMed] [Google Scholar]
- 24.Latz E; Xiao TS; Stutz A Activation and regulation of the inflammasomes. Nat Rev Immunol 2013, 13, 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rathinam VA; Vanaja SK; Fitzgerald KA Regulation of inflammasome signaling. Nat Immunol 2012, 13, 333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu A; Magupalli VG; Ruan J; Yin Q; Atianand MK; Vos MR; Schröder GF; Fitzgerald KA; Wu H; Egelman EH Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 2014, 156, 1193–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y; Fu TM; Lu A; Witt K; Ruan J; Shen C; Wu H Cryo-EM structures of ASC and NLRC4 CARD filaments reveal a unified mechanism of nucleation and activation of caspase-1. Proc Natl Acad Sci U S A 2018, 115, 10845–10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X; Zhang Z; Ruan J; Pan Y; Magupalli VG; Wu H; Lieberman J Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbate A; Toldo S; Marchetti C; Kron J; Van Tassell BW; Dinarello CA Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ Res 2020, 126, 1260–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao W; Yeretssian G; Doiron K; Hussain SN; Saleh M The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem 2007, 282, 36321–9. [DOI] [PubMed] [Google Scholar]
- 31.Yin Y; Yan Y; Jiang X; Mai J; Chen NC; Wang H; Yang XF Inflammasomes are differentially expressed in cardiovascular and other tissues. Int J Immunopathol Pharmacol 2009, 22, 311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Netea MG; Nold-Petry CA; Nold MF; Joosten LA; Opitz B; van der Meer JH; van de Veerdonk FL; Ferwerda G; Heinhuis B; Devesa I; Funk CJ; Mason RJ; Kullberg BJ; Rubartelli A; van der Meer JW; Dinarello CA Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood 2009, 5, 113, 2324–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toldo S; Mezzaroma E; McGeough MD; Peña CA; Marchetti C; Sonnino C; Van Tassell BW; Salloum FN; Voelkel NF; Hoffman HM; Abbate A; Independent roles of the priming and the triggering of the NLRP3 inflammasome in the heart. Cardiovasc Res 2015, 105, 203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toldo S; Mauro AG; Cutter Z; Abbate A Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 2018, 315, H1553–H1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bauernfeind FG; Horvath G; Stutz A; Alnemri ES; MacDonald K; Speert D; Fernandes-Alnemri T; Wu J; Monks BG; Fitzgerald KA; Hornung V; Latz E Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 2009, 183, 787–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juliana C; Fernandes-Alnemri T; Kang S; Farias A; Qin F; Alnemri ES Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem 2012, 287, 36617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghonime MG; Shamaa OR; Das S; Eldomany RA; Fernandes-Alnemri T; Alnemri ES; Gavrilin MA; Wewers MD Inflammasome priming by lipopolysaccharide is dependent upon ERK signaling and proteasome function. J Immunol 2014, 192, 3881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Py BF; Kim MS; Vakifahmetoglu-Norberg H, Yuan J Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell 2013, 49, 331–8. [DOI] [PubMed] [Google Scholar]
- 39.Huang WQ; Wei P; Lin RQ, Huang F Protective Effects of Microrna-22 Against Endothelial Cell Injury by Targeting NLRP3 Through Suppression of the Inflammasome Signaling Pathway in a Rat Model of Coronary Heart Disease. Cell Physiol Biochem 2017, 43, 1346–1358. [DOI] [PubMed] [Google Scholar]
- 40.Yu SY; Dong B; Tang L; Zhou SH LncRNA MALAT1 sponges miR-133 to promote NLRP3 inflammasome expression in ischemia-reperfusion injured heart. Int J Cardiol 2018, 254:50. [DOI] [PubMed] [Google Scholar]
- 41.Song N; Li T Regulation of NLRP3 Inflammasome by Phosphorylation. Front Immunol 2018, 9, 2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weber ANR; Bittner ZA; Shankar S; Liu X; Chang TH; Jin T; Tapia-Abellán A Recent insights into the regulatory networks of NLRP3 inflammasome activation. J Cell Sci 2020, 133, 248344. [DOI] [PubMed] [Google Scholar]
- 43.Pasqua T; Pagliaro P; Rocca C; Angelone T; Penna C Role of NLRP-3 Inflammasome in Hypertension: A Potential Therapeutic Target. Curr Pharm Biotechnol 2018, 19, 708–714. [DOI] [PubMed] [Google Scholar]
- 44.Pavillard LE; Marín-Aguilar F; Bullon P; Cordero MD Cardiovascular diseases, NLRP3 inflammasome, and western dietary patterns. Pharmacol Res 2018, 131, 44–50. [DOI] [PubMed] [Google Scholar]
- 45.Yang X; Sun J; Kim TJ; Kim YJ; Ko SB; Kim CK; Jia X; Yoon BW Pretreatment with low-dose fimasartan ameliorates NLRP3 inflammasome-mediated neuroinflammation and brain injury after intracerebral hemorrhage. Exp Neurol 2018, 310, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rong L; Sun S; Zhu F; Xu Q; Li H; Gao Q; Zhang W; Tang B; Zhang H; Wang H; Kang P Effects of irbesartan on myocardial injury in diabetic rats: The role of NLRP3/ASC/Caspase-1 pathway. J Renin Angiotensin Aldosterone Syst 2020, 21, 1470320320926049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao J; Xie Q; Wei T; Huang C; Zhou W; Shen W Nebivolol Improves Obesity-Induced Vascular Remodeling by Suppressing NLRP3 Activation. J Cardiovasc Pharmacol 2019, 73, 326–333. [DOI] [PubMed] [Google Scholar]
- 48.Tozaki-Saitoh H; Sasaki I; Yamashita T; Hosoi M; Kato TA; Tsuda M Involvement of exchange protein directly activated by cAMP and tumor progression locus 2 in IL-1β production in microglial cells following activation of β-adrenergic receptors. J Pharmacol Sci 2020, 143, 133–140. [DOI] [PubMed] [Google Scholar]
- 49.Qiu Z; Lei S; Zhao B; Wu Y; Su W; Liu M; Meng Q; Zhou B; Leng Y; Xia ZY NLRP3 Inflammasome Activation-Mediated Pyroptosis Aggravates Myocardial Ischemia/Reperfusion Injury in Diabetic Rats. Oxid Med Cell Longev 2017, 2017, 9743280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mariathasan S; Weiss DS; Newton K; McBride J; O'Rourke K; Roose-Girma M; Lee WP; Weinrauch Y; Monack DM; Dixit VM Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 2006, 440, 228–32. [DOI] [PubMed] [Google Scholar]
- 51.Pétrilli V; Papin S; Dostert C; Mayor A; Martinon F; Tschopp J Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ 2007, 14, 1583–9. [DOI] [PubMed] [Google Scholar]
- 52.Mezzaroma E; Toldo S; Farkas D; Seropian IM; Van Tassell BW; Salloum FN; Kannan HR; Menna AC; Voelkel NF; Abbate A The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A 2011, 108, 19725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He Y; Zeng MY; Yang D; Motro B; Núñez G NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 2016, 530, 354–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kimura M; Okano Y Identification and assignment of the human NIMA-related protein kinase 7 gene (NEK7) to human chromosome 1q31.3. Cytogenet Cell Genet 2001, 94, 33–8. [DOI] [PubMed] [Google Scholar]
- 55.Murakami T; Ockinger J; Yu J; Byles V; McColl A; Hofer AM; Horng T Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A 2012, 109, 11282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee GS; Subramanian N; Kim AI; Aksentijevich I; Goldbach-Mansky R; Sacks DB; Germain RN; Kastner DL; Chae JJ The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 2012, 492, 123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X; Hong S; Qi S; Liu W; Zhang X; Shi Z; Chen W; Zhao M; Yin X NLRP3 Inflammasome Is Involved in Calcium-Sensing Receptor-Induced Aortic Remodeling in SHRs. Mediators Inflamm 2019, 2019, 6847087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu W; Sun J; Guo Y; Liu N; Ding X; Zhang X; Chi J; Kang N; Liu Y; Yin X Calhex231 ameliorates myocardial fibrosis post myocardial infarction in rats through the autophagy-NLRP3 inflammasome pathway in macrophages. J Cell Mol Med 2020, 24, 13440–13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lima H Jr.; Jacobson LS; Goldberg MF; Chandran K; Diaz-Griffero F; Lisanti MP; Brojatsch J Role of lysosome rupture in controlling Nlrp3 signaling and necrotic cell death. Cell Cycle 2013, 12, 1868–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chu J; Thomas LM; Watkins SC; Franchi L; Núñez G; Salter RD Cholesterol-dependent cytolysins induce rapid release of mature IL-1beta from murine macrophages in a NLRP3 inflammasome and cathepsin B-dependent manner. J Leukoc Biol 2009, 86, 1227–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martinon F; Pétrilli V; Mayor A; Tardivel A; Tschopp J Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–41. [DOI] [PubMed] [Google Scholar]
- 62.Pazár B; Ea HK; Narayan S; Kolly L; Bagnoud N; Chobaz V; Roger T; Lioté F; So A; Busso N Basic calcium phosphate crystals induce monocyte/macrophage IL-1beta secretion through the NLRP3 inflammasome in vitro. J Immunol 2011, 186, 2495–502. [DOI] [PubMed] [Google Scholar]
- 63.Duewell P; Kono H; Rayner KJ; Sirois CM; Vladimer G; Bauernfeind FG; Abela GS; Franchi L; Nuñez G; Schnurr M; Espevik T; Lien E; Fitzgerald KA; Rock KL; Moore KJ; Wright SD; Hornung V; Latz E NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klionsky DJ; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun Q; Fan J; Billiar TR; Scott MJ Inflammasome and autophagy regulation - a two-way street. Mol Med 2017, 23, 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu X; He L; Cai Y; Zhang G; He Y; Zhang Z; He X; He Y; Zhang G; Luo J Induction of autophagy contributes to the myocardial protection of valsartan against ischemia–reperfusion injury. Mol Med Rep 2013, 8, 1824–1830. [DOI] [PubMed] [Google Scholar]
- 67.Wu X; He L; Chen F; He X; Cai Y; Zhang G; Yi Q; He M; Luo J Impaired autophagy contributes to adverse cardiac remodeling in acute myocardial infarction. PLoS One 2014, 9, e112891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang D; He Y; Ye X; Cai Y; Xu J; Zhang L; Li M; Liu H; Wang S; Xia Z Activation of autophagy inhibits nucleotide-binding oligomerization domain-like receptor protein 3 inflammasome activation and attenuates myocardial ischemia-reperfusion injury in diabetic rats. J Diabetes Investig 2020, 11, 1126–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meng Z; Song MY; Li CF; Zhao JQ shRNA interference of NLRP3 inflammasome alleviate ischemia reperfusion-induced myocardial damage through autophagy activation. Biochem Biophys Res Commun 2017, 494, 728–735. [DOI] [PubMed] [Google Scholar]
- 70.Wen H; Gris D; Lei Y; Jha S; Zhang L; Huang MT; Brickey WJ; Ting JP Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 2011, 12, 408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davidson SM; Adameová A; Barile L; Cabrera-Fuentes HA; Lazou A; Pagliaro P; Stensløkken KO; Garcia-Dorado D; EU-CARDIOPROTECTION COST Action (CA16225). Mitochondrial and mitochondrial-independent pathways of myocardial cell death during ischaemia and reperfusion injury. J Cell Mol Med 2020, 24, 3795–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dan Dunn J; Alvarez LA; Zhang X; Soldati T Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol 2015, 6, 472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schofield JH; Schafer ZT Mitochondrial Reactive Oxygen Species and Mitophagy: A Complex and Nuanced Relationship. Antioxid Redox Signal 2020, 34, 517–530. [DOI] [PubMed] [Google Scholar]
- 74.Zhong Z; Liang S; Sanchez-Lopez E; He F; Shalapour S; Lin XJ; Wong J; Ding S; Seki E; Schnabl B; Hevener AL; Greenberg HB; Kisseleva T; Karin M New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 2018, 560, 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iyer SS; He Q; Janczy JR; Elliott EI; Zhong Z; Olivier AK; Sadler JJ; Knepper-Adrian V; Han R; Qiao L; Eisenbarth SC; Nauseef WM; Cassel SL; Sutterwala FS Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 2013, 39, 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhong Z; Liang S; Sanchez-Lopez E; He F; Shalapour S; Lin XJ; Wong J; Ding S; Seki E; Schnabl B; Hevener AL; Greenberg HB; Kisseleva T; Karin M New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 2018, 560, 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park S; Juliana C; Hong S; Datta P; Hwang I; Fernandes-Alnemri T; Yu JW; Alnemri ES The mitochondrial antiviral protein MAVS associates with NLRP3 and regulates its inflammasome activity. J Immunol 2013, 191, 4358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Madamanchi NR; Runge MS Mitochondrial dysfunction in atherosclerosis. Circ Res 2007, 100, 460–73. [DOI] [PubMed] [Google Scholar]
- 79.Adlam VJ; Harrison JC; Porteous CM; James AM; Smith RA; Murphy MP; Sammut IA Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J 2005, 19, 1088–95. [DOI] [PubMed] [Google Scholar]
- 80.Abel ED; Doenst T Mitochondrial adaptations to physiological vs. pathological cardiac hypertrophy. Cardiovasc Res 2011, 90, 234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alhawiti NM; Al Mahri S; Aziz MA; Malik SS; Mohammad S TXNIP in Metabolic Regulation: Physiological Role and Therapeutic Outlook. Curr Drug Targets 2017, 18, 1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamawaki H; Berk BC Thioredoxin: a multifunctional antioxidant enzyme in kidney, heart and vessels. Curr Opin Nephrol Hypertens 2005, 14, 149–53. [DOI] [PubMed] [Google Scholar]
- 83.Byon CH; Han T; Wu J; Hui ST Txnip ablation reduces vascular smooth muscle cell inflammation and ameliorates atherosclerosis in apolipoprotein E knockout mice. Atherosclerosis 2015, 241, 313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao P; He FF; Tang H; Lei CT; Chen S; Meng XF; Su H; Zhang C NADPH oxidase-induced NALP3 inflammasome activation is driven by thioredoxin-interacting protein which contributes to podocyte injury in hyperglycemia. J Diabetes Res 2015, 2015, 504761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Y; Lian K; Zhang L; Wang R; Yi F; Gao C; Xin C; Zhu D; Li Y; Yan W; Xiong L; Gao E; Wang H; Tao L TXNIP mediates NLRP3 inflammasome activation in cardiac microvascular endothelial cells as a novel mechanism in myocardial ischemia/reperfusion injury. Basic Res Cardiol 2014, 109, 415. [DOI] [PubMed] [Google Scholar]
- 86.Hansson GK; Robertson AK; Söderberg-Nauclér C Inflammation and atherosclerosis. Annu Rev Pathol 2006, 1, 297–329. [DOI] [PubMed] [Google Scholar]
- 87.Moore KJ; Sheedy FJ; Fisher EA Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 2013, 13, 709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kawaguchi M; Takahashi M; Hata T; Kashima Y; Usui F; Morimoto H; Izawa A; Takahashi Y; Masumoto J; Koyama J; Hongo M; Noda T; Nakayama J; Sagara J; Taniguchi S; Ikeda U Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 2011, 123, 594–604. [DOI] [PubMed] [Google Scholar]
- 89.Toldo S; Marchetti C; Mauro AG; Chojnacki J; Mezzaroma E; Carbone S; Zhang S; Van Tassell B; Salloum FN; Abbate A Inhibition of the NLRP3 inflammasome limits the inflammatory injury following myocardial ischemia-reperfusion in the mouse. Int J Cardiol 2016, 209, 215–20. [DOI] [PubMed] [Google Scholar]
- 90.Seropian IM; Toldo S; van Tassell BW; Abbate A Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol 2014, 63, 1593–1603. [DOI] [PubMed] [Google Scholar]
- 91.Westman PC; Lipinski MJ; Luger D; Waksman R; Bonow RO; Wu E; Epstein SE Inflammation as a Driver of Adverse Left Ventricular Remodeling After Acute Myocardial Infarction. J Am Coll Cardiol 2016, 67, 2050–60. [DOI] [PubMed] [Google Scholar]
- 92.Takahashi M Cell-Specific Roles of NLRP3 Inflammasome in Myocardial Infarction. J Cardiovasc Pharmacol 2019, 74, 188–193. [DOI] [PubMed] [Google Scholar]
- 93.Sandanger Ø; Ranheim T; Vinge LE; Bliksøen M; Alfsnes K; Finsen AV; Dahl CP; Askevold ET; Florholmen G; Christensen G; Fitzgerald KA; Lien E; Valen G; Espevik T; Aukrust P; Yndestad A The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc Res 2013, 99, 164–74. [DOI] [PubMed] [Google Scholar]
- 94.Luo B; Wang F; Li B; Dong Z; Liu X; Zhang C; An F Association of nucleotide-binding oligomerization domain-like receptor 3 inflammasome and adverse clinical outcomes in patients with idiopathic dilated cardiomyopathy. Clin Chem Lab Med 2013, 51, 1521–8. [DOI] [PubMed] [Google Scholar]
- 95.Zeng C; Duan F; Hu J; Luo B; Huang B; Lou X; Sun X; Li H; Zhang X; Yin S; Tan H NLRP3 inflammasome-mediated pyroptosis contributes to the pathogenesis of non-ischemic dilated cardiomyopathy. Redox Biol 2020, 34, 101523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gan W; Ren J; Li T; Lv S; Li C; Liu Z; Yang M The SGK1 inhibitor EMD638683, prevents Angiotensin II-induced cardiac inflammation and fibrosis by blocking NLRP3 inflammasome activation. Biochim Biophys Acta Mol Basis Dis 2018, 1864, 1–10. [DOI] [PubMed] [Google Scholar]
- 97.Wang Y; Wu Y; Chen J; Zhao S; Li H Pirfenidone attenuates cardiac fibrosis in a mouse model of TAC-induced left ventricular remodeling by suppressing NLRP3 inflammasome formation. Cardiology 2013, 126, 1–11. [DOI] [PubMed] [Google Scholar]
- 98.Lian D; Lai J; Wu Y; Wang L; Chen Y; Zhang Y; Boini KM; Huang Y; Chen Y Cathepsin B-Mediated NLRP3 Inflammasome Formation and Activation in Angiotensin II -Induced Hypertensive Mice: Role of Macrophage Digestion Dysfunction. Cell Physiol Biochem 2018, 50, 1585–1600. [DOI] [PubMed] [Google Scholar]
- 99.Willeford A; Suetomi T; Nickle A; Hoffman HM; Miyamoto S; Brown JH CaMKIIδ-mediated inflammatory gene expression and inflammasome activation in cardiomyocytes initiate inflammation and induce fibrosis. JCI Insight 2018, 3, e97054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Suetomi T; Willeford A; Brand CS; Cho Y; Ross RS; Miyamoto S; Brown JH Inflammation and NLRP3 Inflammasome Activation Initiated in Response to Pressure Overload by Ca2+/Calmodulin-Dependent Protein Kinase II δ Signaling in Cardiomyocytes Are Essential for Adverse Cardiac Remodeling. Circulation 2018, 138, 2530–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Renu K; Abilash VG; Pichiah TPB; Arunachalam S Molecular mechanism of doxorubicin-induced cardiomyopathy - An update. Eur J Pharmacol 2018, 818, 241–253. [DOI] [PubMed] [Google Scholar]
- 102.Toldo S; Goehe RW; Lotrionte M; Mezzaroma E; Sumner ET; Biondi-Zoccai GG; Seropian IM; Van Tassell BW; Loperfido F; Palazzoni G; Voelkel NF; Abbate A, Gewirtz DA Comparative cardiac toxicity of anthracyclines in vitro and in vivo in the mouse. PLoS One 2013, 8, e58421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Singla DK; Johnson TA; Tavakoli Dargani Z Exosome Treatment Enhances Anti-Inflammatory M2 Macrophages and Reduces Inflammation-Induced Pyroptosis in Doxorubicin-Induced Cardiomyopathy. Cells 2019, 8, 1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marchetti C; Toldo S; Chojnacki J; Mezzaroma E; Liu K; Salloum FN; Nordio A; Carbone S; Mauro AG; Das A; Zalavadia AA; Halquist MS; Federici M; Van Tassell BW; Zhang S; Abbate A; Pharmacologic Inhibition of the NLRP3 Inflammasome Preserves Cardiac Function After Ischemic and Nonischemic Injury in the Mouse. J Cardiovasc Pharmacol. 2015, 66, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mezzaroma E; Mikkelsen RB; Toldo S; Mauro AG; Sharma K; Marchetti C; Alam A; Van Tassell BW; Gewirtz DA; Abbate A Role of Interleukin-1 in Radiation-Induced Cardiomyopathy. Mol Med 2015, 21, 210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Christersdottir T; Pirault J; Gisterå A; Bergman O; Gallina AL; Baumgartner R; Lundberg AM; Eriksson P; Yan ZQ; Paulsson-Berne G; Hansson GK; Olofsson PS; Halle M Prevention of radiotherapy-induced arterial inflammation by interleukin-1 blockade. Eur Heart J 2019, 40, 2495–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li X; Cui W; Hull L; Wang L; Yu T; Xiao M IL-18 binding protein (IL-18BP) as a novel radiation countermeasure after radiation exposure in mice. Sci Rep 2020, 10, 18674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ballak DB; Stienstra R; Tack CJ; Dinarello CA; van Diepen JA IL-1 family members in the pathogenesis and treatment of metabolic disease: Focus on adipose tissue inflammation and insulin resistance. Cytokine 2015, 75, 280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dinarello CA Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr 2006, 83, 447S–455S. [DOI] [PubMed] [Google Scholar]
- 110.Reilly SM; Saltiel AR Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol 2017, 13, 633–643. [DOI] [PubMed] [Google Scholar]
- 111.Vandanmagsar B; Youm YH; Ravussin A; Galgani JE; Stadler K; Mynatt RL; Ravussin E; Stephens JM; Dixit VD The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 2011, 17, 179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Camell CD; Günther P; Lee A; Goldberg EL; Spadaro O; Youm YH; Bartke A; Hubbard GB; Ikeno Y; Ruddle NH; Schultze J; Dixit VD Aging Induces an Nlrp3 Inflammasome-Dependent Expansion of Adipose B Cells That Impairs Metabolic Homeostasis. Cell Metab 2019, 30, 1024–1039.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Youm YH; Grant RW; McCabe LR; Albarado DC; Nguyen KY; Ravussin A; Pistell P; Newman S; Carter R; Laque A; Münzberg H; Rosen CJ; Ingram DK; Salbaum JM; Dixit VD Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab 2013, 18, 519–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jia G; Hill MA; Sowers JR Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ Res 2018, 122, 624–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Luo B; Li B; Wang W; Liu X; Xia Y; Zhang C; Zhang M; Zhang Y; An F NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS One 2014, 9, e104771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cai J; Lu S; Yao Z; Deng YP; Zhang LD; Yu JW; Ren GF; Shen FM; Jiang GJ Glibenclamide attenuates myocardial injury by lipopolysaccharides in streptozotocin-induced diabetic mice. Cardiovasc Diabetol 2014, 13, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cai L; Li W; Wang G; Guo L; Jiang Y; Kang YJ Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes 2002, 51, 1938–48. [DOI] [PubMed] [Google Scholar]
- 118.Ralston JC; Lyons CL; Kennedy EB; Kirwan AM; Roche HM Fatty Acids and NLRP3 Inflammasome-Mediated Inflammation in Metabolic Tissues. Annu Rev Nutr 2017, 37, 77–102. [DOI] [PubMed] [Google Scholar]
- 119.Feng H; Gu J; Gou F; Huang W; Gao C; Chen G; Long Y; Zhou X; Yang Mv Liu S; Lü S; Luo Q; Xu Y High Glucose and Lipopolysaccharide Prime NLRP3 Inflammasome via ROS/TXNIP Pathway in Mesangial Cells. J Diabetes Res 2016, 2016, 6973175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Han Y; Xu X; Tang C; Gao P; Chen X; Xiong X; Yang M; Yang S; Zhu X; Yuan S; Liu F; Xiao L; Kanwar YS; Sun L Reactive oxygen species promote tubular injury in diabetic nephropathy: The role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol 2018, 16, 32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen W; Zhao M; Zhao S; Lu Q; Ni L; Zou C; Lu L; Xu X; Guan H; Zheng Z; Qiu Q Activation of the TXNIP/NLRP3 inflammasome pathway contributes to inflammation in diabetic retinopathy: a novel inhibitory effect of minocycline. Inflamm Res 2017, 66, 157–166. [DOI] [PubMed] [Google Scholar]
- 122.Buckley LF; Viscusi MM; Van Tassell BW; Abbate A Interleukin-1 blockade for the treatment of pericarditis. Eur Heart J Cardiovasc Pharmacother 2018, 4, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mauro AG; Bonaventura A; Vecchie’ A; Mezzaroma E; Carbone S; Narayan P; Potere N; Cannatà A; Paolini JF; Bussani R; Montecucco F; Sinagra G; Van Tassell BW; Abbate A; Toldo S The role of NLRP3 inflammasome in pericarditis: potential for therapeutic approaches. JACC BTS, 2021, 6, 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Klein AL; Lin D; Cremer PC; Nasir S; Luis SA; Abbate A; Ertel A; LeWinter M; Beutler A; Fang F; Paolini JF; Efficacy and safery of rilonacept for recurrent pericarditis: results from a phase II clinical trial. Heart 2020, 107, 488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Klein AL; Imazio M; Cremer P; Brucato A; Abbate A; Fang F; Insalaco A; LeWinter M; Lewis BS; Lin D; Luis SA; Nicholls SJ; Pano A; Wheeler A; Paolini JF; RHAPSODY Investigators. Phase 3 Trial of Interleukin-1 Trap Rilonacept in Recurrent Pericarditis. N Engl J Med 2021, 384, 31–41. [DOI] [PubMed] [Google Scholar]
- 126.Brucato A; Imazio M; Gattorno M; Lazaros G; Maestroni S; Carraro M; Finetti M; Cumetti D; Carobbio A; Ruperto N; Marcolongo R; Lorini M; Rimini A; Valenti A; Erre GL; Sormani MP; Belli R; Gaita F; Martini A Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: the AIRTRIP randomized clinical trial. JAMA 2016, 316, 1906–1912. [DOI] [PubMed] [Google Scholar]
- 127.Wohlford GF; Buckley LF; Vecchié A; Dinesh K; Markley R; Trankle C; Chiabrando JG; de Chazal HM; Van Tassell BW; Abbate A Acute effects of interleukin-1 blockade using anakinra in patients with acute pericarditis. J Cardiovasc Pharmacol 2020, 76, 50–52. [DOI] [PubMed] [Google Scholar]
- 128.Toldo S; Kannan H; Bussani R; Anzini M; Sonnino C; Sinagra G; Merlo M; Mezzaroma E; De-Giorgio F; Silvestri F; Van Tassell BW; Baldi A; Abbate A Formation of the inflammasome in acute myocarditis. Int J Cardiol 2014, 171, e119–21. [DOI] [PubMed] [Google Scholar]
- 129.Wang Y; Gao B; Xiong S Involvement of NLRP3 inflammasome in CVB3-induced viral myocarditis. Am J Physiol Heart Circ Physiol 2014, 307, H1438–47. [DOI] [PubMed] [Google Scholar]
- 130.Wang Y; Jia L; Shen J; Wang Y; Fu Z; Su SA; Cai Z; Wang JA; Xiang M Cathepsin B aggravates coxsackievirus B3-induced myocarditis through activating the inflammasome and promoting pyroptosis. PLoS Pathog 2018, 14, e1006872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kron J; Ellenbogen KA Cardiac sarcoidosis: contemporary review. J Cardiovasc Electrophysiol 2015, 26, 104–9. [DOI] [PubMed] [Google Scholar]
- 132.Kron J; Mauro AG; Bonaventura A; Toldo S; Salloum FN; Ellenbogen KA; Abbate A Inflammasome Formation in Granulomas in Cardiac Sarcoidosis. Circ Arrhythm Electrophysiol 2019, 12, e007582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Interleukin-1 Blockade for Treatment of Cardiac Sarcoidosis (MAGiC-ART). https://clinicaltrials.gov/ct2/show/NCT04017936.
- 134.Di Nisio M; van Es N; Büller HR Deep vein thrombosis and pulmonary embolism. Lancet 2016, 388, 3060–3073. [DOI] [PubMed] [Google Scholar]
- 135.Colling ME; Tourdot BE; Kanthi Y Inflammation, infection and venous thromboembolism. Circ Res 2021, 128, 2917–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Takahashi M NLRP3 inflammasome as a key driver of vascular disease. Cardiovasc Res 2021, 00, 1–14. [DOI] [PubMed] [Google Scholar]
- 137.Gupta N; Sahu A; Prabhakar A; Chatterjee T; Tyagi T; Kumari B; Khan N; Nair V; Bajaj N; Sharma M; Ashraf MZ Activation of NLRP3 inflammasome complex potentiates venous thrombosis in resonse to hypoxia. Proc Natl Acad Sci USA 2017, 114, 4763–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yadav V; Chi L; Zhao R; Tourdot BE; Yalavarthi S; Jacobs BN; Banka A; Liao H; Koonse S; Anyanwu AC; Vosovatti SH; Holinstat MA; Kahlenberg JM; Knight JS; Pinsky DJ; Kanthi Y ENTPD-1 disrupts inflammasome IL-1β-driven venous thrombosis. J Clin Invest 2019, 129, 2872–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Campos J; Ponomaryov T; De Prendergast A; Whitworth K; Smith CW; Khan AO; Kavanagh D; Brill A Neutrophil extracellular traps and inflammasomes cooperatively promote venous thrombosis in mice. Blood Adv 2021, 5, 2319–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang Y; Cui J; Zhang G; Wu C; Abdel-Latif A; Smyth SS; Shiroishi T; Mackman N; Wei Y; Tao M; Li Z Inflammasome activation promotes thrombosis through pyroptosis. Blood Adv 2021, 5, 2619–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu C; Lu W; Zhang Y; Zhang G; Shi X; Hisada Y; Grover SP; Zhang X; Li L; Xiang B; Shi J; Li X; Daugherty A; Smyth SS; Kirchhofer D; Shiroishi T; Shao F; Mackman N; Wei Y; Li Z Inflammasome activation triggers blood clotting and host death through pyroptosis. Immunity 2019, 50, 1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rabinovich A; Cohen JM; Cushman M; Kahn SR; BioSOX Investigators. Association between inflammation markers, anatomic extent of deep venous thrombosis, and venous symptoms after deep venous thrombosis. J Vasc Surg Venous Lymphat Disord 2015, 3, 347–353 [DOI] [PubMed] [Google Scholar]
- 143.Roumen-Klappe EM; Janssen MCH; Van Rossum J; Holewijn S; Van Bokhoven MMJA; Kaasjager K; Wollersheim H; Den Heijer M; Inflammation in deep vein thrombosis and the development of post-thrombotic syndrome: a prospective study. J Thromb Haemost 2009, 7, 582–587. [DOI] [PubMed] [Google Scholar]
- 144.Jara-Palomares L; Solier-Lopez A; Elias-Hernandez T; Asensio-Cruz MI; Blasco-Esquivias I; Sanchez-Lopez L; Rodriguez de la Borbolla M; Arellano-Orden E; Suarez-Valdivia L; Marin-Romero S; Marin-Barrera L; Ruiz-Garcia A; Montero-Romero E; Navarro-Herrero S; Lopez-Campos JL; Serrano-Gotarredona MP; Praena-Fernandez JM; Sanchez-Diaz JM; Otero-Candelera R D-dimere and high-sensitivity C-reactive protein levels to predict venous thromboembolism recurrence after discontinuation of anticoagulation for cancer-associated thrombosis. Br J Cancer 2018, 119, 915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kunutsor SK; Seidu S; Blom AW; Khunti K; Laukkanen JA Serum C-reactive protein increases the risk of venous thromboembolism: a prospective study and meta-analysis of published prospective evidence. Eur J Epidemiol 2017, 32, 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zheng M; Williams EP; Malireddi RSK; Karki R; Banoth B; Burton A; Webby R; Channappanavar R; Jonsson CB; Kanneganti TD Impaired NLRP3 inflammasome activation/pyroptosis leads to robust inflammatory cell death via caspase-8/RIPK3 during coronavirus infection. J Biol Chem 2020, 295, 14040–14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Toldo S; Bussani R; Nuzzi V; Bonaventura A; Mauro AG; Cannatà A; Pillappa R; Sinagra G; Nana-Sinkam P; Sime P; Abbate A Inflammasome formation in the lungs of patients with fatal COVID-19. Inflamm Res 2021, 70, 7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rodrigues TS; de Sá KSG; Ishimoto AY; Becerra A; Oliveira S; Almeida L; Gonçalves AV; Perucello DB; Andrade WA; Castro R; Veras FP; Toller-Kawahisa JE; Nascimento DC; de Lima MHF; Silva CMS; Caetite DB; Martins RB; Castro IA; Pontelli MC; de Barros FC; do Amaral NB; Giannini MC; Bonjorno LP; Lopes MIF; Santana RC; Vilar FC; Auxiliadora-Martins M; Luppino-Assad R; de Almeida SCL; de Oliveira FR; Batah SS; Siyuan L; Benatti MN; Cunha TM; Alves-Filho JC; Cunha FQ; Cunha LD; Frantz FG; Kohlsdorf T; Fabro AT; Arruda E; de Oliveira RDR; Louzada-Junior P; Zamboni DS Inflammasomes are activated in responde to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med 2021, 218, e20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Theobald SJ; Simonis A; Georgomanolis T; Kreer C; Zehner M; Eisfeld HS; Albert MC; Chhen J; Motameny S; Erger F; Fischer J; Malin JJ; Gräb J; Winter S; Pouikli A; David F; Böll; Koehler P; Vanshylla K; Gruell H; Suárez I; Hallek M; Fätkenheuer G; Jung N; Cornely OA; Lehmann C; Tessarz P; Altmüller J; Nürnberg P; Kashkar H; Klein F; Koch M; Rybniker J Long-lived macrophage reprogramming drives spike protein-mediated inflammasome activation in COVID-19. EMBO Mol Med 2021, 13, e14150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Courjon J; Dufies O; Robert A; Bailly L; Torre C; Chirio D; Contenti J; Vitale S; Loubatier C; Doye A; Pomares-Estran C; Gonfrier G; Lotte R; Munro P; Visvikis O; Dellamonica J; Giordanengo V; Carles M; Charvet-Yvan L; Ivanov S; Auberger P; Jacquel A; Boyer L Heterogeneous NLRP3 inflammasome signature in circulating myeloid cells as a biomarker of COVID-19 severity. Blood Adv 2021, 5, 1523–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Campbell GR; To RK; Hanna J; Spector SA SARS-CoV-2, SARS-CoV-2, and HIV-1 derived ssRNA sequences activate the NLRP3 inflammasome in human macrophages through a non-classical pathway. iScience 2021, 24, 102295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Olajide OO; Iwuanyanwy VU; Lepiarz-Raba I; Al-Hindawi A Induction of exaggerated cytokine production in human peripheral blood mononuclear cells by a recombinant SARS-CoV-2 spike glycoprotein S1 and its inhibition by dexamethasone. Inflammation 2021, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Potere N; Candeloro M; Porreca E; Marinari S; Federici C; Auciello R; Di Nisio M Direct oral anticoagulant plasma levels in hospitalized COVID-19 patients treated with dexamethasone. J Thromb Thrombolysis 2021, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ratajczak MZ; Bujko K; Ciechanowicz A; Sielatycka K; Cymer M; Marlicz W; Kucia M SARS-CoV-2 entry receptor ACE2 is expressed on very small CD45- precursor of hematopoietic and endothelial cells and in response to virus spike protein activates the Nlrp3 inflammasome. Stem Cell Rev Rep 2021, 17, 266–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cauchois R; Koubi M; Delarbre D; Manet C; Carvelli J; Blasco VB; Jean R; Fouche L; Bornet C; Pauly V; Mazodier K; Pestre V; Jarrot PA; Dinarello CA; Kaplasnki G Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc Natl Acad Sci USA 2020, 117, 18951–18953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cavalli G; Larcher A; Tomelleri A; Campochiaro C; Della-Torre E; De Luca G; Farina N; Boffini N; Ruggeri A; Poli A; Scarpellini P; Rovere-Querini P; Tresoldi M; Salonia A; Montorsi F; Landoni G; Castagna A; Ciceri F; Zangrillo A; Dagna L Interleukin-1 and interleukin-6 inhibition compared with standard management in patients with COVID-19 and hyperinflammation: a cohort study. Lancet Rheumatol 2021, 3, e253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ucciferri C; Auricchio A; Di Nicola M; Potere N; Abbate A; Cipollone F; Vecchiet J; Falasca K Canakinumab in a subgroup of patients with COVID-19. Lancet Rheumatol 2020, 2, e457–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kyriazopoulou E; Poulakou G; Milionis H; Metallidis S; Adamis G; Tsiakos K; Fragkou A; Rapti A; Damoulari C; Fantoni M; Kalomenidis I; Chrysos G; Angheben A; Kainis I; Alexiou Z; Castelli F; Serino FS; Tsilika M; Bakakos P; Nicastri E; Tzavara V; Kostis E; Dagna L; Koufargyris P; Dimakou K; Savvanis S; Tzatzagou G; Chini M; Cavalli G; Bassetti M; Katrini K; Kotsis V; Tsoukalas G; Selmi C; Bliziotis I; Samarkos M; Doumas M; Ktena S; Masgala A; Papanikolaou I; Kosmidou M; Myrodia DM; Argyraki A; Cardellino CS; Koliakou K; Katsigianni EI; Rapti V; Giannitsioti E; Cingolani A; Micha S; Akinosoglou K; Liatsis-Douvitsas O; Symbardi S; Gatselis N; Mouktaroudi M; Ippolito G; Florou E; Kotsaki A; Netea MG; Eugen-Olsen J; Kyprianou M; Panagopoulos P; Dalekos GN; Giamarellos-Bourboulis EJ Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med 2021, 27, 1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Caricchio R; Abbate A; Gordeev I; Meng J; Hsue PY; Neogi T; Arduino R; Fomina D; Bogdanov R; Stepanenko T; Ruiz-Seco P; Gónzalez-ía A; Chen Y; Li Y; Whelan S; Noviello S; CAN-COVID Investigators. Effect of canakinumab vs placebo on survival without invasive mechanical ventilation in patients hospitalized with severe COVID-19: a randomized clinical trial. JAMA 2021, 326, 230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Potere N; Batticciotto A; Vecchié A; Porreca E; Cappelli A; Abbate A; Dentali F; Bonaventura A The role of IL-6 and IL-6 blockade in COVID.19. Expert Rev Clin Immunol 2021, 1–17. [DOI] [PubMed] [Google Scholar]
- 161.Xu X; Han M; Li T; Sun W; Wang D; Fu B; Zhou Y; Zheng X; Yang Y; Li X; Zhang X; Pan A; Wei H Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA 2020, 117, 10979–10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Potere N; Di Nisio M; Cibelli D; Scurti R; Frattari A; Porreca E; Abbate A; Parruti G Interleukin-6 receptor blockade with subcutaneous tocilizumab in severe COVID-19 pneumonia and hyperinflammation: a case-control study. Ann Rheum Dis 2021, 89, 1–2. [DOI] [PubMed] [Google Scholar]
- 163.Di Nisio M; Potere N; Candeloro M; Spacone A; Pieramati L; Ferrandu G; Rizzo G; La Vella M; Di Carlo S; Cibelli D; Parruti G; Levi M; Porreca E Interleukin-6 receptor blockade with subcutaneous tocilizumab improves coagulation activity in patients with COVID-19. Eur J Int Med 2021, 83, 34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Potere N; Di Nisio M; Rizzo G; La Vella M; Polilli E; Agostinone A; Spacone A; Di Carlo S; Costantini A; Abbate A; Porreca E; Parruti G Low-dose subcutaneous tocilizumab to prevent disease progression in patients with moderate COVID-19 pneumonia and hyperinflammation. Int J Infect Dis 2020, 100, 421–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Potere N; Di Nisio M; Cibelli D; Scurti R; Frattari A; Porreca E; Abbate A; Parruti G Response to: ‘Correspondence on ‘Interleukin-6 receptor blockade with subcutaneous tocilizumab in severe COVID-19 pneumonia and hyperinflammation: a case-control study’ by Potere et al’ by Buckley. Ann Rheum Dis 2020, annrheumdis-2020-218715. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 166.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Shankar-Hari M; Vale CL; Godolphin PJ; Fisher D; Higgins JPT; Spiga F; Savovic J; Tierney J; Baron G; Benbenshty JS; Berry LR; Broman N; Biasi Cavalcanti A; Colman R; De Buyser S; Derde LP; Domingo P; Omar SD; Fernandez-Cruz A; Feuth T; Garcia F; Garcia-Vicuna R; Gonzalez-Alvaro I; Gordon AC; Haynes R; Hermine O; Horby PW; Horick NK; Kumar K; Lambrecht BN; Landray MJ; Leal L; Lederer DJ; Lorenzi E; Mariette X; Merchante N; Misnan NA; Mohan SV; Nivens MC; Oksi J; Perez-Molina JA; Pizov R; Porcher R; Postma S; Rajasuriar R; Ramanan AV; Ravaud P; Reid PD; Rutgers A; Sancho-Lopez A; Seto TB; Sivapalasingam S; Singh Soin A, Staplin N; Stone JH; Strohbehn GW; Sunden-Cullberg J; Tosse-Cisneros J; Tsai LW; van Hoogstraten H; van Meerten T; Cordeiro Veiga V; Westerweel PE; Murthy S; Diaz JV; Marshall JC; Sterne JAC Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA 2021, 326, 499–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.NIH COVID-19 Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/; accessed on August 25, 2021.
- 168.WHO Therapeutics and COVID-19: living guideline. https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.2; accessed on August 25, 2021.
- 169.Luzi L; Pozza G Glibenclamide: an old drug with a novel mechanism of action? Acta Diabetol 1997, 34, 239–44. [DOI] [PubMed] [Google Scholar]
- 170.Lamkanfi M; Mueller JL; Vitari AC; Misaghi S; Fedorova A; Deshayes K; Lee WP; Hoffman HM; Dixit VM Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol 2009, 187, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Marchetti C; Chojnacki J; Toldo S; Mezzaroma E; Tranchida N; Rose SW; Federici M; Van Tassell BW; Zhang S; Abbate A A novel pharmacologic inhibitor of the NLRP3 inflammasome limits myocardial injury after ischemia-reperfusion in the mouse. J Cardiovasc Pharmacol 2014, 63, 316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Quader M; Mezzaroma E; Kenning K; Toldo S Targeting the NLRP3 inflammasome to reduce warm ischemic injury in donation after circulatory death heart. Clin Transplant 2020, 34, e14044. [DOI] [PubMed] [Google Scholar]
- 173.Quader M; Mezzaroma E; Wickramaratne N; Toldo S Novel approach to improve circulatory death donor heart function. JTCVS Tech 2021. In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Carbone S; Mauro AG; Prestamburgo A; Halquist MS; Narayan P; Potere N; Mezzaroma E; Van Tassell BW; Abbate A; Toldo S An Orally Available NLRP3 Inflammasome Inhibitor Prevents Western Diet-Induced Cardiac Dysfunction in Mice. J Cardiovasc Pharmacol 2018, 72, 303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fulp J; He L; Toldo S; Jiang Y; Boice A; Guo C; Li X; Rolfe A; Sun D; Abbate A; Wang XY; Zhang S Structural Insights of Benzenesulfonamide Analogues as NLRP3 Inflammasome Inhibitors: Design, Synthesis, and Biological Characterization. J Med Chem 2018, 61, 5412–5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Perregaux DG; McNiff P; Laliberte R; Hawryluk N; Peurano H; Stam E; Eggler J; Griffiths R; Dombroski MA; Gabel CA. Identification and characterization of a novel class of interleukin-1 post-translational processing inhibitors. J Pharmacol Exp Ther 2001, 299, 187–97. [PubMed] [Google Scholar]
- 177.Coll RC; Robertson AA; Chae JJ; Higgins SC; Muñoz-Planillo R; Inserra MC; Vetter I; Dungan LS; Monks BG; Stutz A; Croker DE; Butler MS; Haneklaus M; Sutton CE; Núñez G; Latz E; Kastner DL; Mills KH; Masters SL; Schroder K; Cooper MA; O'Neill LA A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 2015, 21, 248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Coll RC; Hill JR; Day CJ; Zamoshnikova A; Boucher D; Massey NL; Chitty JL; Fraser JA; Jennings MP; Robertson AAB; Schroder K MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat Chem Biol 2019, 15, 556–559. [DOI] [PubMed] [Google Scholar]
- 179.Daniels MJ; Rivers-Auty J; Schilling T; Spencer NG; Watremez W; Fasolino V; Booth SJ; White CS; Baldwin AG; Freeman S; Wong R; Latta C; Yu S; Jackson J; Fischer N; Koziel V; Pillot T; Bagnall J; Allan SM; Paszek P; Galea J; Harte MK; Eder C; Lawrence CB; Brough D Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer's disease in rodent models. Nat Commun 2016, 7, 12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dempsey C; Rubio Araiz A; Bryson KJ; Finucane O; Larkin C; Mills EL; Robertson AAB; Cooper MA; O'Neill LAJ; Lynch MA Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-beta and cognitive function in APP/PS1 mice. Brain Behav Immun 2017, 61, 306–316. [DOI] [PubMed] [Google Scholar]
- 181.Vande Walle L; Stowe IB; Šácha P; Lee BL; Demon D; Fossoul A; Van Hauwermeiren F; Saavedra PHV; Šimon P; Šubrt V; Kostka L; Stivala CE; Pham VC; Staben ST; Yamazoe S; Konvalinka J; Kayagaki N; Lamkanfi M MCC950/CRID3 potently targets the NACHT domain of wild-type NLRP3 but not disease-associated mutants for inflammasome inhibition. PLoS Biol 2019, 17, e3000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.van der Heijden T; Kritikou E; Venema W; van Duijn J; van Santbrink PJ; Slütter B; Foks AC; Bot I; Kuiper J NLRP3 Inflammasome Inhibition by MCC950 Reduces Atherosclerotic Lesion Development in Apolipoprotein E-Deficient Mice-Brief Report. Arterioscler Thromb Vasc Biol 2017, 37, 1457–1461. [DOI] [PubMed] [Google Scholar]
- 183.van Hout GP; Bosch L; Ellenbroek GH; de Haan JJ; van Solinge WW; Cooper MA; Arslan F; de Jager SC; Robertson AA; Pasterkamp G; Hoefer IE The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur Heart J 2017, 38, 828–836. [DOI] [PubMed] [Google Scholar]
- 184.Gao R; Shi H; Chang S; Gao Y; Li X; Lv C; Yang H; Xiang H; Yang J; Xu L; Tang Y The selective NLRP3-inflammasome inhibitor MCC950 reduces myocardial fibrosis and improves cardiac remodeling in a mouse model of myocardial infarction. Int Immunopharmacol 2019, 74, 105575. [DOI] [PubMed] [Google Scholar]
- 185.Cheng P; Yang G; Zhao X; Zeng W; Sun D; Zeng L; Li G; Guan G; Tan J; Zhu C Precisely and Efficiently Enzyme Response Microspheres with Immune Removal Escape Loaded with MCC950 Ameliorate Cardiac Dysfunction in Acute Myocardial Infarction. J Biomed Nanotechnol 2020, 16, 153–165. [DOI] [PubMed] [Google Scholar]
- 186.Liang L; Zhang G; Li H; Cheng C; Jin T; Su C; Xiao Y; Bradley J; Peberdy MA; Ornato JP; Mangino MJ; Tang W Combined Therapy With Polyethylene Glycol-20k and MCC950 Preserves Post-Resuscitated Myocardial Function in a Rat Model of Cardiac Arrest and Cardiopulmonary Resuscitation. J Am Heart Assoc 2021, 10, e019177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang H; Sun X; Hodge HS; Ferrario CM; Groban L NLRP3 inhibition improves heart function in GPER knockout mice. Biochem Biophys Res Commun 2019, 514, 998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pavillard LE; Cañadas-Lozano D; Alcocer-Gómez E; Marín-Aguilar F; Pereira S; Robertson AAB; Muntané J; Ryffel B; Cooper MA; Quiles JL; Bullón P; Ruiz-Cabello J; Cordero MD NLRP3-inflammasome inhibition prevents high fat and high sugar diets-induced heart damage through autophagy induction. Oncotarget 2017, 8, 99740–99756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yao C; Veleva T; Scott L Jr.; Cao S; Li L; Chen G; Jeyabal P; Pan X; Alsina KM; Abu-Taha I Dr.; Ghezelbash S; Reynolds CL; Shen YH; LeMaire SA; Schmitz W; Müller FU; El-Armouche A; Tony Eissa N; Beeton C; Nattel S; Wehrens XHT; Dobrev D; Li N Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation 2018, 138, 2227–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Krishnan SM; Ling YH; Huuskes BM; Ferens DM; Saini N; Chan CT; Diep H; Kett MM; Samuel CS;Kemp-Harper BK; Robertson AAB; Cooper MA; Peter K; Latz E; Mansell AS; Sobey CG; Drummond GR; Vinh A Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunxtion in salt-sensitive hypertension. Cardiovasc Res 2019, 115, 776–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ren P; Wu D; Appel R; Zhang L; Zhang C; Luo W; Robertson AAB; Cooper MA; Coselli JS; Milewicz DM; Shen YH; LeMaire SA Targeting the NLRP3 Inflammasome With Inhibitor MCC950 Prevents Aortic Aneurysms and Dissections in Mice. J Am Heart Assoc 2020, 9, e014044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Juliana C; Fernandes-Alnemri T; Wu J; Datta P; Solorzano L; Yu JW; Meng R; Quong AA; Latz E; Scott CP; Alnemri ES Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J Biol Chem 2010, 285, 9792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liu Y; Lian K; Zhang L; Wang R; Yi F; Gao C; Xin C; Zhu D; Li Y; Yan W; Xiong L; Gao E; Wang H; Tao L TXNIP mediates NLRP3 inflammasome activation in cardiac microvascular endothelial cells as a novel mechanism in myocardial ischemia/reperfusion injury. Basic Res Cardiol 2014, 109, 415. [DOI] [PubMed] [Google Scholar]
- 194.Kim YS; Kim JS; Kwon JS; Jeong MH; Cho JG; Park JC; Kang JC; Ahn Y BAY 11-7082, a nuclear factor-kappaB inhibitor, reduces inflammation and apoptosis in a rat cardiac ischemia-reperfusion injury model. Int Heart J 2010, 51, 348–53. [DOI] [PubMed] [Google Scholar]
- 195.Marchetti C; Swartzwelter B; Gamboni F; Neff CP; Richter K; Azam T; Carta S; Tengesdal I; Nemkov T; D'Alessandro A; Henry C; Jones GS; Goodrich SA; St Laurent JP; Jones TM; Scribner CL; Barrow RB; Altman RD; Skouras DB; Gattorno M; Grau V; Janciauskiene S; Rubartelli A; Joosten LAB; Dinarello CA OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc Natl Acad Sci U S A 2018, 115, E1530–E1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Marchetti C; Swartzwelter B; Koenders MI; Azam T; Tengesdal IW; Powers N; de Graaf DM; Dinarello CA; Joosten LAB NLRP3 inflammasome inhibitor OLT1177 suppresses joint inflammation in murine models of acute arthritis. Arthritis Res Ther 2018, 20, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sánchez-Fernández A; Skouras DB; Dinarello CA; López-Vales R OLT1177 (Dapansutrile), a Selective NLRP3 Inflammasome Inhibitor, Ameliorates Experimental Autoimmune Encephalomyelitis Pathogenesis. Front Immunol 2019, 10, 2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lonnemann N; Hosseini S; Marchetti C; Skouras DB; Stefanoni D; D'Alessandro A; Dinarello CA; Korte M The NLRP3 inflammasome inhibitor OLT1177 rescues cognitive impairment in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A 2020, 117, 32145–32154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Toldo S; Mauro AG; Cutter Z; Van Tassell BW; Mezzaroma E; Del Buono MG; Prestamburgo A; Potere N; Abbate A The NLRP3 Inflammasome Inhibitor, OLT1177 (Dapansutrile), Reduces Infarct Size and Preserves Contractile Function After Ischemia Reperfusion Injury in the Mouse. J Cardiovasc Pharmacol 2019, 73, 215–222. [DOI] [PubMed] [Google Scholar]
- 200.Aliaga J; Bonaventura A; Mezzaroma E; Dhakal Y; Mauro AG; Abbate A; Toldo S Preservation of contractile reserve and diastolic function by inhibiting the NLRP3 inflammasome with OLT1177 (Dapansutrile) in a mouse model of severe ischemic cardiomyopathy due to non-reperfused anterior wall myocardial infarction. Molecules 2021, 26, 3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Klück V; Jansen TLTA; Janssen M; Comarniceanu A; Efdé M; Tengesdal IW; Schraa K; Cleophas MCP; Scribner CL, Skouras DB, Marchetti C, Dinarello CA, Joosten LAB Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol 2020, 2, e270–e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wohlford GF; Van Tassell BW; Billingsley HE; Kadariya D; Canada JM; Carbone S; Mihalick VL; Bonaventura A; Vecchié A; Chiabrando JG; Bressi E; Thomas G; Ho AC; Marawan AA; Dell M; Trankle CR; Turlington J; Markley R; Abbate A A Phase IB, Randomized, Double-Blinded, Dose Escalation, Single Center, Repeat Dose Safety and Pharmacodynamics Study of the Oral NLRP3 Inhibitor Dapansutrile in Subjects with NYHA II-III Systolic Heart Failure. J Cardiovasc Pharmacol 2020, 77, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mauro AG; Thurber C; Abbate A Colchicine in acute myocardial infarction: “teaching new tricks to an old dog”. Transl Med 2015, 5, e133. [Google Scholar]
- 204.Leung YY; Yao Hui LL; Kraus VB Colchicine-Update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum 2015, 45, 341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Misawa T; Takahama M; Kozaki T; Lee H; Zou J; Saitoh T; Akira S Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol 2013, 14, 454–60. [DOI] [PubMed] [Google Scholar]
- 206.Weng JH; Koch PD; Luan HH; Tu HC; Shimada K; Ngan I; Ventura R; Jiang R; Mitchison TJ Colchicine acts selectively in the liver to induce hepatokines that inhibit myeloid cell activation. Nat Metab 2021, 3, 513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fujisue K; Sugamura K; Kurokawa H; Matsubara J; Ishii M; Izumiya Y; Kaikita K; Sugiyama S Colchicine Improves Survival, Left Ventricular Remodeling, and Chronic Cardiac Function After Acute Myocardial Infarction. Circ J 2017, 81, 1174–1182. [DOI] [PubMed] [Google Scholar]
- 208.Nidorf SM; Eikelboom JW; Budgeon CA; Thompson PL Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 2013, 61, 404–410. [DOI] [PubMed] [Google Scholar]
- 209.Tardif JC; Kouz S; Waters DD; Bertrand OF; Diaz R; Maggioni AP; Pinto FJ; Ibrahim R; Gamra H; Kiwan GS; Berry C; López-Sendón J; Ostadal P; Koenig W; Angoulvant D; Grégoire JC; Lavoie MA; Dubé MP; Rhainds D; Provencher M; Blondeau L; Orfanos A; L'Allier PL; Guertin MC; Roubille F Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med 2019, 381, 2497–2505. [DOI] [PubMed] [Google Scholar]
- 210.Nidorf SM; Fiolet ATL; Mosterd A; Eikelboom JW; Shut A; The SHK; Xu X; Ireland MA; Lenderink T; Larchem D; Hoogslang P; Jerzewski A; Nierop P; Whelan A; Hendriks R; Swart H; Schaap J; Kuijper AFMM van Hessen MWJ; Saklani P; Tan I; Thompson AG; Morton A; Judkins C; Bax WA; Dirksen M; Alings M; Hankey GJ; Budgeon CA; Tijssen JGP; Cornel JH; Thompson PL Colchicine in Patients with Chronic Coronary Disease. New Engl J Med 2020, 383, 1838–1847. [DOI] [PubMed] [Google Scholar]
- 211.Deftereos S; Giannopoulos G; Angelidis C; Alexopoulos N; Filippatos G; Papoutsidakis N; Sianos G; Goudevenos J; Alexopoulos D; Pyrgakis V; Cleman MW; Manolis AS; Tousoulis D; Lekakis J Anti-Inflammatory Treatment With Colchicine in Acute Myocardial Infarction: A Pilot Study. Circulation 2015, 132, 1395–403. [DOI] [PubMed] [Google Scholar]
- 212.Mewton N; Roubille F; Bresson D; Prieur C; Bouleti C; Bochaton T; Ivanes F; Dubreuil O; Biere L; Hayek A; Derimay F; Akodad M; Alos B; Haider L; El Jonhy N; Daw R; De Bourguignon C; Dhelens C; Finet G; Bonnefoy-Cudraz E; Bidaux G; Boutitie F; Maucort-Boulch D; Croisille P; Rioufol G; Prunier F; Angoulvant D Effect of Colchicine on Myocardial Injury in Acute Myocardial Infarction. Circulation 2021, 144, 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tong DC; Quinn S; Nasis A; Hiew C; Roberts.Thomson P; Adams H; Sriamareswaran R; Htun NM; Wilson W; Stub D; van Gaal W; Howes L; Collins N; Yong A; Bhindi R; Whitbourn R; Lee A; Hengel C; Asrress K; Freeman M; Amerena J; Wilson A; Layland J Colchicine in patients with acute coronary syndrome: the Australian COPS randomized clinical trial. Circulation 2020, 142, 1890–1900. [DOI] [PubMed] [Google Scholar]
- 214.Vaidya K; Arnott C; Martínez GJ; Ng B; McCormack S; Sullivan DR; Celermajer DS; Patel S Colchicine Therapy and Plaque Stabilization in Patients With Acute Coronary Syndrome: A CT Coronary Angiography Study. JACC Cardiovasc Imaging 2018, 11, 305–316. [DOI] [PubMed] [Google Scholar]
- 215.Defteros S; Giannopoulos G; Angelidis C; Alexopoulos N; Filippatos G; Papoutsidakis N; Sianos G; Goudevenos J; Alexopoulos D; Pyrgakis V; Cleman MW; Manolis AS; Tousoulis D; Lekakis J Anti-inflammatory treatment with colchicine in acute myocardial infarction: a pilot study. Circulation 2015, 132, 1395–403. [DOI] [PubMed] [Google Scholar]
- 216.Imazio M; Brucato A; Cemin R; Ferrua S; Belli R; Maestroni S; Trinchero R; Spodick DH; Adler Y; CORP (COlchicine for Recurrent Pericarditis) Investigators. Colchicine for recurrent pericarditis (CORP): a randomized trial. Ann Intern Med 2011, 155, 409–14. [DOI] [PubMed] [Google Scholar]
- 217.Imazio M; Belli R; Brucato A; Cemin R; Ferrua S; Beqaraj F; Demarie D; Ferro S; Forno D; Maestroni S; Cumetti D; Varbella F; Trinchero R; Spodick DH; Adler Y Efficacy and safety of colchicine for treatment of multiple recurrences of pericarditis (CORP-2): a multicenter, double-lind, placebo-controlled, randomized trial. Lancet 2014, 383, 2232–7. [DOI] [PubMed] [Google Scholar]
- 218.Production Kimura H. and physiological effects of hydrogen sulfide. Antioxid Redox Signal 2014, 20, 783–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Salloum FN Hydrogen sulfide and cardioprotection--Mechanistic insights and clinical translatability. Pharmacol Ther 2015, 152, 11–7. [DOI] [PubMed] [Google Scholar]
- 220.Toldo S; Das A; Mezzaroma E; Chau VQ; Marchetti C; Durrant D; Samidurai A; Van Tassell BW; Yin C; Ockaili RA; Vigneshwar N; Mukhopadhyay ND; Kukreja RC; Abbate A; Salloum FN Induction of microRNA-21 with exogenous hydrogen sulfide attenuates myocardial ischemic and inflammatory injury in mice. Circ Cardiovasc Genet 2014, 7, 311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gao C; Xu DQ; Gao CJ; Ding Q; Yao LN; Li ZC; Chai W An exogenous hydrogen sulphide donor, NaHS, inhibits the nuclear factor kappaB inhibitor kinase/nuclear factor kappab inhibitor/nuclear factor-kappaB signalling pathway and exerts cardioprotective effects in a rat hemorrhagic shock model. Biol Pharm Bull 2012, 35, 7029–34 [DOI] [PubMed] [Google Scholar]
- 222.Castelblanco M; Lugrin J; Ehirchiou D; Nasi S; Ishii I; So A; Martinon F; Busso N Hydrogen sulfide inhibits NLRP3 inflammasome activation and reduces cytokine production both in vitro and in a mouse model of inflammation. J Biol Chem 2018, 293, 2546–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lin HB; Wei GS; Li FX; Guo WJ; Hong P; Weng YQ; Zhang QQ; Xu SY; Liang WB; You ZJ; Zhang HF Macrophage-NLRP3 inflammasome activation exacerbates cardiac dysfunction after ischemic stroke in a mouse model of diabetes. Neurosci Bull 2020, 36, 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Cocco M; Garella D; Di Stilo A; Borretto E; Stevanato L; Giorgis M; Marini E; Fantozzi R; Miglio G; Bertinaria M Electrophilic warhead-based design of compounds preventing NLRP3 inflammasome-dependent pyroptosis. J Med Chem 2014, 57, 10366–82. [DOI] [PubMed] [Google Scholar]
- 225.Mastrocola R; Penna C; Tullio F; Femminò S; Nigro D; Chiazza F; Serpe L; Collotta D; Alloatti G; Cocco M; Bertinaria M; Pagliaro P; Aragno M; Collino M Pharmacological Inhibition of NLRP3 Inflammasome Attenuates Myocardial Ischemia/Reperfusion Injury by Activation of RISK and Mitochondrial Pathways. Oxid Med Cell Longev 2016, 2016, 5271251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Cocco M; Miglio G; Giorgis M; Garella D; Marini E; Costale A; Regazzoni L; Vistoli G; Orioli M; Massulaha-Ahmed R; Détraz-Durieux I; Groslambert M; Py BF; Bertinaria M Design, Synthesis, and Evaluation of Acrylamide Derivatives as Direct NLRP3 Inflammasome Inhibitors. ChemMedChem 2016, 11, 1790–803. [DOI] [PubMed] [Google Scholar]
- 227.Yajima N; Takahashi M; Morimoto H; Shiba Y; Takahashi Y; Masumoto J; Ise H; Sagara J; Nakayama J; Taniguchi S; Ikeda U Critical role of bone marrow apoptosis-associated speck-like protein, an inflammasome adaptor molecule, in neointimal formation after vascular injury in mice. Circulation 2008, 117, 3079–87. [DOI] [PubMed] [Google Scholar]
- 228.Usui F; Shirasuna K; Kimura H; Tatsumi K; Kawashima A; Karasawa T; Hida S; Sagara J; Taniguchi S; Takahashi M Critical role of caspase-1 in vascular inflammation and development of atherosclerosis in Western diet-fed apolipoprotein E-deficient mice. Biochem Biophys Res Commun 2012, 425, 162–8. [DOI] [PubMed] [Google Scholar]
- 229.Menu P; Pellegrin M; Aubert JF; Bouzourene K; Tardivel A; Mazzolai L; Tschopp J Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis 2011, 2, e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Butts B; Gary RA; Dunbar SB; Butler J Methylation of apoptosis-associated speck-like protein with a caspase recruitment domain and outcomes in heart failure. J Card Fail 2016, 22, 340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Butts B; Butler J; Dunbar SB; Corwin E; Gary RA Effects of exercise on ASC methylation and IL-1 cytokines in heart failure. Med Sci Sports Exerc 2018, 50, 1757–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Desu HL; Plastini M; Illiano P; Bramlett HM; Dietrich DW; de Rivero Vaccari JP; Brambilla R; Keane RW IC100: a nove anti-ASC monoclonal antibody improves functional outcomes in an animal model of multiple sclerosis. J Neuroinflammation 2020, 17, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lilienthal GM; Rahmöller J; Petry J; Bartsch YC; Leliavski A; Ehlers M Potential of murine IgG1 and human IgG4 to inhibit the classical complement and Fcγ receptor activation pathways. Front Immunol 2018, 8, 958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Merkle S; Frantz S; Schön MP; Bauersachs J; Buitrago M; Frost RJA; Schmitteckert EM; Lohse MJ; Engelhardt S A role for caspase-1 in heart failure. Circ Res 2007, 100, 645–53. [DOI] [PubMed] [Google Scholar]
- 235.Pomerantz BJ; Reznikov LL; Harken AH; Dinarello CA Inhibition of caspase 1 reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1beta. Proc Natl Acad Sci U S A 2001, 98, 2871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Audia JP; Yang XM; Crockett ES; Housley N; Haq EU; O'Donnell K; Cohen MV; Downey JM; Alvarez DF Caspase-1 inhibition by VX-765 administered at reperfusion in P2Y12 receptor antagonist-treated rats provides long-term reduction in myocardial infarct size and preservation of ventricular function. Basic Res Cardiol 2018, 113, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Yang XM; Downey JM; Cohen MV; Housley NA; Alvarez DF; Audia JP The Highly Selective Caspase-1 Inhibitor VX-765 Provides Additive Protection Against Myocardial Infarction in Rat Hearts When Combined With a Platelet Inhibitor. J Cardiovasc Pharmacol Ther 2017, 22, 574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Shi H; Gao Y; Dong Z; Yang J; Gao R; Li X; Zhang S; Ma L; Sun X; Wang Z; Zhang F; Hu K; Sun A; Ge J GSDMD-mediated cardiomyocyte pyroptosis promotes myocardial I/R injury. Circ Res 2021, 129, 383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Vromman A; Ruvkun V; Shvartz E; Wojtkiewicz G; Santos Masson G; Tesmenitsky Y; Folco E; Gram H; Nahrendorf M; Swirski FK; Sukhova GK; Libby P Stage-dependent differential effects of interleukin-1 isoforms on experimental atherosclerosis. Eur Heart J 2019, 40, 2482–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ridker PM; Howard CP; Walter V; Everett B; Libby P; Hensen J; Thuren T; CANTOS Pilot Investigative Group. Effects of interleukin-1β inhibition with cancakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation 2012, 126, 2739–48. [DOI] [PubMed] [Google Scholar]
- 241.Ridker PM; Everett BM; Thuren T; MacFadyen JG; Chang WH; Ballantyne C; Fonseca F; Nicolau J; Koenig W; Anker SD; Kastelein JJP; Cornel JH; Pais P; Pella D; Genest J; Cifkova R; Lorenzatti A; Forster T; Kobalava Z; Vida-Simiti L; Flather M; Shimokawa H; Ogawa H; Dellborg M; Rossi PRF; Troquay RPT; Libby P; Glynn RJ; CANTOS Trial Group. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017, 377, 1119–1131. [DOI] [PubMed] [Google Scholar]
- 242.Wohlford GF 4th; Van Tassell BW; Ravindra K; Abbate A; COLCOT and CANTOS: piecing together the puzzle of inflammation and cardiovascular events. Minerva Cardioangiol 2020, 68, 5–8. [DOI] [PubMed] [Google Scholar]
- 243.Abbate A; Salloum FN; Van Tassell BW; Vecile E; Toldo S; Seropian I; Mezzaroma E; Dobrina A Alterations in the interleukin-1/interleukin-1 receptor antagonist balance modulate cardiac remodeling following myocardial infarction in the mouse. PLoS One 2011, 6, e27923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Van Tassell BW; Varma A; Salloum FN; Das A; Seropian IM; Toldo S; Smithson L; Hoke NN; Chau VQ; Robati R; Abbate A Interleukin-1 trap attenuates cardiac remodeling after experimental acute myocardial infarction in mice. J Cardiovasc Pharmacol 2010, 55, 117–22. [DOI] [PubMed] [Google Scholar]
- 245.Abbate A; Salloum FN; Vecile E; Das A; Hoke NN; Straino S; Biondi-Zoccai GG; Houser JE; Qureshi IZ; Ownby ED; Gustini E; Biasucci LM; Severino A; Capogrossi MC; Vetrovec GW; Crea F; Baldi A; Kukreja RC; Dobrina A Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation 2008, 117, 2670–83. [DOI] [PubMed] [Google Scholar]
- 246.Salloum FN; Chau V; Varma A; Hoke NN; Toldo S; Biondi-Zoccai GG; Crea F; Vetrovec GW; Abbate A Anakinra in experimental acute myocardial infarction--does dosage or duration of treatment matter? Cardiovasc Drugs Ther 2009, 23, 129–35. [DOI] [PubMed] [Google Scholar]
- 247.Toldo S; Schatz AM; Mezzaroma E; Chawla R; Stallard TW; Stallard WC; Jahangiri A; Van Tassell BW; Abbate A Recombinant human interleukin-1 receptor antagonist provides cardioprotection during myocardial ischemia reperfusion in the mouse. Cardiovasc Drugs Ther 2012, 26, 273–6. [DOI] [PubMed] [Google Scholar]
- 248.Mauro AG; Mezzaroma E; Torrado J; Kundur P; Joshi P; Stroud K; Quaini F; Lagrasta CA; Abbate A; Toldo S Reduction of Myocardial Ischemia-Reperfusion Injury by Inhibiting Interleukin-1 Alpha. J Cardiovasc Pharmacol 2017, 69, 156–160. [DOI] [PubMed] [Google Scholar]
- 249.Sager HB; Heidt T; Hulsmans M; Dutta P; Courties G; Sebas M; Wojtkiewicz GR; Tricot B; Iwamoto Y; Sun Y; Weissleder R; Libby P; Swirski FK; Nahrendorf M Targeting Interleukin-1β Reduces Leukocyte Production After Acute Myocardial Infarction. Circulation 2015, 132, 1880–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Toldo S; Mezzaroma E; Van Tassell BW; Farkas D; Marchetti C; Voelkel NF; Abbate A Interleukin-1β blockade improves cardiac remodelling after myocardial infarction without interrupting the inflammasome in the mouse. Exp Physiol 2013, 98, 734–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Toldo S; Mezzaroma E; Bressi E; Marchetti C; Carbone S; Sonnino C; Van Tassell BW; Abbate A Interleukin-1β blockade improves left ventricular systolic/diastolic function and restores contractility reserve in severe ischemic cardiomyopathy in the mouse. J Cardiovasc Pharmacol 2014, 64, 1–6. [DOI] [PubMed] [Google Scholar]
- 252.Abbate A; Van Tassell BW; Seropian IM; Toldo S; Robati R; Varma A; Salloum FN; Smithson L; Dinarello CA Interleukin-1β modulation using a genetically engineered antibody prevents adverse cardiac remodeling following acute myocardial infarction in the mouse. Eur J Heart Fail 2010, 12, 319–22. [DOI] [PubMed] [Google Scholar]
- 253.Abbate A; Kontos MC; Grizzard JD; Biondi-Zoccai GG; Van Tassell BW; Robati R; Roach LM; Arena RA; Roberts CS; Varma A; Gelwix CC; Salloum FN; Hastillo A; Dinarello CA; Vetrovec GW; VCU-ART Investigators. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study). Am J Cardiol 2010, 105, 1371–1377.e1. [DOI] [PubMed] [Google Scholar]
- 254.Abbate A; Kontos MC; Abouzaki NA; Melchior RD; Thomas C; Van Tassell BW; Oddi C; Carbone S; Trankle CR; Roberts CS; Mueller GH; Gambill ML; Christopher S; Markley R; Vetrovec GW; Dinarello CA; Biondi-Zoccai G Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies). Am J Cardiol 2015, 115, 288–92. [DOI] [PubMed] [Google Scholar]
- 255.Abbate A; Trankle CR; Buckley LF; Lipinski MJ; Appleton D; Kadariya D; Canada JM; Carbone S; Roberts CS; Abouzaki N; Melchior R; Christopher S; Turlington J; Mueller G; Garnett J; Thomas C; Markley R; Wohlford GF; Puckett L; Medina de Chazal H; Chiabrando JG; Bressi E; Del Buono MG; Schatz A; Vo C; Dixon DL; Biondi-Zoccai GG; Kontos MC; Van Tassell BW Interleukin-1 Blockade Inhibits the Acute Inflammatory Response in Patients With ST-Segment-Elevation Myocardial Infarction. J Am Heart Assoc 2020, 9, e014941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Abbate A; Wohlford GF; Del Buono MG; Chiabrando JG; Markley R; Turlington J; Kadariya D; Trankle CR; Biondi-Zoccai G; Lipinski MJ; Van Tassell BW Interleukin-1 blockade with Anakinra and heart failure following ST-segment elevation myocardial infarction: results from a pooled analysis of the VCUART clinical trials. Eur Heart J Cardiovasc Pharmacother 2021, pvab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Morton AC; Rothman AM; Greenwood JP; Gunn J; Chase A; Clarke B; Hall AS; Fox K; Foley C; Banya W; Wang D; Flather MD; Crossman DC The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur Heart J 2015, 36, 377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Van Tassell BW; Toldo S; Mezzaroma E; Abbate A Targeting interleukin-1 in heart disease. Circulation 2013, 128, 1910–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Liberale L; Bonetti NR; Puspitasari YM; Schwarz L; Akhmedov A; Montecucco F; Ruschitzka F; Beer JH; Lüscher TF; Simard J; Libby P; Camici GG Postischemic administration of IL-1α neutralizing antibody reduces brain damage and neurological deficit in experimental stroke. Circulation 2020, 142, 187–189. [DOI] [PubMed] [Google Scholar]
- 260.Van Tassell BW; Arena RA; Toldo S; Mezzaroma E; Azam T; Seropian IM; Shah K; Canada J; Voelkel NF; Dinarello CA; Abbate A Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS One 2012, 7, e33438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Trankle CR; Canada JM; Cei L; Abouzaki N; Oddi-Erdle C; Kadariya D; Christopher S; Viscusi M; Del Buono M; Kontos MC; Arena R; Van Tassell B; Abbate A Usefulness of Canakinumab to Improve Exercise Capacity in Patients With Long-Term Systolic Heart Failure and Elevated C-Reactive Protein. Am J Cardiol 2018, 122, 1366–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Van Tassell BW; Canada J; Carbone S; Trankle C; Buckley L; Oddi Erdle C; Abouzaki NA; Dixon D; Kadariya D; Christopher S; Schatz A; Regan J; Viscusi M; Del Buono M; Melchior R; Mankad P; Lu J; Sculthorpe R; Biondi-Zoccai G; Lesnefsky E; Arena R; Abbate A Interleukin-1 Blockade in Recently Decompensated Systolic Heart Failure: Results From REDHART (Recently Decompensated Heart Failure Anakinra Response Trial). Circ Heart Fail 2017, 10, e004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Interleukin-1 Blockade In Recently Decompensated Heart Failure - 2 (REDHART2). https://clinicaltrials.gov/ct2/show/NCT03797001.
- 264.Van Tassell BW; Arena R; Biondi-Zoccai G; Canada JM; Oddi C; Abouzaki NA; Jahangiri A; Falcao RA; Kontos MC; Shah KB; Voelkel NF; Dinarello CA; Abbate A Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am J Cardiol 2014, 113, 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Van Tassell BW; Trankle CR; Canada JM; Carbone S; Buckley L; Kadariya D; Del Buono MG; Billingsley H; Wohlford G; Viscusi M; Oddi-Erdle C; Abouzaki NA; Dixon D; Biondi-Zoccai G; Arena R; Abbate A IL-1 Blockade in Patients With Heart Failure With Preserved Ejection Fraction. Circ Heart Fail 2018, 11, e005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Ikonomidis I; Makavos G; Katsimbri P; Boumpas DT; Parissis J; Iliodromitis E Imaging Risk in Multisystem Inflammatory Diseases. JACC Cardiovasc Imaging 2019, 12, 2517–2537. [DOI] [PubMed] [Google Scholar]
- 267.Shin JI; Lee KH; Joo YH; Lee JM; Jeon J; Jung HJ; Shin M; Cho S; Kim TH; Park S; Jeon BY; Jeong H; Lee K; Kang K; Oh M; Lee H; Lee S; Kwon Y; Oh GH; Kronbichler A Inflammasomes and autoimmune and rheumatic diseases: A comprehensive review. J Autoimmun 2019, 103, 102299. [DOI] [PubMed] [Google Scholar]
- 268.Pironti G; Bersellini-Farinotti A; Agalave NM; Sandor K; Fernandez-Zafra T; Jurczak A; Lund LH; Svensson CI; Andersson DC Cardiomyopathy, oxidative stress and impaired contractility in a rheumatoid arthritis mouse model. Heart 2018, 104, 2026–2034. [DOI] [PubMed] [Google Scholar]
- 269.Del Buono M; Abbate A; Toldo S Interplay of inflammation, oxidative stress and cardiovascular disease in rheumatoid arthritis. Heart 2018, 104, 1991–1992. [DOI] [PubMed] [Google Scholar]
- 270.Ikonomidis I; Lekakis JP; Nikolaou M; Paraskevaidis I; Andreadou I; Kaplanoglou T; Katsimbri P; Skarantavos G; Soucacos PN; Kremastinos DT Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation 2008, 117, 2662–9. [DOI] [PubMed] [Google Scholar]
- 271.Ikonomidis I; Tzortzis S; Andreadou I; Paraskevaidis I; Katseli C; Katsimbri P; Pavlidis G; Parissis J; Kremastinos D; Anastasiou-Nana M; Lekakis J Increased benefit of interleukin-1 inhibition on vascular function, myocardial deformation, and twisting in patients with coronary artery disease and coexisting rheumatoid arthritis. Circ Cardiovasc Imaging 2014, 7, 619–28. [DOI] [PubMed] [Google Scholar]
- 272.O'Brien LC; Mezzaroma E; Van Tassell BW; Marchetti C; Carbone S; Abbate A; Toldo S Interleukin-18 as a therapeutic target in acute myocardial infarction and heart failure. Mol Med 2014, 20, 221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ji Q; Zeng Q; Huang Y; Shi Y; Lin Y; Lu Z; Meng K; Wu B; Yu K; Chai M; Liu Y; Zhou Y Elevated plasma IL-37, IL-18, and IL-18BP concentrations in patients with acute coronary syndrome. Mediators Inflamm 2014, 2014, 165742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Youssef AA; Chang LT; Hang CL; Wu CJ; Cheng CI; Yang CH; Sheu JJ; Chai HT; Chua S; Yeh KH; Yip HK Level and value of interleukin-18 in patients with acute myocardial infarction undergoing primary coronary angioplasty. Circ J 2007, 71, 703–8. [DOI] [PubMed] [Google Scholar]
- 275.Naito Y; Tsujino T; Fujioka Y; Ohyanagi M; Okamura H; Iwasaki T Increased circulating interleukin-18 in patients with congestive heart failure. Heart 2002, 88, 296–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Venkatachalam K; Prabhu SD; Reddy VS; Boylston WH; Valente AJ; Chandrasekar B Neutralization of interleukin-18 ameliorates ischemia/reperfusion-induced myocardial injury. J Biol Chem 2009, 284, 7853–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Gu H; Xie M; Xu L; Zheng X; Yang Y; Lv X The protective role of interleukin-18 binding protein in a murine model of cardiac ischemia/reperfusion injury. Transpl Int 2015, 28, 1436–44. [DOI] [PubMed] [Google Scholar]
- 278.Quader M; Mezzaroma E; Kenning K; Toldo S Modulation of Interleukin-1 and −18 Mediated Injury in Donation after Circulatory Death Mouse Hearts. J Surg Res 2021, 257, 468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Hillestad V; Espe EK; Cero F; Larsen KO; Sjaastad I; Nygård S; Skjønsberg OH; Christensen G IL-18 neutralization during alveolar hypoxia improves left ventricular diastolic function in mice. Acta Physiol (Oxf) 2015, 213, 492–504. [DOI] [PubMed] [Google Scholar]
- 280.Xiao H; Li H; Wang JJ; Zhang JS; Shen J; An XB; Zhang CC; Wu JM; Song Y; Wang XY; Yu HY; Deng XN; Li ZJ; Xu M; Lu ZZ; Du J; Gao W; Zhang AH; Feng Y; Zhang YY IL-18 cleavage triggers cardiac inflammation and fibrosis upon β-adrenergic insult. Eur Heart J 2018, 39, 60–69. [DOI] [PubMed] [Google Scholar]
- 281.Elhage R; Jawien J; Rudling M; Ljunggren HG; Takeda K; Akira S; Bayard F; Hansson GK Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc Res 2003, 59, 234–40. [DOI] [PubMed] [Google Scholar]
- 282.Imai T; Oikawa Y; Shimada A; Oguchi S; Takamiya Y; Katsuki T; Okubo Y; Osaki T; Tahara H; Matsushima Y; Miyazaki J; Itoh H Proatherogenic effect of interleukin-18 is exerted with high-fat diet, but not with normal diet in spontaneously hyperlipidemic mice. J Atheroscler Thromb 2011, 18, 1090–101. [DOI] [PubMed] [Google Scholar]
- 283.Bhat OM; Kumar PU; Giridharan NV; Kaul D; Kumar MJ; Dhawan V Interleukin-18-induced atherosclerosis involves CD36 and NF-κB crosstalk in Apo E−/− mice. J Cardiol 2015, 66, 28–35. [DOI] [PubMed] [Google Scholar]
- 284.Pejnovic N; Vratimos A; Lee SH; Popadic D; Takeda K; Akira S; Chan WL Increased atherosclerotic lesions and Th17 in interleukin-18 deficient apolipoprotein E-knockout mice fed high-fat diet. Mol Immunol 2009, 47, 37–45. [DOI] [PubMed] [Google Scholar]
- 285.Carbone S; Lee PJ; Mauro AG; Mezzaroma E; Buzzetti R; Van Tassell B; Abbate A; Toldo S Interleukin-18 mediates cardiac dysfunction induced by western diet independent of obesity and hyperglycemia in the mouse. Nutr Diabetes 2017, 7, e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Netea MG; Joosten LA; Lewis E; Jensen DR; Voshol PJ; Kullberg BJ; Tack CJ; van Krieken H; Kim SH; Stalenhoef AF; van de Loo FA; Verschueren I; Pulawa L; Akira S; Eckel RH; Dinarello CA; van den Berg W; van der Meer JW Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med 2006, 12, 650–6. [DOI] [PubMed] [Google Scholar]
- 287.Murphy AJ; Kraakman MJ; Kammoun HL; Dragoljevic D; Lee MK; Lawlor KE; Wentworth JM; Vasanthakumar A; Gerlic M; Whitehead LW; DiRago L; Cengia L; Lane RM; Metcalf D; Vince JE; Harrison LC; Kallies A; Kile BT; Croker BA; Febbraio MA; Masters SL IL-18 Production from the NLRP1 Inflammasome Prevents Obesity and Metabolic Syndrome. Cell Metab 2016, 23, 155–64. [DOI] [PubMed] [Google Scholar]
- 288.Ridker PM From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res 2016, 118, 145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Ridker PM; MacFadyen JG; Thuren T; Libby P Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1β inhibition with canakinumab: further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis. Eur Heart J 2020, 41, 2153–2163. [DOI] [PubMed] [Google Scholar]
- 290.Ridker PM; Rane M Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ Res 2021, 128, 1728–1746. [DOI] [PubMed] [Google Scholar]
- 291.Broch K; Anstensrud AK; Woxholt S; Sharma K; Tøkkefseb IM; Bendz B; Aakhus S; Ueland T; Amundsen BH; Damås JK; Berg ES; Bjørkelund E; Bendz C; Hopp E; Kleveland O; Stensæth KH; Opdahl A; Kløw NE; Seljeflot I; Andersen GØ; Wiseth R; Aukrust P; Gullestad L Randomized trial of interleukin-6 receptor inhibition in patients with acute ST-segment elevation myocardial infarction. J Am Coll Cardiol 2021, 77, 1845–1855. [DOI] [PubMed] [Google Scholar]
- 292.Ridker PM; Devalaraja M; Baeres FMM; Engelmann MDM; Hovingh GK; Ivkovic M; Lo L; Kling D; Pergola P; Raj D; Libby P; Davidson M; RESCUE Investigators. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomized, placebo-controlled, phase 2 trial. Lancet 2021, 397, 2060–2069. [DOI] [PubMed] [Google Scholar]
- 293.George MJ; Jasmin NH; Cummings VT; Rochard-Loendt A; Launchbury F; Wollard K; Turner-Stokes T; Diaz AIG; Lythgoe M; Stuckey DJ; Hingorani AD; Girloy DW Selective interleukin-6 trans-signaling blockade is more effective than pantantagonism in reperfused myocardial infarction. JACC Basic Transl Sci 2021, 6, 431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]