Abstract
Low-grade fibromyxoid sarcoma (LGFMS) is an uncommon mesenchymal tumor usually arising in the lower extremities and trunk. Only rare examples in the head and neck region have been reported. Fifteen cases of head and neck LGFMS were retrieved. MUC4 was performed on all cases. Results for smooth muscle actins, β-catenin, desmin, S100 protein, Epithelial membrane antigen (EMA) and STAT6 immunohistochemistry, as well as FUS rearrangement status, were recorded when available. Sites included neck (8), supraclavicular region (4) and orbit (1), parapharyngeal space (1) and lower lip (1). The age of the patients ranged from 3 to 97 years (median, 26 years). Tumors displayed classical morphologic features of LGFMS, as described. All cases (15/15) were positive for MUC4, and all cases tested (4/4) harbored FUS rearrangement. Variable positivity for EMA was identified in one case. Follow-up was available in 11 patients, ranging from 2 to 240 months (mean 71.4 months; median, 44 months). Three tumors recurred locally; none metastasized. In conclusion, although distinctly uncommon, LGFMS may arise in the head and neck region and should be distinguished from other more common spindle cell tumors in these locations. The morphologic, immunohistochemical and molecular genetic features of head/neck LGFMS are identical to those occurring elsewhere. The long-term metastatic risk of LGFMS in these locations remains to be fully elucidated.
Keywords: Low-grade fibromyxoid sarcoma, Head and neck, Supraclavicular, Orbit, Lip, Parapharyngeal space, MUC4
Introduction
Low grade fibromyxoid sarcoma is an uncommon malignant tumor of fibroblastic lineage characterized by MUC4 expression and FUS/EWSR1-CREB3L2/CREB3L1 fusions, which commonly involves deep soft tissues of the proximal extremities and trunk followed by the abdomen, pelvis and retroperitoneum [1–4]. Head and neck involvement is rare, and, to the best of our knowledge, approximately 45 cases have been reported [5–38] (Table 1). First case of head and neck LGFMS was reported in a case series by Evan [8]. Most of these are either single case reports or cases with incomplete information reported as part of large series [8]. In fact, only five dedicated small case series with up to 4 patients have been published [16, 20, 28, 33, 35]. Given its rarity, LGFMS arising in the head and neck may cause diagnostic confusion with other more common spindle cell lesions in this region including peripheral nerve sheath tumors, solitary fibrous tumor and spindle cell squamous cell carcinoma. Herein, we describe the clinicopathologic features of 15 classical cases of LGFMS occurring in the head and neck with emphasis on differential diagnoses at these sites.
Table 1.
Reported cases of Head and Neck LGFMS (n = 45)
| References | Age/Sex | Site | Tumor size (cm) | IHC profile | Follow up | |
|---|---|---|---|---|---|---|
| 1 | Evans [5] | 26/M | Neck | N/A | N/A | Multiple recurrences. Alive at 44 years |
| 2 | Papadimitriou et al. [6] | 3Y10m/M | Jaw | N/A | N/A |
Local recurrence at 3 months; Local recurrence and lung mets at 15 years NED 2.5 years |
| 3 | Lane et al. [7] | 36/M | Anterior Neck, Left | N/A | N/A | NED at 55 months |
| 4 | Folpe et al. [8]; 4 cases | N/A | Head/neck | N/A | N/A | N/A |
| 5 | Zamenik and Michal [9] | 41/M | Left supraclavicular region | 6 | Bcl 2, Vimentin + | N/A |
| 6 | Botev et al. [10] | 57/F | Left parotid | 12 | N/A | NED at 36 months |
| 7 | Guillou et al. [11] | 22/M | Neck | 7 | FUS exon 7-CREB3L2 exon 5 by PCR | NED at 38 months |
| 8 | Marglani et al. [12] | 40/F | Sternocleidomastoid muscle | N/A | Vimentin, CD68, MSA, desmin, and SMA + ; EMA, S100, neurofilament 70, 200, 200, and CD34 - | NED at 12 months |
| 9 | Merchant [13] | 57/M | Thyroid | N/A | N/K | NED at 13 months |
| 10 | Wu et al. [14] | 4/F | Left angle of jaw | 2.2 | Vimentin + , EMA focal weak + , CD31, CD34, Desmin, S100, ASMA - | Local recurrence at 2 years. NED at 5 years |
| 11 | Tang et al. [15] | 1Y10M | Cheek | 8 | Vimentin + , S100, Desmin, CD10 - , ASMA focal + | NED after 6 months |
| 12 | Rekhi et al. [16] | 27/M | Jaw | 7.5 | Vimentin, calponin + , S100, desmin, SMA, myogenin, myo-D1− | NED after 22 months |
| 13 | Rekhi et al. [16] | 31/M | Face | 2 | CD34 + , S100− | N/A |
| 14 | Rekhi et al. [16] | 69/M | Neck | 7 | S100, EMA- | 2 Recurrences after 10 years |
| 15 | Evans [17] | 26/M | Neck | N/A | N/A | Multiple recurrences. Mets to lung and chest wall. NED at 44 years |
| 16 | Evans [17] | 9/M | Neck | N/A | N/A | Multiple recurrences followed by radiation after last; lung and chest wall mets at 25 years treated with multiple resections. Died of metastatic disease at 42 years |
| 17 | Abe et al. [18] | 84/F | Forehead | 3 | Vimentin + , Desmin, ASMA, CD34, CD56, S100, CK - | Local recurrence at 15 months. NED at 1 year |
| 18 | He et al. [19] | 14/M | Cheek | 8 | ASMA focal + , Factor VIII, CD34, CD68, S100, Desmin, EMA, CK - | NA |
| 19 | Maretty-Nielsen et al. [20] | 53/M | Neck | 2 | FISH for FUS -ve | NED after 91 months |
| 20 | Maretty-Nielsen et al. [20] | 35/M | Neck | 4 | FISH for FUS -ve | NED after 63 months |
| 21 | Dong and Zang [21] | 65/M | Left lobe of thyroid | 3 | Vimentin, P53, Bcl2 and ASMA + CD99, CK19, Galectin-3, Calcitonin, CD34 - | Vertebral mets at 14 months. NED at 25 months |
| 22 | Lee et al. [22] | 65/F | Parotid areas (masseter muscle) | 3 | Vimentin, P53, Bcl2 and ASMA + CK19, Galectin-3, Calcitonin - | NED at 1 years |
| 23 | Soma et al. [23] | 40/M | Hard palate | 5 | Not performed | NED at 4 months |
| 24 | Mastoraki et al. [24] | 81/M | Lower neck and supraclavicular region | 6.6 | SMA and calponin focally + , HHF-35, S100, CD34, CD57, Desmin and beta-catenin - | Recurrence at 2 years |
| 25 | Chaudhuri et al. [25] | 35/M | Left body of Mandible | 3x | Vimentin + , S100, desmin, SMA, CD34, CD31, CD68, CK AE1/AE3, EMA - | N/A |
| 26 | Spalthoff et al. [26] | 16/M | Maxilla | 3 |
vimentin + , CD117 weak + , SMA, CD31, CD34, S100, desmin - FISH for FUS + |
NED at 6 months |
| 27 | Tatari et al. [27] | 70/F | Right supraclavicular area | 8.5 | CK, MUC4, CD34 + , SMA, Desmin, S100 - | N/A |
| 28 | Cowan et al.[28] | 43/F | Facial skin | N/A | MUC 4 + , P40, beta-catenin, CK, MSA, S100 - | NED at 12 months in one patient. Other was alive at 10 months wd pulmonary and LN mets |
| 29 | Cowan et al. [28] | 45/M | Mandible | N/A | MUC 4 + , P40, beta-catenin,CK, MSA, S100 - | NED at 12 months in one patient. Other was alive at 10 months wd pulmonary and LN mets |
| 30 | Cowan et al. [28] | 73/M | Larynx | N/A | MUC4 + , P40, beta-catenin, CK, MSA, S100 - | NED at 12 months in one patient. Other was alive at 10 months wd pulmonary and LN mets |
| 31 | Cowan et al. [28] | 6/M | Posterior cervical spine | N/A | MUC4 + , P40, beta-catenin, CK, MSA, S100 - | NED at 12 months in one patient. Other was alive at 10 months wd pulmonary and LN mets |
| 32 | Rao et al. [29] | 18/F | Orbit | 2.4 | CD34 + , S100, Desmin, EMA, SMA - | NED at 41 months |
| 33 | Kumari et al. [30] | 44/F | External auditory canal | 2 | Vimentin, EMA, S100 (focally), Bcl2; CKAE1, SMA, Desmin-, MUC4 + | NED at 18 months |
| 34 | Pellini et al. [31] | 17/M | Tongue | 2 | MUC4, Vimentin + , CK, EMA, ASMA, Desmin, CD34, CD56 -; FUS-CREB3L2 + | NED at 6 months |
| 35 | Kanato et al. [32] | 18/F | Buccal mucosa | 5 | Vimentin + | NED at 18 months |
| 36 | Chetverikova and Kasenomm [33] | 33/F | Skull base | 4.9 and 4.2 (× 2) | S100 and EMA, with focal MUC4 + | Recurrence after 15 years |
| 37 | Chetverikova and Kasenomm [33] | 17/F | Skull base | 2.5 |
S100, EMA, SMA, CD34, Bcl-2 - MUC4 + |
NED at 36 months |
| 38 | Park et al. [34] | 78/M | Supraclavicular | 9 | CD34, MDM2, CDK4, S100, Actin, Desmin, Beta-catenin, and MUC4 - | NED at 12 months |
| 39 | Koucky et al. [35] | 57/F | Maxillary sinus | N/A | N/K | NED at 148 months |
| 40 | Koucky et al. [35] | 74/M | Maxillary sinus | N/A | N/K | NED at 65 months |
| 41 | Toro et al. [36] | 57/M | Right parapharyngeal space | 8 | MUC4, Vimentin and Focally EMA + , α-actin, desmin, myogenin, CD34, CKAE1/AE3, S100 protein, GFAP, p63, STAT6 - | NED at 24 months |
| 42 | Deewani et al. [37] | 15/F | Right parapharyngeal space | 7 | MUC4 + , EMA, CKAE1/AE3, TLE1, α-smooth muscle actin, beta-catenin, CD34 and S100 - | NED at 1 month |
| 43 | Doblan [38] | 56/M | Maxilla | N/A | Vimentin, MUC4 + , S100, CD34, desmin, actin, keratin and EMA - | NED 3 months |
NED no evidence of disease; N/A not available; wd with; LN lymph node; mets metastasis; N/K not known; FISH fluorescent in situ hybridization; PCR polymerase chain reaction; MSA muscle specific antigen; SMA smooth muscle actin
Materials and Methods
The study was approved by the institutional review boards of the participating institutions. The surgical pathology and consultation archives of Mayo Clinic and Aga Khan University Hospital were searched for cases of “low-grade fibromyxoid sarcoma”. Tumors arising in the head and neck were segregated. H&E slides were then reviewed to confirm the diagnosis. A representative paraffin block from each tumor was selected for MUC4 immunohistochemistry. The results of previously performed immunostains including smooth muscle actins, β-catenin, desmin, S100 protein, EMA and STAT6 were noted. FUS rearrangement status was recorded when available.
Immunohistochemistry
Immunohistochemistry for MUC4 (mouse monoclonal antibody, clone 8G7; RTU, Cell Marque, Rocklin, California) was performed on 4-μm thick sections prepared from formalin-fixed, paraffin-embedded tissue sections.
Results
Fifteen cases of LGFMS occurring in eight females and seven males ranging from 3 to 97 years of age (median 26 years) were identified from sites including the neck (8), supraclavicular region (4), orbit (1), parapharyngeal space (1) and lower lip (1) (Table 2). Of the neck masses, five involved the posterior aspect of the neck, two arose in the lateral neck (1 right, 1 left), and a single case presented as an anterior mass adjacent to the thyroid. The parapharyngeal case has been previously reported [37]. Twelve were primary tumors, and 3 represented local recurrences. Two of the recurrent cases were initially misdiagnosed on the primary resections and classified as “neurofibroma” and “nodular fasciitis”. Of the cases sent for expert consultation (Cases 7–15), the submitting pathologists’ diagnoses included solitary fibrous tumor, neural tumor, clear cell sarcoma of soft tissue, ‘spindle cell neoplasm,’ ‘atypical spindle cell neoplasm,’ spindle cell carcinoma and metastatic Gastrointestinal stromal tumor (GIST).
Table 2.
Clinicopathological features of head and neck low-grade fibromyxoid sarcoma (n = 15)
| Case | Age (years)/Gender | Site | Size (cm) | Primary/Recurrent | MUC4 | FISH for FUS | Collagen rosettes | Original diagnosis+ or submitting pathologist’s diagnosis^ | Recurrence (Time to first recurrence in months) | Length of follow up (months) | Last known status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 13/M | Cervico-dorsal | 5 | Recurrent | + | NP | + | Neurofibroma+ | + (72) | 156 | AWOD |
| 2 | 19/M | Nape of neck | 3.5 | Primary | + | NP | − | LGFMS+ | − | 66 | AWOD |
| 3 | 32/M | Supraclavicular | 4.6 | Primary | + | NP | − | LGFMS+ | − | 44 | AWOD |
| 4 | 15/F | Parapharyngeal | 6 | Primary | + | NP | − | LGFMS+ | − | 11 | AWOD |
| 5 | 22/F | Supraclavicular | NK | Primary | + | NP | − | LGFMS+ | − | 2 | AWOD |
| 6 | 34/F | Posterior neck | 6.3 | Recurrent | + | NP | − | Nodular fasciitis+ | + (60) | 216 | AWOD |
| 7 | 10/M | Orbit | 1.7 | Recurrent | + | NP | − | LGFMS^ | + (15) | 15 | AWOD |
| 8 | 17/F | Posterior neck | 3 | Primary | + | NP | − | LGFMS^ | − | 72 | AWOD |
| 9 | 58/F | Adjacent to thyroid gland | 2.7 | Primary | + | NP | − | Spindle cell neoplasm^ | NK | 0 | NK |
| 10 | 28/M | Neck (left) | 1.5 | Primary | + | + | − | None^ | − | 36 | AWOD |
| 11 | 28/F | Neck (right) | 1.5 | Primary | + | + | − | LGFMS^ | − | 36 | AWOD |
| 12 | 97/F | Supraclavicular | 0.7 | Primary | + | + | − | Solitary fibrous tumor, neural tumor, metastatic GIST; spindle cell carcinoma^ | − | 48 | AWD* |
| 13 | 26/F | Posterior neck | 7.1 | Primary | + | NP | + | Atypical spindle cell neoplasm^ | NK | 0 | NK |
| 14 | 3/M | Lower lip | 0.8 | Primary | + | + | − | LGFMS vs. clear cell sarcoma of soft tissue^ | NK | 0 | NK |
| 15 | 30/M | Supraclavicular | 3.1 | Primary | + | NP | + | Low-grade spindle cell neoplasm^ | NK | 0 | NK |
FISH fluorescence in situ hybridization; NP not performed; LGFMS low-grade fibromyxoid sarcoma; GIST gastrointestinal stromal tumor; Y Yes; N No; NK not known; N/A not applicable; R recurrence; AWOD Alive without disease; AWD Alive with disease
*Patient was treated with local radiation only
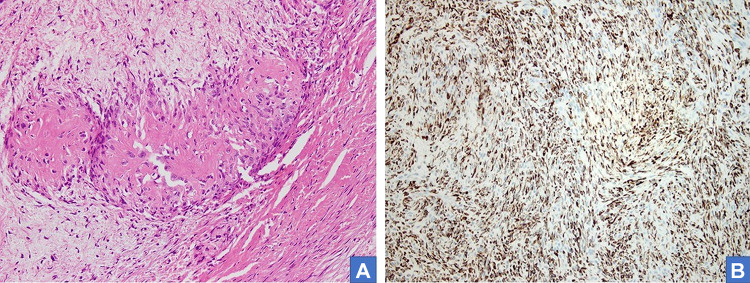
The tumors ranged in size from 1.5 cm to 7.1 cm (median, 3.1 cm). Sectioning revealed circumscribed lobulated masses with grey firm cut surfaces which were focally myxoid in appearance (Fig. 1). Histologically, all appeared circumscribed (Figs. 2A-B) with characteristic alternating hyalinized and myxoid zones and an abrupt transition between the two patterns (Fig. 2C). Tumor cells were arranged in short fascicles or occasionally in a storiform pattern, containing ovoid to elongated normochromatic nuclei and indistinct cytoplasm (Fig. 2D). Focal mild cytologic atypia was appreciated, but mitotic activity was sparse. Arcades of thin-walled vessels were noted within the myxoid zones. Two cases showed sclerotic stroma with cords of epithelioid cells, resembling hybrid low-grade fibromyxoid sarcoma- sclerosing epithelioid fibrosarcoma (Fig. 2A). Scattered giant collagen rosettes were seen in three cases (Fig. 3A). Focal calcification was noted in one case.
Fig. 1.
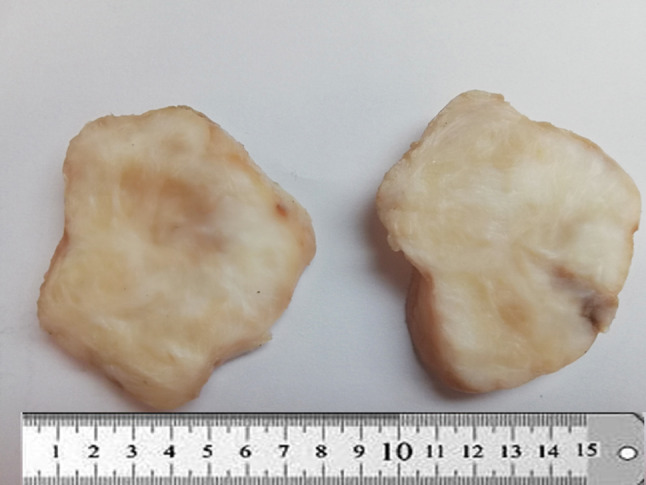
Gross appearance of low-grade fibromyxoid sarcoma. The tumor is circumscribed with firm grey, white cut surface
Fig. 2.
LGFMS of Neck. A relatively circumscribed tumor in the dermis with unremarkable overlying epidermis (A H&E*, 10×). Tumor interface with adnexal structures (B H&E*, 20×). Transition between alternate myxoid and collagenized areas (C H&E*, 40×). Whorled areas containing arcade of vessels and short fascicles of relatively bland spindle cells (D H&E*, 40×) *Hematoxylin and eosin
Fig. 3.
Hematoxylin and eosin-stained section shows higher magnification of giant collagen rosettes (A H&E*, 40×). MUC positivity in tumor cells (B)
By immunohistochemistry, all 15 cases were diffusely MUC4-positive. (Table 1; Fig. 3B). Limited EMA expression was present in one case. Smooth muscle actin, desmin, S100 protein, CD34, STAT6 and β-catenin were negative. FISH studies detected FUS rearrangement in four tested cases.
Follow-up was available for 11 patients, ranging from 2 to 240 months in duration (median, 44 months). Local recurrences were seen in three patients (at 15, 60 and 72 months); two of these tumors were initially misclassified as neurofibroma and nodular fasciitis. Ten of the 11 patients are currently alive without disease. One patient was treated with local radiation only and has stable disease. No metastases were noted.
Discussion
Low-grade fibromyxoid sarcoma is a rare sarcoma that commonly involves the deep soft tissues of extremities and trunk. In our series, the neck was involved in seven cases, while four tumors arose supraclavicularly; one case each involved the cervico-dorsal region, parapharyngeal space, orbit and lower lip. The latter three cases represent the second known cases of parapharyngeal and orbital LGFMS and the first case involving the lip. While the demographics and pathologic features of our cases were similar to previously reported LGFMS at more common sites, the chief significance of this tumor in the head and neck is its potential to be confused with other more common lesion, including (myo)fibroblastic and peripheral nerve sheath tumors, as well as spindle cell squamous cell carcinoma (Table 3).
Table 3.
Summary of differential diagnoses of low-grade fibromyxoid sarcoma
| Tumor | Key histologic features | Immunohistochemistry | Most common genetic aberrations | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MUC4 | Cytokeratin | SMA | beta-catenin | STAT6 | CD34 | S100 | EMA | |||
| Low-grade fibromyxoid sarcoma |
Alternating fibrous and myxoid areas Whorled growth pattern Bland to mildly atypical cells Arcades of small vessels Giant collagen rosettes (30%) |
+ | − | − | − | − | − | − | ± | FUS- CREBB3L2 fusion |
| Spindle cell squamous cell carcinoma |
Cytologic atypia and nuclear pleomorphism Surface squamous dysplasia |
− | + | − | − | − | − | − | + | |
| Desmoid fibromatosis |
Long sweeping fascicles of bland spindle cells Infiltrative growth pattern Large thin walled gaping blood vessels with perivascular edema |
− | − | + | + | − | − | − | − | CTNNB1 mutation |
| Solitary Fibrous Tumor |
Patternless arrangement of spindled to ovoid cells Variably collagenous stroma Prominent branching and hyalinized staghorn-shaped blood vessels |
− | − | − | − | + | + | − | − | NAB2- STAT6 fusion |
| Dermatofibrosarcoma protuberans |
Dermal based Uniform spindle cells arranged in storiform pattern Prominent infiltration of subcutaneous fat |
− | − | ± | − | − | + | − | − | COL1A1-PDGFB fusion |
| Neurofibroma |
Haphazardly arranged bland spindle cells with wavy nuclei -Loose myxoid and collagenized stroma Associated perineurial cells, fibroblasts and mast cells |
− | − | − | − | − | + | + | + | Biallelic inactivation of NF1 |
| Soft tissue perineurioma |
Storiform or whorled growth pattern Slender spindle cells with elongated tapered nuclei and bipolar cytoplasmic processes Collagenous to focally myxoid stroma |
− | − | − | − | − | ± | − | + | Deletion of 22q12 and mutation of NF2 |
| Nodular fasciitis |
Loose arrangement of plump spindled cells Microcystic stromal changes Extravasated red blood cells No hyperchromasia or cytologic atypia |
− | − | + | − | − | − | − | − | USP6 rearrangement |
While myofibroblastic/fibroblastic lesions including desmoid fibromatosis, solitary fibrous tumor, dermatofibrosarcoma protuberans and nodular fasciitis may exhibit some histologic overlap with LGFMS, attention to low power architecture may be particularly helpful in differentiating these tumors. Desmoid fibromatosis is composed of infiltrative long sweeping fascicles of spindled cells, spanning an entire 10 × microscopic field. Solitary fibrous tumors consist of ovoid to spindle cells in short nondescript fascicles, often admixed with stromal collagen and a prominent branching and hyalinized vasculature. Dermatofibrosarcoma protuberans are highly infiltrative dermal-based neoplasms with a characteristic storiform architecture. Both solitary fibrous tumor and dermatofibrosarcoma protuberans are diffusely positive for CD34, a marker typically negative in LGFMS. The former also shows nuclear STAT6 immunoreactivity. Histologic examination of nodular fasciitis reveals a loose arrangement of myofibroblasts with areas of microcystic degeneration. Typically, a combination of immunohistochemical stains including MUC4 should result in appropriate classification.
Due to their deceptively bland cytology, LGFMS may also mimic benign nerve sheath lesions such as neurofibroma, perineurioma and solitary encapsulated neuroma. Neurofibromas consist of a mixed population of nerve sheath elements such as S100/SOX10 positive Schwann cells, fibroblasts and perineurial cells, while perineuriomas are composed solely of EMA positive perineurial cells. Solitary encapsulated neuromas are benign cutaneous nerve sheath lesions with a nodular architecture and predilection for the face. While neurofibromas, perineuriomas, solitary encapsulated neuromas and LGFMS may show some degree of EMA positivity, the perineurial component of nerve sheath tumors exhibit long thin cytoplasmic processes. Furthermore, nerve sheath tumors are MUC4 negative and generally lack the discrete alternating myxoid and hyalinized zones of LGFMS [2].
In the head and neck region, the differential of a spindle cell lesion with cytologic atypia includes spindle cell squamous cell carcinoma. Pathologists should be aware that LGFMS may occasionally express EMA. Thorough examination of the overlying epithelium for dysplasia or carcinoma in situ may provide helpful clues to the diagnosis. Additionally, as the majority of patients in our series were adolescents or young adults, caution should be exercised when considering the diagnosis of LGFMS in the elderly population. In cases where carcinoma is a consideration, multiple keratins as well as fluorescence in situ hybridization for FUS rearrangement, can be employed. MUC4, however, is not useful in this scenario as salivary gland carcinomas and squamous cell carcinomas of the head and neck commonly express MUC4 [39, 40].
Two cases in our series contained foci resembling Sclerosing epithelioid fibrosarcoma (SEF) (hybrid LGFMS/SEF). Foci with SEF-like morphology are composed of clusters and cords of epithelioid cells deposited in a densely hyalinized stroma. In addition to carcinoma, this morphology may raise the possibility of other entities including ossifying fibromyxoid tumor, sclerosing rhabdomyosarcoma and osteosarcoma. Fortunately, the majority of hybrid LGFMS/SEF appear to have similar immunophenotypic and genetic profiles to LGFMS showing MUC4 expression and FUS-CREB3L2 fusions [11].
Finally, the differential diagnosis of LGFMS includes very recently described spindle cell mesenchymal tumors with PRRX1-NCOA1/2 fusions [41, 42]. At low power PRRX1-NCOA1/2 fibroblastic tumors show alternating hyalinized and myxoid zones reminiscent of LGFMS. Additionally, the hyalinized regions often contain ovoid cells embedded in a densely eosinophilic stroma, mimicking SEF. Since MUC4 was negative on tested cases in the two small series by Lacambra and Dermawan [41, 42], either MUC4 immunohistochemistry or molecular testing for the PRRX1-NCOA1/2 fusions should help in definitive classification.
The natural history of LGFMS in these unusual anatomical locations appears to be similar to that of their counterparts elsewhere, with low rates of local recurrence and/or metastasis in the first 5 years. Review of the head and neck literature reveals local recurrence in 9 (out of 37) patients in whom follow up was available [5, 6, 14, 16–18, 24, 33]. Additional long-term follow-up is necessary in our cohort to determine whether patients experience late adverse events. As in other locations, LGFMS of the head/neck are easily misdiagnosed as various benign tumors, potentially resulting in less than adequate excision and an increased risk for local recurrence.
In conclusion, we report the clinicopathologic features of 15 cases of head and neck LGFMS, including a singular lesion of the lip. Although the morphologic, immunohistochemical and molecular genetic feature of head/neck LGFMS are identical to those occurring in other locations, the rarity of LGFMS in these locations carries with it significant potential for misdiagnosis, as well as a potentially confusing, site-specific differential diagnosis.
Author Contributions
All authors contributed significantly to qualify as coauthor.
Funding
The authors did not receive support from any organization for the submitted work.
Data Availability
Yes.
Code Availability
Not applicable
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Ethical approval was sought.
Consent to Participate
Consent was obtained in patients in whom follow up was available.
Consent to Publish
Informed consent was obtained from patients for publication.
Financial interests
The authors declare they have no financial interests.
Research Involving Human and Animal Rights
Research didn’t directly involve Human Participants and/or Animals.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sandra Gjorgova Gjeorgjievski, Email: gjorgos@ccf.org.
Karen Fritchie, Email: fritchk@ccf.org.
Judith Jebastin Thangaiah, Email: jebastin.thangaiah.judith@mayo.edu.
Andrew L. Folpe, Email: folpe.andrew@mayo.edu
Nasir Ud Din, Email: nasir.uddin@aku.edu.
References
- 1.Doyle LA, Mertens F. Low-grade fibromyxoid sarcoma. In World Health Organisation Classification of Soft Tissue and Bone Tumours, 5th edn; WHO Classification of Tumours Editorial Board, IARC Press, Lyon, France, 2019; pp. 127–29.
- 2.Doyle LA, Wang WL, Dal Cin P, Lopez-Terrada D, Mertens F, Lazar AJF, Fletcher CDM, Hornick JL. MUC4 is a sensitive and extremely useful marker for sclerosing epithelioid fibrosarcoma: association with FUS gene rearrangement. Am J Surg Pathol. 2012;36:1444–1451. doi: 10.1097/PAS.0b013e3182562bf8. [DOI] [PubMed] [Google Scholar]
- 3.Mertens F, Fletcher CDM, Antonescu CR, Coindre JM, Colecchia M, Domanski HA, Downs-Kelly E, Fisher C, Goldblum JR, et al. Clinicopathologic and molecular genetic characterization of low-grade fibromyxoid sarcoma, and cloning of a novel FUS/CREB3L1 fusion gene. Lab Invest. 2005;85:408–415. doi: 10.1038/labinvest.3700230. [DOI] [PubMed] [Google Scholar]
- 4.Ud Din N, Ahmad Z, Zreik R, Horvai A, Folpe AL, Fritchie F. Abdominopelvic and retroperitoneal low-grade fibromyxoid sarcoma: a clinicopathologic study of 13 cases. Am J Clin Pathol. 2018;29:128–134. doi: 10.1093/ajcp/aqx137. [DOI] [PubMed] [Google Scholar]
- 5.Evans HL. Low-grade fibromyxoid sarcoma. A report of 12 cases. Am J Surg Pathol. 1993;17:595–600. doi: 10.1097/00000478-199306000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Papadimitriou JC, Ord RA, Drachenberg CB. Head and neck fibromyxoid sarcoma: clinicopathological correlation with emphasis on peculiar ultrastructural features related to collagen processing. Ultrastruct Pathol. 1997;21:81–87. doi: 10.3109/01913129709023250. [DOI] [PubMed] [Google Scholar]
- 7.Lane KL, Shannon RJ, Weiss SW. Hyalinizing spindle cell tumor with giant rosettes: a distinctive tumor closely resembling low grade fibromyxoid sarcoma. Am J Surg Pathol. 1997;21:1481–1488. doi: 10.1097/00000478-199712000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Folpe AL, Lane KL, Paull G, Weiss SW. Low-grade fibromyxoid sarcoma and hyalinizing spindle cell tumor with giant rosettes: a clinicopathologic study of 73 cases supporting their identity and assessing the impact of high-grade areas. Am J Surg Pathol. 2000;24:1353–1360. doi: 10.1097/00000478-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Zamecnık M, Michal M. Low-grade fibromyxoid sarcoma: a report of eight cases with histologic, immunohistochemical, and ultrastructural study. Ann Diagn Pathol. 2000;4(4):207–217. doi: 10.1053/adpa.2000.8122. [DOI] [PubMed] [Google Scholar]
- 10.Botev B, Casale M, Vincenzi B, D’Ascanio L, Santini D, Esposito V, Di Marino MP, Baldi A, Rinaldi V, Tonini G, Salvinelli F. A giant sarcoma of the parotid gland: a case report and review of the literature. Vivo. 2006;20:907–910. [PubMed] [Google Scholar]
- 11.Guillou L, Benhattar J, Gengler C, Gallagher G, Ranchère-Vince D, Collin F, Terrier P, Terrier-Lacombe MJ, Leroux A, et al. Translocation-positive low-grade fibromyxoid sarcoma: clinicopathologic and molecular analysis of a series expanding the morphologic spectrum and suggesting potential relationship to sclerosing epithelioid fibrosarcoma: a study from the French Sarcoma Gro. Am J Surg Pathol. 2007;31:1387–1402. doi: 10.1097/PAS.0b013e3180321959. [DOI] [PubMed] [Google Scholar]
- 12.Marglani O, Commons S, Lamothe A. Radiation-induced low grade fibromyxoid sarcoma of the sternocleidomastoid muscle. J Otolaryngol. 2007;36:E73–E75. [PubMed] [Google Scholar]
- 13.Merchant SH. Low grade fibromyxoid sarcoma: a report of a case with epithelioid cell morphology, masquerading as a papillary thyroid carcinoma. Acta Cytol. 2009;53:689–692. doi: 10.1159/000325411. [DOI] [PubMed] [Google Scholar]
- 14.Wu X, Petrovic V, Torode IP, Chow CW. Low grade fibromyxoid sarcoma: problems in the diagnosis and management of a malignant tumour with bland histological appearance. Pathology. 2009;41:155–160. doi: 10.1080/00313020802579276. [DOI] [PubMed] [Google Scholar]
- 15.Tang Z, Zhou ZH, Lv CT, Qin LY, Wang Y, Tian G, Luo XL, Zhu Q, Xu XG. Low-grade fibromyxoid sarcoma: clinical study and case report. J Oral Maxillofac Surg. 2010;68:873–884. doi: 10.1016/j.joms.2009.04.136. [DOI] [PubMed] [Google Scholar]
- 16.Rekhi B, Deshmukh M, Jambhekar NA. Low-grade fibromyxoid sarcoma: a clinicopathologic study of 18 cases, including histopathologic relationship with sclerosing epithelioid fibrosarcoma in a subset of cases. Ann Diagn Pathol. 2011;15:303–311. doi: 10.1016/j.anndiagpath.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Evans HL. Low-grade fibromyxoid sarcoma: a clinicopathologic study of 33 cases with long-term follow-up. Am J Surg Pathol. 2011;35:1450–1462. doi: 10.1097/PAS.0b013e31822b3687. [DOI] [PubMed] [Google Scholar]
- 18.Abe Y, Hashimoto I, Nakanishi H. Recurring facial low-grade fibromyxoid sarcoma in an elderly patient: a case report. J Med Invest. 2012;59:266–269. doi: 10.2152/jmi.59.266. [DOI] [PubMed] [Google Scholar]
- 19.He KF, Jia J, Zhao YF. Low-grade fibromyxoid sarcoma with cystic appearance and osseous metaplasia in the cheek: a case report and review of the literature. J Oral Maxillofac Surg. 2013;71:1143–1150. doi: 10.1016/j.joms.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Maretty-Nielsen K, Baerentzen S, Keller J, Dyrop HB, Safwat A. Low-grade fibromyxoid sarcoma: incidence, treatment strategy of metastases, and clinical significance of the FUS gene. Sarcoma. 2013 doi: 10.1155/2013/256280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong W, Zhang H. Low-grade fibromyxoid sarcoma of the thyroid: a case report. Ann Acad Med Singap. 2013;42:55–56. [PubMed] [Google Scholar]
- 22.Lee EJ, Hwang HJ, Byeon HK, Park HS, Choi HS. A low grade fibromyxoid sarcoma masseter muscle: a case report. J Med Case Rep. 2015;9:176. doi: 10.1186/s13256-015-0658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soma S, Bhat S, Shetty SK. Low grade fibromyxoid sarcoma of the palate: a case report. J Clin Diagn Res. 2015;9:XD01_2. doi: 10.7860/JCDR/2015/14670.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mastoraki A, Strigkos T, Tatakis FP, Christophi A, Smyrniotis V. Recurrent low-grade fibromyxoid sarcoma of the neck: report of a case and review of the literature. Indian J Surg Oncol. 2015;6:296–299. doi: 10.1007/s13193-015-0429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhuri K, Kasimsetty CR, Lingappa A, Gujjar PV. Low-grade fibromyxoid sarcoma involving the mandible: a diagnostic dilemma. J Oral Maxillofac Pathol. 2016;20:33. doi: 10.4103/0973-029X.185914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spalthoff S, Bredt M, Gellrich NC, Jehn P. A rare pathology: low-grade fibromyxoid sarcoma of the maxilla. J Oral Maxillofac Surg. 2016;74(219):e1–10. doi: 10.1016/j.joms.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Tatari MM, Elhariti L, Abou-elfadl M, Abada RL, Rouadi S, Roubal M, Mahtar M. Low grade fibromyxoid sarcoma of the neck: a case report. Ann Clin Case Rep. 2016;1:1056. [Google Scholar]
- 28.Cowan ML, Thompson LD, Leon ME, Bishop JA. Low-grade fibromyxoid sarcoma of the head and neck: a clinicopathologic series and review of the literature. Head Neck Pathol. 2016;10:161–166. doi: 10.1007/s12105-015-0647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao R, Honavar SG, Mulay K, Reddy VA. Primary orbital low-grade fibromyxoid sarcoma – A case report. Indian J Ophthalmol. 2019;67:568–570. doi: 10.4103/ijo.IJO_353_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumari K, Thota R, Chaudhary HL, Sharma MC, Thakar A, Singh G. Low-grade fibromyxoid sarcoma of the external auditory canal: a rare pathology and unusual location. Head Neck Pathol. 2019 doi: 10.1007/s12105019010304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pellini R, De Virgilio A, Petruzzi G, Pichi B, Mercante G, Malvezzi L, Vidiri A, Spriano G. Low-grade fibromyxoid sarcoma of the tongue: a rare nosological entity. Otorinolaringologia. 2019;69:188–191. doi: 10.23736/S0392-6621.18.02205-1. [DOI] [Google Scholar]
- 32.Kanato T, Kalyani S, Lailyang T, Santosh D, Rebecca T, Charai H. Low grade fibromyxoid sarcoma in oral cavity: a rare case report. Indian J Otolaryngol Head Neck Surg. 2019;71(Suppl1):25–26. doi: 10.1007/s12070-015-0946-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chetverikova E, Kasenõmm P. Low-grade fibromyxoid sarcoma of the lateral skull base: presentation of two cases. Case Rep Otolaryngol. 2019;2019:7917040. doi: 10.1155/2019/7917040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park JM, Lim HR, Kim JH, Dong Lee DH. Giant low-grade fibromyxoid sarcoma in the neck. Korean J Otorhinolaryngol Head Neck Surg. 2020;63:432–435. doi: 10.3342/kjorl-hns.2019.00857. [DOI] [Google Scholar]
- 35.Koucky V, Kalferta D, Novakovab DK, Plzaka J. Low-grade fibromyxoid sarcoma of the maxillary sinus. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2020 doi: 10.5507/bp.2020.032. [DOI] [PubMed] [Google Scholar]
- 36.Toro C, Costa P, Vecchio GM, Magro G. Low-grade fibromyxoid sarcoma of the parapharyngeal space: a case report and review of the literature. Oral Maxillofac Surg Cases. 2020;6:100152. doi: 10.1016/j.omsc.2020.100152. [DOI] [Google Scholar]
- 37.Deewani H, Suhail A, Uddin N. Low grade fibromyxoid sarcoma of the parapharyngeal space- An unusual location. BMJ Case Rep. 2021;14(5):e237083. doi: 10.1136/bcr-2020-237083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doblan A. Low-grade fibromyxoid sarcomas with the maxillary sinus localization: a case report and review of the literature. Indian J Otolaryngol Head Neck Surg. 2021 doi: 10.1007/s12070-021-02562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taverna C, Baněčková M, Lorenzon M, Palomba A, Franchi A, Skalova A, Agaimy A. MUC4 is a valuable marker for distinguishing secretory carcinoma of the salivary glands from its mimics. Histopathology. 2020 doi: 10.1111/his.14251. [DOI] [PubMed] [Google Scholar]
- 40.Weed DT, Gomez-Fernandez C, Bonfante E, Lee TD, Pacheco J, Carvajal ME, Goodwin WJ, Carraway KL. MUC4 (sialomucin complex) expression in salivary gland tumors and squamous cell carcinoma of the upper aerodigestive tract. Otolaryngol Head Neck Surg. 2001;124(2):127–141. doi: 10.1067/mhn.2001.112575. [DOI] [PubMed] [Google Scholar]
- 41.Lacambra MD, Weinreb I, Demicco EG, Chow C, Sung YS, Swanson D, To KF, Wong KC, Antonescu CR, Dickson BC. PRRX-NCOA1/2 rearrangement characterizes a distinctive fibroblastic neoplasm. Genes Chromosomes Cancer. 2019;58:705–712. doi: 10.1002/gcc.22762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dermawan JK, Azzato EM, Thangaiah JJ, Gjorgova-Gjeorgievski S, Rubin BP, Folpe AL, Agaimy A, Fritchi KJ. PRRX1-NCOA1 rearranged fibroblastic tumor: clinicopathologic, immunohistochemical and molecular genetic study of six cases of a potentially under-recognized, distinctive mesenchymal tumor. Histopathology. 2021 doi: 10.1111/his.14454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Yes.
Not applicable