Abstract
Prostate-specific membrane antigen (PSMA) is a transmembrane glycoprotein that is overexpressed in the prostate gland and prostate cancer. PSMA has been recently used in positron emission tomography/computed tomography (PET/CT) imaging and targeted alpha-radiation therapy (TAT) for prostate cancer. Recently, the tubarial gland, a type of minor salivary gland that is described as a new organ situated in the pharynx, is reported to express PMSA. Here, we studied the expression of PSMA in common benign and malignant salivary gland tumors. We performed immunohistochemistry for PSMA in 55 salivary gland tumors comprising 10 pleomorphic adenomas, 10 Warthin tumors, 9 basal cell adenomas, 9 adenoid cystic carcinomas, 9 mucoepidermoid carcinomas, and 8 salivary duct carcinomas. PSMA was expressed in 97% of benign tumors and 77% of malignant tumors. Moreover, PSMA was expressed in 59% of normal salivary glands adjacent to the tumor. PSMA is relatively expressed in salivary gland tumors and salivary glands. Therefore, salivary gland neoplasm, and normal salivary gland, possibly demonstrate the accumulation of PSMA in PET/CT. Thus, we need to monitor the side effects in the salivary glands during TAT for prostate cancer.
Keywords: Salivary gland neoplasms, PSMA, Immunohistochemistry, Positron emission tomography, Mucoepidermoid carcinoma, Salivary duct carcinoma
Introduction
A majority of the patients with prostate cancer generally show an excellent prognosis, but some patients suffer from nodal and distant metastases [1]. Positron emission tomography/computed tomography (PET/CT) using agents targeting prostate-specific membrane antigen (PSMA) has been recently established (PSMA PET/CT) and used as a tool for detecting metastatic lesions or disease relapse [2]. PMSA is a transmembrane glycoprotein comprising the extracellular domain, transmembrane region, and intracellular domain (Fig. 1) [2, 3]. It is expressed on both the non-neoplastic prostatic epithelial cells and prostate cancer cells and is a highly sensitive and specific immunohistochemical marker for prostate cancer [4]. Furthermore, radiotherapy involving alpha rays, i.e., targeted alpha therapy (TAT) utilizing PMSA, has been developed for the treatment of metastatic castration-resistant prostate cancer (mCRPCa) [2]. In this therapy, PMSA-expressing cancer cells are destroyed by alpha rays.
Fig. 1.
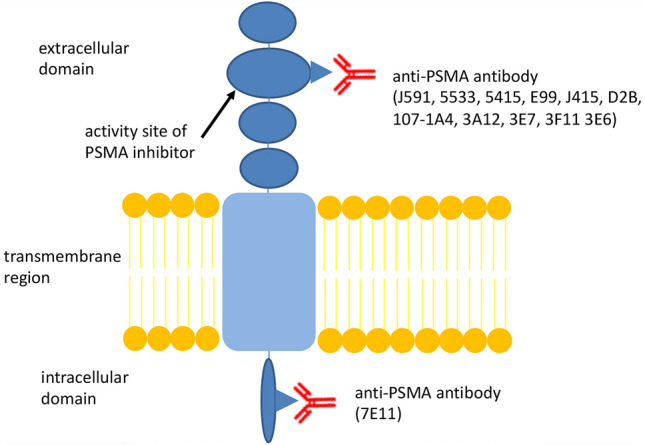
The schema of prostate-specific membrane antigen (PSMA) receptor
However, the tubarial gland, a type of minor salivary gland that is described by Valstar et al. as a new organ situated in the pharynx, is reported to express PMSA, and thus, is at risk for radiotherapy [5]. Therefore, this study investigated whether the salivary gland tumors and normal salivary glands express PMSA.
Materials and Methods
We collected 55 salivary gland tumors, including 43 non-neoplastic salivary glands adjacent to the tumors, resected at the Oita University Hospital between January 2010 and March 2021. There were 29 benign tumors: 10 pleomorphic adenomas (PA), 10 Warthin tumors (WT), and 9 basal cell adenomas (BCA); and 26 malignant tumors: 9 adenoid cystic carcinomas (ACC), 9 mucoepidermoid carcinomas (MC), and 8 salivary duct carcinomas (SDC) (Table 1). The non-neoplastic salivary glands were obtained from 28 parotid glands, 3 submandibular glands, and 13 minor salivary glands of the oral cavity. Formalin-fixed paraffin-embedded tissue blocks showing representative histology were selected for each case and cut at a thickness of 4 µm. Immunohistochemical staining was performed manually. Briefly, the sections were deparaffinized in xylene and rehydrated in graded alcohol. Endogenous peroxidase activity was abolished by incubation with 3% hydrogen peroxide for 20 min at room temperature. Antigens were retrieved by autoclaving in a pH 9.0 citrate buffer. The slides were then incubated with anti-PSMA antibody (anti-PSMA antibody, clone 3E6, DAKO, Denmark, Cat. No. M3620) for 2 h at room temperature. The immunoreaction was visualized using a streptavidin-labeled biotin peroxidase complex system (Nichirei, Tokyo, Japan). Reactivity was scored using a three-graded scoring of colorimetric intensity and population of positive cells. For intensity scoring, strong staining and no staining were scored as 2 + and 0, respectively. Weak staining was scored as 1 + . In population scoring, positive staining of 2 + was detected in > 50% of the cells and 1 + in 49% to 10% of the cells. The specimen with no staining was scored as 0. The case in which the product of the intensity score and population score was > 2 was defined as PSMA-positive. The study was approved by the institutional ethics committee and review board of the Oita University, Japan (Approval Number: 2096).
Table 1.
Tumors, tumor location, and prostate-specific membrane antigen (PSMA) positivity
| Tumor | Location | Case number | PSMA positive case (%) | Matched cases of the background salivary gland (%) |
|---|---|---|---|---|
| PA | Parotid gland | 7 | 7 (100) | 3 (43) |
| Submandibular gland | 2 | 2 (100) | 2 (100) | |
| Soft palate | 1 | 1 (100) | 1 (100) | |
| WT | Parotid gland | 10 | 10 (100) | 1 (10) |
| BCA | Parotid gland | 9 | 8 (89) | 6 (75) |
| ACC | Tongue | 3 | 2 (67) | 1 (50) |
| Oral floor | 2 | 2 (100) | 2 (100) | |
| Parotid gland | 1 | 1 (100) | 0 (0) | |
| Submandibular gland | 1 | 1 (100) | 0 (0) | |
| Buccal mucosa | 1 | 1 (100) | 1 (100) | |
| Soft palate | 1 | 1 (100) | 1 (100) | |
| MC | Parotid gland | 3 | 2 (67) | 1 (50) |
| Gingiva | 3 | 2 (67) | 1 (50) | |
| Soft palate | 1 | 1 (100) | 1 (100) | |
| Hard palate | 1 | 1 (100) | 1 (100) | |
| Tongue | 1 | 1 (100) | 1 (100) | |
| SDC | Parotid gland | 7 | 5 (71) | 3 (60) |
| Submandibular gland | 1 | 0 (0) | 0 (0) | |
| Total | 55 | 48 (87) | 26 (54) |
PA pleomorphic adenoma, WT Warthin tumor, BCA basal cell adenoma, ACC adenoid cystic carcinoma, MC mucoepidermoid carcinoma, SDC salivary duct carcinoma
Results
The tumors and locations are summarized in Table 1. The results of PSMA immunostaining are indicated in Tables 2 and 3, and Figs. 2, 3 and 4. Eighty-seven percent of the tumors were PSMA-positive. The positive ratios for PA and WT were the highest (100% each), followed by those for BCA, ACC, and MC (89%, 89%, and 78%, respectively). SDC had a relatively low positive ratio (67%). PSMA expression was detected in both luminal and myoepithelial cells in most of the cases; however, myoepithelial cells in PA and BCA had stronger staining than luminal cells (Figs. 2, 3, and 4). The proportion of tumors with a 2 + population score was as follows: PA, 50%; WT, 100%; BCA, 56%; ACC, 33%; MC, 33%; and SDC, 50% (Table 3). Considering the contrast effect for PSMA-PET/CT, we evaluated the multiplication of the intensity and population scores (Table 4). The higher the score, the greater the contrast effect. The highest score of 4, which was product of the intensity score 2 + and population score 2 + , was observed in 20% of PA, 56% of BCA, 22% of ACC, 33% of MC, and 50% of SDC (Table 4). PA showed multifocal positive staining, but the area of chondroid differentiation was weakly positive or negative. All WT showed a diffusely positive, faint reaction.
Table 2.
Prostate-specific membrane antigen (PSMA) intensity score in tumors
| Intensity score | PA (%) | WT (%) | BCA (%) | ACC (%) | MC (%) | SDC (%) |
|---|---|---|---|---|---|---|
| 2 | 5 (50) | 0 (0) | 6 (75) | 3 (33) | 4 (44) | 4 (50) |
| 1 | 5 (50) | 10 (100) | 2 (25) | 5 (56) | 3 (33) | 1 (13) |
| 0 | 0 (0) | 0 (0) | 1 (11) | 1 (11) | 2 (22) | 3 (38) |
PA pleomorphic adenoma, WT Warthin tumor, BCA basal cell adenoma, ACC adenoid cystic carcinoma, MC mucoepidermoid carcinoma, SDC salivary duct carcinoma
Table 3.
Prostate-specific membrane antigen (PSMA) area score for tumors
| Area score | PA (%) | WT (%) | BCA (%) | ACC (%) | MC (%) | SDC (%) |
|---|---|---|---|---|---|---|
| 2 | 5 (50 | 10 (100) | 5 (56) | 3 (33) | 3 (33) | 4 (50) |
| 1 | 5 (50) | 0 (0) | 3 (33) | 5 (56) | 4 (44) | 1 (13) |
| 0 | 0 (0) | 0 (0) | 1 (11) | 1 (11) | 2 (22) | 3 (38) |
PA pleomorphic adenoma, WT Warthin tumor, BCA basal cell adenoma, ACC adenoid cystic carcinoma, MC mucoepidermoid carcinoma, SDC salivary duct carcinoma
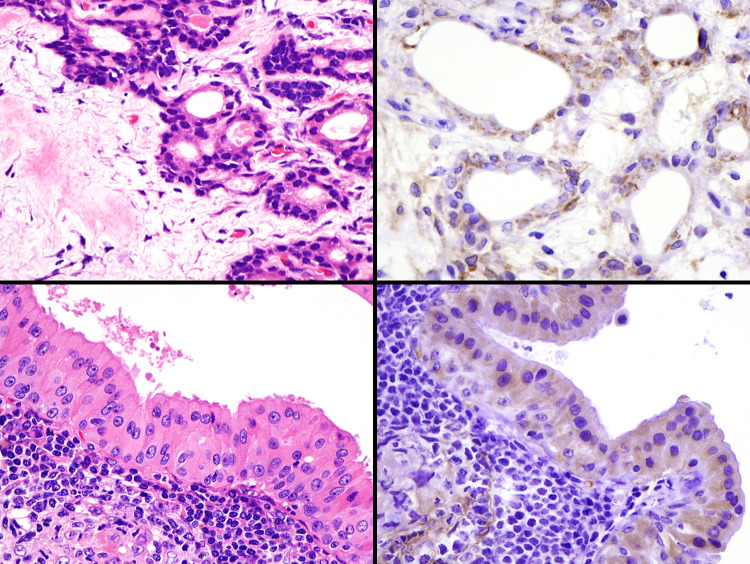
Fig. 2.
Immunohistochemical findings of prostate-specific membrane antigen (PSMA) in pleomorphic adenoma (PA, upper) and Warthin tumor (WT, lower). Many cases show positive staining for myoepithelial and luminal cells in PA. All WTs show diffusely positive, but faint, staining
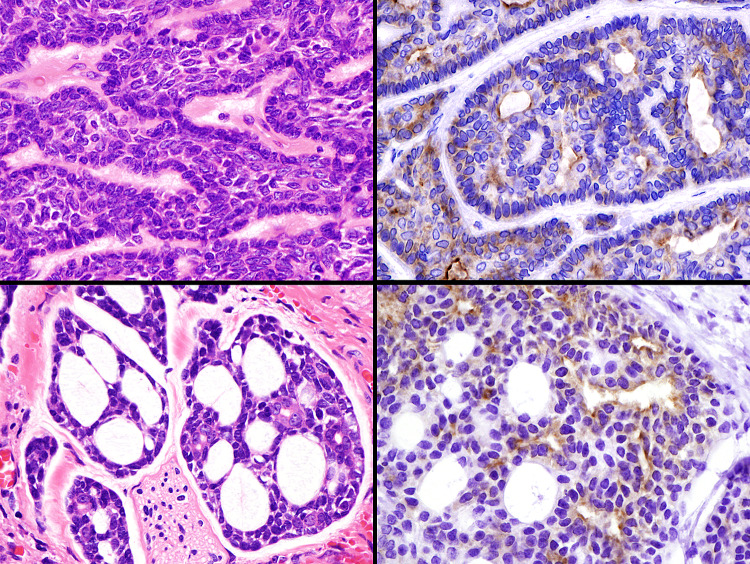
Fig. 3.
Immunohistochemical findings of prostate-specific membrane antigen (PSMA) in basal cell adenoma (upper) and adenoid cystic carcinoma (lower). Many cases show positive staining for myoepithelial and luminal cells
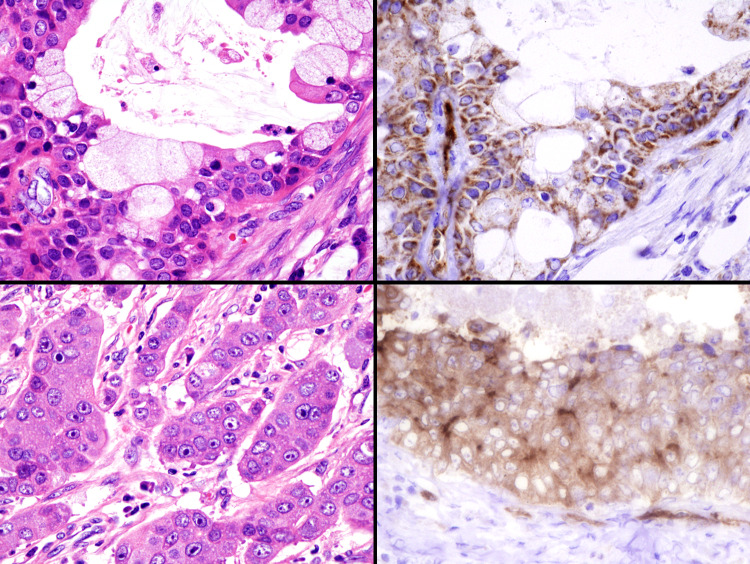
Fig. 4.
Immunohistochemical findings of prostate-specific membrane antigen (PSMA) in mucoepidermoid carcinoma (upper) and salivary duct carcinoma (lower). Many cases show positive staining for tumor cells
Table 4.
Prostate-specific membrane antigen (PSMA) intensity × area score for tumors
| Intensity x Area | PA (%) | WT (%) | BCA (%) | ACC (%) | MC (%) | SDC (%) |
|---|---|---|---|---|---|---|
| 4 | 2 (20) | 0 (0) | 5 (56) | 2 (22) | 3 (33) | 4 (50) |
| 2 | 6 (60) | 10 (100) | 1 (13) | 2 (22) | 1 (11) | 0 (0) |
| 1 | 2 (20) | 0 (0) | 2 (25) | 4 (44) | 3 (33) | 1 (13) |
| 0 | 0 (0) | 0 (0) | 1 (11) | 1 (11) | 2 (22) | 3 (38) |
PA pleomorphic adenoma, WT Warthin tumor, BCA basal cell adenoma, ACC adenoid cystic carcinoma, MC mucoepidermoid carcinoma, SDC; salivary duct carcinoma
The positive ratio of the non-neoplastic area varied (Table 5, Fig. 5). The minor salivary gland tended to show a high percentage, while major salivary gland showed a lower percentage. The positive ratio of tumor and adjacent non-neoplastic salivary gland was 60% (6/10), 10% (1/10), 75% (6/8), 63% (5/8), 71% (5/7), and 60% (3/5) for PA, WT, BCA, ACC, MC, and SDC, respectively”. (Tables 1 and 5). The proportion of WT, a benign tumor that mainly developed in the parotid gland (serous gland), was significantly low.
Table 5.
Positivity of prostate-specific membrane antigen (PSMA) in the normal salivary glands
| Location | PSMA + | Total | Positivity (%) | No data |
|---|---|---|---|---|
| Parotid gland | 14 | 33 | 42 | 9 |
| Submandibular gland | 2 | 3 | 67 | 1 |
| Tongue | 2 | 3 | 67 | 1 |
| Soft palate | 3 | 3 | 100 | 0 |
| Gingiva | 1 | 2 | 50 | 0 |
| Oral floor | 2 | 2 | 100 | 0 |
| Hard palate | 1 | 1 | 100 | 0 |
| Buccal mucosa | 1 | 1 | 100 | 0 |
| 26 | 48 | 59 |
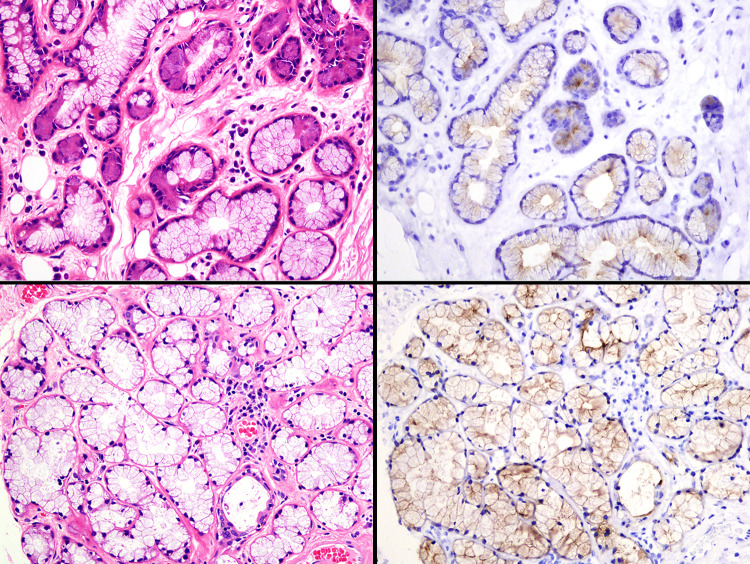
Fig. 5.
Immunohistochemical findings of prostate-specific membrane antigen (PSMA) in the normal salivary glands: submandibular gland (upper) and sublingual gland (lower). Many cases show positive staining for mucinous gland cells
Discussion
The tubarial gland, recently discovered by Valstar et al., is histopathologically considered to be a seromucinous gland [5]. This newly identified minor salivary gland showed positive accumulation in PSMA PET/CT scan, and PSMA expression in this organ was identified immunohistologically [5]. Valstar et al. also described the positivity of the parotid and submandibular glands in PSMA PET/CT scans [5]. But there are few reports of it, and it might be premature to define it as a new organ [6]. The tumors arising in these salivary glands are expected to show PSMA expression, but there are only a few reports describing the expression of PSMA in the salivary gland tumors, except for ACC [7]. In contrast, positive accumulation in PSMA PET/CT scan has been reported in a wide variety of tumors, including adenocarcinoma of the gastrointestinal tract [8–11], hepatocellular carcinoma [12], thyroid cancer [13, 14], and renal cancer [15]. In this study, we evaluated the expression of PSMA in the benign and malignant salivary gland tumors and tumor-adjacent normal salivary glands using immunohistochemistry. Expression of PSMA was frequently detected in both benign and malignant tumors, while only poorly in the normal salivary glands, especially major salivary glands. Cases in which PSMA was expressed in both tumor and normal salivary glands were limited. These results suggest that the salivary gland tumors can be detected by PSMA PET/CT scan. The tumors with high intensity scores and high population scores are expected to show more amplified signals in PSMA PET/CT scans, but it is unclear whether immunohistochemical results concur with those of PSMA PET/CT scan. This is because the antibody used in this study (3E6) was not used as the contrast agent ligand for PSMA PET/CT scan (Fig. 1). However, the target of 3E6 is possibly similar to that of 3E7, which has been used in the PSMA PET/CT scan, and the relationship between immunohistochemistry using 3E6 and PSMA PET/CT scan in prostatic adenocarcinoma has been reported. Therefore, PSMA PET/CT scans are likely to be used in the diagnosis of salivary gland tumors [16].
Further, PSMA has attracted attention in radioligand therapy or TAT, and in imaging to detect early metastasis in the past few years [17]. Moreover, TAT is expected to be used as a therapy against mCRPCa, because it is a lethal form of the disease. Prostate cancers have been treated with a high dose-rate remote after-loading system (RALS) since some time. For mCRPCa, the treatment response has been predicted to TAT involving radiolabeled PSMA inhibitors, such as a significant delay in growth or DNA double-stranded breaks in tumors. We demonstrated the expression of PSMA in benign and malignant salivary gland tumors and the normal salivary glands, along with TAT that is effective in these tumors. As the normal salivary glands express PSMA, the function of the salivary glands may be affected, and dysphagia or xerostomia may be caused by TAT with PSMA [5]. These side effects should be considered when patients present with these symptoms.
In conclusion, we studied the immunohistochemical reactivity of PSMA for the common benign and malignant salivary gland tumors, along with analyses of the major and minor salivary glands adjacent to the tumors. Several benign (28 cases, 97%) and malignant tumors (20 cases, 77%) tested positive for PSMA, and the normal salivary glands (26 cases, 59%) were also positive. Therefore, salivary gland tumors have the potential to demonstrate accumulation of PSMA PET/CT, especially BCA, ACC, MC, and SDC. Moreover, we need to pay attention to the side effects of dysphagia and xerostomia as the radiolabeled PSMA inhibitors used in TAT for mCRPCa may be effective in the normal salivary glands.
Author Contributions
HN summarized this paper. YK, TK, HK, and TD checked all research progress and the manuscript.
Funding
Not applicable
Data Availability
Data available on request from the corresponding author.
Declarations
Conflict of interest
Not applicable
Ethical Approval
The study is approved by the institutional ethics committee (approval number: 2096).
Consent to Participate
The study is retrospective and the patriciate’s consent is obtained through opt-out.
Consent for Publication
Approve.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Donswijk ML, van Leeuwen PJ, Vegt E, Cheung Z, Heijmink SWTPJ, van der Poel HG, et al. Clinical impact of PSMA PET/CT in primary prostate cancer compared to conventional nodal and distant staging: a retrospective single center study. BMC Cancer. 2020;20:723. doi: 10.1186/s12885-020-07192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tateishi U. Prostate-specific membrane antigen (PSMA)-ligand positron emission tomography and radioligand therapy (RLT) of prostate cancer. Jpn J Clin Oncol. 2020;50:349–356. doi: 10.1093/jjco/hyaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czerwińska M, Bilewicz A, Kruszewski M, Wegierek-Ciuk A, Lankoff A. Targeted radionuclide therapy of prostate cancer-from basic research to clinical perspectives. Molecules. 2020;25:1743. doi: 10.3390/molecules25071743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oh WJ, Chung AM, Kim JS, Han JH, Hong SH, Lee JY, et al. Differential immunohistochemical profiles for distinguishing prostate carcinoma and urothelial carcinoma. J Pathol Transl Med. 2016;50:345–354. doi: 10.4132/jptm.2016.06.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valstar MH, de Bakker BS, Steenbakkers RJHM, de Jong KH, Smit LA, Klein Nulent TJW, et al. The tubarial salivary glands: a potential new organ at risk for radiotherapy. Radiother Oncol. 2021;154:292–298. doi: 10.1016/j.radonc.2020.09.034. [DOI] [PubMed] [Google Scholar]
- 6.Iwanaga J, Ibaragi S, Nakano K, Takeshita Y, Tubbs RS. No convincing evidence for the presence of tubarial salivary glands: a letter to the editor regarding “The tubarial salivary glands: a potential new organ at risk for radiotherapy”. Radiother Oncol. 2021;154:321–322. doi: 10.1016/j.radonc.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Klein Nulent TJW, van Es RJJ, Krijger GC, de Bree R, Willems SM, de Keizer B. Prostate-specific membrane antigen PET imaging and immunohistochemistry in adenoid cystic carcinoma- a preliminary analysis. Eur J Nucl Med Mol Imaging. 2017;44:1614–1621. doi: 10.1007/s00259-017-3737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malik D, Kumar R, Mittal BR, Singh H, Bhattacharya A, Sood A, et al. 68Ga-labelled PSMA (prostate specific membrane antigen) expression in signet-ring cell gastric carcinoma. Eur J Nucl Med Mol Imaging. 2018;45:1276–1277. doi: 10.1007/s00259-018-3993-4. [DOI] [PubMed] [Google Scholar]
- 9.Arçay A, Eiber M, Langbein T. Incidental finding of colon carcinoma related to high uptake in 18F-PSMA-1007 PET. Clin Nucl Med. 2020;45:561–562. doi: 10.1097/RLU.0000000000003081. [DOI] [PubMed] [Google Scholar]
- 10.Soeda F, Watabe T, Kato H, Uemura M, Nonomura N. Duodenal adenocarcinoma mimicking metastasis of prostate cancer on 18F-prostate-specific membrane Antigen-1007 PET/CT. Clin Nucl Med. 2021;46:49–51. doi: 10.1097/RLU.0000000000003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sirtl S, Todica A, Ilhan H, Zorniak M, Bartenstein P, Mayerle J. Incidental finding of a PSMA-positive pancreatic cancer in a patient suffering from a metastasized PSMA-positive prostate cancer. Diagnostics (Basel) 2021;11:129. doi: 10.3390/diagnostics11010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuyumcu S, Has-Simsek D, Iliaz R, Sanli Y, Buyukkaya F, Akyuz F, et al. Evidence of prostate-specific membrane antigen expression in hepatocellular carcinoma using 68Ga-PSMA PET/CT. Clin Nucl Med. 2019;44:702–706. doi: 10.1097/RLU.0000000000002701. [DOI] [PubMed] [Google Scholar]
- 13.Bertagna F, Albano D, Giovanella L, Bonacina M, Durmo R, Giubbini R, et al. 68Ga-PSMA PET thyroid incidentalomas. Hormones (Athens) 2019;18:145–149. doi: 10.1007/s42000-019-00106-8. [DOI] [PubMed] [Google Scholar]
- 14.Arora S, Damle NA, Parida GK, Singhal A, Nalli H, Dattagupta S, et al. Recurrent medullary thyroid carcinoma on 68Ga-prostate-specific membrane antigen PET/CT: Exploring new theranostic avenues. Clin Nucl Med. 2018;43:359–360. doi: 10.1097/RLU.0000000000002010. [DOI] [PubMed] [Google Scholar]
- 15.Ahn T, Roberts MJ, Abduljabar A, Joshi A, Perera M, Rhee H, et al. A review of prostate-specific membrane antigen (PSMA) positron emission tomography (PET) in renal cell carcinoma (RCC) Mol Imaging Biol. 2019;21:799–807. doi: 10.1007/s11307-018-01307-0. [DOI] [PubMed] [Google Scholar]
- 16.Kuten J, Fahoum I, Savin Z, Shamni O, Gitstein G, Hershkovitz D, et al. Head-to-head comparison of 68Ga-PSMA-11 with 18F-PSMA-1007 PET/CT in staging prostate cancer using histopathology and immunohistochemical analysis as a reference standard. J Nucl Med. 2020;61:527–532. doi: 10.2967/jnumed.119.234187. [DOI] [PubMed] [Google Scholar]
- 17.Chakravarty R, Siamof CM, Dash A, Cai W. Targeted α-therapy of prostate cancer using radiolabeled PSMA inhibitors: a game changer in nuclear medicine. Am J Nucl Med Mol Imaging. 2018;8:247–267. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the corresponding author.