Abstract
Juvenile xanthogranuloma (JXG) is the most common form of non-Langerhans cell histiocytosis and oral mucosal involvement is exceedingly rare. Histiocytic disorders harbor activating mutations in MAPK pathway, including the report of BRAF V600E in JXG of extracutaneous site. However, no information is available for oral JXG. Herein, the clinicopathological and immunohistochemical features of five new oral JXG were evaluated in conjunction with literature review. Also, we assessed the BRAF V600E in oral samples. Five oral JXG were retrieved from pathology archives. Morphological and immunohistochemical analyses were performed. The BRAF V600E status was determined with TaqMan allele-specific qPCR. The series comprised of three female and two male patients, most of them adults, with a median age of 39 years (range 13–68 years). Clinically, the lesions appeared as asymptomatic solitary nodules, measuring until 2.5 cm, with more incident to the buccal mucosa. Morphologically, most of the cases presented classical histological features of JXG, with histiocytic cells consistent with the non-Langerhans cell immunophenotype. BRAF V600E was not detected in the cases tested. This is the first and largest published series of oral JXG affecting adults and a Brazilian population. The molecular pathogenesis of oral JXG remains unknown. Clinicians and pathologists must recognize JXG to avoid misdiagnoses with oral benign or malignant lesions.
Keywords: Juvenile xanthogranuloma, non-Langerhans cell histiocytosis, Histiocytic disorders, Oral cavity, BRAF V600E, Mutation
Introduction
Non-Langerhans cell histiocytosis (non-LCH) constitute a group of disorders infiltrated by histiocytes that do not meet the immunophenotype of Langerhans cells, i.e., expression of CD1a, S-100 and detection of Birbeck granules [1, 2]. Juvenile xanthogranuloma (JXG) is the most common form of non-LCH of childhood that mainly occurs in the cutaneous sites of normolipemic infants and children, rarely affecting adults [1, 2].
JXG rarely affects the oral cavity, with less than 40 cases reported in the English‐language literature [3]. From these, the oral "adult-type" xanthogranulomas are still more rare [3]. Morphologically, JXG are characterized by the presence of numerous foamy histiocytes exhibiting varying degrees of cytoplasmic vacuolization and Touton-type multinucleated giant cells, a feature of JXG [3–6]. Microscopic variants have been described [5, 7, 8], and may represent a challenging diagnosis, even for experienced pathologists [3, 5].
The etiology of oral JXG is uncertain, although it is suggested that this entity has a reactive nature and self-limiting behavior [4, 5]. The molecular pathogenesis of JXG is also little understood [9]. Some studies have investigated the genetic basis of cutaneous and extracutaneous JXG [9–14], however, no data is available for oral lesions. Histiocytic disorders have been associated with activating mutations in MAPK pathway genes [9–14], including the report of BRAF V600E in JXG of extracutaneous site [9, 14]. However, the presence or not of BRAF V600E mutation in oral lesions is unknown.
In this study, we report the clinicopathological and immunohistochemical features of five new cases of oral JXG, in conjunction with a literature review. We also investigated the BRAF V600E status in the current cases.
Materials and Methods
Samples
Five oral JXG cases were recovered from two Brazilian oral pathology services: Federal University of Rio de Janeiro (n = 4), and Federal University of Minas Gerais (n = 1). Clinical and sociodemographic data were collected from the medical records. Hematoxylin and eosin-stained sections were obtained from each case. The morphological features of the five included cases were re-evaluated by the oral pathologists included in the study.
Immunohistochemistry Analysis
Immunohistochemistry analyses were performed by the streptavidin–biotin peroxidase complex technique on formalin-fixed paraffin-embedded tissue, using standard protocols for the following antibodies: CD68 (clone PG‐M1, dilution 1:500), CD163 (clone PG-M, dilution 1:500), factor XIIIa (clone E980.1, dilution 1:300), CD10 (clone 56C6, dilution 1:500), S-100 protein (polyclonal, dilution 1:10,000), and Ki-67 (clone MIB1, dilution 1:100). All antibodies were obtained from Dako (Glostrup, Denmark). Internal positive controls and negative controls were considered in all reactions.
BRAF V600E Analysis
BRAF V600E mutation detection was performed in all five cases. Genomic DNA was isolated from paraffin blocks using a kit (Qiamp DNA FFPE Tissue Kit; Qiagen, Hilden, Germany) and DNA quantification was performed by spectrophotometry (NanoDrop 2000; Thermo Fisher Scientific, Wilmington, USA). The BRAF V600E mutation was assessed by allele-specific qPCR using TaqMan probes BRAF_476_mu and BRAF_rf (Applied Biosystems, Foster City, USA). Reactions were run on the StepOne Real-Time PCR System (Applied Biosystems, Waltham, Massachusetts, USA), and the mutation status was determined by the Mutation Detector Software (Life Technologies Corporation, Carlsbad, USA).
Literature Review
All papers included in the literature review were case reports, case series, or retrospective studies of patients with JXG in the oral cavity published in the English language with sufficient clinical and histopathological data for a definitive diagnosis. The following information was collected when available: year of publication, number of reported cases, sociodemographic data of patients, clinical characteristics of the lesion, time of evolution, treatment performed, recurrence, and follow-up period. Histopathological and immunohistochemical data were also extracted.
Descriptive and quantitative analyses were performed using the Statistical Package for the Social Sciences for Windows 20.0 (SPSS, Inc., Chicago, IL, USA). Continuous variables were expressed by median and range (minimum and maximum value). Categorical variables were expressed as the absolute number of cases and percentage values.
Results
Current Cases
Five cases of oral JXG were retrieved. Figures 1 and 2 illustrate the main clinicopathological findings in cases 2 and 4. The series comprised of three females (60.0%) and two males (40.0%), with a median age of 39 years (range 13–68 years) and a 1.5:1 female-to-male ratio. The majority of patients were Caucasian (n = 3, 50.0%). The buccal mucosa was the most affected site (n = 3 cases; 60.0%) followed by the tongue (n = 1, 20.0%) and the lip (n = 1, 20.0%). All cases presented clinically as solitary, well-circumscribed sessile nodules with smooth surfaces of durations varying from 1 to 12 months (median 4.5 months) and measuring from 1.0 to 2.5 cm (median 1.5 cm). The color varied between normochromic (n = 3, 60.0%), reddish (n = 1, 20.0%), and yellow (n = 1, 20.0%) (Figs. 1A and 2A). Three patients were asymptomatic (60.0%), and pain was related by two (40.0%). Cutaneous lesions were not detected. The clinical differential diagnoses included lipoma, pleomorphic adenoma, and traumatic fibroma. All lesions were treated through simple excision, with no data available for follow-up.
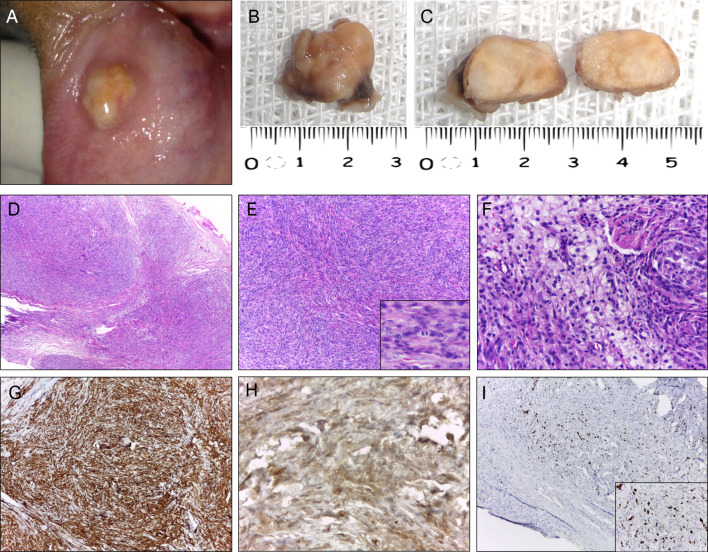
Fig. 1.
Clinicopathologic aspects of classic juvenile xanthogranuloma (case 4). A Clinical aspect of the lesion showing a pedunculated, pink nodule in the tip of the tongue. B, C Macroscopic aspect of surgical specimen displaying grayish color and homogenous yellow cut surfaces. D Low power photomicrograph showing a dense proliferation of histiocytic mononuclear cells (hematoxylin–eosin stain, original magnification ×100). E Note the multinucleated giant cell characterized by an arc of nuclei toward the outer membrane (hematoxylin–eosin stain, original magnification ×400). F. Mononuclear and giant cells showing strong and diffuse cytoplasmic positivity for CD63 (IHC, original magnification ×200; inset ×400) and G CD163 (IHC, original magnification ×200; inset ×400). H Focal positivity for Factor XIIIa (IHC, original magnification ×400). I Ki-67 (MIB-1) labeling index was approximately 5% (IHC, original magnification ×200; inset ×400)
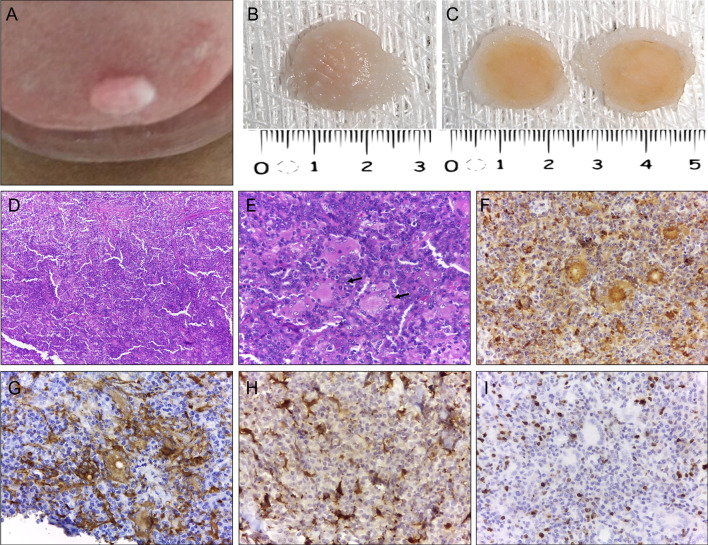
Fig. 2.
Clinicopathologic aspects of non-lipidized juvenile xanthogranuloma (case 2). A Clinical aspect of the lesion showing a sessile, yellowish nodule in the lower labial mucosa. The underlying mucosa was intact. B, C Macroscopic aspect of surgical specimen displaying typical yellowish‐brown color and homogenous grayish-yellow cut surfaces. D Low power photomicrograph showing a well-circumscribed nodule composed predominantly of spindle cells in a multilobular pattern (hematoxylin–eosin stain, original magnification ×100). E Note the solid proliferation of spindle cells arranged in fascicular and storiform growth pattern and a few mitosis (hematoxylin–eosin stain, original magnification ×200; inset ×400). F Detail of epithelioid histiocytes showing marked cytoplasmic vacuolization (hematoxylin–eosin stain, original magnification ×400) and G Note strong and diffuse cytoplasmic positivity for CD63 (IHC, original magnification ×200; inset ×400) and H Factor XIIIa (IHC, original magnification ×400) I Numerous tumor cells positive for Ki-67 (IHC, original magnification ×200; inset ×400)
Gross examination of oral JXG included well-defined soft tissue fragments of yellowish to brownish coloration, firm in consistency, with smooth yellowish-gray cut surfaces (Figs. 1B, C, 2B, C). Morphologically, 80% (4/5) presented as the classical form of JXG and were characterized by a diffuse, nonencapsulated, lymphohistiocytic proliferation in a dense connective tissue (Fig. 1D). The mononuclear component exhibited spindle to elongated cells with indistinct cytoplasmic borders and round to ovoid nuclei, containing dense chromatin and small nucleoli. Multinucleated cells with nuclei arranged around a central eosinophilic cytoplasm and pale, foamy cytoplasm on the periphery (Touton-type giant cells) were also observed (Fig. 1E). Small lymphocytes, plasma cells, and occasional eosinophils were scattered throughout the lesion. Necrosis was absent in all cases. One case (case 2) (Fig. 2) lacked the presence of xanthomatous cells and was classified as a non-lipidized variant of JXG, which displayed a compact proliferation of spindle and epithelioid mononuclear cells in a fibrous stroma. Mononuclear cells were arranged in a storiform pattern and exhibited eosinophilic cytoplasm, irregular or round nucleus with vesicular chromatin, nucleoli generally inconspicuous or small, and brisk mitotic activity (Fig. 2D, E). There were few Touton-type multinucleated giant cells. Also, focal areas showed a background with scattered small lymphocytes, plasma cells, and clusters of foamy and epithelioid histiocytes (Fig. 2F).
All current cases were submitted for immunohistochemical analysis and positivity to CD68 was verified in all. Other immunomarkers investigated were CD163 (3/5), factor XIIIa (5/5), S-100 (5/5), CD10 (1/5) and Ki-67 (5/5). In all five cases, the mononuclear and multinucleated cells expressed strong and diffuse cytoplasmic expression for CD68 (Figs. 1F and 2G). Histiocytic and Touton giant cells (Fig. 1G) were also positive for CD163 (3/3), while CD10 positivity (1/1) was restricted to the mononuclear component. Most cases (4/5) presented focal positivity for factor XIIIa (Fig. 1H). However, the non-lipidized variant showed strong and diffuse positivity for factor XIIIa (Fig. 2H). All cases were negative for protein S-100. In most cases (4/5), the Ki-67 labeling index was low (< 5%) (Fig. 1I); however, case 2 (Fig. 2I) had a relatively higher rate of about 25% and 10% in the superficial and deep regions, respectively.
Considering the BRAF status, in all five cases investigated, the BRAF V600E mutation was absent.
Literature Review
A total of 29 publications in the English language reporting 37 documented cases of oral JXG were selected through electronic search.
Table 1 provides an overview of the data reported in the literature (1974–2021) and compares them with the five new cases reported in the present study. Overall, of the 37 published oral JXG cases, 25 (67.6%) cases occurred in males and 12 (32.4%) in females (male-to-female ratio, 2.1:1). The affected individuals' median age was 9 years (ranging from congenital to 64 years). Individuals in the first and second decades of life were most affected (n = 20, 54.1%, and n = 9, 20.3%, respectively). Most cases occurred in the gingiva (n = 11, 29.8%), followed by the tongue (n = 10, 27.0%), and the palate (n = 8, 21.6%). Other anatomical sites included the lips, buccal mucosa, alveolar ridge, and anterior mandibular vestibule. Most of cases presented as yellowish-brown nodules (n = 14, 53.9%), often asymptomatic (n = 22, 88.0%). Nevertheless, pain was mentioned in three cases (12%). Three cases (8.1%) presented concomitant skin lesions, and none reported visceral involvement. The size of the lesions ranged from 0.3 to 5.0 cm (largest diameter), with a median of 1.4 cm. The duration time of the lesions varied from 3 days to 24 months (median 4 months). In addition, one congenital case was reported.
Table 1.
Comparison of clinical and demographic findings of the literature review with the current series of oral juvenile xanthogranuloma
| Variable | Literature cases | References | Current cases | Total |
|---|---|---|---|---|
| Sex | ||||
| Male | 25 (67.6%) | [3–8, 16, 17, 20, 21, 23, 24, 26, 27, 29, 32, 34, 35, 37, 38] | 2 (40%) | 27 (64.3%) |
| Female | 12 (32.4%) | [6, 8, 18, 19, 22, 25, 28, 30, 31, 33, 36] | 3 (60%) | 15 (35.7%) |
| M:F ratio | 2.1:1 | 1:1.5 | 1.8:1 | |
| Age group (years) | ||||
| 0–9 | 20 (54.1%) | [3, 6, 8, 19, 20, 22, 26, 28, 30, 33–36, 38] | – | 20 (47.6%) |
| 10–19 | 9 (24.3%) | [6, 8, 18, 24, 31, 32, 37] | 1 (20%) | 10 (23.8%) |
| 20–29 | – | – | 1 (20%) | 1 (2.4%) |
| 30–39 | 4 (10.8%) | [16, 17, 21, 27] | 1 (20%) | 5 (11.9%) |
| 40–49 | 1 (2.7%) | [30] | – | 1 (2.4%) |
| 50–59 | 1 (2.7%) | [4] | 1 (20%) | 2 (4.8%) |
| 60–69 | 2 (5.4%) | [7, 29] | 1 (20%) | 3 (7.1%) |
| Median | 9 | 39 | 10.5 | |
| Range | 0–64 | 13–68 | 0–68 | |
| Anatomic location | ||||
| Gingiva | 11 (29.8%) | [4, 8, 18–22, 36, 37] | – | 11 (26.2%) |
| Tongue | 10 (27%) | [2, 8, 25–31] | 1 (20%) | 11 (26.2%) |
| Palate | 8 (21.6%) | [28, 33, 35–38] | – | 8 (19%) |
| Lip | 3 (8.1%) | [5, 6, 34] | 1 (20%) | 4 (9.5%) |
| Buccal mucosa | 3 (8.1%) | [8, 16, 17] | 3 (60%) | 6 (14.3%) |
| Alveolar ridge mucosa | 1 (2.7%) | [7] | – | 1 (2.4%) |
| Anterior mandibular vestibule | 1 (2.7%) | [32] | – | 1 (2.4%) |
| Symptomatology | ||||
| Asymptomatic | 22 (88%) | [3–8, 17, 19, 21–23, 25, 27, 29, 32, 34–36] | 3 (60%) | 25 (83.3%) |
| Pain | 3 (12%) | [26, 29, 31] | 2 (40%) | 5 (16.7%) |
| NI | 12 (32.43%) | [6, 18, 20, 24, 26, 28, 33, 37, 38] | – | 12 (28.57%) |
| Size (cm) | ||||
| Median | 1.4 | [3–6, 16, 17, 20, 21, 23–29, 31–38] | 1.5 | 1.5 |
| Range | 0.3–5 | 1.0–2.5 | 0.3–5.0 | |
| NI | 10 (27.0%) | [7, 8, 18, 19, 22, 30] | – | 11(26.19%) |
| Color | ||||
| Yellowish-brown | 14 (53.9%) | [3, 4, 6–8, 18–20, 24, 26, 32–34] | 1 (20%) | 15 (48.4%) |
| Reddish-pink | 6 (23.1%) | [5, 17, 22, 23, 30, 37] | 1 (20%) | 7 (22.6%) |
| Normal color | 4 (15.4%) | [17, 21, 29, 31] | 3 (60%) | 7 (22.6%) |
| Purple | 1 (3.8%) | [36] | – | 1 (3.2%) |
| White | 1 (3.8%) | [24] | – | 1 (3.2%) |
| NI | 11 (29.72%) | [8, 16, 24, 25, 27, 28, 38] | – | 11 (26.19%) |
| Skin lesions | ||||
| Yes | 3 (8.1%) | [20, 26, 36] | – | 3 (7.1%) |
| No | 34 (91.9%) | [3–8, 16–19, 21–25, 27–30, 32–35, 37, 38] | 5 (100%) | 39 (92.9%) |
| Visceral involvement | ||||
| No | 37 (100%) | [3–8, 16–38] | NI | 37 (100%) |
| Treatment | ||||
| Conservative surgical excision | 31 (96.9%) | [3–8, 16–19, 21–23, 25, 27–29, 31–38] | 5 (100%) | 36 (97.3%) |
| Spontaneous regression after incisional biopsy | 1 (3.1%) | [30] | – | 1 (2.7%) |
| NI | 5 (13.51%) | [20, 24, 26] | – | 5 (11.90%) |
| Recurrence | ||||
| Yes | 4 (13.8%) | [8, 19, 24, 35] | – | 4 (11.8%) |
| No | 25 (86.2%) | [3–6, 16–18, 21–23, 25, 28–32, 34, 36–38] | 5 (100%) | 30 (88.2%) |
| NI | 8 (21.62%) | [7, 20, 24, 26, 27, 33, 36] | – | 8 (19.04%) |
NI not informed
Simple excision was the treatment in 31 cases (83.8%); however, five cases (16.12%) lacked this information and one regressed spontaneously after incisional biopsy. The follow-up time was available for 21 cases (56.8%), with a median of 12 months (range 1–84 months). Four (14.8%) cases exhibited recurrence. Macroscopically, six published cases described the color of the specimens as white and yellowish or reddish-brown.
Microscopically, most of the oral JXG (n = 32, 86.5%) showed the classic presentation of histiocytes with cytoplasm that was eosinophilic, vacuolated or xanthomatous, and often accompanied by Touton-type giant cells. However, five cases (13.5%) reported variations in microscopic findings, such as lack of lipidization and Touton giant cells, the presence of a monomorphous population of cells, histiocytes with plasmacytoid appearance, and areas of cytological atypia, elevated mitosis or material resembling amyloid in the stroma.
Of 29 published studies, 20 (68.9%) submitted cases for immunohistochemical analysis. CD-68 (17/20) and S-100 (16/20) were the antibodies most used, followed by Vimentin (8/20), CD1a (8/20) and alpha-1-antitrypsin (6/20). The positivity to CD68 and negativity to CD1a was seen in all the cases tested. The S-100 marker showed focal to diffuse positivity in only three studies. Other markers used to stain the cells of histiocytic/macrophage nature were, lysozyme (5/20,) HAM-56 (3/20) and CD163 (1/20). Another immunomarker also used in the published studies was factor XIIIa and a weak positivity in a focal to diffuse pattern was mentioned.
Discussion
Since JXG was first described [15], only 42 cases (including the current series) were published as arising from the oral cavity [3–8, 16–38]. Similar to the cutaneous JXG [1, 2], most oral cases affect children in the first decade of life. Interestingly, in our five-case series, in contrast to the incidence of this condition in pediatric age range, only one patient was in childhood. This is the first and largest series published affecting adults and a Brazilian population. The term xanthogranuloma appears to be the nomenclature most appropriate for lesions in adulthood [3, 5]; however, the term JXG is still the most recognized.
Although a slight predilection for females in the present series was observed, an overall analysis of oral JXG revealed a two times predominance for males, which was the same gender predilection in cutaneous cases [1, 2]. Regarding location, although occurrence in the buccal mucosa is exceedingly rare [8, 16, 17], this site was the most affected in the present series. The gingiva [3, 4, 18–23] and tongue [8, 24–31], however, are the main sites reported in the literature.
Considering the clinical presentation, oral JXG commonly manifests as a painless submucosal nodule of a yellowish-brown color [3, 4, 7, 8, 18–20, 24, 26, 32–34]. Interestingly, in our cases with a normochromic or reddish covering mucosa, the macroscopic appearance of the lesions after excision showed a yellowish appearance. Of the six published cases describing the macroscopic color of the specimens [4, 16, 21, 33, 35, 36], four reported the same yellowish finding [4, 16, 21, 33]. This finding suggests that this color seems to be a common feature of JXG in the oral cavity. Although oral lesions were usually small [4, 18, 20], cases exceeding 4 cm in the tongue, cheek and palate were reported [16, 31, 37].
Unlike cutaneous JXG [1, 2], oral lesions usually do not regress spontaneously [4, 6]. Therefore, surgical removal is mentioned as necessary, since even these lesions show indolent clinical behavior. Interestingly, only one oral case underwent spontaneous healing after incisional biopsy [30]. The recurrence rate of oral lesions showed to be relatively low and related to incomplete surgical excision.
Oral JXG shares clinical similarity with several benign conditions [4, 6, 23, 37] of different etiologies, including other xanthomas (verruciform xanthoma), true neoplasms (lipoma or granular cell tumor, odontogenic fibroma), developmental lesions (Fordyce granules, lymphoid aggregate, dermoid and epidermoid cysts), reactive/inflammatory conditions (abscess, fibrous hyperplasia, pyogenic granuloma or ossifying fibroma), and manifestations of systemic disease [4–7, 23, 27, 30, 32]. Salivary gland disorders, such as mucocele, pleomorphic adenoma or mucoepidermoid carcinoma, were reported as a differential diagnosis in cases localized in the palate [37, 38]. Therefore, accurate diagnosis requires careful morphological evaluation.
Morphologically, the classic hallmark of JXG is the presence of numerous foam cells accompanied or not by Touton-type giant cells [3, 6–8, 24, 26, 27, 30], as evidenced in our series. Morphological differential diagnosis from the classical pattern includes mucous extravasation phenomena with abundant vacuolated macrophages, foreign body reactions, verruciform xanthoma [6], and Langerhans cell histiocytosis (LCH) [3, 6]. Clinical history and careful histological analysis are sufficient to exclude the first three differential diagnoses; however, the exclusion of LCH could require immunohistochemistry analysis. Histiocytes in JXG are positive for CD68, CD163, antitrypsin, anti-chymotrypsin, and negative for CD1a, S-100 protein, and langerin/CD207, unlike Langerhans cells that are positive for both markers [4, 6, 8, 26, 33]. In addition, eosinophils are common findings in LCH, whereas giant Touton cells are characteristic of JXG, although not exclusive. Moreover, the absence of Birbeck granules in the ultrastructural evaluation helps rule out LCH [8, 32, 34, 35].
On the other hand, the morphologic differential diagnosis of the unusual variants of JXG classified as mononuclear [7], non-lipidized [5], and early JXG [8] is challenging due to the significant overlap of morphological findings with several benign and malignant soft tissue tumors [5], and should be supported by immunohistochemistry to avoid misdiagnosis. The mononuclear variant is characterized by numerous foamy cells exhibiting cytoplasmic vacuolization and the absence of or few multinucleated giant cells. For this reason, it can be misdiagnosed as melanoma, balloon cell nevus, or LCH [3, 5, 7]. In turn, the non-lipidized variant consists of a prominent spindle cell component arranged in a storiform pattern with a relative absence of inflammatory cells, foamy histiocytes, foreign body giant cells, and multinucleated Touton cells, and usually show a high mitotic index [5], similar to case 2. On the other hand, early lesions of JXG are composed of predominantly bland histiocytes exhibiting little or no cytoplasmic vacuolization, and no giant Touton cells. In addition, the stroma can exhibit an eosinophilic and hyalinized appearance similar to amyloid, however, negative for Congo red [8]. Thus, careful clinical and morphological evaluation is essential to ensure a correct diagnosis.
CD-68 [3–8, 14, 17, 22, 23, 26–29, 33, 34, 36] and S-100 [5–7, 17, 23, 26, 27, 29, 34, 35, 38] are the main markers that applied in the JXG cases in the literature. All current cases were investigated and showed positivity to CD68 and negativity to S-100, ruling out LCH. Another histiocytic marker applied in the current cases was CD163, although, in the literature, other markers, such as alpha-1-antitrypsin [4, 6, 8, 33, 34, 38], lysozyme [5, 26, 33–35] and HAM-56 [5, 26, 35], are more often used to display the histiocytic/macrophagic nature of the cells. It is suggested that factor XIIIa immunostaining is associated with the precursor cell of many of the non-LCH, which are more positive in histiocytes in the early stages of maturation. Thus, the pattern of immunopositivity for factor XIIIa in the literature [3–5, 8, 26] is variable from focal to diffuse. In this investigation, it varied from a focal positivity in spindle cells in the classic morphology, to diffuse and strong in the non-lipidized variant, as previously described by other authors [3, 5, 8]. The stain to Ki-67 [3, 5, 6, 26] is not usually applied once most lesions show low index; however, some examples [3, 5], such our case 2, could show higher mitotic index, suggesting the possibility of malignancy. Thus, it should be interpreted with caution in conjunction with clinical features to avoid misdiagnosis.
The molecular pathogenesis of oral JXG is unknown; however, histiocytic disorders have been associated with activating MAPK pathway mutations [10–14]. On the basis that BRAF V600E was reported in another type of non-LCH [10] and in 3/3 JXG of extracutaneous site [9], we hypothesized if oral JXG also share the same mutation. The absence of BRAF V600E in the present series does not rule out that BRAF or other genes associated with the MAPK pathway could be involved in the pathogenesis of oral JXG. Also, the small number of samples evaluated reflect the rarity of the disease in the oral location. Additional studies could elucidate the genetic basis of these oral lesions.
In summary, this is the first and largest published series of oral JXG affecting adults and a Brazilian population. A BRAF series of oral JXG was investigated for the first time and the molecular pathogenesis of oral JXG remains unknown. Clinicians and pathologists must recognize JXG to avoid misdiagnoses with oral benign or malignant lesions.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interest
No conflicts of interest were declared concerning the publication of this article.
Ethical Approval
This study was approved by the local research ethics committee (CAAE 44358821.4.0000.5149) and carried out following the Helsinki Declaration for study involving human subjects.
Informed Consent
The patients provided an informed consent declaration to permit the use of images and medical information and the manuscript is in accordance with the Institutional Ethics committee.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Carolina Peres Mota and John Lennon Silva Cunha have contributed equally to this work.
References
- 1.Weitzman S, Jaffe R. Uncommon histiocytic disorders: the non-Langerhans cell histiocytoses. Pediatr Blood Cancer. 2005;45(3):256–264. doi: 10.1002/pbc.20246. [DOI] [PubMed] [Google Scholar]
- 2.Luder CM, Nordmann TM, Ramelyte E, Mühleisen B, Kerl K, Guenova E, et al. Histiocytosis - cutaneous manifestations of hematopoietic neoplasm and non-neoplastic histiocytic proliferations. J Eur Acad Dermatol Venereol. 2018;32(6):926–934. doi: 10.1111/jdv.14794. [DOI] [PubMed] [Google Scholar]
- 3.Sánchez-Romero C, Cuenca Arriaga AI, Paes de Almeida O, Gutiérrez Cortés E. Oral juvenile xanthogranuloma in a child: clinical, histological and immunohistochemical profile of a rare entity. J Cutan Pathol. 2018;45(7):515–521. doi: 10.1111/cup.13152. [DOI] [PubMed] [Google Scholar]
- 4.Consolaro A, Sant'Ana E, Lawall MA, Consolaro MF, Bacchi CE. Gingival juvenile xanthogranuloma in an adult patient: case report with immunohistochemical analysis and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2009;107(2):246–252. doi: 10.1016/j.tripleo.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 5.Batista AC, Mendonça EF, Arantes Elias LS, Andrade BA, Almeida OP, León JE. Nonlipidized juvenile xanthogranuloma: an unusual variant with a potential diagnostic pitfall. Int J Pediatr Otorhinolaryngol. 2012;76(2):295–299. doi: 10.1016/j.ijporl.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Israel MS, Carlos R, Pires FR. Oral juvenile xanthogranuloma: report of two cases. Pediatr Dent. 2017;39(3):238–240. [PubMed] [Google Scholar]
- 7.Fabrizi G, Massi G. Mononuclear variant of juvenile xanthogranuloma in the oral cavity of an adult patient. Br J Dermatol. 2001;144(4):909–911. doi: 10.1046/j.1365-2133.2001.04161.x. [DOI] [PubMed] [Google Scholar]
- 8.Flaitz C, Allen C, Neville B, Hicks J. Juvenile xanthogranuloma of the oral cavity in children: a clinicopathologic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2002;94(3):345–352. doi: 10.1067/moe.2002.122340. [DOI] [PubMed] [Google Scholar]
- 9.Techavichit P, Sosothikul D, Chaichana T, Teerapakpinyo C, Thorner PS, Shuangshoti S. BRAF V600E mutation in pediatric intracranial and cranial juvenile xanthogranuloma. Hum Pathol. 2017;69:118–122. doi: 10.1016/j.humpath.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 10.Haroche J, Charlotte F, Arnaud L, von Deimling A, Hélias-Rodzewicz Z, Hervier B, et al. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. 2012;120(13):2700–2703. doi: 10.1182/blood-2012-05-430140. [DOI] [PubMed] [Google Scholar]
- 11.Chakraborty R, Hampton OA, Shen X, Simko SJ, Shih A, Abhyankar H, et al. Mutually exclusive recurrent somatic mutations in MAP2K1 and BRAF support a central role for ERK activation in LCH pathogenesis. Blood. 2014;124(19):3007–3015. doi: 10.1182/blood-2014-05-577825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakraborty R, Hampton OA, Abhyankar H, Zinn DJ, Grimes A, Skull B, et al. Activating MAPK1 (ERK2) mutation in an aggressive case of disseminated juvenile xanthogranuloma. Oncotarget. 2017;8(28):46065–46070. doi: 10.18632/oncotarget.17521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoyt BS, Yan S, Linos KD, Momtahen S, Sriharan A, Tran TN, et al. BRAF V600E mutations are not an oncogenic driver of solitary xanthogranuloma and reticulohistiocytoma: testing may be useful in screening for Erdheim-Chester disease. Exp Mol Pathol. 2019;111:104320. doi: 10.1016/j.yexmp.2019.104320. [DOI] [PubMed] [Google Scholar]
- 14.Picarsic J, Pysher T, Zhou H, Fluchel M, Pettit T, Whitehead M, et al. BRAF V600E mutation in juvenile xanthogranuloma family neoplasms of the central nervous system (CNS-JXG): a revised diagnostic algorithm to include pediatric Erdheim-Chester disease. Acta Neuropathol Commun. 2019;7(1):168. doi: 10.1186/s40478-019-0811-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adamson NF. Congenital xanthoma multiplex in a child. Br J Dermatol. 1905;17:222–223. [Google Scholar]
- 16.Costa F, Cian R, Robiony M, Zerman N, Politi M. Unilateral swelling of the cheek. J Oral Maxillofac Surg. 2008;66(2):342–348. doi: 10.1016/j.joms.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 17.Chen CY, Sung CL, Mu-Yen H, Wen-Chen W, Li-Min L, Yuk-Kwan C. An adult juvenile xanthogranuloma in the buccal mucosa. J Dent Sci. 2015;10(3):334–337. doi: 10.1016/j.jds.2013.02.028. [DOI] [Google Scholar]
- 18.Kjaerkeim A, Stokke T. Juvenile xanthogranuloma of the oral cavity. An electron microscopic study. Oral Surg Oral Med Oral Pathol. 1974;38(3):414–425. doi: 10.1016/0030-4220(74)90369-7. [DOI] [PubMed] [Google Scholar]
- 19.Christensen RE, Jr, Hertz RS, Cherrick HM. Intraoral juvenile xanthogranuloma. Oral Surg Oral Med Oral Pathol. 1978;45(4):586–590. doi: 10.1016/0030-4220(78)90040-3. [DOI] [PubMed] [Google Scholar]
- 20.Ossoff RH, Levin DL, Esterly NB, Tucker GF. Intraoral and cutaneous juvenile xanthogranuloma. Ann Otol Rhinol Laryngol. 1980;89(3 Pt 1):268–270. doi: 10.1177/000348948008900317. [DOI] [PubMed] [Google Scholar]
- 21.Takeda Y, Suzuki A, Fujioka Y, Takayama K. Xanthogranuloma of the oral cavity in adult. A case report and review of the literature. Acta Pathol Jpn. 1986;36(10):1565–1570. doi: 10.1111/j.1440-1827.1986.tb02827.x. [DOI] [PubMed] [Google Scholar]
- 22.Vasconcelos FO, Oliveira LA, Naves MD, Castro WH, Gomez RS. Juvenile xanthogranuloma: case report with immunohistochemical identification of early and late cytomegalovirus antigens. J Oral Sci. 2001;43(1):21–25. doi: 10.2334/josnusd.43.21. [DOI] [PubMed] [Google Scholar]
- 23.Kim YK, Han D, Yang WI, Park JH, Cho ES, Kim DW. Gingival juvenile xanthogranuloma. Korean J Oral Maxillofac Pathol. 2019;43(5):203–207. doi: 10.17779/KAOMP.2019.43.5.010. [DOI] [Google Scholar]
- 24.Cohen DM, Brannon RB, Davis LD, Miller AS. Juvenile xanthogranuloma of the oral mucosa. Oral Surg Oral Med Oral Pathol. 1981;52(5):513–523. doi: 10.1016/0030-4220(81)90364-9. [DOI] [PubMed] [Google Scholar]
- 25.Patel AV, Meechan JG, Soames JV. Juvenile xanthogranuloma of the oral cavity: a case report. Int J Paediatr Dent. 1993;3(1):43–45. doi: 10.1111/j.1365-263X.1993.tb00046.x. [DOI] [PubMed] [Google Scholar]
- 26.Sangüeza OP, Salmon JK, White CR, Jr, Beckstead JH. Juvenile xanthogranuloma: a clinical, histopathologic and immunohistochemical study. J Cutan Pathol. 1995;22(4):327–335. doi: 10.1111/j.1600-0560.1995.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 27.Satow SJ, Zee S, Dawson KH, Gown AM, Oda D, Worthington P. Juvenile xanthogranuloma of the tongue. J Am Acad Dermatol. 1995;33(2 Pt 2):376–379. doi: 10.1016/0190-9622(95)91438-2. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro NL, Malis DJ, Charon CC, Billman GF, Kearns DB. Giant juvenile xanthogranuloma of the tongue. Am J Otolaryngol. 1999;20(4):241–244. doi: 10.1016/S0196-0709(99)90007-8. [DOI] [PubMed] [Google Scholar]
- 29.Tanyeri H, Weisenberg E, Friedman M. Juvenile xanthogranuloma of the tongue. Otolaryngol Head Neck Surg. 2000;123(5):641–642. doi: 10.1067/mhn.2000.109661. [DOI] [PubMed] [Google Scholar]
- 30.Villa A, Mariani U, Villa F. Lingual juvenile xanthogranuloma in a woman: a case report. J Med Case Rep. 2011;5:30. doi: 10.1186/1752-1947-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baik FM, Andeen NK, Schmechel SC, Futran ND. A large juvenile xanthogranuloma within the tongue. Otolaryngol Head Neck Surg. 2014;150(2):332–333. doi: 10.1177/0194599813514534. [DOI] [PubMed] [Google Scholar]
- 32.Kwan CY, Min LL, Chung LC, Hang YY. Intraoral juvenile xanthogranuloma. A case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 1996;81(4):450–453. doi: 10.1016/S1079-2104(96)80022-9. [DOI] [PubMed] [Google Scholar]
- 33.Tagawa T, Inui M, Murata M. Palatal juvenile xanthogranuloma. A case report. Int J Oral Maxillofac Surg. 1996;25(6):453–4. doi: 10.1016/S0901-5027(96)80082-8. [DOI] [PubMed] [Google Scholar]
- 34.Shimoyama T, Horie N, Ide F. Juvenile xanthogranuloma of the lip: case report and literature review. J Oral Maxillofac Surg. 2000;58(6):677–679. doi: 10.1016/S0278-2391(00)90167-8. [DOI] [PubMed] [Google Scholar]
- 35.Kawashiri S, Kumagai S, Nakagawa K, Yamamoto E, Imai K. Juvenile xanthogranuloma occurring in the oral cavity: case report and histopathological findings. J Oral Pathol Med. 1997;26(10):484–487. doi: 10.1111/j.1600-0714.1997.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 36.Collins L, Banks R, Robinson M. Juvenile xanthogranuloma: unusual intraoral finding. Br J Oral Maxillofac Surg. 2015;53(1):86–88. doi: 10.1016/j.bjoms.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 37.Dissanayaka WL, Jayasooriya PR, Wickramasuriya G, Dias DK, Tilakaratne WM. Oral juvenile xanthogranuloma: report of two cases and literature review. Oral Surg. 2010;3:22–35. [Google Scholar]
- 38.Palacios J, Rodriguez-Peralto JL, Contreras F. Congenital oral juvenile xanthogranuloma: report of a case. J Oral Maxillofac Surg. 1987;45(8):707–709. doi: 10.1016/0278-2391(87)90314-4. [DOI] [PubMed] [Google Scholar]